Abstract
Background
The mechanical properties of extracellular matrix grafts that are intended to augment or replace soft tissues should be comparable to the native tissue. Such grafts are often used in fiber-reinforced tissue applications that undergo multi-axial loading and therefore knowledge of the anisotropic and nonlinear properties are needed, including the moduli and Poisson's ratio in two orthogonal directions within the plane of the graft. The objective of this study was to measure the tensile mechanical properties of several marketed grafts: Alloderm, Restore, CuffPatch, and OrthADAPT.
Methods
The degree of anisotropy and nonlinearity within each graft was evaluated from uniaxial tensile tests and compared to their native tissue.
Results
The Alloderm graft was anisotropic in both the toe and linear-region of the stress-strain response, was highly nonlinear, and generally had low properties. The Restore and CuffPatch grafts had similar stress-strain responses, were largely isotropic, had a linear-region modulus of 18 MPa, and were nonlinear. OrthADAPT was anisotropic in the linear region (131 vs 47 MPa) and was highly nonlinear. The Poisson ratio for all grafts was between 0.4 and 0.7, except for the parallel orientation of Restore which was greater than 1.0.
Interpretation
Having an informed understanding of how the available grafts perform mechanically will allow for better assessment by the physician for which graft to apply depending upon its application.
Keywords: anisotropy, nonlinearity, tensile mechanics, graft material
Introduction
Extracellular matrix graft materials are indicated in several clinical procedures to reinforce or replace soft-tissue mechanical function. Currently several materials are approved, in clinical trials, or in development (Aurora et al., 2007, Chen et al., 2009). As these grafts are primarily intended to augment or replace the biomechanical function of the soft tissue structure, their mechanical properties are important. These grafts, and certainly their tissue applications, are nonlinear, with a low-stiffness toe-region and a higher stiffness linear-region. Moreover, as grafts are often used in clinical applications requiring repair of fiber-reinforced tissue, such as tendon and ligament, knowledge of the anisotropic and nonlinear properties are needed, including the moduli and Poisson's ratio in two orthogonal directions within the plane of the graft. Recently Derwin and coworkers directly compared the tensile mechanics of four graft materials, reporting elastic moduli an order of magnitude lower than native tendon (Derwin et al., 2006). While providing key subfailure material properties, that study did not measure, Poisson's ratio, anisotropy, or nonlinearity. Therefore, the objective of this study was to measure the tensile mechanical properties of one allograft: Alloderm, and three xenografts: Restore, Cuffpatch, and OrthADAPT marketed for use in soft tissue applications. The degree of anisotropy and nonlinearity within each graft was evaluated to gain an overall understanding of the material properties of grafts under uniaxial tensile testing.
Alloderm (LifeCell Corp., Branchburg, NJ, USA) is an acellular dermal matrix that is derived from skin and processed to remove all cells and antigenic factors. Alloderm is used in bladder reconstruction (Choe et al., 2001, Lemer et al., 1999), facial plastic surgery and reconstruction (Sclafani et al., 2002, Vural et al., 2006), breast reconstruction (Bindingnavele et al., 2007, Zienowicz and Karacaoglu, 2007), and skin grafting (Gore, 2005, Sheridan et al., 1998, Sclafani et al., 2000). GraftJacket is a subset of Alloderm produced by (Life Cell Corp., Branchburg, NJ) where its mechanical properties have been compared to other ECM grafts (Barber and Aziz-Jacobo, 2009, Barber et al., 2006) intended for tendon augmentation. Additionally GraftJacket has been used in both animal models (Lee, 2007, Lee, 2008, Lee, 2004) and human studies (Bond et al., 2008, Burkehead et al., 2007, Dopirak et al., 2007). Restore Orthobiologic Implant (DePuy Orthopaedics, Warsaw, IN, USA) and CuffPatch (Arthrotek, Warsaw, IN, USA) are composed of acellularized porcine small intestine submucosa. These graft materials have potential for use in rotator cuff tendon repair (Derwin et al., 2006, Dejardin et al., 2001). Restore is composed of ten non-crosslinked layers that are chemically disinfected and sterilized; each layer is oriented 20° relative to the other and laminated. CuffPatch is composed of eight layers that are purified, crosslinked with water-soluble carbodiimide, and laminated. The mechanical properties of Restore and CuffPatch have been described (Barber et al., 2006, Derwin et al., 2006, Johnson et al., 2007). In addition, Restore has been studied for use in ligament and tendon augmentation in several animal models (Badylak et al., 1995, Dejardin et al., 2001, Derwin et al., 2004, Perry et al., 2007, Zalavras et al., 2006) and limited human studies (Iannotti et al., 2006, Malcarney et al., 2005, Walton et al., 2007). OrthADAPT (Pegasus Biologics Inc.,Irvine, CA, USA) is composed of collagen from equine pericardium that is sterilized and crosslinked using a technique known as UltiFix, which is a non-glutaraldehyde method that fixes tissue by linking the amine and the carboxyl moieties through amide bonds (Girardot and Girardot, 1996). OrthADAPT is planned for use in ligament and tendon repair (Barber and Aziz-Jacobo, 2009, Johnson et al., 2007) and soft-tissue reinforcement of defects in organ walls (e.g., abdominal and thoracic walls) (Johnson et al., 2007).
