Abstract
In vertebrates, fibrinolysis is primarily carried out by the serine protease plasmin (Pm), which is derived from activation of the zymogen precursor, plasminogen (Pg). One of the most distinctive features of Pg/Pm is the presence of five homologous kringle (K) domains. These structural elements possess conserved Lys-binding sites (LBS) that facilitate interactions with substrates, activators, inhibitors and receptors. In human Pg (hPg), K2 displays weak Lys affinity, however the LBS of this domain has been implicated in an atypical interaction with the N-terminal region of a bacterial surface protein known as PAM (Pg-binding group A streptococcal M-like protein). A direct correlation has been established between invasiveness of group A streptococci and their ability to bind Pg. It has been previously demonstrated that a 30-residue internal peptide (VEK-30) from the N-terminal region of PAM competitively inhibits binding of the full-length parent protein to Pg. We have attempted to determine the effects of this ligand-protein interaction on the regulation of Pg zymogen activation and conformation. Our results show minimal effects on the sedimentation velocity coefficients (S°20,w) of Pg when associated to VEK-30 and a direct relationship between the concentration of VEK-30 or PAM and the activation rate of Pg. These results are in contrast with the major conformational changes elicited by small-molecule activators of Pg, and point towards a novel mechanism of Pg activation that may underlie group A streptococcal (GAS) virulence.
Keywords: Plasminogen, streptokinase, group A streptococci, M-like protein, PAM
1. INTRODUCTION
In order for invasive pathogens to migrate beyond the site of infection, host physiological barriers such as the extracellular matrix, the basement membrane, and encapsulating fibrin networks must be overcome. To circumvent these impediments, proteolytic enzymes can facilitate dissemination of the microorganism. Along these lines, recruitment of a host protease to the bacterial surface represents a particularly effective mechanism for enhancing invasiveness [1-3].
Group A streptococcus (GAS)1 is highly adapted to human hosts and affects over 700 million people worldwide each year. While the majority of these cases are classified as mild, a host of severe and aggressive invasive GAS infections befall approximately 18 million individuals per year [4]. Two of the more distinctive features of GAS are the production of cell wall-attached M proteins and the secretion of streptokinase (SK). The former minimize phagocytosis and mediate GAS binding to a number of host proteins, while the latter is a highly efficient activator of human plasminogen [5]. Plasminogen (Pg) is the zymogen of the serine protease plasmin (Pm) and circulates in blood and in most extravascular fluids. Fully active Pm is generated as a result of cleavage of R561-V562 peptide bond and cleavage of the N-terminal 77-residues of the Pg precursor. Pm is the primary fibrinolytic agent in mammalian systems, but has also been implicated in processes such as cell migration, wound healing, angiogenesis, and the growth and metastasis of tumors [6]. The critical nature of activated host Pg in bacterial infectivity in humans has been reinforced through work demonstrating that mice expressing the transgene for hPg exhibit a striking increase in mortality when infected with human GAS isolates in comparison to littermate controls [7, 8].
M proteins belong to a family of well-characterized surface proteins that form α-helical coiled-coils on the bacterial surface. One member of the M protein family, Pg-binding group A streptococcal M-like protein (PAM), is a 43 kDa single polypeptide chain that binds Pg or Pm with high affinity. Its role as a virulence factor in concert with human Pg/Pm has been confirmed in a study in which a GAS strain expressing a mutated version of PAM deficient in Pg-binding was found to be markedly less virulent than the strain expressing wt-PAM in mice carrying the hPg transgene [9]. Contained within the N-terminal region of PAM are two tandem repeats that direct the binding of PAM to Pg. Despite the obvious absence of a C-terminal lysine residue within this span, the PAM docking site on Pg has been localized to the lysine-binding site (LBS) of kringle 2 (K2), the weakest of the lysine-binding kringles [10]. Binding studies have established that a 30-residue peptide (VEK-30) that overlaps with this internal Pg-binding sequence, corresponding to PAM residues 85-113 with a non-native Tyr appended to the C-terminus as a spectrophotometric handle, is a convenient, readily synthesized surrogate ligand for full-length PAM [10,11]. Crystal structures of K2 and K1-K3 in complex with VEK-30 have revealed that the through-space orientations of the Arg17, His18, and Glu20 side-chains of VEK-30 are roughly isosteric with the charged moieties of a C-terminal lysine [12,13]. Additionally, the finding that the binding of VEK-30 to K1-K3 results in the disappearance of K3 electron density [13] suggests that a large conformational change may be operative in Pg-PAM functionality, akin to that observed for Pg in the presence of small-molecule activators such as 6-aminohexanoic acid (6-AHA) [14, 15]. Preliminary data from a recent study, in which VEK-30 was found to augment SK-mediated activation of Pg, is likewise supportive of this premise [16]. The present study was thus undertaken to determine if structural alterations attend the binding of VEK-30 to Pg, as evaluated by the effects of the bacterial peptide on the hydrodynamic volume and activation rate of Pg. The effects of full-length wt-PAM on Pg activation properties were also examined. The results of this study point towards a physiological scenario in which VEK-30 and PAM effect subtle structural changes in Pg that render it more susceptible to the action of the urokinase-type Pg activator (uPA) and SK, potentially facilitating the invasive character of PAM-expressing GAS strains.
2. EXPERIMENTAL
2.1. Proteins and peptides
Wild type (wt) human Glu1-Pg was a gift from Enzyme Research (South Bend, IN). SK from Streptococcus equisimilis was expressed and purified as described [17]. Wt-PAM (GAS strain NS13) and the mutants K98A/K111A (KK-PAM), R101A/H102A (RH-PAM) and K98A/R101A/H102A/E104A/K111A (KRHEK-PAM) were expressed and purified as described in an earlier study [18]. VEK-30 and a scrambled version of VEK-30 (sVEK-30), consisting of identical amino acid composition but randomized primary structure, were synthesized as previously published [12].
2.2. Analytical ultracentrifugation
All experiments were conducted in a Beckman XL-I Optima™ analytical ultracentrifuge (Beckman-Coulter, Fullerton, CA) operated at 20 °C. Sedimentation coefficients of wt Pg in the presence and absence of VEK-30 were determined under previously specified conditions [19]. The concentration of Pg was 0.5 mg/mL. Runs conducted in the presence of VEK-30 employed Pg:VEK-30 ratios of 1:5 and 1:20 (mol:mol). Analysis of the sedimentation velocity curves using Beckman XL-A/XL-I software (version 4.0) generated the Sapp values. For the determination of the apparent molecular weight (Mw,app) of PAM by sedimentation equilibrium, the protein was dissolved to a final concentration of 22 μM in 10 mM HEPES/100 mM NaCl pH 7.4, and rotated at speeds of 10,000 and 15,000 rpm. The data were analyzed using aforementioned software provided by Beckman, as was the simulation of the radius vs. absorbance curve for purely monomeric PAM. The experimentally derived apparent molecular weight was determined from global fitting of the data from three separate scans at the two indicated speeds.
