Abstract
Background
To determine the 5-year recurrence-free survival in patients with high-risk prostate cancer after neoadjuvant combination chemotherapy followed by surgery. Secondary endpoints included safety, pathologic effects of chemotherapy and predictors of disease recurrence.
Patients and Methods
Fifty seven patients were enrolled in a Phase I/II study of weekly docetaxel 35 mg/m2 and escalating mitoxantrone to 4 mg/m2 prior to prostatectomy. Patients were treated with 16 weeks of chemotherapy administered weekly on a 3 of every 4 week schedule. A tissue micro-array, constructed from the prostatectomy specimens served to facilitate the exploratory evaluation of biomarkers. The primary end point was relapse-free survival. Relapse was defined as a confirmed serum prostate-specific antigen (PSA) > 0.4 ng/ml.
Results
Of the 57 patients, 54 received 4 cycles of docetaxel and mitoxantrone prior to radical prostatectomy. Grade 4 toxicities were limited to leucopenia, neutropenia and hyperglycemia. Serum testosterone levels remained stable after chemotherapy. Negative surgical margins were attained in 67% of cases. Lymph node involvement was detected in 18.5% of cases. With a median follow-up of 63 months, 27 of 57 (47.4%) patients recurred. The Kaplan-Meier relapse-free survival at 2 years was 65.5% (95%CI 53.0% to 78.0%) and 49.8% at 5 years (95%CI 35.5% to 64.1%). Pretreatment serum PSA, lymph node involvement, and post-chemotherapy tissue VEGF expression were independent predictors of early relapse.
Conclusions
Preoperative chemotherapy with docetaxel and mitoxantrone is feasible. Approximately half of the high risk patients remain relapse free at 5 years and clinical and molecular predictors of early relapse were identified.
Keywords: prostate cancer, neoadjuvant chemotherapy, chemotherapy prior to prostatectomy, docetaxel, mitoxantrone
INTRODUCTION
Recent advances have lead to improvements in diagnostic and therapeutic strategies for prostate cancer; however, treatment failure after primary therapy occurs in 35–40% of cases.1, 2 The implementation of risk stratification models have allowed for the identification of prostate cancer patients that are at greatest risk for recurrence after primary therapy.3, 4 After radical prostatectomy, disease relapse may occur locally, at distant sites or both.5, 6 Effective systemic therapy regimens capable of eradicating microscopic metastases and improving local control are needed to improve the outlook for patients with high-risk, localized prostate cancer.
Randomized controlled trials of neoadjuvant androgen suppressive therapy have shown that three months of preoperative androgen suppression significantly alters tumor phenotype and surgical margin status, but does not reduce the risk of prostate cancer recurrence.7–9 Complete pathologic responses, a validated marker in other tumor types, have only rarely been observed with androgen suppression alone. Longer androgen suppression prior to prostatectomy has not shown a clinical benefit to date.10 These results strongly suggests that castration-resistant prostate cancer (CRPC) clones are present at the time of initial diagnosis. Therapeutic agents that are active against disseminated prostate cancer, and in particular, CRPC, are likely to be necessary to improve outcomes in high-risk localized prostate cancer.
The use of systemic chemotherapeutic agents has only recently become standard treatment for patients with metastatic CRPC. Mitoxantrone and docetaxel are both active in advanced prostate cancer. 11, 12 The availability of active cytotoxic agents stimulated their experimental application in combination with surgery in high-risk prostate cancer patients.13 Pre-operative treatment also has the unique advantage of allowing assessment of tumor response and collection of pre- and post-treatment tumor tissue for molecular interrogation.
To date, several groups have tested the safety and preliminary efficacy of preoperative taxane-based chemotherapy in patients with high-risk disease.14-18 These studies have consistently shown that taxanes can be safely administered in the pre-operative setting. In studies of single-agent docetaxel, PSA reductions were observed in the majority of patients, although pathologic complete responses were not observed. The addition of androgen suppression therapy to systemic taxane treatment has also been evaluated.16, 18 In these studies, both PSA and pathologic changes have been typical of the expected responses with hormonal therapies. It is somewhat encouraging that, in a recent study by Chi et al., complete pathologic responses were observed in 3% of patients treated with a combination of androgen suppression and docetaxel.19 The contribution of chemotherapy to this outcome is unclear since it was studied in combination with hormonal therapy and not in isolation.