Materials and Methods
Four graft materials were evaluated: Alloderm, Restore, CuffPatch, and OrthADAPT. One graft from each type was hydrated at 4°C in 1% phosphate buffered saline 24 hours prior to mechanical testing. Tensile test samples were then prepared by cutting the full-thickness graft to 5 × 30 mm parallel sided samples using a custom-built razor die in two orthogonal directions aligned parallel (n=4-7) and perpendicular (n=4-7) to the graft edges (Figure 1). Post-test strain analysis demonstrated that this geometry had homogeneous mid-substance strain. Thus, while this study cannot provide information on variability within a single graft's material source or manufacturing process, it is designed to address mechanical behaviors (anisotropy, nonlinearity) within a single graft type.
Figure 1.
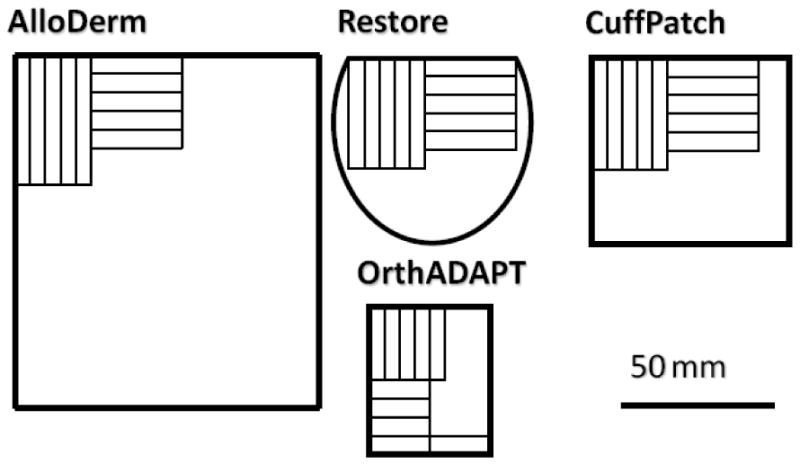
Size and shape of each graft material with the location, size, and orientation of test samples. Five grafts were evaluated: AlloDerm, Restore, CuffPatch, and OrthADAPT.
Following sample preparation, sand paper was glued to the end of each sample for gripping and black enamel markers were applied using an air-brush to provide contrast for optical strain analysis as previously described (Lynch et al., 2003). The sample width and length was determined by the average of three measurements taken with digital calipers, the resulting grip to grip aspect ratio was 3:1. Sample thickness was measured from calibrated images using a dissecting microscope (MZ6; Leica, Wetzlar, Germany), and measured using ImageJ (National Institutes of Health, Bethesda, MD, USA) (Nerurkar et al., 2007).
Mechanical testing was performed in uniaxial tension using an Instron 5542 testing system (Canton, MA, USA). The sample was placed in custom-built grips and tested in a 1% phosphate buffered saline bath according to the following protocol: (1) a nominal tare load corresponding to 50 kPa stress was applied at a strain rate of 0.1% strain/s and held for 5 min; (2) 15 preconditioning cycles to 0.1% strain were applied at a rate of 0.05% strain/s; and (3) a quasi-static constant elongation was applied until failure at a rate of 0.1% strain/s, all strain rates were based on the grip-to-grip specimen length (Nerurkar et al., 2007). Images were acquired at a rate of 5 images/s during elongation to failure using a 1.4 megapixel CCD camera (Basler Vision Technologies; Basler, Germany) and custom-written LabView (National Instruments; Austin, TX, USA) image capture program for strain analysis.
