Abstract
Maintenance of genomic stability is critical for all cells. Homologous recombination (HR) pathways promote genome stability using evolutionarily conserved proteins such as RecA, SSB, and RecQ, the E. coli homologue of five human proteins at least three of which suppress genome instability and cancer. A previous report indicated that RecQ promotes the net accumulation in cells of intermolecular HR intermediates (IRIs), a net effect opposite that of the yeast and two human RecQ homologues. Here we extend those conclusions. We demonstrate that cells that lack both UvrD, an inhibitor of RecA-mediated strand exchange, and RecG, a DNA helicase implicated in IRI resolution, are inviable. We show that the uvrD recG cells die a “death-by-recombination” in which IRIs accumulate blocking chromosome segregation. First, their death requires RecA HR protein. Second, the death is accompanied by cytogenetically visible failure to segregate chromosomes. Third, FISH analyses show that the unsegregated chromosomes have completed replication, supporting the hypothesis that unresolved IRIs prevented the segregation. Fourth, we show that RecQ and induction of the SOS response are required for the accumulation of replicated, unsegregated chromosomes and death, as are RecF, RecO, and RecJ. ExoI exonuclease and MutL mismatch-repair protein are partially required. This set of genes is similar but not identical to those that promote death-by-recombination of ΔuvrD Δruv cells. The data support models in which RecQ promotes the net accumulation in cells of IRIs and RecG promotes resolution of IRIs that form via pathways not wholly identical to those that produce the IRIs resolved by RuvABC. This implies that RecG resolves intermediates other than or in addition to standard Holliday junctions resolved by RuvABC. The role of RecQ in net accumulation of IRIs may be shared by one or more of its human homologues.
Keywords: RecQ, genome instability, homologous recombination, SOS response, mismatch repair, replication fork
1. Introduction
The RecQ family of DNA helicases is highly conserved throughout evolution, and many RecQ homologues play roles in preventing genomic instability. Humans have five known homologues of E. coli RecQ. The loss of three of them, BLM, WRN, and RECQ4, are associated respectively with Bloom, Werner and Rothmund-Thomson cancer-predisposition syndromes (reviewed [1]). Although the other two human RecQ homologues, RECQ1 and RECQ5, are not currently associated with specific syndromes, these proteins also inhibit genome instability and tumorigenesis in mammals [2, 3], and so are also probable candidates for human cancer-prevention proteins. How RecQ homologues protect cells from genome instability and resulting cancer susceptibility is an important problem. Though there may be several different cellular processes upon which RecQ homologues impinge, one common one is homologous recombination (HR), which can have both genome stabilizing and destabilizing effects (reviewed [1].)
DNA repair via HR proceeds via a few general steps (reviewed [4, 5]). First various proteins expose single-stranded (ss)DNA in a damaged DNA molecule. This can occur at, e.g., a double-strand break or end, or single-strand gap, and is often associated with problems at a replication fork. Next, exposed ssDNA is coated by RecA or a RecA homologue. RecA-coated ssDNA then engages a duplex DNA molecule, usually a sister chromosome, exchanging strands in a region of base complementarity. This strand exchange links the two molecules in an intermolecular HR intermediate (IRI). Finally, IRIs must be “resolved” to produce separate chromosomes so that repaired chromosomes may then be segregated.
Biochemically, RecQ homologues display activities compatible with roles both in forming IRIs and in their resolution. Sometimes the same protein possesses both kinds of activities. For example, E. coli RecQ can both promote strand invasion by RecA and disrupt RecA-mediated strand-invasion events in vitro [6]. RecQ can also function in a complex with Topoisomerase III and SSB to resolve intermediates formed from converging replication forks in vitro [7]. Similarly, the budding yeast homologue of RecQ, Sgs1, and human BLM can form complexes with Top3 to resolve converging recombination intermediates (double Holliday junctions) [8, 9]. Human RECQ4 displays ATP-dependent strand-annealing activity, but has no known helicase activity [10], but BLM and WRN appear to have both strand annealing and DNA unwinding (opposing) activities in vitro [11].
Given the numerous activities and substrates of RecQ homologues in vitro, it has been informative to understand their overall, dominant or net effects on HR in living cells. These fall into two classes depending on whether they promote net accumulation or net reduction of IRIs in cells. For example, although elegant in vivo studies demonstrate that one role of yeast Sgs1 is to help expose ssDNA at double-strand ends prior to loading of RecA homologue Rad51 [12–14], a role that leads to formation of IRIs, the net or overall effect of Sgs1 is the opposite. Sgs1 promotes net reduction of IRIs in cells. Cells lacking Sgs1 and the Rad51-removal protein Srs2 are inviable, and their inviability requires Rad51 [15]. This implies that they die from excess accumulation of Rad51-promoted IRIs that prevent chromosome segregation. Similarly, human WRN reduces overall IRI levels, in that WRN-deficient cells also die a RAD51-dependent “death by recombination” in which chromosome segregation fails [16, 17]. The death can be averted by production of a bacterial Holliday-junction (IRI) resolvase, indicating excess IRIs as its cause [17]. Also, human BLM protein is thought to reduce IRI levels both because of its biochemical activity in resolving model IRI DNA substrates [18, 19] and also because BLM cells show increased levels of micronuclei [20] and anaphase bridges which are thought to result from chromosome-segregation defects [21].
Whereas Sgs1, WRN and BLM exemplify one paradigm in HR—net removal of IRIs—E. coli RecQ appears to exemplify an opposite paradigm: promotion of net accumulation of IRIs in cells. First, the loss of recQ restored viability to ΔtopB parETs cells. When raised to the restrictive temperature, ΔtopB parETs cells die a death that is accompanied by chromosome-segregation failure, and is caused by the loss of an apparent TopoIII-dependent IRI-resolution pathway redundant with RuvABC [22]. Therefore, the fact that loss of recQ restored viability to, or relieved the inviability of, topB parETs cells implies that the presence of RecQ protein promoted the net accumulation of IRIs in cells (though the data were interpreted otherwise in that paper [22]). Second, cells that lack the demonstrated IRI-resolution system RuvABC and the RecA/IRI-inhibitor protein UvrD die a RecA-dependent “death by recombination” in which unresolved IRIs prevent chromosome segregation [23]. The chromosomes had demonstrably completed replication, implying that their failure to segregate was not from incomplete replication but rather from being joined in unresolved IRIs. Moreover, loss of RecQ allowed Δruv ΔuvrD cell viability, indicating a role for RecQ in net IRI accumulation [23]. This is a second paradigm for RecQ proteins in HR opposite that of Sgs1, WRN and BLM.
We suggested that organisms with multiple RecQ homologues might possess homologues that affect net IRI levels oppositely, some acting like Sgs1, WRN and BLM, and others acting like E. coli RecQ [23]. Such division of labor appears to be the case in Arabidopsis, in which RECQ4B appears to promote HR and the accumulation of IRIs, whereas the closely related RECQ4A promotes resolution of IRIs that accumulate [24].
The strongest demonstration that RecQ acts oppositely of some other RecQ homologues in cells used the special mutant genetic background of cells lacking uvrD and ruvA or ruvB or ruvC Holliday-junction-resolution proteins [23]. In this report, we generalize and extend those conclusions by examining the effect of RecQ in cells that lack uvrD and recG, a different putative Holliday-junction resolution protein.
RecG is a branch-migration helicase [25], the in vivo function of which is less certain than that of the Ruv proteins. RecG is redundant with Ruv in conjugational and transduction HR assays [26], and so was thought to be a Holliday-junction-resolution protein like Ruv. However other functions for RecG in replication-fork management have been suggested such as promoting Holliday-junction formation through replication-fork reversal [27].
The logic of this study is the following. All of recombinational DNA repair proceeds via heteroduplex DNA intermediates often between molecules (IRIs). Because unresolved IRIs kill cells by preventing chromosome segregation, proteins that promote net accumulation of IRIs in cells promote the death of mutants that lack known IRI removal proteins. In this study we identify many proteins responsible for death of IRI-removal-impaired uvrD recG cells. This identifies pathways that lead to net accumulation of IRIs in cells, specifically IRIs normally processed by RecG. This was done previously for cells lacking the uvrD and ruvA or ruvB or ruvC Holliday-junction-resolution proteins [23]. That study produced a list of proteins, including RecQ, that promote net accumulation of IRIs resolved by Ruv. Because RuvABC resolve Holliday junctions (reviewed [28]), the proteins identified lead to net accumulation specifically of IRIs containing Holliday junctions. In this study we demonstrate that uvrD recG cells are also inviable and characterize a large number of proteins that contribute to this inviability. The results confirm, in a different genetic background, that RecQ promotes net accumulation of IRIs and show that this causes “death-by-recombination” of uvrD recG cells. Moreover, the proteins identified that promote inviability of uvrD recG cells are overlapping but not identical with those identified previously as promoting death of uvrD ruv mutants. Therefore, we infer that RecG resolves IRIs not identical to (other than or in addition to) those resolved by Ruv. Moreover, the proteins identified here show the pathways leading to the specific DNA intermediates processed by RecG.
2. Materials and methods
2.1. E. coli strains and methods
All strains and plasmids used in this study are described in Table 1. Strains were created using P1 transduction as described [29] and verified using PCR, sensitivity to ultraviolet light and other phenotypic characteristics. Quantitative transductions were performed using concentrations of phage chosen in which transductants were linearly proportional to P1 particles added and transductants per particle was used to assess transduction efficiency. Transductants were incubated at 32°C on Luria Bertani Herskowitz (LBH) plates containing 50 μg/ml kanamycin (Kan) and 0.5% sodium citrate (Citrate) as per [23]. Colony forming units (cfu) were scored after 24 hours of growth using a Microbiology International ProtoCOL counter.