As docetaxel and mitoxantrone exert anti-tumor effects through distinct cellular mechanisms, this combination has potential for synergistic activity against prostate cancer. Based on the experience with similar agents in breast cancer, incomplete cross-resistance between these two agents would be expected. Indeed, evidence suggests that docetaxel is active in patients previously exposed to mitoxantrone and that mitoxantrone retains some activity in patients whose cancer has progressed after docetaxel.20 Based on these lines of evidence, we developed a regimen that combines both of these agents. Previously we reported the results of mitoxantrone dose escalation to 4 mg/m2 with a fixed dose of docetaxel (35 mg/m2) in our initial Phase I cohort. Here we report the pathologic, biomarker and clinical outcomes for this multimodality approach to the treatment of high-risk prostate cancer.
METHODS
Patients
Eligibility criteria for this study included: histologically confirmed adenocarcinoma of the prostate, prostatectomy planned as primary therapy, 10-year minimum life expectancy, and at least one of the following high-risk features: clinical stage T2c or surgically resectable T3a, or serum PSA ≥ 15 ng/ml, or Gleason grade ≥ 4+3 (i.e. 4+3, 4+4, or any 5 elements). Additional requirements included a negative radionuclide bone scan and a pelvic computerized tomographic scan to rule out pelvic nodal involvement (CT required only in patients with a PSA ≥ 40 ng/ml), ECOG performance status ≤1, and left ventricular ejection fraction ≥ 50% by multiple gated acquisition technetium (MUGA) scan. Exclusion criteria were: any prior therapy for prostate cancer, any significant active medical illness, a second malignancy other than non-melanoma skin cancer within 5 years, ≥ grade 2 peripheral neuropathy, hypersensitivity to drugs formulated with polysorbate-80, significant contraindications to corticosteroids, white blood cell count < 3000/mm3, neutrophil count < 1500/mm3, platelet count < 100,000/mm3, conjugated bilirubin > upper limit of normal (ULN), alkaline phosphatase > 4.0 × ULN, alanine transaminase (ALT) > 2.0 × ULN or ALT > 1.5 × ULN concomitant with alkaline phosphatase > 2.5 × ULN.
Written informed consent was obtained from all patients. The protocol was approved by the Institutional Review Boards of all participating institutions.
Treatment
Docetaxel was administered at a fixed dose of 35 mg/m2, while mitoxantrone was dose escalated in the first 10 patients, who received mitoxantrone at doses of 2–5 mg/m214 and was given at a dose of 4 mg/m2 in all remaining patients. Patients received four 28-day cycles of chemotherapy with docetaxel and mitoxantrone administered as three weekly doses followed by a 1-week break. Dexamethasone 4 mg po was given 12 hr and 1 hr prior to and 12 hr after treatment. In patients who weighed more than 130% of their ideal body weight (IBW, defined as 50 kg + 2.3 kg/inch for each inch of height over 5 feet),21 body surface was calculated based on adjusted weight, defined as IBW + 0.5 (Actual weight - IBW).
Dose Modifications
Hematologic Events
Scheduled doses with both chemotherapeutic agents were withheld for platelet count < 75,000/mm3 or neutrophil count < 1000/mm3 until count levels recovered to above these parameters. The mitoxantrone dose was reduced by 25% if recovery took longer than 1 week, if counts were below these parameters for two consecutive doses, or for any grade 4 hematologic toxicity. The dose of docetaxel was reduced by 25% if a patient again met these criteria despite the mitoxantrone dose reduction. Patients who had a platelet count < 75,000/mm3, a neutrophil count < 1000/mm3 that persisted longer than 2 weeks, or had any grade 4 hematologic toxicity despite the 25% dose reduction of both agents received no additional chemotherapy. Hematologic growth factors were allowed at the treating physician’s discretion; however, growth factor support could not serve as a replacement for the pre-planned dose reductions.
Non-Hematologic Events
The dose of docetaxel and mitoxantrone was to be reduced by 25% for aspartate transferase (AST) of 1.6 – 5 × upper limit of normal (ULN). Therapy was withheld for bilirubin > ULN, alkaline phosphatase > 5 times ULN, or ALT > 5 times ULN. Therapy was resumed at a 25% dose reduction in patients whose liver function tests recovered within 3 weeks. Mitoxantrone therapy was to be discontinued for any clinical evidence of cardiotoxicity that was associated with a > 10% decline in ejection fraction. For all other persistent and clinically significant ≥ grade 3 non-hematologic toxicities, therapy was to be withheld until resolution of toxicity and treatment resumed with a 25% reduction in the dose of both agents.