2.3. Pg Activation assays
Activation of full length Glu1-Pg was followed at 25 °C in a Spectramax Plus 384 plate reader (Molecular Devices, Sunnyvale, CA) at 405 nm. The zymogen (27-270 nM) was incubated with 250 μM of the chromogenic substrate, S-2251 (Chromogenix, Milan, Italy) and varying concentrations of VEK-30 or PAM proteins, as indicated. After 10 min, Pg activation was initiated by the addition of Abbokinase (human uPA for injection, Abbott Laboratories, Chicago, IL) or by either an equimolar amount of SK (with respect to the molar concentration of Pg) or a catalytic amount of preformed SK-Pm. The latter was made immediately prior to use by mixing 3 μM each of Glu-Pg and SK (molar ratio) in 10 mM HEPES/100 mM sodium acetate, pH 7.4, and incubating for 30 min at room temperature. These conditions were sufficient for complete conversion to of Pg to Pm, as confirmed by performing SDS-PAGE on the samples under reducing conditions in which the light and heavy chains of Pm, but not single-chain of the Pg zymogen, were observed. All activation assay reactions consisted of a total volume of 200 μL in either 10 mM HEPES/100 mM NaCl, pH 7.4 or 10 mM HEPES/100 mM sodium acetate, pH 7.4. All the data presented correspond to the average of duplicate assays. Each experiment was repeated at least twice.
2.4. Surface Plasmon Resonance (SPR)
All the experiments were carried out in a BIAcore X instrument (BIAcore AB, Uppsala, Sweden). Pg or wt-PAM proteins were coupled to CM-5 sensor chips by the amine coupling method, to a level of 200-1000 resonance units (RU). All assays were carried out at 25 °C in HBS-EP buffer (BIAcore). Progress curves for binding were obtained by injecting PAM proteins (0.5 nM-50 nM) over immobilized Pg. Regeneration of the chip surface was accomplished by injecting 100 μL of 10 mM glycine pH 1.5. The entire set of sensorgrams for each experiment was X- and Y-transformed and the binding data were fitted to a 1:1 Langmuir binding model using the BIAevaluation 4.1 software (BIAcore AB). Association and dissociation rate constants (kon and koff) were fitted globally and separately. From these data, the equilibrium dissociation constants (Kd) were calculated. Validation of the Kd values derived from the kinetic data was achieved through evaluation of steady-state binding data by plotting response at equilibrium versus analyte concentration.
3. RESULTS
3.1 Effects of the bacterial PAM peptide on the sedimentation properties of Pg
The present study was undertaken to determine if the interaction between the PAM peptide and full length Pg had discernible consequences on the tertiary structure of the latter. For this purpose, sedimentation velocity analyses were performed on full-length Pg in the presence and absence of VEK-30 in Cl−- and acetate-containing buffers. Cl− is considered to be a negative effector of Pg activation that promotes the closed, tight conformation of the zymogen, while acetate ion is much less effective in this regard [20]. In contrast to Cl−, C-terminal lysine analogs (such as 6-AHA) are considered to be positive effectors of activation, due to their ability promote the open, relaxed conformation of Pg [14]. This is dramatically illustrated by the approximately 1 unit decrease in sedimentation coefficient (S°20,w) of Pg when saturating amounts of 6-AHA are added to Pg in Cl−-containing buffer [14,19,20]. As can be concluded from the data summarized in Table 1, the effect of a 5-fold molar excess of VEK-30 on Pg conformation is negligible in Cl− buffer and small, but significant, in acetate buffer. At a 20:1 molar ratio of VEK-30 to Pg, the reduction of S°20,w in acetate buffer is even more pronounced. However, the modest changes result from the binding of VEK-30 are likely at a locus not related to the primary VEK-30 binding site in the K2 binding pocket. This conjecture is based on calculations using the estimated Kd of VEK-30 for K2, which is in the range of 25-50 nM [11,21]. At a 5:1 molar ratio of VEK-30 to Pg, which corresponds to an absolute concentration ratio of 28 μM (VEK-30) to 5.5 μM (Pg), the K2 binding site is already greater than 99% saturated by VEK-30. Hence, the 0.84 unit reduction in S°20,w that attends Pg in the presence of a 20:1 molar ratio of VEK-30 to Pg suggests that VEK-30 may be acting nonspecifically at a site ancillary to the primary K2 site in acetate buffer. This conjecture is reinforced by the decrease in S°20,w of Pg observed in acetate buffer at a 1:20 molar ratio of Pg to sVEK-30, a randomized variant of the VEK-30 parent..
Table 1.
Effects of VEK-30 on the sedimentation coefficient (S°20,w) of Glu1-Pga.
| Analyte | Buffer | |
|---|---|---|
| Tris-HCl/NaCl | Tris-OAc/NaOAc | |
| Pg | 5.60 ± 0.03 | 5.15 ± 0.08 |
| Pg:VEK-30 (1:5) | 5.61 ± 0.03 | 4.98 ± 0.06* |
| Pg:VEK-30 (1:20) | 5.52 ± 0.05 | 4.31 ± 0.08* |
| Pg:sVEK-30 (1:20) | NDb | 4.86 ± 0.08* |
Values are the average of at least 2 separate experiments ± SEM.
Values denoted with an asterisk represent significant (P < 0.05) changes in S°20,w compared to the corresponding buffer conditions in the absence of VEK-30.
Not determined.
3.2. VEK-30 stimulates the uPA-catalyzed activation of Pg
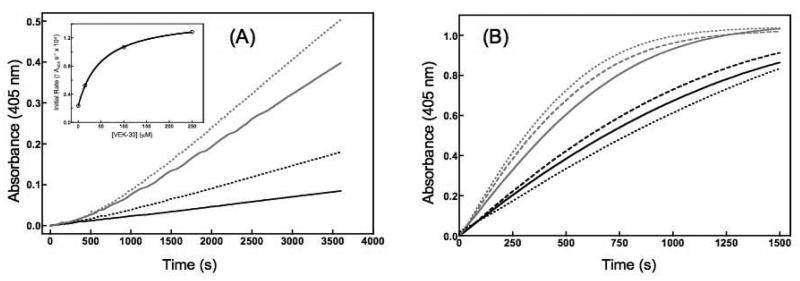
VEK-30 was utilized in a series of Pg activation assays in which human uPA and bacterial SK were used as activators of Pg. The primary data of Fig. 1A reveal that increasing the concentration of VEK-30 results in an increase in the rate of Pm generation in both Cl−- and acetate-containing buffer. However, the enhancement in Pm generation resulting from the presence of VEK-30 is more pronounced for the assays conducted in Cl− buffer. This is illustrated by the nearly 7-fold rate enhancement effected by 75 μM VEK-30 in Cl− buffer compared with the 2.3-fold increase stimulated by the same VEK-30 concentration in acetate buffer (Fig. 1B). However, while not as efficacious in potentiating uPA-mediated Pg activation in acetate buffer, VEK-30 is more potent in acetate conditions, manifesting an EC50 value of 1.8 ± 0.4 μM. In Cl− buffer, this value is increased to 16.5 ± 0.9 μM (Fig. 1B, inset). In the presence of 1 mM 6-AHA, the rate-enhancements of Pm generation catalyzed by uPA were only 2.2-fold and 1.1-fold in Cl− and acetate buffers, respectively (data not shown). Hence, across both buffer systems, VEK-30 is a more potent stimulator of Pg conversion to Pm than 6-AHA, the canonical small-molecule activator of Pg.