Stress (σ) was calculated as applied force divided by cross-sectional area. The two-dimensional Langrangian strain (E) was calculated from optical images using Vic2D software (O'Connell et al., 2007) (Correlated Solutions, Inc. - Columbia, SC, USA) within a square central region containing approximately 25% of the sample length. The Poisson's ratio was calculated as the ratio of transverse/longitudinal strain (Lynch et al., 2003). An exponential equation was used to fit the stress-strain response σ = A(eBE −1)(Sarver et al., 2003, Yin and Elliott, 2004). The modulus was calculated as the derivative of this exponential equation (ABeBE) in the toe-region (Etoe=0 strain) and in the linear-region (Elinear=18% strain, except for OrthADAPT which was calculated at Elinear=10% strain). Linear-region modulus was calculated at 0.9 times failure strain for samples that failed below 18% strain (n=3 out of 32).
Each graft material was individually evaluated for anisotropy and nonlinearity by performing a two tailed t-test. A graft material was defined as anisotropic if the linear-region modulus was different between parallel and perpendicular orientations. A graft material was defined as nonlinear if the modulus was different between the toe- and linear-region as defined by a two tailed paired t-test. For both of these cases, significance was set at P<0.05 and a trend toward significance was set as P<0.1.
Results
For all graft materials the stress-strain response was well described using the exponential function, σ = A(eBE −1) with an average R2 of 0.99 (Figure 2, n=32). The average toe-region modulus for all materials ranged from 0.59 to 11.54 MPa (Figure 3, Table 1). The linear-region modulus was below 50 MPa for all grafts except the parallel orientation for Alloderm and OrthADAPT, which had a linear-region modulus of 221 and 130 MPa respectively (Figure 4). The Poisson ratio for all grafts was between 0.35 and 0.87, except for one orientation of Restore which was greater than 1.0 (Figure 5).
Figure 2.
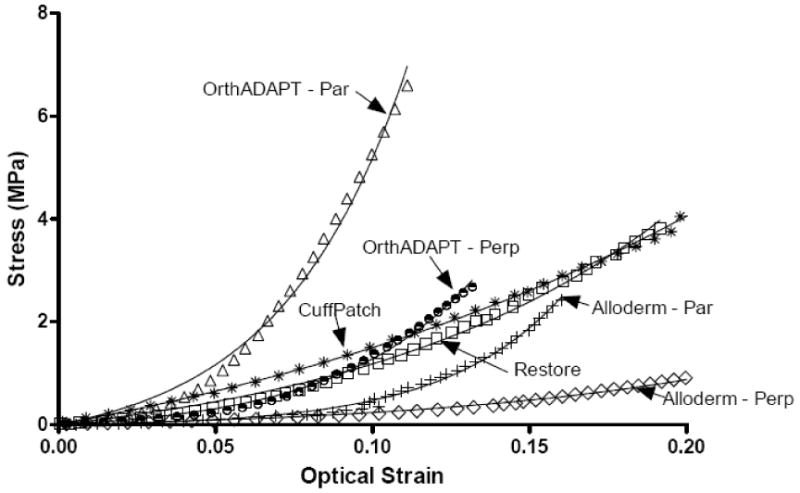
Representative stress-strain response for each graft material. Symbol = experimental data and solid line = model curve fit. Alloderm and OrthADAPT (par)allel and (per)pendicular are displayed as their toe and linear region modulus are statistically different between orientations.
Figure 3.
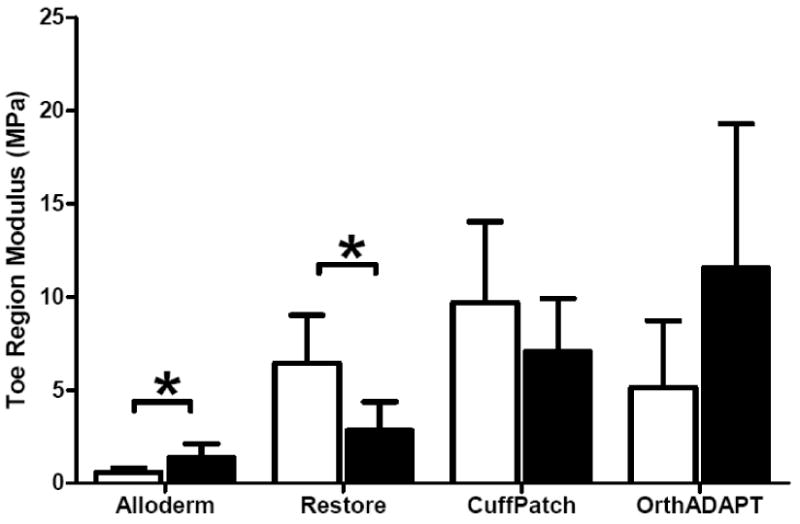
Average toe-region modulus for each graft material, where error bars represent the standard deviation. Samples were aligned in two planar orthogonal orientations described as parallel (black bars) and perpendicular (white bars). Significant differences with orientation denoted by * P<0.05
Table 1.