Patient Monitoring
At week 1 of each 4-week cycle, a chemistry panel, PSA and complete blood count with automated differential were collected. A complete blood count was also obtained at week 2 and 3, during each drug treatment visit. Serum testosterone was measured before chemotherapy and after 4 cycles of treatment. A physical examination and assessment of toxicity was performed every 4 weeks. Peri-operative surgical morbidities, operative time, blood loss, and pathologic findings were recorded in all patients. After completion of chemotherapy and prostatectomy, patients are to be followed with serum PSA tests every 3 months and regular clinic visits for 5 years or until evidence of relapse.
Translational Studies
Pre- and post-chemotherapy plasma vascular endothelial growth factor (VEGF) were measured. Tumor expression of VEGF, nuclear Ki67, nuclear and cytoplasmic p16, and CD10 were characterized in a tissue microarray constructed from formalin fixed prostatectomy specimens. Detailed methods for these analyses are provided in Supplemental Data.
Statistical Design
The primary endpoint for this study was to determine the 5-year relapse-free survival following combination chemotherapy and prostatectomy. Relapse was defined as the detection and confirmation of a serum PSA > 0.4 ng/ml or any other clinical evidence of disease or initiation of any prostate-cancer directed therapy. Sample size and power calculations were previously described.14
For exploratory outcomes analyses, Gleason scores were categorized into either three categories (6 versus 7 versus 8 and higher) or two categories (6 and 7 versus 8 and higher). Pathologic and clinical T-stages were each split into T1–2 versus T3–4. Continuous variables were dichotomized into high and low groups based on median values. Nuclear and cytoplasmic p16 were split into groups with greater or less than 20% staining. In addition, each of these variables were analyzed following stratification for surgical node status. Pre-and post-chemotherapy testosterone levels and serum VEGF levels were compared using a paired t-test. PSA fluctuations during chemotherapy was calculated by determining the percent change from baseline. Values that remained between +20% and −20% from baseline were considered stable.
Time-dependent analyses were carried out using the Kaplan-Meier method. The effect of covariates on time-dependent outcome variables was examined using the log rank test in univariate analyses and the Cox proportional Hazards Ratio in multivariate analyses. Predictor variables tested included patient age, lymph node status, surgical margin status, biopsy Gleason score, pathological Gleason score, clinical and pathologic T stage, cancer tissue VEGF % intensity, pre-chemotherapy plasma VEGF, baseline PSA, PSA density (serum PSA/prostate volume), PSA pattern of change on therapy, prostate volume, nuclear Ki67, nuclear and cytoplasmic p16 and CD10 staining.
RESULTS
Patients
Between January 2001 and November 2004, 57 patients with high-risk localized prostate cancer were recruited from the clinics at all study sites. All screened patients were eligible. The median age was 63 (range 49–74). Median ECOG performance status was 0. The median serum PSA was 12.2 ng/ml (range 1.4–58.6 ng/ml) and the median biopsy Gleason score was 8 (range 6–10). The pre-treatment characteristics are summarized in Table 1. The median follow-up for recurrence-free patients was 63 months (range 7 to 88 months). The median follow-up for all patients (until last visit or until relapse) is 55 months, (range 7 to 88 months).
Table 1.
Patient characteristics prior to therapy
| Number | 57 |
| Age | |
| Median | 63 |
| Range | 49–74 |
| ECOG PS | |
| 0 | 54 |
| 1 | 3 |
| PSA | |
| Median | 12.2 |
| Range | 1.4–58.6 |
| Clinical Stage (AJCC 2002 criteria) | |
| T1c | 7 |
| T2a | 7 |
| T2b | 10 |
| T2c | 19 |
| T3a | 11 |
| T3b | 2 |
| T4 | 1 |
| Biopsy Gleason Score | |
| 6 | 5 |
| 7 | 22 |
| 8 | 17 |
| 9 | 12 |
| 10 | 1 |
Treatment
Of the 57 patients, 54 completed 4 cycles of therapy and had surgery. Two patients completed fewer than 4 cycles of therapy before going on to surgery due to the following factors: one patient had persistent grade 2 nausea after 8 of 12 planned doses and one patient developed grade 2 neuropathy after 9 of 12 planned doses of treatment. One patient was incarcerated out of the reach of our health care system and was lost to follow-up.