Table 1.
Escherichia coli K-12 strains and plasmids used in this study.
| Plasmid/Strain | Relevant genotype | Source or reference |
|---|---|---|
| pCP20 | FLP recombinase vector, temperature-sensitive origin, AmpR, CamR | [49] |
| pML104-3 | red+ gam+ under IPTG promoter, temperature-sensitive origin, SpecR, RecA+ | S. Elledge |
| BW26355 | ΔrecA635::FRTKanFRT | CGSC7651a; [49] |
| JW0525 | ΔintD::FRTKanFRT | [50] |
| JW1852 | ΔruvC::FRTKanFRT | [50] |
| JW1993 | ΔxonA::FRTKanFRT | [50] |
| JW2597 | ΔrecN::FRTKanFRT | [50] |
| JW3627 | ΔrecG::FRTKanFRT | [50] |
| JW4128 | ΔmutL::FRTKanFRT | [50] |
| MG1655 | sequenced wild-type E. coli K-12 | [51] |
| N2731 | recG258::Tn10miniKan | [44] |
| RDK1541 | recO1504::Tn5 | [52] |
| RTC0013 | ΔrecB::Kan | [53] |
| RTC0016 | ΔrecG::Kan | [53] |
| SMR6319 | 594 hsdrK− mK+ | [23] |
| SMR8976 | SMR6319 ΔuvrD404::FRTcatFRT | [23] |
| SMR8977 | SMR6319 ΔuvrA402::Gen | [23] |
| SMR8982 | SMR6319 ΔmutL482::FRT | [23] |
| SMR8986 | SMR6319 ΔmutL482::FRT ΔuvrA402::Gen | [23] |
| SMR8987 | ΔrecF1904::FRTcatFRT | [23] |
| SMR8991 | SMR6319 recA200(Ts) | [23] |
| SMR9801 | SMR6319 lexA3(Ind−) | [23] |
| SMR9805 | SMR6319 recAo281 lexA3(Ind−) | [23] |
| SMR9809 | SMR6319 recAo281 | [23] |
| SMR9811 | SMR6319 ΔuvrD404::FRTcatFRT metE163::Tn10 | [23] |
| SMR9812 | SMR6319 ΔuvrD404::FRTcatFRT ΔrecQ1906::FRT metE163::Tn10 | [23] |
| SMR9837 | SMR6319 sulA211 | [23] |
| SMR9847 | SMR6319 ΔrecJ::FRTKanFRT | [23] |
| SMR9932 | MG1655 recG258::Tn10miniKan | MG1655 x P1(N2731) |
| SMR10394 | SMR6319 ΔxonA::FRTKanFRT | SMR6319 x P1(JW1993) |
| SMR10395 | SMR6319 ΔxonA::FRT | SMR10394 x pCP20 |
| SMR10401 | SMR6319 ΔrecG::Kan | SMR6319 x P1(RTC0016) |
| SMR10407 | SMR6319 ΔruvC::FRTKanFRT | SMR6319 x P1(JW1852) |
| SMR10408 | SMR6319 ΔruvC::FRT | SMR10407 x pCP20 |
| SMR10411 | SMR6319 ΔrecF1904::FRTcatFRT | SMR6319 x P1(SMR8987) |
| SMR10416 | SMR6319 ΔruvC::FRT ΔxonA::FRTKanFRT | SMR10408 x P1(JW1993) |
| SMR10417 | SMR6319 ΔruvC::FRT ΔxonA::FRT | SMR10416 x pCP20 |
| SMR10419 | SMR6319 recG258::Tn10miniKan | SMR6319 x P1(SMR9932) |
| SMR10423 | SMR6319 ΔrecF::FRT | SMR10411 x pCP20 |
| SMR10424 | SMR6319 [pML104-3] | SMR6319 x pML104-3 |
| SMR10425 | SMR6319 ΔrecA635::FRTKanFRT | SMR6319 x P1(BW26355) |
| SMR10426 | SMR6319 ΔrecA::FRT | SMR10425 x pCP20 |
| SMR10427 | SMR6319 ΔrecA::FRT [pML104-3] | SMR10426 x pML104-3 |
| SMR10428 | SMR6319 ΔrecA::FRT ΔrecG::Kan [pML104-3] | SMR10427 x P1(RTC0016) |
| SMR10434 | SMR6319 ΔrecJ::FRT | SMR9847 x pCP20 |
| SMR10437 | SMR6319 ΔrecF::FRT recG258::Tn10miniKan | SMR10423 x P1(SMR9932) |
| SMR10438 | SMR6319 ΔxonA::FRT recG258::Tn10miniKan | SMR10395 x P1(SMR9932) |
| SMR10439 | SMR6319 ΔrecJ::FRT recG258::Tn10miniKan | SMR10434 x P1(SMR9932) |
| SMR10440 | SMR6319 lexA3(Ind−) recG258::Tn10miniKan | SMR9801 x P1(SMR9932) |
| SMR10441 | SMR6319 recAo281 lexA3(Ind−) recG258::Tn10miniKan | SMR9805 x P1(SMR9932) |
| SMR10442 | SMR6319 recAo281 recG258::Tn10miniKan | SMR9809 x P1(SMR9932) |
| SMR10443 | SMR6319 sulA211 recG258::Tn10miniKan | SMR9837 x P1(SMR9932) |
| SMR10678 | ruvA60 rus-1 ΔintD::FRTKanFRT | TNM759 x P1(JW0525) |
| SMR10698 | SMR6319 ΔrecG::Kan [pML104-3] | SMR10401 x pML104-3 |
| SMR10729 | SMR6319 ΔrecN::FRTKanFRT | SMR6319 x P1(JW2597) |
| SMR10730 | SMR6319 ΔrecN::FRT | SMR10729 x pCP20 |
| SMR10734 | SMR6319 ΔrecN::FRT recG258::Tn10miniKan | SMR10730 x P1(SMR9932) |
| SMR10735 | SMR6319 ΔrecF::FRT recG258::Tn10miniKan ΔuvrD::FRTcatFRT | SMR10437 x P1(SMR9811) |
| SMR10736 | SMR6319 ΔxonA::FRT recG258::Tn10miniKan ΔuvrD::FRTcatFRT | SMR10438 x P1(SMR9811) |
| SMR10737 | SMR6319 ΔrecJ::FRT recG258::Tn10miniKan ΔuvrD::FRTcatFRT | SMR10439 x P1(SMR9811) |
| SMR10738 | SMR6319 recG258::Tn10miniKan ΔuvrD::FRTcatFRT ΔrecQ::FRT metE163::Tn10 | SMR10419 x P1(SMR9812) |
| SMR10739 | SMR6319 recA200(Ts) recG258::Tn10miniKan | SMR8991 x P1(SMR9932) |
| SMR10740 | SMR6319 recA200(Ts) recG258::Tn10miniKan ΔuvrD::FRTcatFRT | SMR10739 x P1(SMR9811) |
| SMR10741 | SMR6319 ΔintD::FRTKanFRT | SMR6319 x P1(JW0525) |
| SMR10742 | SMR6319 ΔintD::FRTKanFRT rus-1 | SMR6319 x P1(SMR10678) |
| SMR10743 | SMR6319 ΔintD::FRT | SMR10741 x pCP20 |
| SMR10744 | SMR6319 ΔintD::FRT rus-1 | SMR10742 x pCP20 |
| SMR10745 | SMR6319 ΔintD::FRT recG258::Tn10miniKan | SMR10743 x P1(SMR9932) |
| SMR10746 | SMR6319 ΔintD::FRT rus-1 recG258::Tn10miniKan | SMR10744 x P1(SMR9932) |
| SMR11115 | SMR6319 ΔuvrA402::Gen recG258::Tn10miniKan | SMR8977 x P1(SMR9932) |
| SMR11116 | SMR6319 ΔmutL482::FRT recG258::Tn10miniKan | SMR8982 x P1(SMR9932) |
| SMR11117 | SMR6319 ΔmutL482::FRT ΔuvrA402::Gen recG258::Tn10miniKan | SMR8986 x P1(SMR9932) |
| SMR11132 | SMR6319 ΔrecG::FRTKanFRT | SMR6319 x P1(JW3627) |
| SMR11133 | SMR6319 ΔrecG::FRT | SMR11132 x pCP20 |
| SMR11134 | SMR6319 recO1504::Tn5 | SMR6319 x P1(RDK1541) |
| SMR11135 | SMR6319 ΔrecG::FRT recO1504::Tn5 | SMR11133 x P1(RDK1541) |
| SMR11186 | SMR6319 ΔrecB::Kan | SMR6319 x P1(RTC0013) |
| SMR11187 | SMR6319 ΔrecG::FRT ΔrecB::Kan | SMR11133 x P1(RTC0013) |
| SMR11188 | SMR6319 ΔrecG::FRT [pML104-3] | SMR11133 x pML104-3 |
| SMR11189 | SMR6319 ΔrecB::Kan [pML104-3] | SMR11186 x pML104-3 |
| SMR11190 | SMR6319 ΔrecG::FRT ΔrecB::Kan [pML104-3] | SMR11187 x pML104-3 |
| SMR11301 | SMR6319 lexA3(Ind−) ΔmutL::FRTKanFRT | SMR9801 x P1(JW4128) |
| SMR11303 | SMR6319 recAo281 lexA3(Ind−) ΔmutL::FRTKanFRT | SMR9805 x P1(JW4128) |
| SMR11305 | SMR6319 recAo281 ΔmutL::FRTKanFRT | SMR9809 x P1(JW4128) |
| SMR11318 | SMR6319 lexA3(Ind−) ΔmutL::FRT | SMR11301 x pCP20 |
| SMR11319 | SMR6319 recAo281 lexA3(Ind−) ΔmutL::FRT | SMR11303 x pCP20 |
| SMR11320 | SMR6319 recAo281 ΔmutL::FRT | SMR11305 x pCP20 |
| SMR11323 | SMR6319 ΔmutL::FRTKanFRT | SMR6319 x P1(JW4128) |
| SMR11325 | SMR6319 lexA3(Ind−) ΔmutL::FRT recG258::Tn10miniKan | SMR11318 x P1(SMR9932) |
| SMR11327 | SMR6319 recAo281 lexA3(Ind−) ΔmutL::FRT recG258::Tn10miniKan | SMR11319 x P1(SMR9932) |
| SMR11329 | SMR6319 recAo281 ΔmutL::FRT recG258::Tn10miniKan | SMR11320 x P1(SMR9932) |
| TNM759 | rus-1 ruvA60 | [39] |
CGSC – The Coli Genetic Stock Center, Yale
2.2. Cotransduction assay
Cotransductions were performed as in [23]. P1 transductions were performed and cells plated on LBH plates containing 10 μg/ml tetracycline (Tet) and 0.5% Citrate at 32°C, or the temperature stated. Following 48 hours of growth, between 100 and 200 colonies for each genotype were purified by streaking on LBH-Tet-Citrate plates. The transduction plates were then incubated for an additional 48h to confirm that no additional colonies formed. The streaked colonies were incubated for 48h before being replica plated onto LBH-Citrate plates containing 25 μg/ml chloramphenicol (Cam). Cotransductant frequencies are calculated as % CamR/TetR patches. For cotransduction into recipients containing the recA200Ts allele, transductions were performed at 30°C to allow recombination, then plating and subsequent incubations were at 42°C. For strains carrying pML104-3, the plasmid was maintained with 100 μg/ml spectinomycin (Spec) at 30°C during growth and cotransduction. Lambda red gam induction was performed using 0.4 mM IPTG for one hour prior to cotransduction. The cells were then plated onto medium lacking Spec at 37°C to allow the plasmid to be lost. All CamR TetR cfu were screened and verified to be SpecS.