Figure 1.
Effects of VEK-30 on Pm generation by uPA in Cl−- and acetate-containing buffers. Glu1-Pg (27 nM) was activated by 20 nM of uPA in the presence of VEK-30. (A) Plots of absorbance at 405 nm versus time depicting the activity of generated Pm on the chromogenic substrate S-2251 (250 μM). Assays were performed in 10 mM HEPES/100 mM NaCl buffer, pH 7.4 (solid lines) or 10 mM HEPES/NaOAc buffer, pH 7.4 (dotted lines). For each buffer condition, the concentrations of VEK-30 included in the assays were, from bottom to top, 0, 0.9, 3, 15, and 75 μM. Inset, linearity of the rate of Pg activation at time points corresponding to ≤ 15% substrate consumption. Closed and open circles represent the data points obtained from assays conducted in NaCl and NaOAc buffers, respectively, while the lines correspond to the fits of those data obtained from linear regression analysis. (B) Fold increases in the rate of VEK-30 stimulated Pm generation in the presence of 10 mM HEPES/100 mM NaCl buffer, pH 7.4 (black bars) and 10 mM HEPES/100 mM NaOAc buffer, pH 7.4 (gray bars) as assessed from the initial rate data of companion panel A. Initial rates accompanying uPA-mediated Pm activation in the absence of VEK-30 in both Cl−- and acetate-containing buffers were used as the reference for the calculation of fold changes. Inset, concentration-response curves corresponding to the initial rates of Pm generation as a function of VEK-30 concentration. Data were fit by non-linear regression to the equation for one-site direct binding. The EC50 values (± SE for the best fit) for VEK-30 derived from the depicted fits are 16.5 ± 0.9 μM and 1.8 ± 0.4 μM for the experiments conducted in Cl− (black squares) and acetate (gray squares), respectively.
3.3. VEK-30 increases the SK-mediated activation of Pg
From the physiological and pathological standpoints, the effects of VEK-30 on human Pg activation by SK, a Pg activator secreted by GAS bacteria that lacks inherent protease activity, are of particular interest. The data shown in Fig. 2A reveals that the enhancement, by VEK-30, of SK-mediated Pg activation occurs in a concentration-dependent manner in Cl− buffer—a trend similar to the one observed when uPA was used as the activator. At 150 μM, the highest VEK-30 concentration employed, the enhancement of SK-mediated Pg activation is over 12-fold (Fig. 2B). In contrast, the potentiating effect of VEK-30 shows minor concentration dependence in acetate conditions (Fig. 2A and Fig. 2C). The EC50 value for VEK-30 as an SK potentiator in Cl− is 79 ± 11 μM, significantly higher than that observed in the corresponding uPA assay. In Cl− buffer, the randomized sVEK-30 was unable to replicate the effects of the wt peptide (Fig. 2B). In contrast, sVEK-30 evoked a small concentration-dependent on Pg activation similar to that observed with the wt peptide (Fig. 2B).
Figure 2.
Effects of VEK-30 and sVEK-30 on the activation of Glu1-Pg by SK in Cl−- and acetate-containing buffers. (A) Plots of absorbance at 405 nm versus time depicting the activity of generated Pm on the chromogenic substrate S-2251 (250 μM). Activation of Glu1-Pg (27 nM) by the addition of SK (20 nM) was carried out in 10 mM HEPES/100 mM NaCl, pH 7.4 (solid lines), or 10 mM HEPES/100mM NaOAc buffer, pH 7.4 (dotted lines). For assays conducted in Cl−-containing buffer, the concentrations of VEK-30 used were, from bottom to top, 0, 0.3, 1.5, 15, 45, and 75 μM. For assays conducted in acetate-containing buffer, the traces depicted correspond to VEK-30 concentrations of 0, 1.5, 15, and 150 μM (bottom to top). Inset, linearity of the rate of Pg activation at time points corresponding to ≤ 15% substrate consumption. Closed squares represent the data points obtained from assays conducted in NaCl buffer, while the lines correspond to the fits of those data obtained from linear regression analysis. (B) The time course of Pm generation catalyzed by SK in the presence of sVEK-30 in Cl−- and acetate-containing buffers. Experimental conditions were the same as those described in Figure 2A. Assays conducted in Cl−-containing buffer are represented by the bottom grouping of traces, while those experiments conducted in acetate-containing buffer are denoted by the top three traces. For each buffer condition the concentrations of sVEK-30 used were 0 μM (solid lines), 15 μM (dashed lines), and 150 μM (dotted lines). (C) Fold increases in the rate of VEK-30-stimulated Pm generation in the presence of 10 mM HEPES/100 mM NaCl buffer, pH 7.4 (gray bars) and 10 mM HEPES/100 mM NaOAc buffer, pH 7.4 (white bars) as assessed from the initial rate data of companion panel A. Initial rates accompanying SK-mediated Pm activation in the absence of VEK-30 in both Cl−- and acetate-containing buffers were used as the reference for the calculation of fold changes. Inset, concentration-response curve corresponding to the initial rates of Pm generation as a function of VEK-30 concentration in Cl−-containing buffer. Data were fit by non-linear regression to the equation for one-site direct binding. The EC50 value (± SE for the best fit) for VEK-30 derived from the depicted fit is 78.8 ± 11.6 μM.
