Abstract
The transcription factor Sox9 has been implicated in inner ear formation in several species. To investigate the long-term consequences of Sox9 depletion on inner ear development we analyzed the inner ear architecture of Sox9-depleted Xenopus tadpoles generated by injection of increasing amounts of Sox9 morpholino antisense oligonucleotides. We found that Sox9-depletion resulted in major defects in the development of vestibular structures, semicircular canals and utricle, while the ventrally located saccule was less severely affected in these embryos. Consistent with this phenotype we observed a specific loss of the dorsal expression of Wnt3a expression in the otic vesicle of Sox9 morphants, associated with an increase in cell death and a reduction in cell proliferation in the region of the presumptive otic epithelium. We propose that in addition to its early role in placode specification, Sox9 is also required for the maintenance of progenitors in the otic epithelium.
Keywords: Sox9, vestibular, auditory, semicircular canals, utricle, saccule, Xenopus
Introduction
The vertebrate inner ear is the organ responsible for the sense of hearing, balance and detection of acceleration. It develops from a thickening of the head ectoderm known as the otic placode that invaginates and eventually detaches from the surface ectoderm to form the otic vesicle or otocyst. Differential growth and intricate morphogenetic movements will shape the otic vesicle into a highly specialized sensory organ. In the fully developed inner ear the dorsally located utricle and semicircular canals constitute the vestibular apparatus (pars superior), structures that have been highly conserved during evolution. In contrast on the ventral aspect of the inner ear the auditory chambers (pars inferior) went through more extensive modifications (Riley and Phillips, 2003). For example the auditory organ in mammals is the cochlea, while in amphibians the auditory system includes the saccule a low-frequency vibration/sound detector, and the ampibian papilla and basilar papilla, a low- to mid-frequency and a high-frequency sound detector, respectively (Smotherman and Narins, 2000).
Analysis of mouse inner ear mutants has permitted the identification of a number of genes that are critical for the formation of the different components of the inner ear (reviewed in Fekete, 1996; Fekete, 1999; Fekete and Wu, 2002). These genes at early developmental stages often show restricted expression pattern in the developing otic epithelium. For example, Pax2 is expressed in the ventromedial aspect of the otic vesicle, and targeted deletion of Pax2 resulted in agenesis of the cochlear duct (Torres et al., 1996). Mouse embryos carrying a mutation in Hmx3, a gene expressed in the dorsolateral aspect of the otocyst, failed to develop semicircular canals (Wang et al., 1998; Hadrys et al., 1998). These results indicate that the otocyst is a mosaic structure in which the development of its various elements is under independent genetic control (Fekete, 1999).
Sox9, a member of the Sox family of transcriptional regulators, is expressed in the otic vesicle of several species (Liu et al., 2003; Saint-Germain et al., 2004; Bagheri-Fam et al., 2006). Interference with Sox9 function in Xenopus by injection of morpholino antisense oligonucleotides resulted in the downregulation of otic-specific genes, and most of these embryos failed to develop a morphologically recognizable otic vesicle (Saint-Germain et al., 2004). Similarly, loss of Sox9a and Sox9b function in zebrafish resulted in a complete absence or severe reduction of the otic vesicle (Liu et al., 2003; Yan et al., 2005). In contrast to these studies, conditional inactivation of Sox9 in the prospective otic epithelium indicated that Sox9 is not essential for the initial otic specification in the mouse, instead Sox9 is cell-autonomously required for placode invagination, presumably by regulating the adhesive properties of the placodal cells (Barrionuevo et al., 2008). In humans, heterozygous SOX9 mutations result in campomelic dysplasia (CD), a pathology characterized by dwarfism, craniofacial defects, bowing of the long bones and sex reversal (Foster et al., 1994; Wagner et al. 1994; OMIM 114290). These symptoms are often associated with sensorineural deafness, and malformations of the inner ear canals (Tokita et al., 1979; Houston et al., 1981; Savarirayan et al., 2003).
To investigate the long-term consequences of Sox9 depletion on inner ear development we analyzed the inner ear architecture of Sox9-depleted Xenopus tadpoles generated by injection of increasing amounts of Sox9 morpholino antisense oligonucleotides. We found that Sox9-depletion resulted in severe defects in the development of the vestibular structures of the inner ear, while the saccule was less severely affected. Consistent with this phenotype we observed a loss of progenitors in the region of the presumptive otic epithelium of Sox9 morphants. We propose that in addition to its role in otic placode specification Sox9 is also involved in the maintenance of progenitors in the otic epithelium.