2.3. Temperature-shift assay
Synchronous death of cells upon temperature shift to 30°C was performed by growing cultures to saturation in LBH at 42°C and then plating dilutions on pre-warmed LBH plates at 42°C and 30°C. Cfu were scored after 24 hours incubation. Plates were then placed back at 42°C/30°C for an additional 24 hours to verify that no slow-growing colonies appeared.
2.4. Statistical Analyses
Unless otherwise stated, strains indicated as inviable or having restoration of viability are statistically significantly different from the appropriate control strains with p≤ 0.01. Statistical analyses were performed using SigmaStat and measured by ANOVA with Fisher LSD post-hoc analysis.
2.5. Microscopy and fluorescence in situ hybridization
Chromosome-segregation analyses were as described [23]. Cells were visualized using a Zeiss Axio Imager microscope equipped with 100× oil objective and DAPI filter and Hamamatsu camera.
Fluorescence In Situ Hybridization (FISH) was as described [23]. Probes were 6 kb DNA fragments PCR amplified (Phusion DNA polymerase, New England Biolabs) from strain MG1655 DNA. Primers for the ori and ter probes were as described [30]. Probes were visualized using the Oregon Green filter and Rhodamine filter with the above microscope. Foci were scored on separate channels using ImageJ. Merged images were processed using Axiovision.
3. Results
3.1. Inviability of ΔrecG ΔuvrD double mutants
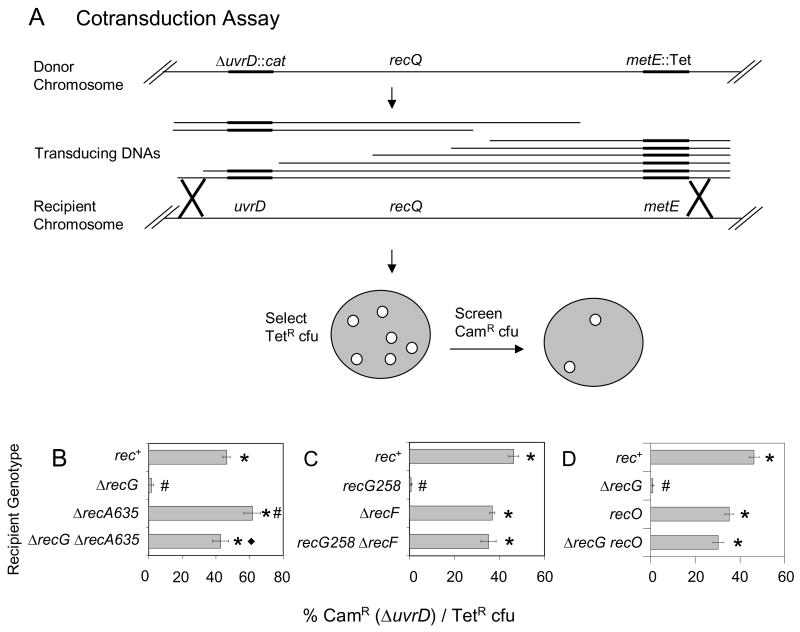
We tested whether the net effect in cells of RecG is reduction of IRI levels by determining whether ΔrecG ΔuvrD cells have a growth defect. If RecG acted similarly to Ruv in IRI resolution [31], then we might expect a ΔrecG ΔuvrD strain to have a growth defect due to excessive accumulation of IRIs, which can block chromosome segregation leading to death by recombination [23]. To test for synthetic lethality (inviability of the double mutant) between ΔrecG and ΔuvrD, we used phage P1-mediated cotransduction (Fig. 1A; e.g., [23]) which measures the viability of recipient cells cotransduced with two linked antibiotic resistance markers, one of which disrupts a gene of interest, in our case UvrD. The frequency with which both markers are cotransduced is determined by the distance between the markers. When an antibiotic marker disrupts a gene that is synthetically lethal with a mutation in the recipient strain’s genome, the frequency of cfu containing both antibiotic resistances will be reduced. We find that a deletion of uvrD can be efficiently cotransduced with a linked TetR marker into a recombination-proficient (rec+) background, but cannot be cotransduced into cells lacking RecG, indicating that the ΔrecG ΔuvrD double mutant is inviable (Fig. 1B).
Fig. 1.
Demonstration of inviability of recG ΔuvrD double mutants by cotransduction and requirements for RecA, RecF and RecO for the inviability. (A) Cotransduction assay. Phage P1 grown on a donor strain with two linked antibiotic resistances (SMR9811) recombine into a recipient strain. Transductants are selected for the non-lethal marker (TetR) and screened for those that also contain the potentially lethal marker (ΔuvrD::cat). recQ is located between uvrD and the linked Tet marker, so for examining a requirement for RecQ (Fig. 2) P1 grown on SMR9812, a strain that is ΔrecQ, is used. (B) ΔrecG ΔuvrD cells are inviable and RecA is required for their inviability. ΔuvrD cannot be cotransduced into a ΔrecG recipient (SMR10698), but can into rec+ (SMR10424), ΔrecA (SMR10427) and ΔrecA ΔrecG (SMR10428) recipients carrying pML104-3. (C) RecF is required for recG ΔuvrD inviability. ΔuvrD cannot be cotransduced into recG258 (SMR10419), but can into rec+ (SMR6319), ΔrecF (SMR10423) and ΔrecF recG258 (SMR10437) recipients. (D) RecO is required for ΔrecG ΔuvrD inviability. ΔuvrD cannot be cotransduced into ΔrecG (SMR11133), but can into recO (SMR11134) and recO ΔrecG (SMR11135) recipients. Mean ± SEM of 3 experiments for B, C and D. * indicates a significant difference from recG (B–D). # indicates a significant difference from rec+ (B–D); ◆ indicates a significant difference from the ΔrecA single mutant (B, p = 0.02) of the double mutant indicated.
3.2. Recombination proteins are required for ΔrecG ΔuvrD inviability
If the synthetic lethality between RecG and UvrD was due to accumulated toxic IRIs, such as D-loops and/or Holliday junctions, then removing proteins that promote formation or longevity of these intermediates might relieve the inviability. RecA is the major recombinase in cells and also activates induction of the SOS DNA-damage response. Because RecA is required for transduction, to test the hypothesis that recA deletion might allow viability of ΔrecG ΔuvrD cells, we used ΔrecA strains carrying recA+ on plasmid pML104-3, which has a temperature-sensitive origin of replication and is lost at 37°C. The cotransduction was performed at 30°C and then plated at 37°C to switch the cells into a ΔrecA ΔrecG ΔuvrD state. We found that the recA deletion allowed viability of ΔrecG ΔuvrD cells (Fig. 1B; p= 0.02 for the difference between ΔrecG and ΔrecG ΔrecA recipients). Thus RecA function is required for the inviability of ΔrecG ΔuvrD double mutants.