The mechanism of Glu1-Pg activation by SK is a unique process characterized by the following scheme [22-25]:
The individual steps of this process involve the formation of a 1:1 SK•Pg encounter complex that subsequently undergoes a set of conformational alterations that results in the generation of intermediates SK•Pg* and SK•Pg’. These species possess serine protease activity despite retention of the R561-V562 peptide bond, a molecular feature that distinguishes Pg from Pm. Further processing, not entirely understood, produces SK•Pm complexes capable of acting as conventional Pg activators to produce Pm. Because SK-Pg*, SK-Pg’ SK-hPm and Pm all possess the ability to cleave the chromogenic substrate S-2251 [22,24,25], it is conceivable that the effects of VEK-30 on the activation profiles depicted in Fig. 2 result from VEK-30 enhancement of the proteolytic activity of the aforementioned hydrolytic species rather than rendering Glu1-Pg more activatable. To these possibilities, we first investigated the effects of increasing levels of peptide on the activation of Glu1-Pg (50 nM) in Cl−-containing buffer by a catalytic amount (1.5 nM) of pre-formed SK-Pm complex. As shown in Fig 3A, a direct correlation was observed between the activation rate of Pg and the concentration of VEK-30. In contrast, increasing concentrations of VEK-30 had a negligible effect on the initial rates of S-2251 hydrolysis catalyzed by SK-Pm and Pm (Fig. 3B). Taken together, these data suggest a process in which potentiation, by VEK-30, of the rate of substrate hydrolysis observed in the presence of equimolar amounts of Pg and SK (Fig. 2) arises from a direct effect between Pg and the bacterial peptide, and does not support a mechanism in which the incipient catalytic species, viz., SK-Pm and Pm, are affected by VEK-30.
Figure 3.
The effects of VEK-30 on the rate of S-2251 conversion in the presence of catalytically active components of the Pg-SK system in Cl−-containing buffer. (A) Increasing amounts of VEK-30 enhance the rate of S-2251 (250 μM) hydrolysis in the presence of catalytic amounts of pre-formed SK-Pm complex (1.5 nM) and Glu1-Pg (50 nM). The VEK-30 concentrations represented, from bottom to top, are 0, 15, 100, and 250 μM. Inset, Plot of the concentration-response data corresponding to the initial rates of S-2251 hydrolysis as a function of VEK-30. Data were fit by non-linear regression to the equation for one-site direct binding. The EC50 value (± SE for the best-fit) for VEK-30 derived from these fitted data is 50.4 ± 1.3 μM. (B) Hydrolytic activity of 45 nM preformed SK-Pm complex (black traces) and 60 nM Pm (gray traces) on S-2251 (250 μM) in the presence of 0 μM (solid lines), 15 μM (dashed lines), and 150 μM (dotted lines) VEK-30. All experiments were conducted in 10 mM HEPES/100 mM NaCl, pH 7.4.
3.4. Full-length PAM protein is a potent stimulator of SK-mediated Pg activation
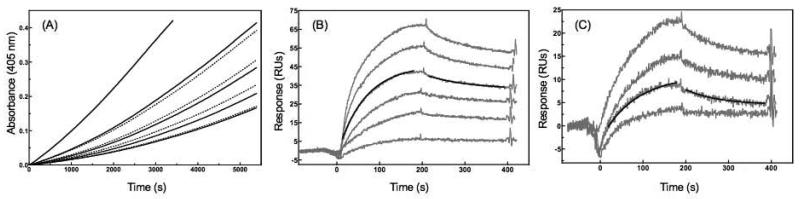
Previously studies have identified VEK-30 as a high-affinity (Kd ~50 nM) ligand for K2 of Pg, making it a convenient peptide analog of the PAM parent [11,21]. However, because PAM displays an affinity for Pg that is approximately 50-fold greater than that displayed by VEK-30 [11], it is likely that sequence elements located outside of the segment comprising VEK-30 contribute to the PAM-Pg binding interface. To determine if similar PAM sequence considerations are at play in the SK-mediated activation of Pg, the ability of PAM to potentiate the activation rate of Pg by equimolar SK was evaluated. As shown in Fig. 4A, the full-length bacterial protein was capable of enhancing the activation rate of Pg in a concentration-dependent manner in Cl−-containing buffer. At concentrations as low as 20 nM PAM, a 3.6-fold increase in the activation rate was observed. For VEK-30 potentiation under the same buffer conditions, a comparable increase (3.3-fold) required much higher (15 μM) concentrations of VEK-30 (Fig. 2B). Hence, PAM is approximately 3 orders of magnitude more potent than VEK-30 in stimulating the SK-mediated conversion of Pg to Pm. At 500 nM, the highest concentration of PAM employed, a 220-fold enhancement in the Pg conversion rate was attained (data not shown), whereas the effects of VEK-30 in the submicromolar range were found to be negligible (Fig. 2B). Hence, it can be concluded that PAM residues that lie outside of the VEK-30 sequence are not only responsible for the enhanced Pg affinity displayed by the full-length protein, but also augment the stimulatory properties of the full-length protein, compared to VEK-30, in the context of SK-dependent Pg activation.
Figure 4.
The interaction of Pg with wt-PAM and R101A/H102A-PAM. (A) Effects of recombinant wt-PAM protein (solid lines) and R101A/H102A-PAM (dotted lines) on the activation rate of Pg (80 nM) by equimolar amounts of SK. The concentrations of wt-PAM used were 0, 1, 5, 10 and 20 nM (bottom to top) and the concentrations of R101A/H102A-PAM used were 1, 5, 10, 20 nM (bottom to top). The buffer was 10 mM HEPES/100 mM NaCl, pH 7.4. (B) Real time interaction (gray traces) of wt-PAM (0.1, 6.25, 12.5 and 35 nM, bottom to top) and immobilized Pg studied by SPR. The black traces are representative fits of the association and dissociation phases shown for the 6.25 nM concentration of wt-PAM. (C) Real time interaction (gray traces) of R101A /H102A-PAM (0.1, 6.25, 12.5 and 35 nM, bottom to top) and immobilized Pg studied by SPR. The black traces are representative fits of the association and dissociation phases shown for the 6.25 nM concentration of the mutant version of PAM. For the SPR studies, Pg was immobilized on CM-5 chips (~600 RU) and the interactions with the PAM proteins were monitored at 25 °C using a flow rate of 30 μL/min in HBS-EP buffer.