Average (standard deviation) material property for each graft material. A and B represent curvefit parameters. Samples were aligned in two planar orthogonal directions described as parallel and perpendicular. Statistical analyses performed for toe-region modulus, linear-region modulus and Poisson's ratio within a single graft material, as shown below. Anisotropy: significant differences between parallel and perpendicular orientation denoted by * P≤0.05 and trend by ‡ P≤0.1. Nonlinearity: significant differences between toe-region and linear-region modulus denoted by a P≤0.05 and trend by b P≤0.1.
| Parallel | Perpendicular | ||||
|---|---|---|---|---|---|
| A(MPa) | Alloderm* | 0.02 | (0.01) | 0.12 | (0.07) |
| Restore* | 0.69 | (0.24) | 0.24 | (0.15) | |
| CuffPatch | 3.10 | (3.86) | 0.89 | (0.41) | |
| OrthADAPT | 0.18 | (0.15) | 0.92 | (0.68) | |
| B | Alloderm* | 32.24 | (5.68) | 12.88 | (4.16) |
| Restore | 9.80 | (3.01) | 14.06 | (5.34) | |
| CuffPatch | 7.07 | (4.80) | 8.01 | (1.05) | |
| OrthADAPT* | 32.34 | (7.46) | 15.57 | (6.66) | |
| Toe-region modulus (MPa) at 0% strain | Alloderm* | 0.59a | (0.22) | 1.35a | (0.74) |
| Restore* | 6.44a | (2.58) | 2.82b | (1.54) | |
| CuffPatch | 9.66a | (4.38) | 7.04a | (2.87) | |
| OrthADAPT | 5.13a | (3.60) | 11.54b | (7.76) | |
| Linear-region modulus (MPa) at 18% strain, except OrthADAPT at 10% | Alloderm* | 221.48 | (141.20) | 11.21 | (3.53) |
| Restore | 18.38 | (9.76) | 18.04 | (14.96) | |
| CuffPatch | 35.73 | (21.68) | 30.12 | (12.98) | |
| OrthADAPT | 130.50 | (72.76) | 46.99 | (23.37) | |
| Poisson's ratio | Alloderm | 0.35 | (0.06) | 0.50 | (0.19) |
| Restore* | 0.50 | (0.15) | 1.15 | (0.43) | |
| CuffPatch | 0.65 | (0.11) | 0.65 | (0.09) | |
| OrthADAPT | 0.70 | (0.36) | 0.87 | (0.73) | |
Figure 4.
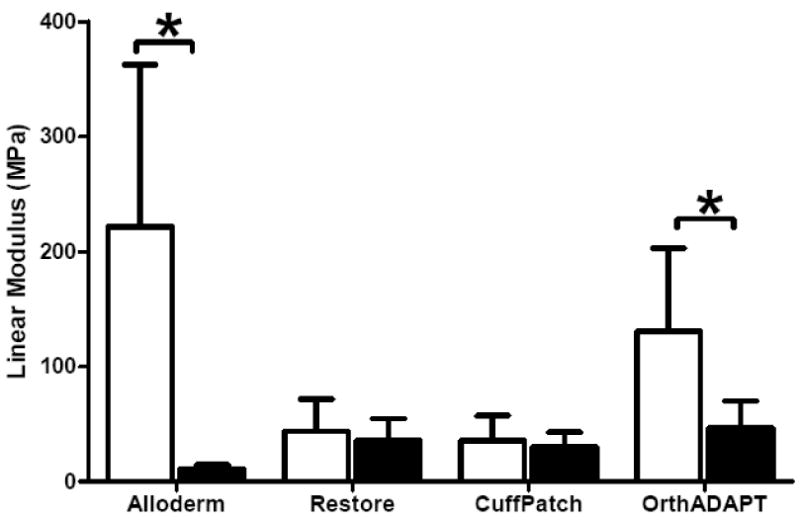
Average linear-region modulus for each graft material, where error bars represent the standard deviation. All samples were aligned in two planar orthogonal orientations described as parallel (black bars) and perpendicular (white bars). Significant differences with orientation denoted by * P<0.05.