Among the 54 patients who received four cycles of therapy and had surgery, 218 of 228 (96%) doses of chemotherapy were delivered as scheduled. All 10 of the missed doses were due to neutropenia. Four patients received erythropoietin (40,000 units weekly) for anemia and no patient received granulocyte colony stimulating factors.
Toxicity
Treatment-related toxicity for the entire 4-cycle course of therapy is detailed by in Table 2. Neutropenia was the most common treatment-related hematologic toxicity. Hyperglycemia, expected with dexamethasone premedications, and onycholysis, commonly reported with weekly docetaxel, were the most common non-hematologic toxicities. One patient suffered a calf venous thrombosis below the popliteal vein while receiving chemotherapy.
Table 2.
Treatment related toxicities > grade 1 (n=57).
| Toxicity | Grade 2 | Grade 3 | Grade 4 | % Occurrence |
|---|---|---|---|---|
| Hematological | ||||
| Neutropenia | 9 | 11 | 7 | 50% |
| Leukopenia | 15 | 16 | 5 | 67% |
| Febrile neutropenia | 0 | 2 | 0 | 4% |
| Thrombocytopenia | 1 | 0 | 0 | 2% |
| Anemia | 3 | 0 | 0 | 6% |
| Non-hematological | ||||
| Hyperglycemia | 11 | 5 | 2 | 33% |
| Onycholysis | 17 | 0 | 0 | 31% |
| Alopecia | 10 | 0 | 0 | 19% |
| Fatigue | 8 | 0 | 0 | 15% |
| Hypophosphatemia | 5 | 2 | 0 | 13% |
| Diarrhea | 3 | 3 | 0 | 11% |
| Hyperlacrimation | 4 | 0 | 0 | 7% |
| Taste Disturbance | 4 | 0 | 0 | 7% |
| Vision Disturbance | 3 | 0 | 0 | 6% |
| Dermatology- skin toxicity | 3 | 0 | 0 | 6% |
| Neuropathy | 2 | 0 | 0 | 4% |
| Hyper phosphatemia | 2 | 0 | 0 | 4% |
| Abdominal Pain | 1 | 1 | 0 | 4% |
| Anorexia | 2 | 0 | 0 | 4% |
| Diaphoresis | 1 | 0 | 0 | 2% |
| Epistaxis | 1 | 0 | 0 | 2% |
| Extra-pyramidal reaction | 1 | 0 | 0 | 2% |
| Fingertip pain | 1 | 0 | 0 | 2% |
| Gastric reflux | 1 | 0 | 0 | 2% |
| Hearing Loss | 1 | 0 | 0 | 2% |
| Indigestion | 1 | 0 | 0 | 2% |
| Nail Pain | 1 | 0 | 0 | 2% |
| Night Sweats | 1 | 0 | 0 | 2% |
| Pain due to biopsy | 1 | 0 | 0 | 2% |
| Paronychia | 1 | 0 | 0 | 2% |
| Pneumonia | 1 | 0 | 0 | 2% |
| Stomatitis | 1 | 0 | 0 | 2% |
| URI | 1 | 0 | 0 | 2% |
| Vomiting | 1 | 0 | 0 | 2% |
| Weight Loss | 1 | 0 | 0 | 2% |
| Cellulitis | 0 | 1 | 0 | 2% |
| Calf thrombosis | 0 | 1 | 0 | 2% |
| Injection site reaction | 0 | 1 | 0 | 2% |
| Nausea | 0 | 1 | 0 | 2% |
Surgery after chemotherapy
Acknowledging the limitations of sample size as well as variations in individual pelvic anatomy and in operative technique amongst different surgeons, observed operative morbidity was comparable to most published data for prostatectomy without prior chemotherapy.22–24 In the absence of direct measures of technical difficulty we evaluated commonly used surrogates. The median operative time was 4.3 hours (mean 4.4 hours, range 2.4 to 6.5 hours). The median estimated blood loss was 900 cc (mean 1023 cc, range 80 to 3100). There was one intra-operative complication, i.e. a rectal injury that was thought to be possibly-related to chemotherapy. Post-operative complications were limited to a wound infection in one patient. There were no post-operative deep venous thromboses.