Based on the crystal structure of this kringle in complexation with VEK-30 [13], a model was proposed in which the LBS of K1-K3 interacts with residues R17, H18, and E20 of VEK-30 (R101, H102, and E104 in PAM numbering). The charged side chains of these residues, contained within a single turn of the VEK-30 helix, assume a spatial arrangement that is isosteric with the charged moieties of C-terminal lysine. Hence, the anionic center of the LBS formed by K2 residues D219 and E221 (Glu1-Pg numbering) interacts with the two basic residues of VEK-30 previously mentioned, while the cationic locus of the K2 binding pocket, R234, forms a salt-bridge with E104 of VEK-30. Experimental confirmation of this model was provided by direct binding studies in which it was shown that binding to K2 was completely abrogated for VEK-30[R101A] and reduced by about 10-fold for the H102A and E104A variants [12,26]. However, with respect to full-length PAM, these specific replacements had minimal effect on the affinity of the resultant variants for Pg [18]. These outcomes are consistent with the very slight diminution of potentiation observed in the kinetic profile of the R101A/H102A PAM mutant (RH-PAM) in comparison to wt-PAM (Fig. 4A). Another PAM mutant was generated in which K98, R101, H102, E104, and K111 were replaced by alanine (KRHEK-PAM). While this version of PAM is devoid of all three residues that form the VEK-30 pseudo C-terminal lysine, it stimulated SK-mediated Pg activation in a manner identical to RH-PAM (data not show). A fourth mutant, K98A/K111A-PAM (KK-PAM), was designed to probe the roles of the two lysines contained within the a1/a2 repeat of PAM, one of which (K98) forms a salt-bridge with D219 of K2 in the VEK-30/K1-K3 crystal structure. The apparent importance of this electrostatic contact in the interaction of the bacterial peptide with K2 was highlighted by binding data which revealed that the affinity of VEK-30[K98A] for K2 was reduced by 20-fold compared to VEK-30 [26]. However, this substitution in full-length PAM yielded a mutant that displayed a profile that was indistinguishable from wt-PAM in the Pg activation assays (data not shown). Collectively, these results suggest that VEK-30 and PAM assume different modalities for binding to K2.
3.5. Direct binding of wt-PAM and variants to Pg
To evaluate the relationship between stimulation of SK-mediated Pg activation and Pg binding for wt-PAM and the three aforementioned PAM variants, we employed SPR in the determination of direct Pg-PAM binding parameters (Fig. 4B and 4C). The Kd values obtained for the binding of KK-PAM and KRHEK-PAM to Pg are similar to those obtained for wt-PAM, in consonance with the similar stimulatory effects these proteins exert in the Pg activation assays (Table 2). Moreover, for these three PAM proteins, the kinetics of association and dissociation are dictated by similar rate constants. In contrast, the affinity of RH-PAM for immobilized Pg is compromised approximately 20-fold compared with wt-PAM. This increase in Kd value is dominated by the kon term, which is decreased 10-fold for the RH variant, whereas the dissociation rate constant for RH-PAM is only 2-fold faster than the value observed for the wt protein. Hence, despite the apparent importance of the pseudo-lysine motif in mediating VEK-30 interactions with K2 of Pg, the specific residues implicated in the K1-K3/VEK-30 structure are not as consequential with respect to the binding and activation of PAM and Pg.
Table 2.
Binding parameters for the interaction of Pg and PAM proteins.
| Soluble analytea | kon (M−1s−1) | koff (s−1) | Kd (nM)b |
|---|---|---|---|
| wt-PAM | (3.4 ± 0.9) × 105 | (7.9 ± 1.7) × 10−3 | 23 ± 11 (33 ± 1) |
| KK-PAM | (2.5 ± 1.2) × 105 | (9.5 ± 0.9) × 10−3 | 38 ± 19 (44 ± 25) |
| RH-PAM | (3.3 ± 1.2) × 104 | (1.4 ± 0.1) × 10−2 | 424 ± 157 (731 ± 440) |
| KRHEK-PAM | (3.4 ± 0.9) × 105 | (1.7 ± 0.7) × 10−2 | 50 ± 241 (50 ± 90) |
Glu1-Pg was covalently immobilized on the CM-5 chip at levels between 200-1000. The injected ligand analyte was varied between 0.5-50 nM.
Kd values were values calculated from the kinetic data (koff/kon). Values in parentheses were determined from the response (RU) at equilibrium versus analyte concentration. Values reported are the averages of at least three independent experiments ± SEM.
3.6. Self-association of PAM
As a member of the M-like protein family, we surmised that PAM likely exists as an extended coiled-coil, since this supersecondary motif has been empirically established for several M and M-like proteins and, owing to the high primary sequence homology shared among these protein families in their central and C-terminal domains, is strongly predicted for other members [27-29]. We have previously determined that neither VEK-30 nor the VEK-30/K1-K3 complex exhibit higher-order structures in solution. However, the N-terminal regions of the M proteins (wherein the VEK-30 sequence is located) display higher variability and therefore lower propensity for adoption of the coiled-coil motif in comparison with their C-terminal segments [1,30]. As shown in Fig. 5, sedimentation equilibrium experiments performed with full-length PAM indeed revealed the existence of a higher-order species characterized by a Mw,app of 72300 (± 5800). This value is significantly higher than the Mw,app of 43600 expected for purely monomeric PAM based on primary sequence—a difference that is highlighted by comparing the fitted experimental data with the simulated curve for the monomeric species (Fig. 5). These results lend support to the conjecture that PAM, like other M and M-like proteins, can self-associate, most likely adopting the form of an extended coiled-coil.
Figure 5.
Sedimentation equilibrium data showing the tendency of PAM (22 μM) to self-associate. The data (○) for this representative scan were collected at 20 °C at a rotor speed of 15,000 rpm. The calculated fit (black line) for these data yielded a Mw,app of 71600. The partial specific volume of PAM was calculated on the basis of amino acid composition and determined to be 0.734 mL/g. A simulation of the fit corresponding to monomeric PAM (Mw,app = 43600) under identical conditions is shown for comparison (gray line).
4. DISCUSSION
Within the realm of protein structural biology, the reversible interconversion of Glu1-Pg between closed and open forms is regarded as one of the more dramatic examples of a ligand-induced conformational change. Several compelling lines of evidence exist in support of a major structural alteration between the Cl−-bound (closed) and 6-AHA-bound (open) Pg forms [15,20,31,32]. Circular dichroism spectroscopy reveals that both Pg forms have the same secondary structure [15], hence the closed state is likely maintained by interdomain contacts that are abrogated upon ligand binding. However, in the absence of a high-resolution structural comparison of apo- and ligand-saturated Pg, the precise interactions mediating the closed versus open forms remain speculative. To date, the crystallographic structures of unbound K1-K3 and the K1-K3/VEK-30 complex are the only multi-kringle structures that have been solved at atomic resolution [12,13]. Comparison of the structures of unbound and VEK-30-bound K1-K3 revealed two major differences [13]. Firstly, in an overlay in which K2 of the apo and bound species were superimposed, a 48° rotation of K1 was noted. Secondly, in contrast to unbound K1-K3, electron density for most residues of K3 was not observed in the VEK-30-complexed structure. Additionally, no electron density was mapped for the 9 latter residues of 11 residue linker region that intervenes between K2 and K3. This lack of electron density was attributed to an increase in the mobility of K3 in excess of the time-scale of the crystallographic experiment, rendering the K3 residues invisible. When considering the changes in K1-K3 structure and mobility that appear to result from VEK-30 binding, it seemed reasonable to posit that these aforementioned conformational changes could also occur in full-length Pg, in addition to other structural effects that might be communicated beyond K1-K3. However, as determined through sedimentation velocity analysis, the presence of 5- and 20-fold molar excesses of VEK-30 failed to exert any effect on the S°20,w of the Cl−-bound, closed form of Pg. Importantly, the differences in the S°20,w value of Pg between 6-AHA and VEK-30 indicate that the mechanism of action of the bacterial peptide, in Cl− buffer, does not replicate that of 6-AHA. A likely explanation for the refractory nature of the closed Pg form in the presence of VEK-30 may hinge on the apparent importance of the interaction between the N-terminal activation peptide (AP) and kringle 5 in maintaining the compact state of the molecule [18,33]. Because K2 is the only Pg kringle that has demonstrable VEK-30 binding [10], it is not surprising that VEK-30, by virtue of its inability to bind K5, is unable to promote the extended conformation of Pg. Despite the persistence of the closed Pg form in the presence of VEK-30, the formation of the VEK-30-Pg complex may trigger local rearrangements that render the R561-V562 bond more susceptible to cleavage and/or could promote exposure of the AP-cleavable region, thereby enhancing the rate of zymogen conversion. These conformational alterations may include the aforementioned changes in the K1-K3 crystal structure that accompany VEK-30 binding [13], i.e., rotation of K1 and an increase in K3 flexibility. These permutations, while significant from the conformational standpoint, are not linked to the closed-open transition in Pg and, thus, are without effect on the S°20,w.