Figure 5.
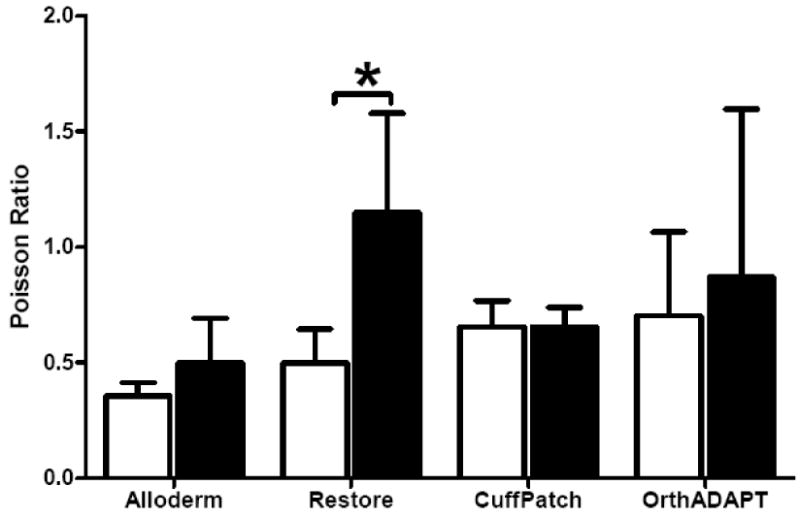
Average Poisson's ratio for each graft material, where error bars represent the standard deviation. Samples were aligned in two planar orthogonal orientations described as parallel (black bars) and perpendicular (white bars). Significant differences with orientation denoted by * P<0.05.
The Alloderm graft was anisotropic in both the toe and linear-region with a 1.3× and 18.8× difference in modulus with orientation (P=0.05 and 0.03). The Alloderm graft was also nonlinear, with a 370× difference between the toe- and linear-region modulus in one orientation and a 7× difference in the other orientation (P<0.05).
The Restore graft exhibited anisotropy within the toe-region with a 1.3× difference in toe-region modulus (P=0.04) between the parallel and perpendicular orientation and a 1.3× difference in the Poisson's ratio (P=0.05). However, there was no significant difference with orientation for the linear-region modulus. The Restore graft displayed non-linearity, as the ratio of the linear- to toe- region modulus was 2.8× and significant in the parallel orientation (P=0.04) and was 6.4× and showed a trend towards significance (P=0.1) in the perpendicular orientation.
The CuffPatch graft was planar isotropic, with no difference in the moduli or Poisson's ratio measured in the parallel and perpendicular orientation (P>0.05). Therefore, the data from both sample orientations were pooled. The CuffPatch graft showed nonlinearity, with a 3× difference between the toe- and linear-region modulus.
The OrthADAPT graft was anisotropic with a 1.8× difference in linear-region moduli between orientations There was no difference in the toe-region moduli or Poisson's ratio measured in the parallel and perpendicular orientation (P>0.05). The OrthADAPT was nonlinear for both orientations, with a 3-24× difference between the toe- and linear-region modulus (significant in the parallel orientation P= 0.008 and trend in the perpendicular orientation P=0.10).
Discussion
This study measured the tensile mechanical properties of the Alloderm, Restore, CuffPatch, and OrthADAPT grafts. Soft tissue grafts are available (or being developed) for a number of applications, yet there are few comprehensive, peer-reviewed, independent measures of their material properties. The majority of available studies are failure tests (Barber et al., 2006, Boguszewski, 2008, Coons and Alan Barber, 2006, Dejardin et al., 2001), which are relevant, but not a suitable indicator of physiological function. In this study, the degree of anisotropy and nonlinearity within each graft was evaluated, which provides critical functional parameters to be compared to the native tissue mechanics when selecting a graft for specific applications. An advantage of this study is applying the same uniaxial tensile test protocol across all materials and using optical analyses to quantify graft strain rather than grip-to-grip strain. The most important limitation is the small sample size, as all test samples were taken from a single fabricated graft. Thus, while this study can determine whether a graft is anisotropic and nonlinear, and can demonstrate general differences between graft materials, it is not intended as a detailed analysis of any one graft and material variability was not evaluated. However, in support of the reported properties as a reasonable representation of the graft population, the linear-region moduli values for three of the grafts studied here were similar to data recently reported by Derwin and coworkers from 6-10 lots for each graft (Derwin et al., 2006).