Effect of chemotherapy on serum testosterone and PSA values
To ensure that any changes in PSA or tumor characteristics were not due to an effect on androgenic steroid production, pre- and post chemotherapy testosterone measurements were compared. There was no change in testosterone with therapy; the mean serum testosterone concentration was 343 ng/dL prior to therapy (95%CI 303.7 – 382.1 ng/dL) and 352 ng/dL after therapy (95%CI 304.7 – 399.3 ng/dL) (n=53, p=0.71).
The use of PSA as an indirect measure of antitumor activity was facilitated by the finding that therapy did not result in reduced testosterone levels. Considerable fluctuation in individual PSA measurements were seen and conventional measures developed in metastatic hormone refractory prostate cancer are not readily applicable in this setting. Hence we examined the overall effect on PSA in each patient. Three distinct patterns emerged. The first group (n = 28, 52%) had a decreasing PSA pattern defined as sustained decline greater than 20%. The second group (n = 12, 22%) had PSA levels that neither increased nor decreased by more than 20% during chemotherapy. The third group (n=14, 26%) had both increases and decreases of over 20% from one time point to the next. No patient developed sustained PSA progression defined as a confirmed increase of >20%.
Initial operative outcome
Pathologic outcome at surgery is summarized in Table 3. Negative margins were attained in 36 of 54 patients (67%, 95% CI 53% to 79%). There were no pathologic complete responses. Post-operative PSA was < 0.2 ng/ml in 43 of 54 patients (80%, 95% CI 66% to 89%).
Table 3.
Pathologic outcomes after neoadjuvant chemotherapy (n=54)
| Negative surgical margin | 36 (67%) |
| Postoperative serum PSA < 0.2 ng/ml | 43 (80%) |
| Nodal metastases present | 10 (18.5%) |
| Pathologic Stage (AJCC 2002) | |
| T2a | 4 |
| T2b | 5 |
| T2c | 16 |
| T3a | 10 |
| T3b | 9 |
| T3c | 7 |
| T4 | 3 |
| Surgical Gleason Score* | |
| 6 | 8 |
| 7 | 25 |
| 8 | 8 |
| 9 | 11 |
| 10 | 1 |
Surgical Gleason score not assigned in one patient
Biomarker studies
Biomarker results are presented in supplementary data. Briefly, the expression of Ki-67, CD10, and p16 and plasma VEGF concentrations were not predictive of clinical outcomes while post-chemotherapy tissue VEGF expression was an independent predictor of early relapse.
Cancer Recurrence
Twenty-seven of 57 (47.4%) patients have experienced disease recurrence. The Kaplan-Meier progression-free survival for the entire study group was 65.5% (95% CI 53.0% to 78.0%) at 2 years and 49.8% (95% CI 35.5% to 64.1%) at 5 years. For lymph node negative patients, 2 and 5 year relapse-free survival probabilities were 77.9% and 58.7%, respectively.
Independent predictors of disease relapse are shown in Table 4. Biopsy Gleason score, PSA density, increased VEGF expression in surgical specimens as well as node involvement with tumor were independently associate with risk of relapse. The profound effect of persistent disease in lymph nodes is illustrated in Figure 1. The effect of tissue VEGF expression is shown in Figure 2. Because the presence of disease in lymph nodes after induction chemotherapy was a dominant predictor of outcome, we also examined predictors of relapse in the subset of patients who did not have node involvement identified at surgery. Only tumor VEGF expression following chemotherapy was associated with early relapse in this subset. PSA decline during chemotherapy was not predictive of VEGF expression or time to relapse.
Table 4.
Predictors of relapse in multivariate analyses
| All Patients | ||
|---|---|---|
| Variable | Hazard Ratio (95% CI) | p |
| Node metastases | 9.7 (2.8–33.4) | <0.001 |
| Biopsy Gleason score | 1.8 (1.1–3.1) | 0.03 |
| PSA density | 6.4 (1.8–22.7) | 0.004 |
| Increased cancer VEGF expression | 3.5 (1.2–10.4) | 0.023 |
| Node-negative patients | ||
| Increased cancer VEGF expression | 3.8 (1.2–12.0) | 0.023 |
Figure 1.