RecF helps RecA load onto ssDNA such as gaps formed at stalled replication forks (reviewed [32]) and therefore helps promote both the strand-exchange and SOS-induction activities of RecA. We find that functional RecF is also required for inviability of recG ΔuvrD cells (Fig. 1C), as is functional RecO (Fig. 1D), which works in a complex with RecF [33]. That is, ΔrecG ΔuvrD cells are viable if recF (Fig. 1C) or recO (Fig. 1D) is also knocked out.
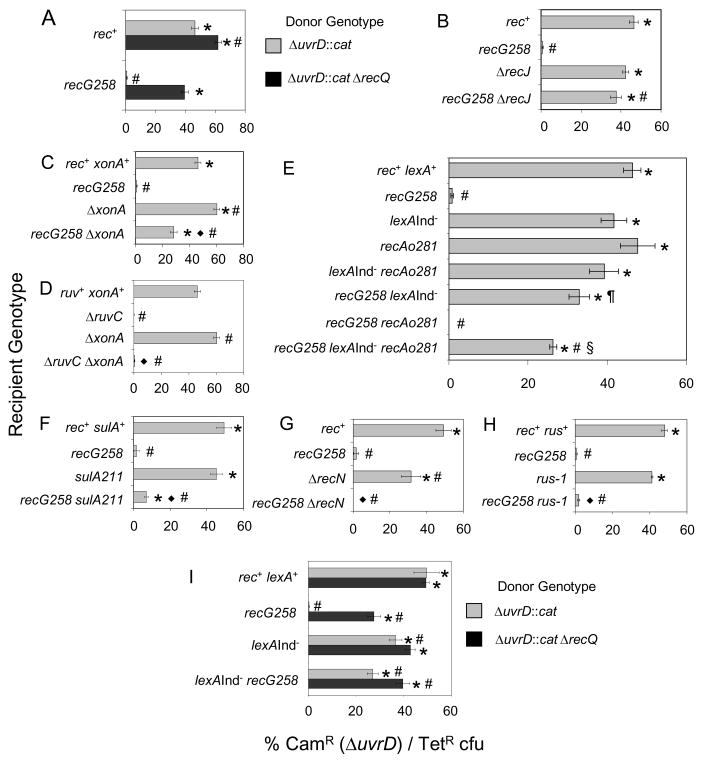
Interestingly, the recG ΔuvrD inviability also requires functional RecQ. That is, recG ΔuvrD ΔrecQ cells are viable (Fig. 2A). Transducing DNAs containing both ΔuvrD and ΔrecQ were efficiently cotransduced with a linked TetR marker into a recG strain whereas DNAs that were ΔuvrD but recQ+ were not (Fig. 2A). Twenty clones that arose as CamR TetR colonies when rec+ cells were cotransduced with the ΔuvrD ΔrecQ containing P1-lysate and 40 when recG was cotransduced were checked by PCR to verify that there was not a high frequency of double recombination that would result in loss of the ΔrecQ mutation. 1/20 CamR TetR colonies that arose after cotransduction into rec+ lost the ΔrecQ mutation, but all 40 of the recG colonies carried ΔrecQ. The requirement for RecQ in the death of recG ΔuvrD cells is similar to that seen previously for RecQ in promoting the net-accumulation of toxic IRIs in Δruv ΔuvrD cells [23].
Fig. 2.
Roles of recombination proteins and the SOS response in the death of recG uvrD cells. Data are results of co-transduction experiments as described in Fig. 1. (A) RecQ is required for recG ΔuvrD inviability. ΔuvrD and ΔrecQ alleles can be cotransduced together into recG258 recipient cells (SMR10419, donor strain SMR9812), but the ΔuvrD mutation alone (donor SMR9811) cannot. rec+ positive-control recipient, SMR6319. (B) RecJ is required for recG ΔuvrD inviability. ΔuvrD can be cotransduced into both ΔrecJ (SMR10434) and ΔrecJ recG258 (SMR10439) recipients. (C) ExoI is partially required for recG ΔuvrD inviability. ΔuvrD can be cotransduced into a ΔxonA recipient (SMR10395) efficiently, and a ΔxonA recG258 recipient (SMR10438) with intermediate efficiency. (D) ExoI is not required for inviability of ΔruvC ΔuvrD cells. ΔuvrD cannot be cotransduced into a ΔxonA ΔruvC recipient (SMR10417). ΔxonA positive control, SMR10395; ruvC negative control, SMR10408. (E) The SOS response, SOS-induced levels of RecA, and induction of another SOS gene(s) promote recG ΔuvrD inviability. Efficient cotransduction of ΔuvrD into “SOS-off” lexA(Ind−) (SMR9801), recAo281 (SMR9809) cells which produce SOS-induced levels of RecA, lexA(Ind−) recAo281 (SMR9805), and lexA(Ind−) recG258 (SMR10440) recipients, indicates that shutting off the SOS response via the lexA(Ind−) mutation partially restores viability to recG ΔuvrD cultures. Cotransduction efficiency is partially reduced from the level in lexA(Ind−) recG258 in a lexA(Ind−) recAo281 recG258 recipient (SMR10441), but is not abolished as in recAo281 recG258 (SMR10442) and recG258 (SMR10419). Therefore, SOS-induced levels of RecA account for some but not all of the contribution of the SOS response to recG ΔuvrD inviability. (F) SulA contributes little to recG ΔuvrD inviability. ΔuvrD is cotransduced poorly into a sulA211 recG258 recipient (SMR10443). sulA211 positive control, SMR9837. (G) RecN is not required for recG ΔuvrD inviability. ΔuvrD cannot be cotransduced into a ΔrecN recG258 recipient (SMR10734). ΔrecN positive control, SMR10730. (H) Activation of expression of Rus endonuclease by the rus-1 mutation does not restore viability to recG ΔuvrD cells. ΔuvrD cannot be cotransduced into recG258 rus-1 recipients (SMR10746). rec+ rus+ control, SMR10743; rus-1 positive control, SMR10744; recG258 negative control, SMR10745. (I) RecQ promotes recG ΔuvrD inviability wholly or partly independently of induction of the SOS response. ΔuvrD and ΔrecQ alleles confer greater viability when cotransduced together into recG258 lexA(Ind−) recipient cells (SMR10440, donor strain SMR9812) than the ΔuvrD alone (donor SMR9811), implying that ΔrecQ promotes recG ΔuvrD inviability wholly or partly independently of the LexA/SOS response. Mean ± SEM of 3 experiments (A–I). * indicates a significant difference from recG; # indicates a significant difference from rec+ (A–I). ◆ indicates a significant difference of each double mutant tested from the following isogenic single mutant: ΔxonA (C, D), sulA (F), recN (G), and rus-1 (H). In (A) recG cotransduced with ΔuvrD ΔrecQ is significantly different from rec+ cotransduced with same. In (E), the § indicated strain is significantly different from all others in the panal while the ¶ indicated strain is significantly different from the lexA(Ind−) single mutant among mutants carrying the lexA(Ind−) allele. In (I) lexA(Ind−) recG cotransduced with ΔuvrD ΔrecQ is significantly different from the same strain cotransduced with ΔuvrD alone. It is also significantly different from recG cotransduced with the ΔrecQ ΔuvrD donor.
RecQ interacts with several proteins either directly or indirectly through an interaction with SSB, single-stranded DNA binding protein [34, 35]. Of the proteins thought to interact with RecQ, single-stranded exonucleases RecJ and ExoI (encoded by the xonA gene) are of particular interest. RecJ 5′ exonuclease could work with RecQ in creating substrates for RecA loading and recombination [36]. We find that either ΔrecJ (Fig. 2B) or ΔxonA (Exonuclease I; Fig. 2C) allowed viability of recG ΔuvrD cells, though in the case of Exonuclease I the rescue is only partial. The requirement for functional RecJ in the death of recG ΔuvrD cells is similar to that seen for Δruv ΔuvrD synthetic lethality, and again supports the hypothesis that recG ΔuvrD cells are undergoing death by recombination. The partial restoration of viability to recG ΔuvrD cultures by lox of ExoI is not seen for the Δruv ΔuvrD synthetic lethality (Fig. 2D). This difference between the recG ΔuvrD and Δruv ΔuvrD situations is discussed below.
3.3. Role of the SOS response in recG ΔuvrD inviability
Cells that experience DNA damage induce an SOS response. During SOS, more than 40 genes normally repressed by LexA transcriptional repressor are upregulated due to degradation of LexA [37]. We find that a lexA(Ind−) allele, which keeps the SOS genes repressed, partially restores viability of recG ΔuvrD cultures (Fig. 2E). ΔuvrD can be cotransduced into a recG lexA(Ind−) recipient strain at an intermediate frequency, indicating that the SOS response contributes to, but is not the only source of, the recG ΔuvrD inviability. Because RecA levels are increased in SOS-induced cells, it was possible that SOS-induced levels of RecA cause the recG ΔuvrD inviability, as was the case for the Δruv ΔuvrD inviability [23]. To test this hypothesis we placed an operator constitutive allele of RecA (recAo281), which is not repressed by LexA, into the lexA(Ind−) background. We find the recAo allele confers some but not all of the inviability blocked by the SOS-off lexA(Ind−) mutation (Fig. 2E). We conclude that SOS-induced levels of RecA, and of one or more additional SOS genes account for the role of the SOS response in death of recG ΔuvrD cells. This differs from what was seen for the Δruv ΔuvrD inviability [23] in which recA was the only SOS gene required at SOS-induced levels for the inviability.