The Alloderm properties were the lowest of the grafts studied, except for the parallel orientation linear modulus (221 MPa), which although very high also had a large standard deviation. The generally low properties are consistent with the clinical use of this material which is in relatively non- to low-load bearing applications. Alloderm exhibited anisotropy, as the toe-region and linear-region moduli for parallel and perpendicular orientations were significantly different from each other. It is well established that skin exhibits anisotropic properties both in vivo (Finlay, 1969, Gibson et al., 1969) and in vitro (Kenedi RM, 1964, Lanir and Fung, 1974, Ridge RM, 1966). There is no observable preferred fiber orientation in the graft, so the parallel and perpendicular orientations were aligned with the graft edges and do not represent any particular anatomic orientation of this material. However, it is notable that these moduli are within the range of previous studies for Alloderm (11.8(0.6) MPa, (Lemer et al., 1999) and ∼2 MPa (Sclafani et al., 2002)) and are similar to the modulus of Graft Jacket (22.5(5.3) MPa) (Derwin et al., 2006) and (54.7-69.0 MPa)(Barber and Aziz-Jacobo, 2009), which is a subset of Alloderm with different thickness. The Alloderm linear modulus is also on the same order as autologous dermis (3.8 MPa), rectus fascia (5.3 MPa), and vaginal mucosa (26.25 MPa) (Choe et al., 2001). Alloderm was also nonlinear. Its low toe region modulus is indicative of the pronounced elongation at the beginning of loading that has been observed in the literature and supports the recommendation of pre-stretching before implantation (Morgan et al., 2004, Vural et al., 2006).
Restore and CuffPatch are often compared to each other due to similar tissue origins of porcine small intestine submucosa. These grafts are labeled for reinforcement of soft tissues repaired by suture or suture anchors during tendon repair surgery, not replacement. They both had similar linear region moduli between 18-36 MPa. These values are similar to previously reported linear region moduli of 14.1(3.6) and 17(2.8) MPa, respectively, for Restore and CuffPatch (Derwin et al., 2006), and 26.3(14.1) MPa modulus for native small intestine submucosa (Raghavan et al., 2005). Notably, although both Restore and CuffPatch have been approved for use in rotator cuff tendon augmentation, their moduli are much lower than the 84-187 MPa values of human intraspinatus tendon (Halder et al., 2000).
OrthADAPT is anisotropic in the linear-region moduli and had the second highest linear-region modulus and most nonlinearity of the grafts studied. The high modulus and nonlinearity are likely due to the collagen content. These values are similar to previously reported linear modulus of 100.6(36) MPa (Barber and Aziz-Jacobo, 2009). These properties provide support for use of OrthADAPT for rotator cuff tendon repair, as its toe and linear modulus are on the same order of magnitude as human supraspinatus tendon of the rotator cuff (Lake et al., 2009). However, this graft may not work well for tendon repair if the crosslinking treatment results in an adverse biological response (Valentin et al., 2006). In addition, although the properties are similar, it is also important that the entire nonlinear response reflects that of native tissue: OrthADAPT transition strain is 10%, which is higher than native supraspinatus tendon transition strain of 3-6% (Lake et al., 2009). Thus for this and other grafts studied here, pre-straining may be appropriate to match the nonlinear response. Finally, OrthADAPT is anisotropic, which is similar to that of the tendon.
In conclusion, the subfailure material properties were measured for several graft materials. This data provides properties for a general comparison across graft materials, with emphasis on anisotropy and nonlinearity. Fiber reinforced tissues such as tendon and ligament are highly anisotropic and nonlinear; it is important for graft materials to match the functional behaviors of the target biological tissues. The small sample size, necessitated by the difficulty in obtaining material, permits only general comparisons across grafts and is not intended to suggest any graft is superior to another. Indeed, the value of these data is for comparison to the nonlinear and anisotropic properties of the tissue being augmented or replaced by the graft material. Having an informed understanding of how the available grafts perform mechanically will allow for better assessment by the physician for which graft to apply depending upon is application.