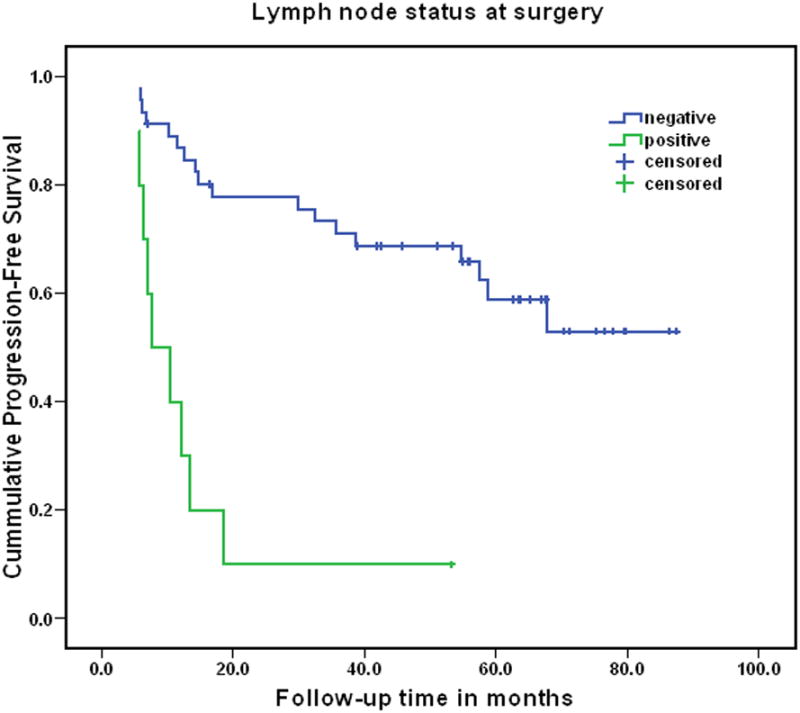
Progression-free survival in patients with pathologically involved vs. uninvolved lymph nodes (log rank p<0.001).
Figure 2.
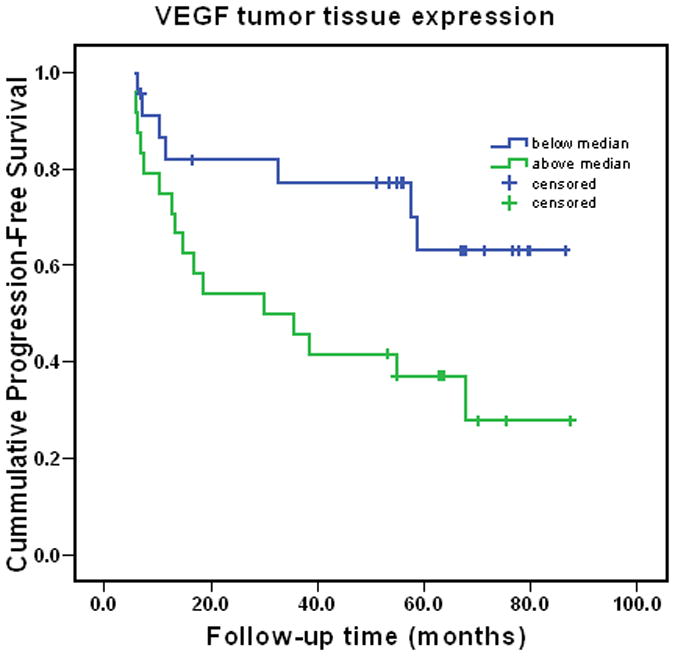
Progression-free survival in patients with tumor VEGF expression above and below the median (log rank p=0.02).
DISCUSSION
Studies of androgen suppression therapy prior to surgery have shown a near uniform resistance to androgen suppression illustrated by the presence of residual tumor in pathologic specimens.7–9 Several groups have therefore explored the use of chemotherapy prior or after surgical removal of the prostate.13, 25
The regimen developed in this study combines the two most active chemotherapy drugs in prostate cancer and was well-tolerated and feasible in a multi-center study of neoadjuvant chemotherapy for high-risk prostate cancer.