One of the proteins induced during the SOS response is SulA, an inhibitor of cell division [4]. We found that SulA plays only a small role in the recG ΔuvrD inviability. ΔuvrD was cotransduced into sulA recG cells at a frequency of 5% (Fig. 2F) which is lower than into recG lexA(Ind−) (33% cotransduction frequency, Fig. 2E), but still significantly greater than into recG alone (p< 0.05, ANOVA). Thus, SulA makes a minor contribution to the role of the SOS response in the recG ΔuvrD inviability. RecN is also upregulated by the SOS response, however, ΔuvrD could not be cotransduced into a recN recG recipient background (Fig. 2G), indicating that functional RecN is not required for the inviability of recG ΔuvrD cells. We conclude that the SOS response promotes the death of recG ΔuvrD cells by upregulation of RecA, SulA and possibly an additional component(s).
RecQ is required for SOS-induction under certain circumstances [38]. To address whether RecQ promotes death of recG ΔuvrD cells solely by helping turn-on SOS, we examined the frequency with which DNA containing ΔuvrD ΔrecQ is cotransduced into a lexA(Ind−) recG background. We find that there is a significant increase over the frequency with which ΔuvrD is cotransduced into lexA(Ind−) recG (Fig. 2I), indicating that RecQ and LexA function in wholly or partially independent pathways promoting inviability of recG ΔuvrD cells. Therefore, RecQ does more in the death pathway than simply helping to activate the SOS response.
3.4. Expression of Rus resolvase does not alleviate the recG ΔuvrD inviability
RusA is a Holliday-junction resolvase encoded in a cryptic prophage which is normally silent in E. coli [39]. RusA can compensate for the loss of Ruv in vitro [39] and can restore viability to ΔuvrD ΔruvC strains (RG Lloyd, personal communication). This demonstrates that ΔuvrD ΔruvC cells die from accumulation of IRIs containing either 4-way (Holliday) or 3-way junctions connecting the entangled chromosomes, because these are the DNA substrates cleaved by RusA [40, 41]. By contrast, we find that expression of RusA via the rus-1 allele did not relieve the recG ΔuvrD inviability (Fig. 2H). This result suggests that the lethal DNA substrate in recG ΔuvrD strains might be an intermediate that is not recognized and resolved by RusA, as indicated by in vitro work [41]. However we cannot rule out the possibility that RusA cannot act on Holliday junctions or other DNA intermediates in the presence of RuvABC which are likely to compete with Rus. RusA has never been shown to have a phenotype in Ruv+ cells.
3.5. Role of mismatch repair but not nucleotide excision repair in the SOS pathway of recG ΔuvrD inviability
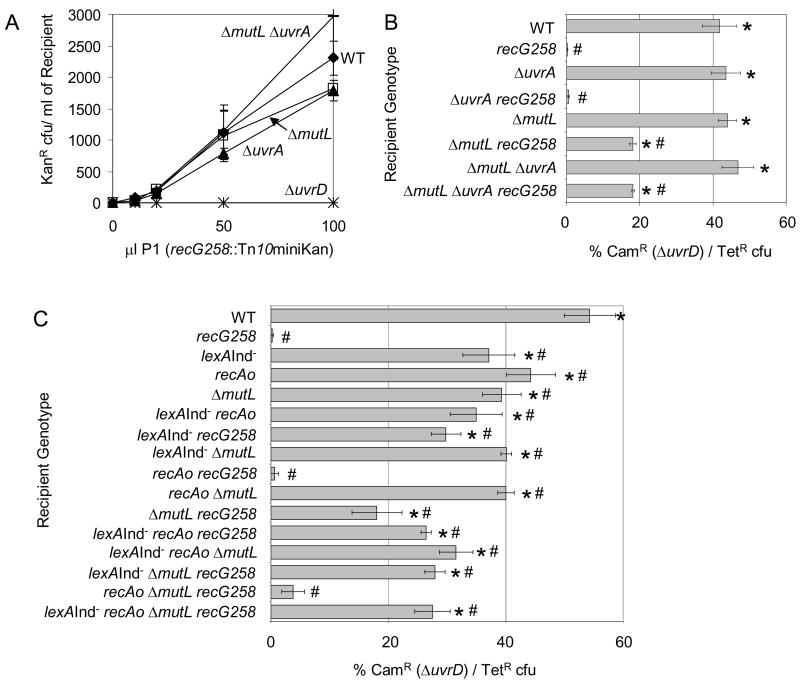
Apart from removing RecA from ssDNA, UvrD functions in both mismatch repair (MMR), which also requires MutL, MutS and MutH, and nucleotide excision repair (NER), which also requires UvrA, UvrB and UvrC proteins [4]. During MMR, MutS binds the DNA mismatch, MutL binds MutS and coordinates with MutH endonuclease to produce a single-strand nick near the mismatched site, which is then usually unwound by UvrD removing the DNA strand containing the incorrect base [4]. Similarly, UvrABC perform early steps in NER whereas UvrD acts later. We had no difficulty creating the double ΔuvrA recG and ΔmutL recG mutants or the triple ΔuvrA ΔmutL recG mutant as illustrated by quantitative transduction (Fig. 3A). Therefore, simply lacking the MMR or NER pathways does not cause inviability in recG cells.
Fig. 3.
Incomplete mismatch repair and not nucleotide excision repair contributes to death of recG ΔuvrD cells. (A) Simple loss of either MMR or NER does not cause inviability of recG cells, in that quantitative transduction of recG258 (SMR9932 donor) into strains mutant for proteins involved in NER (ΔuvrA, SMR8977), MMR (ΔmutL, SMR8982) or both (ΔuvrA ΔmutL, SMR8986) is not impaired relative to MMR- and NER-proficient cells (SMR6319). Therefore, blocking formation of the MMR and NER intermediates created by MutL and UvrA does not create an inviability with recG. ΔuvrD negative control, SMR8976. (B) Although simple loss of either MMR or NER does not cause inviability of recG cells, loss of the MutL step in MMR relieves some of the inviability of recG ΔuvrD cultures. This implies that MMR intermediates initiated by MutL can be lethal to recG cells if UvrD is not present to complete repair and remove those intermediates. ΔuvrD can be cotransduced with intermediate efficiency into a ΔmutL recG258 recipient (SMR11116) indicating a partial requirement for MutL, but not UvrA, in the death of recG ΔuvrD cultures. recG258 SMR10419; ΔuvrA recG258 SMR11115; MMR- and NER-proficient cells SMR6319 (“WT”); ΔuvrA SMR8977; ΔmutL SMR8982; ΔuvrA ΔmutL SMR8986; and ΔmutL ΔuvrA recG258 SMR11117 recipient cells. (C) The combination of incomplete MMR and SOS-induction does not account for all of the recG ΔuvrD inviability. When combined, the partial requirement for MutL and the SOS response does not restore more viability to recG ΔuvrD cells than lexA(Ind−) or ΔmutL alone, or in the presence of SOS-induced levels of RecA. MMR- and SOS-proficient cells SMR6319; recG258 SMR10419; lexA(Ind−) SMR9801; recAo281 SMR9809; ΔmutL SMR11323; lexA(Ind−) recAo281 SMR9805; lexA(Ind−) recG258 SMR10440; lexA(Ind−) ΔmutL SMR11318; recAo281 recG258 SMR10442; recAo281 ΔmutL SMR11320; ΔmutL recG258 SMR11116; lexA(Ind−) recAo281 recG258 SMR10441; lexA(Ind−) recAo281 ΔmutL SMR11319; lexA(Ind−) ΔmutL recG258 SMR11325; recAo281 ΔmutL recG258 SMR11329; lexA(Ind−) recAo281 ΔmutL recG258 SMR11327. Mean ± SEM of 3 experiments (A–C). * indicates a significant difference from recG; # indicates a significant difference from WT (B, C). In (B) the triple ΔmutL ΔuvrA recG258 mutant is significantly different from all of the constituent single and double mutants except for ΔmutL recG258 (p= 0.99). In (C) the quadruple mutant lexA(Ind−) recAo281 ΔmutL recG258 is significantly different from recAo281; lexA(Ind−) ΔmutL; recAo281 recG258; recAo281 ΔmutL (p= 0.01); ΔmutL (p= 0.02), and recAo281 ΔmutL recG258. For WT compared to recAo, p= 0.02.
On the other hand, we find that MutL, but not UvrA, contributes to the ΔuvrD recG inviability (Fig. 3B). ΔuvrD can be transduced into ΔmutL recG cells, albeit at about half the efficiency as when ΔuvrD is transduced into recG+ cells (Fig. 3B). These data imply that incomplete MMR intermediates created by MutSLH but left unresolved in cells lacking UvrD cause some of the inviability of recG ΔuvrD cells. The single-strand-nicked-DNA intermediates created by MutSLH acting upon spontaneous mismatches, perhaps from spontaneous replication errors, appear to underlie some of the problem. Thus, part of the reason for the ΔuvrD inviability with RecG appears to involve incomplete MMR, whereas part is independent of MutL-dependent MMR. We conclude that a function of UvrD beside MMR or NER, probably its role in opposing RecA filaments, is responsible for about half the synthetic lethality of recG ΔuvrD cells, and that the accumulation of MutL-generated MMR intermediates that accumulate in the absence of UvrD underlies the other half.