Results
Sox9 morpholino antisense oligonucleotide
To analyze the long-term consequences of Sox9 depletion on inner ear development we analyzed the inner ear architecture of Sox9-depleted Xenopus tadpoles generated by injection of increasing amounts of Sox9 morpholino antisense oligonucleotide (Sox9MO). The specificity of this translation blocking Sox9MO has been previously demonstrated (Spokony et al., 2002; Saint-Germain et al., 2004). Briefly, we have shown using a number of molecular markers that Sox9MO blocks neural crest and otic placode formation in the context of the whole embryo. Importantly, injection of a 5-bp mismatched morpholino or a standard control morpholino had no effect on the expression of these markers or on subsequent development of the neural crest and the otocyst. Moreover, both the neural crest and the otic placode phenotype of Sox9 morphants could be fully rescued by Sox9 over-expression, using a construct lacking the morpholino recognition motif (Spokony et al., 2002; Saint-Germain et al., 2004). While it has been difficult to document the reduction in endogenous Sox9 protein upon Sox9MO injection due to the lack of appropriate antibodies, we show that in an in vitro transcription/translation assay Sox9MO blocks translation of Sox9 mRNA in a concentration dependent manner (Fig S1).
Abnormal development of the utricle and semicircular canals in Sox9-depleted tadpoles
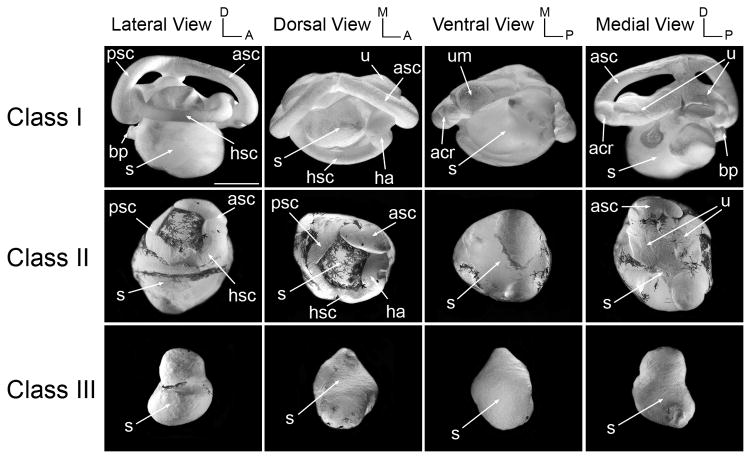
The paint-fill technique is a simple assay allowing for a rapid evaluation of the three-dimensional architecture of the inner ear during embryogenesis. Initially developed to analyze the mammalian inner ear (Martin and Swanson, 1993) this technique has been since then applied to different organisms including Xenopus (Bisonnette and Fekete, 1996; Bever and Fekete, 2002; Bever et al., 2003). To analyze the role of Sox9 on inner ear development embryos received a unilateral injection of 1 ng, 2.5 ng or 5 ng of Sox9MO in one animal ventral blastomere at the 8-cell stage to target the otic vesicle as previously shown (Huang et al., 1998; Saint-Germain et al., 2004). At stage 52 the inner ear structure of these tadpoles was analyzed by the paint-filling technique. As shown in Fig 1, Sox9MO injection resulted in severe abnormality in the vestibular apparatus. The anterior, posterior and horizontal semicircular canals as well as the utricle were misshaped, often reduced in size (class II phenotype) or completely missing (class III phenotype). When present the semicircular canals were often fused to the saccule. Interestingly, in most cases the saccule was present, though reduced in size. The proportion of affected embryos for each dose of Sox9MO is shown in Table 1, and representative cases of the various classes of phenotype are depicted in Fig 1. While the inner ear of most embryos was normal for the lower dose of Sox9MO (97%; n=29), in the case of 2.5 ng of Sox9MO, approximately 50% of the embryos showed defects in the development of the semicircular canals and utricle (class II and III phenotypes; n=50). For the higher dose of Sox9MO (5 ng) the survival rate at stage 52 was extremely low, only 24% of the injected embryos (n=100) survived, among which 96% (n=24) had a class I phenotype (normal inner ear structure; not shown). This indicates that for a dose of 5 ng of Sox9MO the most strongly affected embryos did not survive up to stage 52. For this reason in subsequent studies we primarily focused our analysis on the two lower doses of Sox9MO, 1 ng and 2.5 ng.