Acknowledgments
This study supported by the Penn Center for Musculoskeletal Disorders. Materials provided by Humacyte, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aurora A, Mccarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16:S171–8. doi: 10.1016/j.jse.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Badylak SF, Tullius R, Kokini K, Shelbourne KD, Klootwyk T, Voytik SL, Kraine MR, Simmons C. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res. 1995;29:977–85. doi: 10.1002/jbm.820290809. [DOI] [PubMed] [Google Scholar]
- Barber FA, Aziz-Jacobo J. Biomechanical testing of commercially available soft-tissue augmentation materials. Arthroscopy. 2009;25:1233–9. doi: 10.1016/j.arthro.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Barber FA, Herbert MA, Coons DA. Tendon augmentation grafts: biomechanical failure loads and failure patterns. Arthroscopy. 2006;22:534–8. doi: 10.1016/j.arthro.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Bindingnavele V, Gaon M, Ota KS, Kulber DA, Lee DJ. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007 doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Boguszewski DVD, Bailey NA, Shearn DL, Butler JT. Biomechanical Comparison of Abdominal Wall Hernia Repair Materials. ASME Summer Bioengineering Conference; Marco Island, Florida. 2008. [Google Scholar]
- Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403–409. e1. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Burkehead W, Schiffern S, Krishnan S. Use of GraftJacket as an augmentation for massive rotator cuff tears. Semin Arthroplasty. 2007;18:11–18. [Google Scholar]
- Chen J, Xu J, Wang A, Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices. 2009;6:61–73. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- Choe JM, Kothandapani R, James L, Bowling D. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482–6. doi: 10.1016/s0090-4295(01)01205-5. [DOI] [PubMed] [Google Scholar]
- Coons DA, Alan Barber F. Tendon graft substitutes-rotator cuff patches. Sports Med Arthrosc. 2006;14:185–90. doi: 10.1097/00132585-200609000-00011. [DOI] [PubMed] [Google Scholar]
- Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. Am J Sports Med. 2001;29:175–84. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- Derwin K, Androjna C, Spencer E, Safran O, Bauer TW, Hunt T, Caplan A, Iannotti J. Porcine small intestine submucosa as a flexor tendon graft. Clin Orthop Relat Res. 2004:245–52. doi: 10.1097/01.blo.0000131235.91264.d7. [DOI] [PubMed] [Google Scholar]
- Derwin KA, Baker AR, Spragg RK, Leigh DR, Iannotti JP. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665–72. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- Dopirak R, Bond J, Synder S. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. Int J Shoulder Surg. 2007;1:7–15. [Google Scholar]
- Finlay B. Scanning electron microscopy of the human dermis under uni-axial strain. Biomed Eng. 1969;4:322–7. [PubMed] [Google Scholar]
- Gibson T, Stark H, Evans JH. Directional variation in extensibility of human skin in vivo. J Biomech. 1969;2:201–4. doi: 10.1016/0021-9290(69)90032-3. [DOI] [PubMed] [Google Scholar]
- Girardot JM, Girardot MN. Amide cross-linking: an alternative to glutaraldehyde fixation. J Heart Valve Dis. 1996;5:518–25. [PubMed] [Google Scholar]
- Gore DC. Utility of acellular allograft dermis in the care of elderly burn patients. J Surg Res. 2005;125:37–41. doi: 10.1016/j.jss.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Halder A, Zobitz ME, Schultz F, An KN. Mechanical properties of the posterior rotator cuff. Clin Biomech (Bristol, Avon) 2000;15:456–62. doi: 10.1016/s0268-0033(99)00095-9. [DOI] [PubMed] [Google Scholar]
- Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88:1238–44. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- Johnson W, Inamasu J, Yantzer B, Papangelou C, Guiot B. Comparative in vitro biomechanical evaluation of two soft tissue defect products. J Biomed Mater Res B Appl Biomater. 2007 doi: 10.1002/jbm.b.30816. [DOI] [PubMed] [Google Scholar]
- Kenedi RM, G T, Daly CH, editors. Biomechanics and Related Bio-Engineering Topics. Pergamon Press; Oxford: 1964. [Google Scholar]
- Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile Loading. J Orthop Res. 2009 doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanir Y, Fung YC. Two-dimensional mechanical properties of rabbit skin. II. Experimental results. J Biomech. 1974;7:171–82. doi: 10.1016/0021-9290(74)90058-x. [DOI] [PubMed] [Google Scholar]