The apparent increase in hydrodynamic volume noted for Pg when saturated with VEK-30 in acetate buffer, as evidenced by the decrease in the S°20,w, is difficult to rationalize, but may be related to nonspecific binding of the peptide to a site or sites in Pg that further relaxes, or partially unfolds, the open form of Pg. Because a similar outcome was noted with sVEK-30, this effect is not specific to the VEK-30 sequence and may be related to the preponderance of charged amino acids (9 acidic and 6 basic) that constitute VEK-30 and the randomized variant.
Under saturating VEK-30 concentrations, the rate enhancement of uPA-catalyzed Pg activation is more robust in Cl− (6.8-fold) than in acetate buffer (2.3-fold). These increases mirror those observed for Pg activation in the presence of 6-AHA, in which a 10-fold and 1.2-fold increase in Pg conversion was effected by 6-AHA in Cl− and acetate buffer, respectively [34]. However, because 6-AHA can promote the extended conformation of Pg whereas VEK-30 cannot, the global Pg structural changes produced by these two effectors differ greatly, although these differences do not exclude a scenario in which VEK-30 and 6-AHA can effect similar local changes in the vicinity of the R561-V562 peptide bond.
As observed for uPA activation of Pg in Cl− buffer, the rate of SK-mediated conversion of Pg is augmented in the presence of increasing concentrations of VEK-30. It was also observed that the conversion rate of the closed form of Pg by a catalytic amount of preformed SK-Pm increases as a function of VEK-30 concentration. Because VEK-30 does not alter the rate of substrate hydrolysis catalyzed by SK-Pm or Pm, it can be assumed that the rate accelerations observed in Fig. 2 and Fig. 3B result from the stabilization, by VEK-30, of a more activation-susceptible conformation of Pg in either free or SK-bound form. Unlike the outcome observed for uPA in acetate buffer, the effects of VEK-30 on SK-mediated Pg conversion are modest (Fig. 2). This indicates that in the open, acetate-bound conformation, the equimolar SK:Pg complex is almost maximally disposed with respect to both AP and R561-V562 bond cleavage. Because the slight stimulation in Pg activation exerted by VEK-30 in the acetate buffer system can be recapitulated by sVEK-30 (Fig. 2B), this effect may be nonspecific in character. This result is consistent with the sedimentation behavior of Pg observed in acetate buffer with both VEK-30 and sVEK-30 and may be sourced in a peptide-induced unfolding of Pg related to the highly charged character of both peptide species.
Also of note are the differing EC50 values for VEK-30 enhancement of Pg activation, as mediated by equimolar uPA, equimolar SK, and catalytic SK-Pm in Cl− buffer. These values, derived from the curves depicted in the insets to Figs. 1B (equimolar uPA), 2C (equimolar SK), and 3A (catalytic SK-Pm) are 16.5 μM, 78 μM, and 50 μM, respectively. These disparate EC50 values likely reflect the interaction of VEK-30 with three distinct Pg species, namely, uPA-bound Pg, SK-bound Pg, and free Pg.
As seen in the VEK-30 concentration-response curve for the SK-Pm-catalyzed conversion of Pg to Pm (Fig. 3A, inset), the EC50 value of 50.4 μM fits well to a model of one-site binding, as is also observed for VEK-30 stimulation of Pg activation by uPA and equimolar SK (insets to Figs 1B and 2C, respectively). These observations suggest that in each experiment the stimulatory effects of VEK-30 are exerted on a single species (although passive binding to other species cannot be discounted). Hence, the bacterial peptide most likely does not alter the equilibrium or the rates of the intermediate steps in the Pg activation process carried out by SK. In other words, the step of conformational activation (SK-Pg conversion to SK-Pg*), and the conversions of SK-Pg* to SK-Pg’ and SK-Pg’ to SK-Pm, are not significantly affected by the presence of VEK-30.
The ability of VEK-30 to augment uPA-catalyzed Pg conversion, in addition to SK-mediated Pg activation, demonstrates that PAM-mediated effects on Pg are not specific to the bacterial system. Also, the low EC50 for VEK-30 in the enhancement of uPA-mediated Pg activation points toward a contributory role for host Pg activators in the virulence of PAM-positive GAS strains. In the immediate microenvironment of the infecting bacterium, wherein the effective SK concentration would be high compared with uPA, such a role would likely be minimal. However, because of the tissue remodeling that occurs in invasive GAS infection, the involvement of tPA in this setting may also be of some physiological significance and remains to be explored. The data presented herein suggest that VEK-30 exerts a general effect on the conformation of Pg, possibly by rendering the R561-V562 bond more disposed to cleavage by activators.
An additional point to consider is that the aforementioned EC50 values for VEK-30 in the activation of Pg are 2-3 orders of magnitude higher than the dissociation constants that attend the mere binding of VEK-30 to Pg [11,21]. Several plausible explanations for this discrepancy can be forwarded, including the likelihood that the VEK-30 conformation required for binding is different from that required for activation. In solution, free VEK-30 is highly flexible and samples a large array of conformations [12]. If the population of the activation-promoting confirmation is small relative to the conformation sufficient for binding, this would account for the need for high VEK-30 concentrations in the activator-mediated conversion of Pg to Pm.