Because both the SOS response and incomplete MMR contribute to part of the recG ΔuvrD inviability, we tested whether the loss of these together was sufficient to restore complete viability. We found that the loss of mutL did not significantly affect the frequency of ΔuvrD cotransduction into lexA(Ind−) recG cells (Fig. 3C). These data show that MutL-promoted inviability in recG ΔuvrD cells occurs via the SOS-dependent pathway. That is, if SOS induction is blocked, loss of MutL confers no additional growth-enhancing benefit to cultures. This implies that a MutL-generated DNA intermediate retards growth/viability by activating an SOS response. This, plus the fact that RecQ is required for the inviability independently of a role in SOS induction (Fig. 2I), supports death-by-recombination models for part of the inviability.
3.6. DSE processing by RecB does not contribute to the ΔrecG ΔuvrD inviability
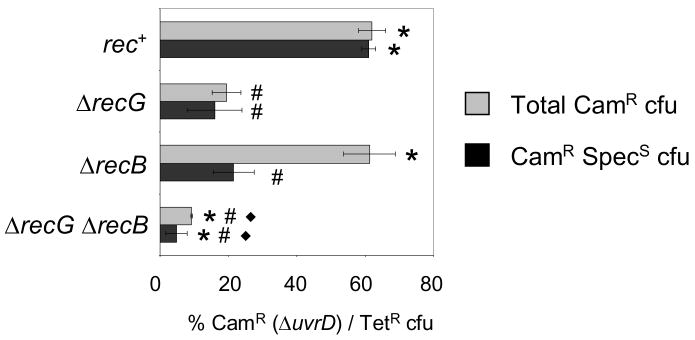
DNA double-strand breaks (DSBs) and ends (DSEs) are recognized by the RecBCD exonuclease complex which also creates substrates for RecA-mediated recombination [42]. To determine whether DSBs/DSEs might be part of the spontaneous DNA damage, failed repair of which kills ΔrecG ΔuvrD cells, we tested whether RecB contributes to the ΔrecG ΔuvrD inviability by cotransduction into strains carrying plasmid pML104-3 which, in addition to encoding RecA and having a temperature-sensitive origin of replication, has the lambda recombination genes red and gam under the control of an IPTG-inducible promoter, allowing recombination. IPTG was added for an hour prior to cotransduction and the cells were plated immediately on Tetracycline plates at 37°C to allow the plasmid to be lost. Fig. 4 shows both the total cotransduction frequency and the adjusted cotransduction frequency of the cells which lost pML104-3 and became SpecS. We found that ΔuvrD was cotransduced into a recB strain similarly to rec+, but many of the colonies retained the plasmid indicating that there is some benefit, probably the over-production of RecA by the plasmid, in the absence of RecB. However, ΔuvrD could not be cotransduced efficiently into ΔrecG ΔrecB cells. We conclude that DNA processing by RecB is not required for the ΔrecG ΔuvrD inviability. Therefore the DNA substrate recombined during death is not a DSE.
Fig. 4.
RecB is not required for death of ΔrecG ΔuvrD cells. ΔuvrD cannot be cotransduced into ΔrecG (SMR11188) or ΔrecB ΔrecG (SMR11190), but can into rec+ (SMR10424) and ΔrecB (SMR11189). All strains carry pML104-3 therefore all TetR cfu were also tested for SpecR to assay the loss of pML104-3 at the restrictive temperature, 37°C. Note that the somewhat higher cotransduction into recG cells seen here is an apparent effect of the altered temperature regiment with higher temperature allowing greater viability (data not shown). Mean ± SEM of 3 experiments. * indicates a significant difference from recG, # indicates a significant difference from WT, and ◆ indicates a significant difference from ΔrecB. For ΔrecG compared to ΔrecG ΔrecB p ≤ 0.05.
3.7. Chromosome-segregation failure but not failed replication during death of recG ΔuvrD cells
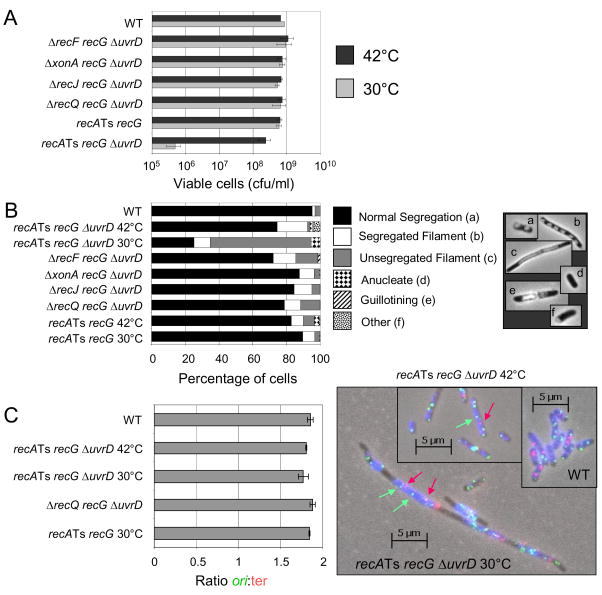
If recG ΔuvrD cells die a death by recombination, we would expect this death to be accompanied by chromosome-segregation failure. We constructed recA(Ts) recG uvrD cells, which are viable at the restrictive temperature (42°C), due to the absence of RecA function, then shifted these cells to permissive temperature to watch them die en masse. When grown at the non-permissive temperature (42°C), recA(Ts) recG uvrD cells are viable and 75% show normal chromosome segregation (Fig. 5A, B). When the cells are switched to the permissive temperature (30°C) for 4 hours, the cells die synchronously (Fig. 5A). During this death, the cells become filamented (elongated) with only 20% having normal chromosome segregation whereas the majority (64%) have unsegregated chromosomes (Fig. 5B). Removing RecF, ExoI, RecJ or RecQ from the cells rescues the chromosome-segregation defect, restoring the cells to ≥ 80% having normal chromosome segregation (Fig. 5B). These data support the hypothesis that RecA, RecQ and RecF promote net accumulation of IRIs in recG ΔuvrD cells.
Fig. 5.
Chromosome-segregation defects but completed replication during synchronous death of recG ΔuvrD cells. (A) Upon shift of recA(Ts) recG ΔuvrD cells (SMR10740) switched to the permissive temperature (30°C, RecA+ phenotype), from the restrictive temperature (42°C, RecA− phenotype), cells die synchronously and show (B) chromosome-segregation defects as indicated by an increased percentage of filamented cells with unsegregated nucleoids. The chromosome-segregation defect is also rescued by the additional mutation of ΔrecF, ΔxonA, ΔrecJ, or ΔrecQ (Strains SMR10735, SMR10736, SMR10737 and SMR10738 respectively). Control recA(Ts) recG cells (SMR10739) do not show chromosome-segregation defects at the permissive temperature. Mean of 3 experiments. SEM is < 5% of cells/category for all genotypes and temps. (C) Completion of chromosome replication in dying recA(Ts) recG ΔuvrD cells shown by fluorescence in situ hybridization (FISH) to ori (green foci) and terminus-proximal (red foci) chromosomal sequences. Left, the ratios of ori:ter foci in cells are unchanged during synchronous death of recA(Ts) recG ΔuvrD (SMR10740) cells at permissive or restrictive temperatures from those of the non-dying wild-type (rec+, SMR6319) control. The ratio of ori:ter is not different from that of rec+ or recA(Ts) recG (SMR10739) cells, or of ΔrecQ recG ΔuvrD (SMR10738) cells. Right, representative example of FISH data. Images are overlays of phase contrast (blue), red (ter hybridization) and green (ori hybridization) exposures. Red arrows, examples of red ter foci; green arrows, examples of green ori foci. Mean ± SEM of 3 experiments for B and C; ≥ 500 cells scored/genotype/temperature/experiment.
RecG is implicated not only in dissociating D-loops but also in regressing stalled replication forks [27]. We therefore wanted to test the possibility that the death seen in recG ΔuvrD cells is not death by recombination but rather due to replication failure. An accumulation of stalled replication forks that remain unregressed in the absence of RecG would be expected to cause the chromosome-segregation defect seen. To test this possibility, we used fluorescence in situ hybridization (FISH) with probes to the E. coli replication origin and terminus to determine whether or not replication is completed normally. If chromosome replication were incomplete then switching recA(Ts) recG ΔuvrD cells to the permissive temperature should result in an increase in the ratio of origins:termini in cells from the approximately 2:1 seen in normally replicating cells, such as wild-type or recA(Ts) recG ΔuvrD cells at the restrictive temperature. Instead, we see that switching the cells to the permissive temperature did not alter the ratio of origin:terminus foci significantly. All populations of cells showed 1.8 ori:ter as expected of normally replicating cells and seen for wild-type cells (Fig. 5C). We conclude that chromosome replication is completed normally during RecA-, RecQ-, RecF-dependent death of recG ΔuvrD cells. The observation of failed chromosome segregation with completed chromosome replication supports a death by accumulation of unresolved IRIs.
4. Discussion
4.1. RecQ promotes death by recombination in recG uvrD cells
We have shown that cells lacking RecG and UvrD are inviable due to a chromosome-segregation defect (Fig. 5B) that occurs despite completion of chromosome replication (Fig. 5C), implicating a build-up of unresolved IRIs as a cause of their death. Although an SOS response is partially required for the death (Fig. 2E), RecA (Fig. 1B) and RecQ (Fig. 2A) promote this death-by-recombination in roles independent of SOS induction (Fig. 2E and I), strongly supporting the idea that RecQ promotes a net accumulation in cells of unresolved IRIs. Whereas this conclusion was drawn previously from results in cells lacking RuvABC and UvrD [23], the current data generalize this conclusion to a different strain background showing that it is general property of RecQ not peculiar o the Δruv ΔuvrD mutant strain background.