- Lee DK. Achilles tendon repair with acellular tissue graft augmentation in neglected ruptures. J Foot Ankle Surg. 2007;46:451–5. doi: 10.1053/j.jfas.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Lee DK. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. J Foot Ankle Surg. 2008;47:8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Lee MS. GraftJacket augmentation of chronic Achilles tendon ruptures. Orthopedics. 2004;27:s151–3. doi: 10.3928/0147-7447-20040102-15. [DOI] [PubMed] [Google Scholar]
- Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497–503. doi: 10.1002/(sici)1520-6777(1999)18:5<497::aid-nau12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Lynch HA, Johannessen W, Wu JP, Jawa A, Elliott DM. Effect of fiber orientation and strain rate on the nonlinear uniaxial tensile material properties of tendon. J Biomech Eng. 2003;125:726–31. doi: 10.1115/1.1614819. [DOI] [PubMed] [Google Scholar]
- Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907–11. doi: 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- Morgan AS, Mciff T, Park DL, Tsue TT, Kriet JD. Biomechanical properties of materials used in static facial suspension. Arch Facial Plast Surg. 2004;6:308–10. doi: 10.1001/archfaci.6.5.308. [DOI] [PubMed] [Google Scholar]
- Nerurkar NL, Elliott DM, Mauck RL. Mechanics of oriented electrospun nanofibrous scaffolds for annulus fibrosus tissue engineering. J Orthop Res. 2007;25:1018–28. doi: 10.1002/jor.20384. [DOI] [PubMed] [Google Scholar]
- O'Connell GD, Johannessen W, Vresilovic EJ, Elliott DM. Human internal disc strains in axial compression measured noninvasively using magnetic resonance imaging. Spine. 2007;32:2860–8. doi: 10.1097/BRS.0b013e31815b75fb. [DOI] [PubMed] [Google Scholar]
- Perry SM, Gupta RR, Van Kleunen J, Ramsey ML, Soslowsky LJ, Glaser DL. Use of small intestine submucosa in a rat model of acute and chronic rotator cuff tear. J Shoulder Elbow Surg. 2007;16:S179–83. doi: 10.1016/j.jse.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Raghavan D, Kropp BP, Lin HK, Zhang Y, Cowan R, Madihally SV. Physical characteristics of small intestinal submucosa scaffolds are location-dependent. J Biomed Mater Res A. 2005;73:90–6. doi: 10.1002/jbm.a.30268. [DOI] [PubMed] [Google Scholar]
- Ridge RM WV. The directional effect of skin -- a Bioengineering study of skin with particular reference to Langer's Lines. Journal of Investigational Dermatology. 1966;46:341–346. [PubMed] [Google Scholar]
- Sarver JJ, Robinson PS, Elliott DM. Methods for quasi-linear viscoelastic modeling of soft tissue: application to incremental stress-relaxation experiments. J Biomech Eng. 2003;125:754–8. doi: 10.1115/1.1615247. [DOI] [PubMed] [Google Scholar]
- Sclafani AP, Mccormick SA, Cocker R. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164–71. doi: 10.1001/archfaci.4.3.164. [DOI] [PubMed] [Google Scholar]
- Sclafani AP, Romo T, 3rd, Jacono AA, Mccormick S, Cocker R, Parker A. Evaluation of acellular dermal graft in sheet (AlloDerm) and injectable (micronized AlloDerm) forms for soft tissue augmentation. Clinical observations and histological analysis. Arch Facial Plast Surg. 2000;2:130–6. doi: 10.1001/archfaci.2.2.130. [DOI] [PubMed] [Google Scholar]
- Sheridan R, Choucair R, Donelan M, Lydon M, Petras L, Tompkins R. Acellular allodermis in burns surgery: 1-year results of a pilot trial. J Burn Care Rehabil. 1998;19:528–30. doi: 10.1097/00004630-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Valentin JE, Badylak JS, Mccabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–86. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- Vural E, Mclaughlin N, Hogue WR, Suva LJ. Comparison of biomechanical properties of alloderm and enduragen as static facial sling biomaterials. Laryngoscope. 2006;116:394–6. doi: 10.1097/01.mlg.0000194691.32551.24. [DOI] [PubMed] [Google Scholar]
- Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am. 2007;89:786–91. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- Yin L, Elliott DM. A biphasic and transversely isotropic mechanical model for tendon: application to mouse tail fascicles in uniaxial tension. J Biomech. 2004;37:907–16. doi: 10.1016/j.jbiomech.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Zalavras CG, Gardocki R, Huang E, Stevanovic M, Hedman T, Tibone J. Reconstruction of large rotator cuff tendon defects with porcine small intestinal submucosa in an animal model. J Shoulder Elbow Surg. 2006;15:224–31. doi: 10.1016/j.jse.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–81. doi: 10.1097/01.prs.0000267340.31742.1. [DOI] [PubMed] [Google Scholar]