Lastly, full-length PAM was found to be a much more potent stimulator of SK-mediated Pg activation than VEK-30, in consonance with the approximately 50-fold increase in the apparent binding affinity of PAM for Pg compared with VEK-30 [11]. The conformational argument presented above could be invoked as one explanation for this disparity. Full-length PAM displays significant α-helicity in solution, and most likely exists as a dimeric coiled-coil (vide infra). As such, the activation-promoting form(s) of PAM may represent a greater sub-population of conformational states due to the enhanced rigidity of PAM compared to VEK-30. Another explanation for the lack of agreement between VEK-30 and PAM, with respect to Pg binding and activation characteristics, may lie in the absence of key residues in VEK-30 that contribute to exosite interactions between Pg and PAM, rendering the interaction tighter and thereby increasing the potency of the bacterial protein in stimulating Pg activation. A recent investigation identified R114 and H115 as being integral to the interaction of PAM with Pg [18], however an earlier study [10] showed that a peptide comprised of PAM residues 85-116 displayed K2 binding properties that were very similar to those of VEK-30 (which lacks R114 and H115). It is possible that in the absence of R101 and H102, the R114/H115 dyad can fulfill the electrostatic role of the former residues with respect to interactions in the K2 pocket. This interpretation is borne out by the results of this study in which alanine substitution of PAM residues R101 and H102 (RH-PAM) effected only a slight reduction (~ 2-fold) in the capacity of the resultant protein to potentiate SK-mediated conversion of the Pg zymogen, and a 20-fold decrease in the affinity of RH-PAM for Pg as assessed by SPR-monitored binding. Additionally, the Pg activation and SPR results obtained with KRHEK-PAM suggest that the role played by Glu104 in VEK-30, i.e., functioning as the carboxylate moiety of the C-terminal lysine isostere, may be assumed by other acidic amino acids in PAM. Candidate residues for satisfying this function could include Asp116 or Asp118. These mechanistic scenarios could also account for the marked reduction of K2 binding observed for VEK-30 peptide variants in which the Arg, His, and Glu residues corresponding to sequence positions 101, 102, and 104 of PAM were replaced by Ala [12, 26]. These peptides lacked the residues corresponding to R114, H115 and the downstream Asp residues in PAM, hence those particular amino acids were not available to function as surrogates for R101, H102, and Glu104, roles they could be assuming in the RH-PAM and KRHEK-PAM mutants. An additional point that obscures comparisons between results obtained with full-length PAM and VEK-30 involves the ability of PAM, but not VEK-30, to self-associate in solution. The crystal structures of VEK-30 bound to K2 and K1-K3 reveal two molecules of each complex in the asymmetric unit, with the interfaces of each dimer residing between two parallel, end-to-end VEK-30 helices [12,13]. These latter outcomes may result from crystal packing interactions that are obviously not operating in solution. It is possible that full-length PAM, despite a strong tendency to self-associate, contains segments of coiled-coil instability, as has been established for other M proteins [35]. Such segments may include that which corresponds to VEK-30, which could bind as a single strand within the K2 binding pocket. A high-resolution structure of PAM complexed with K2 would clearly be invaluable in delineating the molecular features that distinguish the high affinity binding of PAM to K2 from that of the weaker VEK-30/K2 interaction.
ACKNOWLEDGEMENTS
*This work was supported by National Institutes of Health grant HL-013423 (to F.J.C). We thank Raul Juarez for assisting with peptide synthesis and purification.
Footnotes
- 6-AHA
- 6-aminohexanoic acid
- GAS
- group A streptococcus
- PAM
- plasminogen binding group A streptococcal M-like protein
- Pg
- plasminogen
- Pm
- plasmin
- SK
- streptokinase
- uPA
- urokinase-type Pg activator
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Berge A, Sjobring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J. Biol. Chem. 1993;268:25417–25424. [PubMed] [Google Scholar]
- [2].Ploplis VA, Castellino FJ. Nonfibrinolytic functions of plasminogen. Methods. 2000;21:103–110. doi: 10.1006/meth.2000.0981. [DOI] [PubMed] [Google Scholar]
- [3].Walker MJ, McArthur JD, McKay F, Ranson M. Is plasminogen deployed as a Streptococcus pyogenes virulence factor? Trends Microbiol. 2005;13:308–313. doi: 10.1016/j.tim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [4].Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- [5].Ringdahl U, Sjobring U. Analysis of plasminogen-binding M proteins of Streptococcus pyogenes. Methods. 2000;21:143–150. doi: 10.1006/meth.2000.0985. [DOI] [PubMed] [Google Scholar]
- [6].Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb. Haemost. 2005;93:647–654. doi: 10.1160/TH04-12-0842. [DOI] [PubMed] [Google Scholar]
- [7].Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjöbring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- [8].Cole JN, McArthur JD, McKay FC, Sanderson-Smith ML, Cork AJ, Ranson M, Rohde M, Itzek A, Sun H, Ginsburg D, Kotb M, Nizet V, Chhatwal GS, Walker MJ. Trigger for group A streptococcal M1T1 invasive disease. FASEB J. 2006;20:1745–1747. doi: 10.1096/fj.06-5804fje. [DOI] [PubMed] [Google Scholar]
- [9].Sanderson-Smith ML, Dinkla K, Cole JN, Cork AJ, Maamary PG, McArthur JD, Chhatwal GS, Walker MJ. M protein-mediated plasminogen binding is essential for the virulence of an invasive Streptococcus pyogenes isolate. FASEB J. 2008;22:2715–2722. doi: 10.1096/fj.07-105643. [DOI] [PubMed] [Google Scholar]
- [10].Wistedt AC, Kotarsky H, Marti D, Ringdahl U, Castellino FJ, Schaller J, Sjobring U. Kringle 2 mediates high affinity binding of plasminogen to an internal sequence in streptococcal surface protein PAM. J. Biol. Chem. 1998;273:24420–24424. doi: 10.1074/jbc.273.38.24420. [DOI] [PubMed] [Google Scholar]
- [11].Wistedt AC, Ringdahl U, Muller-Esterl W, Sjobring U. Identification of a plasminogen-binding motif in PAM, a bacterial surface protein. Mol. Microbiol. 1995;18:569–578. doi: 10.1111/j.1365-2958.1995.mmi_18030569.x. [DOI] [PubMed] [Google Scholar]