Patients who participated in our study were similar to those who took part in other contemporary taxane-based treatment trials developed for high-risk, localized prostate cancer.16,19 Negative surgical margins, a favorable pathologic finding in men undergoing radical prostatectomy 26 were attained in 67% of our cases. These results compared favorably to high risk patients undergoing surgery alone, where the rates of negative margins range from 35% to 54% only.7–9 Our negative- margin rate in chemotherapy alone treated patients were comparable to the results obtained with chemo-hormonal treatment where margin negative rates were 73% to 78%.16, 19 Further studies are needed to determine if pre-operative cytotoxic chemotherapy has a direct role in improving surgical margin status. Positive lymph nodes were detected in 18.5% of cases in our trial compared to 6% in two other chemo-hormonal neoadjuvant trials.16, 19 There were no pathologic complete responses in the current study, a finding that has been observed in the majority of other neoadjuvant chemotherapy trials.15–18
With a median follow-up of 63 months for non-relapsing patients, the Kaplan-Meier progression-free survival at 2 years and 5 years for all patients was 65.5% and 49.8% respectively, comparable to those reported from studies of neoadjuvant chemo-hormonal therapy. Chi et al. reported 70% relapse-free survival with a follow-up of 43 months while Konety et al. saw a 55% relapse free survival with a median follow-up of 29 months. As was the case with neoadjuvant hormonal therapy, larger studies and long-term follow-up would be required to determine if pre-operative chemotherapy is beneficial and whether the addition of hormonal therapy to chemotherapy in this setting adds any benefit.
We studied predictors of recurrence because early recurrences after surgery are associated with increased mortality.27 In this study, the strongest predictor of treatment failure was the presence of lymphatic disease. These findings support the pursuit of improved imaging methods with the capacity to identify these high-risk patients so alternate strategies can be investigated. Not unexpectedly, pre-treatment PSA was also predictive of disease recurrence
Prostate cancer biomarkers are needed to improve the prediction of disease recurrence for high-risk patients. We identified increased tissue VEGF expression as a new independent predictor of relapse in patients undergoing neoadjuvant chemotherapy followed by prostatectomy. This novel finding merits confirmation. This finding supports the hypothesis that of VEGF signaling may be important in prostate cancer treatment resistance. Although identified in a different setting and not yet confirmed, the finding is encouraging for the ongoing CALGB trial of docetaxel with or without the VEGF antibody, bevacizumab.
An important limitation of this study, is our inability to isolate the effects of tumor biology, surgical skill, and chemotherapy. Only a randomized clinical trial where the systemic therapy is the sole variable can measure its impact on relapse-free survival. The clinical and biomarker risk factor analyses should be interpreted as exploratory.
In conclusion, we demonstrated that the two cytotoxic drugs that are most commonly used in the treatment of advanced prostate cancer can be safely combined. The novel regimen developed here maybe worth evaluation in patients with advanced metastatic disease. We also demonstrated the feasibility of delivering multi-agent chemotherapy prior to prostatectomy. The five-year relapse-free survival results are encouraging despite the absence of complete pathologic responses. Consideration should be given to the evaluation of this multimodality strategy in a randomized controlled trial.
Supplementary Material
Acknowledgments
Supported in part by grant GIA US #16080 from Aventis Pharmaceuticals, grant #031.G0008 from Serono, Inc., and grants 3M01RR00334-33S2 and 1 R01 CA119125-01 from the National Institutes of Health.
We acknowledge the contributions of Drs. Bruce Lowe, Raul Parra, Jeffrey Johnson, Douglas Ackerman, William J. Ellis, Thomas Takayama, Roger Wicklund, and Robert Skinner who, in addition to Drs. Garzotto, Lange, and Lieberman, performed surgery on patients enrolled in the study. We thank Nicola Janeba, Vasko Kaimaktchiev, and Nina Katovic, for technical assistance. We thank Dr. Larry True for support in constructing the tissue microarray. We thank Susanne McGlothlin for editorial assistance.
Footnotes
Dr. Beer has received speaker's honoraria from Aventis, a company that may have a commercial interest in the results of this research. This potential conflict of interest has been reviewed and managed by the VA Conflict of Interest in Research Committee.