Figure 1. Morphological analysis of the inner ear of Sox9-depleted tadpoles at stage 52.
Representative cases of the membranous labyrinth of Sox9-depleted tadpoles inner ears (Sox9MO; 2.5 ng) at stage 52 are shown after injection with latex paint. The overall architecture of the inner ear of these tadpoles has been divided into four classes; Class I: normal inner ear structure; Class II: abnormal semicircular canals and utricle; Class III: semicircular canals and utricle absent, saccule reduced in size; Class IV: no inner ear. aa, anterior ampula; asc, anterior semicircular canal; acr, anterior cristae recess; apr, amphibian papilla recess; bp, basilar papilla; ha, horizontal ampula; hsc, horizontal semicircular canal; psc, posterior semicircular canal; pcr, posterior cristae recess; s, saccule; u, utricle; um, utriclar macula. The scale bar represents 500 μm.
Table I.
Quantification of the inner ear phenotype of Sox9-depleted embryos
| Class | Stage 48 | Stage 52 | |||
|---|---|---|---|---|---|
| 1 ng | 2.5 ng | 5 ng | 1 ng | 2.5 ng | |
| I | 24 (80%) | 32 (64%)* | 25 (54%)** | 28 (97%) | 25 (50%) |
| II | 5 (17%) | 11 (22%) | 14 (30%) | 1 (3%) | 9 (18%) |
| III | 1 (3%) | 5 (10%) | 3 (7%) | 0 (0%) | 15 (30%) |
| IV | 0 (0%) | 2 (4%) | 4 (9%) | 0 (0%) | 1 (2%) |
1 embryo with a smaller size inner ear
3 embryos with a smaller size inner ear, among which 2 were missing the saccule
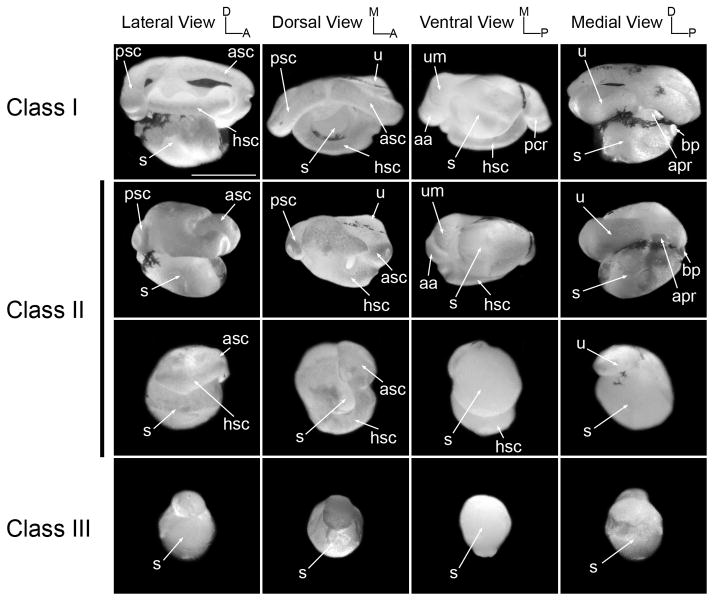
The abnormal development of the semicircular canals observed at stage 52 suggested that the specification of the dorsal aspect of the inner ear was primarily perturbed in these tadpoles. This led us to evaluate inner ears at an earlier time point to determine whether semicircular canals formation was affected in these animals. Canal formation results from the fusion of axial protrusions (Haddon and Lewis, 1991), a process that is completed for all three semicircular canals by stage 47 (Bever et al., 2003). At stage 48 a significant delay was observed in the fusion of the semicircular protrusions in Sox9MO-injected tadpoles, this is especially true for the anterior and posterior semicircular canals (Fig 2; class II, lateral and dorsal views). The proportion of affected embryos (Table 1) was directly correlated to the amount of injected morpholino. At the lower dose of Sox9MO (1 ng) only 20% of the embryos (n=30) showed defects, while for the higher doses of 2.5 ng and 5 ng, 36% (n=50) and 46% (n=46) of the embryos were affected, respectively. The survival rate at stage 48 of embryos injected with 5 ng of Sox9MO was fairly similar to what was observed for uninjected embryos.
Figure 2. Morphological analysis of the inner ear of Sox9-depleted tadpoles at stage 48.