The synthetic lethality of recG and ΔuvrD is similar to what was seen in cells lacking Ruv and UvrD, but some differences in genetic requirements imply some differences in the mechanisms. Unlike death of Δruv ΔuvrD cells, we observed that death of recG ΔuvrD cultures requires an SOS-response component in addition to RecA (Fig. 2E), and partially requires Exo I (Fig. 2C) and MutL (Fig. 3B, C). This death is also not blocked by RusA activation (Fig. 2H). Together these data indicate that there are some differences from the death-by-recombination of Δruv ΔuvrD cells. Presumably, some different DNA substrates accumulate in cells lacking RecG and UvrD than in cells lacking Ruv and UvrD.
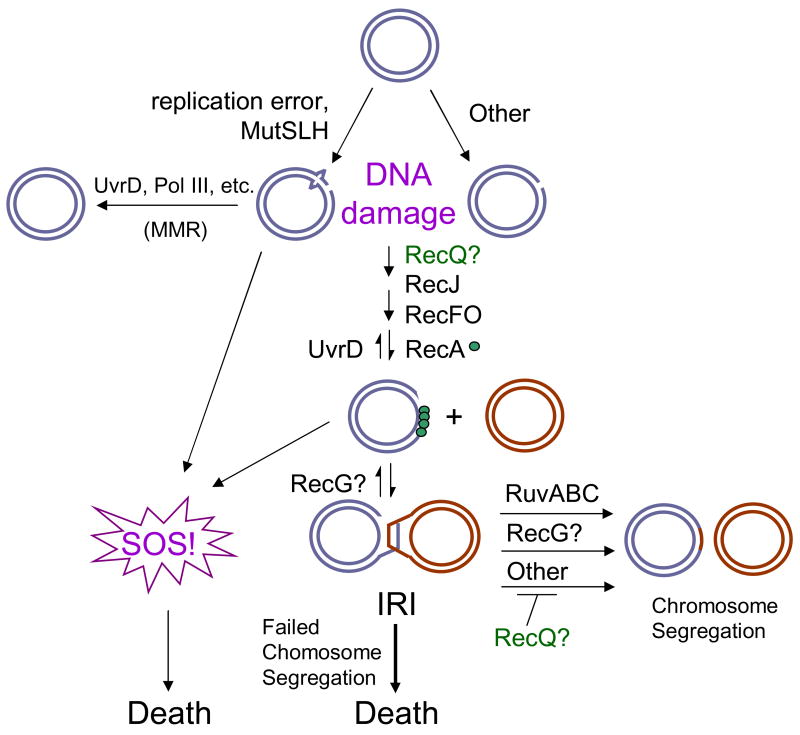
In Fig. 6 we show an elaboration on the general model for death-by-recombination previously presented by Magner et al. [23], adapted for RecQ-promoted death of recG ΔuvrD cells. This model shows RecF, RecO, RecA, RecQ and RecJ working to promote the net accumulation of IRIs in cells. The IRIs can become toxic if they remain unresolved. UvrD acts to oppose RecA [43] and thus effect a net reduction in IRIs in cells. It is unknown whether RecQ promotes net accumulation of IRIs by assisting the formation of IRIs, or alternatively, by opposing a Ruv- and RecG-independent IRI resolution pathway. This is an important aspect of RecQ mechanism of action in HR that awaits future experiments.
Fig. 6.
Models for death of ΔrecG ΔuvrD cells. DNA damage arises from replication errors (mismatches) which are recognized and processed by MutSLH or from other sources. In the presence of UvrD, MMR can be completed to give intact dsDNA. In the absence of UvrD, the damaged DNA is processed by homologous recombination proteins. RecQ and RecJ may function together at this stage to create ssDNA gaps which are substrates for RecF, O and RecA, which can promote both the induction of SOS and the initiation of recombination (formation of IRIs). IRIs are then resolved in multiple pathways, primarily through the action of RuvABC, but also via the action of RecG. Both the persistence of unresolved IRIs and the SOS response can lead to loss of viability (death.) RecG might branch migrate D-loops or IRIs to contribute to their elimination. RecQ might either promote the formation of ssDNA required for IRI formation or inhibit an alternative resolution pathway to Ruv and RecG. Blue and Red circles represent dsDNA.
4.2. Mismatch repair intermediates promote death via SOS in recG cells
The model in Fig. 6 also accommodates our findings that part of the death of recG ΔuvrD cells requires MutL (Fig. 3B), and acts via the SOS component of the death pathway (Fig. 3C). We suggest that MMR intermediates initiated by MutL and left unresolved because of the absence of UvrD lead to SOS induction, which contributes to death in recG cells. This was observed only in recG ΔuvrD cells (here) and not in ruv ΔuvrD cells [23]. Cells lacking recG might be particularly sensitive to death by enhanced SOS induction because they are already partially SOS induced [44]. The loss of UvrD and accumulation of MMR intermediates might push them past a threshold of SOS-induction beyond which cell proliferation is impaired.
4.3. RecG role in net IRI reduction
The data presented here also suggest that different classes (structures) of IRIs can accumulate in cells, some of which are recognized and resolved by RuvABC, others of which are recognized/resolved by RecG. These IRIs accumulated in the absence of UvrD are either too numerous to be resolved by the limiting capacity of RuvABC present in the recG ΔuvrD cells or are unable to switch between resolution pathways. This could be either because they are intermediates not recognized by the Ruv and Rus (Fig. 2H) enzymes (i.e. not Holliday or three-way junctions), or possibly they are bound and sequestered, though not resolved, by the Ruv proteins which are at their capacity for resolution. Although the inability to restore viability to recG ΔuvrD cells with RusA suggests that the toxic substrate is not a Holliday junction, the caveat to this interpretation is that RusA may be unable to act in the presence of RuvABC. To date, all of its phenotypes have been documented in cells lacking Ruv [40, 41]. The Ruv proteins might compete with RusA for their joint substrate. It is possible that some proteins that promote the ΔrecG ΔuvrD inviability such as MutL and ExoI, or even RecQ and RecJ do so by preventing switching between classes of IRIs. Because RecG has been hypothesized to process D-loops [45–47], one possibility for the ΔrecG ΔuvrD inviability is that D-loops form but cannot be branch-migrated into a substrate recognizable by RuvABC or unwound and reannealed to the original strand in a synthesis-dependent stand annealing (SDSA) pathway [4]. The failure to process D-loops might cause them to accumulate and the IRIs joined by them to become toxic.
Although there is no known eukaryotic homolog of E. coli RecG it is likely that there are analogues throughout phylogeny. Most of DNA recombination and repair proteins are highly conserved (notably RecQ and RecA) and it would be surprising if this were not also true of RecG. Like RecQ, RecG has been studied but its role in vivo is still unknown. It was thought to have significant redundancy with other helicases present in E. coli [26]. The differences between the ΔrecG ΔuvrD and Δruv ΔuvrD inviabilities: partial rescue by sulA, ΔxonA and ΔmutL that are not seen for Δruv ΔuvrD [23], may indicate that different DNA substrates, or different IRIs, accumulate in the Δruv ΔuvrD than in the ΔrecG ΔuvrD background, all of which can become toxic if unresolved. This suggests that RecG is not simply a redundant helicase in E. coli, but also, or in addition, resolves IRIs that are poorly recognized/resolved by RuvABC.
4.4. Role of RecQ in net IRI accumulation
RecQ has now been shown to promote death-by-recombination in both the Δruv ΔuvrD [23] and the ΔrecG ΔuvrD (here) backgrounds. Models presented previously show RecQ acting at its preferred substrate, a 5′ DNA end at a replication fork, to produce a substrate for RecA binding, thus leading to IRI formation [23]. It is possible that RecQ, acting in this manner could promote the regression of a stalled replication fork, a substrate that RecG might then be able to recognize and have a role in resetting. RecA and RecF are able to stabilize regressed replication forks [48]. However, our data do not support this as the role for RecQ in ΔrecG ΔuvrD inviability because we saw no evidence for incomplete replication in the dying ΔrecG ΔuvrD cells (Fig. 5C). Though the net effect of RecQ remains an increase in the accumulation of IRIs in cells, how it causes this effect and the substrate(s) it recognizes in vivo remain uncertain. Homologues of E. coli RecQ may be involved in one or both paradigms of RecQ action. As more information comes to light on the different, opposing roles of RecQ homologues in vivo, understanding the RecQ-promoted death-by-recombination pathway(s) and the proteins involved will illuminate how RecQ homologues prevent genomic instability.
Acknowledgments
We thank David Bates for advice and the generous use of the Zeiss microscope, RG Lloyd for communication of unpublished results, and PJ Hastings for comments on the manuscript. This work was supported by National Institutes of Health grant R01-CA85777.