References
- 1.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Kuban DA, Thames HD, Levy LB, et al. Long-term multi-institutional analysis of stage T1-T2 prostate cancer treated with radiotherapy in the PSA era. Int J Radiat Oncol Biol Phys. 2003;57:915–928. doi: 10.1016/s0360-3016(03)00632-1. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Whittington R, Malkowicz SB, et al. Pretreatment nomogram for prostate-specific antigen recurrence after radical prostatectomy or external-beam radiation therapy for clinically localized prostate cancer. J Clin Oncol. 1999;17:168–172. doi: 10.1200/JCO.1999.17.1.168. [DOI] [PubMed] [Google Scholar]
- 4.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998;90:766–771. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 5.Han M, Pound CR, Potter SR, Partin AW, Epstein JI, Walsh PC. Isolated local recurrence is rare after radical prostatectomy in men with Gleason 7 prostate cancer and positive surgical margins: therapeutic implications. J Urol. 2001;165:864–866. [PubMed] [Google Scholar]
- 6.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. Jama. 2004;291:1325–1332. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 7.Aus G, Abrahamsson PA, Ahlgren G, et al. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU Int. 2002;90:561–566. doi: 10.1046/j.1464-410x.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 8.Hurtado-coll A, Goldenberg SL, Klotz L, Gleave ME. Preoperative neoadjuvant androgen withdrawal therapy in prostate cancer: the Canadian experience. Urology. 2002;60:45–51. doi: 10.1016/s0090-4295(02)01570-4. discussion 51. [DOI] [PubMed] [Google Scholar]
- 9.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–116. [PubMed] [Google Scholar]
- 10.Gleave ME, Goldenberg SL, Chin JL, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–506. discussion 506–507. [PubMed] [Google Scholar]
- 11.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 12.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 13.Gleave M, Kelly WK. High-risk localized prostate cancer: a case for early chemotherapy. J Clin Oncol. 2005;23:8186–8191. doi: 10.1200/JCO.2005.03.3068. [DOI] [PubMed] [Google Scholar]
- 14.Beer TM, Garzotto M, Lowe BA, et al. Phase I study of weekly mitoxantrone and docetaxel before prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2004;10:1306–1311. doi: 10.1158/1078-0432.ccr-1021-03. [DOI] [PubMed] [Google Scholar]
- 15.Dreicer R, Magi-Galluzzi C, Zhou M, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63:1138–1142. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 16.Konety BR, Eastham JA, Reuter VE, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol. 2004;171:709–713. doi: 10.1097/01.ju.0000108122.36893.5a. [DOI] [PubMed] [Google Scholar]
- 17.Oh WK, George DJ, Kaufman DS, et al. Neoadjuvant docetaxel followed by radical prostatectomy in patients with high-risk localized prostate cancer: a preliminary report. Semin Oncol. 2001;28:40–44. doi: 10.1016/s0093-7754(01)90153-8. [DOI] [PubMed] [Google Scholar]
- 18.Hussain M, Smith DC, El-Rayes BF, et al. Neoadjuvant docetaxel and estramustine chemotherapy in high-risk/locallyadvanced prostate cancer. Urology. 2003;61:774–780. doi: 10.1016/s0090-4295(02)02519-0. [DOI] [PubMed] [Google Scholar]
- 19.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180:565–570. doi: 10.1016/j.juro.2008.04.012. discussion 570. [DOI] [PubMed] [Google Scholar]
- 20.Michels J, Montemurro T, Murray N, Kollmannsberger C, Nguyen Chi K. First- and second-line chemotherapy with docetaxel or mitoxantrone in patients with hormone-refractory prostate cancer: does sequence matter? Cancer. 2006;106:1041–1046. doi: 10.1002/cncr.21695. [DOI] [PubMed] [Google Scholar]
- 21.Lacy C, Armstrong L, Goldman M, Lance L. Drug Information Handbook. 10. Cleveland, OH: LexiComp Inc; 2002. [Google Scholar]
- 22.Hammerer P, Hubner D, Gonnermann D, Huland H. Perioperative and postoperative complications of pelvic lymphadenectomy and radical prostatectomy in 320 consecutive patients. Urologe A. 1995;34:334–342. [PubMed] [Google Scholar]
- 23.Lerner SE, Blute ML, Lieber MM, Zincke H. Morbidity of contemporary radical retropubic prostatectomy for localized prostate cancer. Oncology (Huntingt) 1995;9:379–382. discussion 382, 385–376, 389. [PubMed] [Google Scholar]
- 24.Tewari A, Srivasatava A, Menon M. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 25.Sonpavde G, Chi KN, Powles T, et al. Neoadjuvant therapy followed by prostatectomy for clinically localized prostate cancer. Cancer. 2007;110:2628–2639. doi: 10.1002/cncr.23085. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–1177. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 27.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. Jama. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