- [12].Rios-Steiner JL, Schenone M, Mochalkin I, Tulinsky A, Castellino FJ. Structure and binding determinants of the recombinant kringle-2 domain of human plasminogen to an internal peptide from a group A Streptococcal surface protein. J. Mol. Biol. 2001;308:705–719. doi: 10.1006/jmbi.2001.4646. [DOI] [PubMed] [Google Scholar]
- [13].Cnudde SE, Prorok M, Castellino FJ, Geiger JH. X-ray crystallographic structure of the angiogenesis inhibitor, angiostatin, bound to a peptide from the group A streptococcal surface protein PAM. Biochemistry. 2006;45:11052–11060. doi: 10.1021/bi060914j. [DOI] [PubMed] [Google Scholar]
- [14].Violand BN, Sodetz JM, Castellino FJ. The effect of epsilon-amino caproic acid on the gross conformation of plasminogen and plasmin. Arch. Biochem. Biophys. 1975;170:300–305. doi: 10.1016/0003-9861(75)90121-6. [DOI] [PubMed] [Google Scholar]
- [15].Mangel WF, Lin B, Ramakrishnan V. Characterization of an extremely large, ligand-induced conformational change in plasminogen. Science. 1990;248:69–73. doi: 10.1126/science.2108500. [DOI] [PubMed] [Google Scholar]
- [16].Fu Q, Figuera-Losada M, Ploplis VA, Cnudde S, Geiger JH, Prorok M, Castellino FJ. The lack of binding of VEK-30, an internal peptide from the group A streptococcal M-like protein, PAM, to murine plasminogen is due to two amino acid replacements in the plasminogen kringle-2 domain. J. Biol. Chem. 2008;283:1580–1587. doi: 10.1074/jbc.M705063200. [DOI] [PubMed] [Google Scholar]
- [17].Sazonova IY, Robinson BR, Gladysheva IP, Castellino FJ, Reed G. alpha Domain deletion converts streptokinase into a fibrin-dependent plasminogen activator through mechanisms akin to staphylokinase and tissue plasminogen activator. J. Biol. Chem. 2004;279:24994–25001. doi: 10.1074/jbc.M400253200. [DOI] [PubMed] [Google Scholar]
- [18].Sanderson-Smith ML, Walker MJ, Ranson M. The maintenance of high affinity plasminogen binding by group A streptococcal plasminogen-binding M-like protein is mediated by arginine and histidine residues within the a1 and a2 repeat domains. J. Biol. Chem. 2006;281:25965–25971. doi: 10.1074/jbc.M603846200. [DOI] [PubMed] [Google Scholar]
- [19].McCance SG, Castellino FJ. Contributions of individual kringle domains toward maintenance of the chloride-induced tight conformation of human glutamic acid-1 plasminogen. Biochemistry. 1995;30:10589–10594. doi: 10.1021/bi00029a035. [DOI] [PubMed] [Google Scholar]
- [20].Urano T, Chibber BAK, Castellino FJ. The reciprocal effects of epsilon-aminohexanoic acid and chloride ion on the activation of human [Glu1]plasminogen by human urokinase. Proc. Natl. Acad. Sci U.S.A. 1987;84:4031–4034. doi: 10.1073/pnas.84.12.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nilsen SL, Prorok M, Castellino FJ. Enhancement through mutagenesis of the binding of the isolated kringle 2 domain of human plasminogen to omega-amino acid ligands and to an internal sequence of a Streptococcal surface protein. J. Biol. Chem. 1999;274:22380–22386. doi: 10.1074/jbc.274.32.22380. [DOI] [PubMed] [Google Scholar]
- [22].McClintock DK, Bell PH. The mechanism of activation of human plasminogen by streptokinase. Biochem. Biophys. Res. Comm. 1971;43:694–702. doi: 10.1016/0006-291x(71)90670-x. [DOI] [PubMed] [Google Scholar]
- [23].Reddy KN, Markus G. Mechanism of activation of human plasminogen by streptokinase. Presence of active center in streptokinase-plasminogen complex. J. Biol. Chem. 1972;247:1683–1691. (1972) [PubMed] [Google Scholar]
- [24].Gonzalez-Gronow M, Siefring GE, Jr., Castellino FJ. Mechanism of activation of human plasminogen by the activator complex, streptokinase-plasmin. J. Biol. Chem. 1978;253:1090–1094. [PubMed] [Google Scholar]
- [25].Collen D, Van Hoef B, Schlott B, Hartmann M, Gührs KH, Lijnen HR. Mechanisms of activation of mammalian plasma fibrinolytic systems with streptokinase and with recombinant staphylokinase. Eur. J. Biochem. 1993;216:307–314. doi: 10.1111/j.1432-1033.1993.tb18147.x. [DOI] [PubMed] [Google Scholar]
- [26].Schenone MM, Warder SE, Martin JA, Prorok M, Castellino FJ. An internal histidine residue from the bacterial surface protein, PAM, mediates its binding to the kringle-2 domain of human plasminogen. J. Pept. Res. 2000;56:438–435. doi: 10.1034/j.1399-3011.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- [27].Phillips GN, Jr., Flicker PF, Cohen C, Manjula BN, Fischetti VA. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fischetti VA. Streptococcal M protein: molecular design and biological behavior. Clin. Microbiol. Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nilson BH, Frick IM, Åkesson P, Forsen S, Björck L, Åkerström B, Wikström M. Structure and stability of protein H and the M1 protein from Streptococcus pyogenes. Implications for other surface proteins of gram-positive bacteria. Biochemistry. 1995;34:13688–13698. doi: 10.1021/bi00041a051. [DOI] [PubMed] [Google Scholar]
- [30].Andre I, Perssin J, Blom AM, Nilsson H, Drakenberg T, Lindahl G, Linse S. Streptococcal M protein: structural studies of the hypervariable region, free and bound to human C4BP. Biochemistry. 2006;45:4559–4568. doi: 10.1021/bi052455c. [DOI] [PubMed] [Google Scholar]
- [31].Violand BN, Byrne R, Castellino FJ. The effect of alpha-,omega-amino acids on human plasminogen structure and activation. J. Biol. Chem. 1978;253:5395–5401. [PubMed] [Google Scholar]
- [32].Marshall JM, Brown AJ, Ponting CP. Conformational studies of human plasminogen and plasminogen fragments: evidence for a novel third conformation of plasminogen. Biochemistry. 1994;33:3599–3606. doi: 10.1021/bi00178a017. [DOI] [PubMed] [Google Scholar]
- [33].Cockell CS, Marshall JM, Dawson KM, Cederholm-Williams SA, Ponting CP. Evidence that the conformation of unliganded human plasminogen is maintained via an intramolecular interaction between the lysine-binding site of kringle 5 and the N-terminal peptide. Biochem. J. 1998;333:99–105. doi: 10.1042/bj3330099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Urano T, De Serrano DS, Chibber BAK, Castellino FJ. The control of the urokinase-catalyzed activation of human glutamic acid 1-plasminogen by positive and negative effectors. J. Biol. Chem. 1987;262:15959–15964. [PubMed] [Google Scholar]
- [35].McNamara C, Zinkernagel AS, Macheboeuf P, Cunningham MW, Nizet V, Ghosh P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science. 2008;319:1405–1408. doi: 10.1126/science.1154470. [DOI] [PMC free article] [PubMed] [Google Scholar]