Abbreviations
- cfu
colony-forming units
- FISH
fluorescence in situ hybridization
- HR
homologous recombination
- IRI
intermolecular recombination intermediate
- ssDNA
single-stranded DNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bohr VA. Rising from the RecQ-age: the role of human RecQ helicases in genome maintenance. Trends Biochem Sci. 2008;33:609–620. doi: 10.1016/j.tibs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, Zheng L, Stark JM, Barnes EL, Chi P, Janscak P, Jasin M, Vogel H, Sung P, Luo G. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S, Brosh RM., Jr Unique and important consequences of RECQ1 deficiency in mammalian cells. Cell Cycle. 2008;7:989–1000. doi: 10.4161/cc.7.8.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg E, Walker G, Siede W, Wood R, Schultz R, Ellenberger T. DNA Repair and Mutagenesis. 2. ASM Press; Washington, D.C: 2005. [Google Scholar]
- 5.Mimitou EP, Symington LS. Nucleases and helicases take center stage in homologous recombination. Trends Biochem Sci. 2009;34:264–272. doi: 10.1016/j.tibs.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Harmon FG, Kowalczykowski SC. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12:1134–1144. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suski C, Marians KJ. Resolution of converging replication forks by RecQ and Topoisomerase III. Mol Cell. 2008;30:779–789. doi: 10.1016/j.molcel.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ira G, Malkova A, Liberi G, Foiani M, Haber JE. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, Topoisomerase IIIalpha, and BLAP75. J Biol Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- 10.Macris MA, Krejci L, Bussen W, Shimamoto A, Sung P. Biochemical characterization of the RECQ4 protein, mutated in Rothmund-Thomson syndrome. DNA Repair (Amst) 2006;5:172–180. doi: 10.1016/j.dnarep.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Machwe A, Xiao L, Groden J, Matson SW, Orren DK. RecQ family members combine strand pairing and unwinding activities to catalyze strand exchange. J Biol Chem. 2005;280:23397–23407. doi: 10.1074/jbc.M414130200. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2008;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangloff S, Soustelle C, Fabre F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nat Genet. 2000;25:192–194. doi: 10.1038/76055. [DOI] [PubMed] [Google Scholar]
- 16.Prince PR, Emond MJ, Monnat RJ., Jr Loss of Werner syndrome protein function promotes aberrant mitotic recombination. Genes Dev. 2001;15:933–938. doi: 10.1101/gad.877001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saintigny Y, Makienko K, Swanson C, Emond MJ, Monnat RJ., Jr Homologous recombination resolution defect in Werner syndrome. Mol Cell Biol. 2002;22:6971–6978. doi: 10.1128/MCB.22.20.6971-6978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachrati CZ, Hickson ID. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popuri V, Bachrati CZ, Muzzolini L, Mosedale G, Costantini S, Giacomini E, Hickson ID, Vindigni A. The Human RecQ helicases, BLM and RECQ1, display distinct DNA substrate specificities. J Biol Chem. 2008;283:17766–17776. doi: 10.1074/jbc.M709749200. [DOI] [PubMed] [Google Scholar]
- 20.Rosin MP, German J. Evidence for chromosome instability in vivo in Bloom syndrome: increased numbers of micronuclei in exfoliated cells. Hum Genet. 1985;71:187–191. doi: 10.1007/BF00284570. [DOI] [PubMed] [Google Scholar]
- 21.Chan KL, North PS, Hickson ID. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 2007;26:3397–3409. doi: 10.1038/sj.emboj.7601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez CR, Yang S, Deibler RW, Ray SA, Pennington JM, Digate RJ, Hastings PJ, Rosenberg SM, Zechiedrich EL. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Mol Microbiol. 2005;58:80–101. doi: 10.1111/j.1365-2958.2005.04812.x. [DOI] [PubMed] [Google Scholar]
- 23.Magner DB, Blankschien MD, Lee JA, Pennington JM, Lupski JR, Rosenberg SM. RecQ promotes toxic recombination in cells lacking recombination intermediate-removal proteins. Mol Cell. 2007;26:273–286. doi: 10.1016/j.molcel.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartung F, Suer S, Puchta H. Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:18836–18841. doi: 10.1073/pnas.0705998104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitby MC, Vincent SD, Lloyd RG. Branch migration of Holliday junctions: identification of RecG protein as a junction specific DNA helicase. EMBO J. 1994;13:5220–5228. doi: 10.1002/j.1460-2075.1994.tb06853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lloyd RG. Conjugational recombination in resolvase-deficient ruvC mutants of Escherichia coli K-12 depends on recG. J Bacteriol. 1991;173:5414–5418. doi: 10.1128/jb.173.17.5414-5418.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGlynn P, Lloyd RG. Rescue of stalled replication forks by RecG: simultaneous translocation on the leading and lagging strand templates supports an active DNA unwinding model of fork reversal and Holliday junction formation. Proc Natl Acad Sci USA. 2001;98:8227–8234. doi: 10.1073/pnas.111008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuzminov A. RuvA, RuvB and RuvC proteins: cleaning-up after recombinational repairs in E. coli. Bioessays. 1993;15:355–358. doi: 10.1002/bies.950150511. [DOI] [PubMed] [Google Scholar]
- 29.Miller J. Generalized transduction; use of P1 in strain construction. In: Miller J, editor. Experiments in molecular genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, New York: 1972. pp. 201–205. [Google Scholar]
- 30.Bates D, Kleckner N. Chromosome and replisome dynamics in E. coli: loss of sister cohesion triggers global chromosome movement and mediates chromosome segregation. Cell. 2005;121:899–911. doi: 10.1016/j.cell.2005.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lloyd RG, Sharples GJ. Processing of recombination intermediates by the RecG and RuvAB proteins of Escherichia coli. Nucleic Acids Res. 1993;21:1719–1725. doi: 10.1093/nar/21.8.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courcelle J. Recs preventing wrecks. Mutat Res. 2005;577:217–227. doi: 10.1016/j.mrfmmm.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Umezu K, Chi NW, Kolodner RD. Biochemical interaction of the Escherichia coli RecF, RecO, and RecR proteins with RecA protein and single-stranded DNA binding protein. Proc Natl Acad Sci USA. 1993;90:3875–3879. doi: 10.1073/pnas.90.9.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shereda RD, Bernstein DA, Keck JL. A central role for SSB in Escherichia coli RecQ DNA helicase function. J Biol Chem. 2007;282:19247–19258. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- 35.Butland G, Peregrin-Alvarez JM, Li J, Yang W, Yang X, Canadien V, Starostine A, Richards D, Beattie B, Krogan N, Davey M, Parkinson J, Greenblatt J, Emili A. Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature. 2005;433:531–537. doi: 10.1038/nature03239. [DOI] [PubMed] [Google Scholar]
- 36.Niu H, Raynard S, Sung P. Multiplicity of DNA end resection machineries in chromosome break repair. Genes Dev. 2009;23:1481–1486. doi: 10.1101/gad.1824209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Courcelle J, Khodursky A, Peter B, Brown PO, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hishida T, Han YW, Shibata T, Kubota Y, Ishino Y, Iwasaki H, Shinagawa H. Role of the Escherichia coli RecQ DNA helicase in SOS signaling and genome stabilization at stalled replication forks. Genes Dev. 2004;18:1886–1897. doi: 10.1101/gad.1223804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandal TN, Mahdi AA, Sharples GJ, Lloyd RG. Resolution of Holliday intermediates in recombination and DNA repair: indirect suppression of ruvA, ruvB, and ruvC mutations. J Bacteriol. 1993;175:4325–4334. doi: 10.1128/jb.175.14.4325-4334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharples GJ, Chan SN, Mahdi AA, Whitby MC, Lloyd RG. Processing of intermediates in recombination and DNA repair: identification of a new endonuclease that specifically cleaves Holliday junctions. EMBO J. 1994;13:6133–6142. doi: 10.1002/j.1460-2075.1994.tb06960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bolt EL, Lloyd RG. Substrate specificity of RusA resolvase reveals the DNA structures targeted by RuvAB and RecG in vivo. Mol Cell. 2002;10:187–198. doi: 10.1016/s1097-2765(02)00560-9. [DOI] [PubMed] [Google Scholar]
- 42.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol Mol Biol Rev. 2008;72:642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veaute X, Delmas S, Selva M, Jeusset J, Le Cam E, Matic I, Fabre F, Petit MA. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 2005;24:180–189. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd RG, Buckman C. Genetic analysis of the recG locus of Escherichia coli K-12 and of its role in recombination and DNA repair. J Bacteriol. 1991;173:1004–1011. doi: 10.1128/jb.173.3.1004-1011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGlynn P, Al-Deib AA, Liu J, Marians KJ, Lloyd RG. The DNA replication protein PriA and the recombination protein RecG bind D-loops. J Mol Biol. 1997;270:212–221. doi: 10.1006/jmbi.1997.1120. [DOI] [PubMed] [Google Scholar]
- 46.Briggs GS, Mahdi AA, Weller GR, Wen Q, Lloyd RG. Interplay between DNA replication, recombination and repair based on the structure of RecG helicase. Philos Trans R Soc Lond B Biol Sci. 2004;359:49–59. doi: 10.1098/rstb.2003.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman-Ohana R, Karunker I, Cohen A. A RecG-independent nonconservative branch migration mechanism in Escherichia coli recombination. J Bacteriol. 1999;181:7199–7205. doi: 10.1128/jb.181.23.7199-7205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Courcelle J, Donaldson JR, Chow KH, Courcelle CT. DNA damage-induced replication fork regression and processing in Escherichia coli. Science. 2003;299:1064–1067. doi: 10.1126/science.1081328. [DOI] [PubMed] [Google Scholar]
- 49.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. 2006;2 doi: 10.1038/msb4100050. 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 52.Kolodner R, Fishel RA, Howard M. Genetic recombination of bacterial plasmid DNA: effect of RecF pathway mutations on plasmid recombination in Escherichia coli. J Bacteriol. 1985;163:1060–1066. doi: 10.1128/jb.163.3.1060-1066.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cirz RT, Chin JK, Andes DR, de Crecy-Lagard V, Craig WA, Romesberg FE. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 2005;3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]