Representative cases of the membranous labyrinth of Sox9-depleted tadpoles inner ears (Sox9MO; 2.5 ng) at stage 48 are shown after injection with latex paint. For phenotypic classes definition and abbreviations see Fig 1. The scale bar represents 500 μm.
Altogether these results indicate that the development of the semicircular canals and the utricle are the most severely affected structures in the inner ear of Sox9-depleted tadpoles, suggesting an important function for Sox9 in the development of the vestibular apparatus. Tadpoles injected with Sox9MO exhibited swimming defects (circling behavior). However because other defects, such as shortened or kinked tails, can also result in a similar phenotype in injected embryos, it is difficult to attribute this circling behavior solely to the observed vestibular defects.
Development of the inner ear sensory organs, otoconia and statoaccoustic ganglion in Sox9-depleted tadpoles
Each chamber of the developing inner ear is associated with a sensory epithelium containing mechanosensory hair cells, which convey auditory and vestibular information (Smotherman and Narins, 1999; 2000). The maculae are the sensory epithelia of the utricle (utricular macula) and saccule (saccular macula), while the sensory epithelia associated with each semicircular canal, are known as the anterior, horizontal and posterior cristae, respectively (Fritzsch et al., 2002). It has been previously shown that at stage 45, the 5 sensory organs can be visualized in the form of thickened epithelia (Quick and Serrano, 2005), expressing variable levels of Bmp4 (Kil and Collazo, 2001). Therefore to assess the development of the sensory organs we analyzed the expression of Bmp4 in Sox9-depleted embryos by in situ hybridization on sections. We found that for a dose of 1 ng of Sox9MO all 5 sensory patches formed normally in all embryos examined (n=10; Fig 3A). In embryos injected with 2.5 ng of Sox9MO the overall size of the otic vesicle was reduced by approximately 50% as compared to the otic vesicle on the uninjected side (an average of 32 sections for the control ear vs. an average of 18 sections for the injected ear; n=10). Moreover, in these embryos the otic vesicle was positioned at a greater distance from the surface ectoderm as compared to control otocysts, which are typically found immediately underneath the ectoderm (Fig 3A, B). This difference may be the result of the loss of the most dorsal structures of the inner ear (pars superior) as described above (Fig 1 and 2). In these tadpoles at least 4 of the 5 sensory organs appeared to form in the proper location, but the thickened epithelia were often reduced in size (Fig 3A). When missing, the sensory organs affected were either the most anterior (anterior crista) or the most posterior (posterior crista). These observations are consistent with the loss of anterior and posterior semicircular canals observed by paint-filling at stage 48 (Fig 2) and stage 52 (Fig 1). While reduced in size, the saccular macula was maintained in its proper location even for the higher dose of Sox9MO further suggesting that Sox9 function is especially critical for the development of the pars superior in Xenopus.
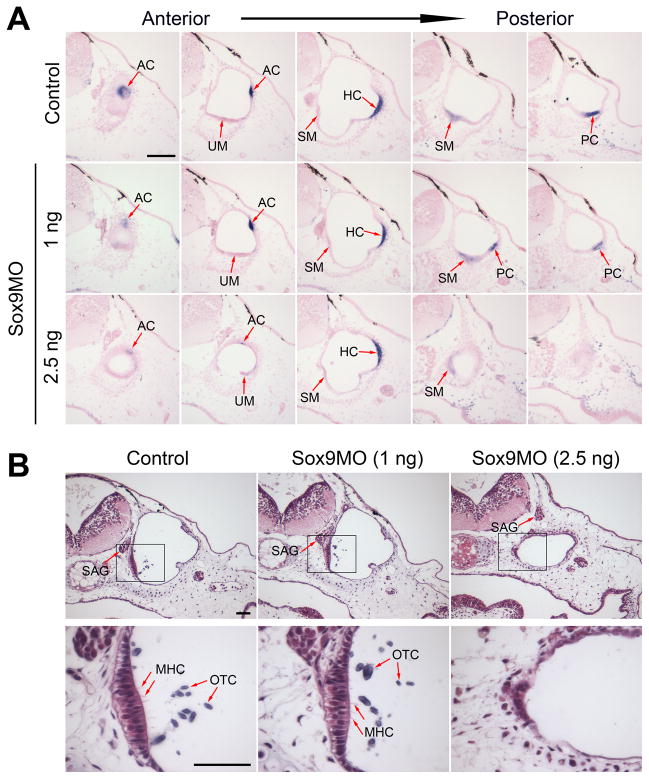
Figure 3. Development of the inner ear sensory organs, otoconia and statoaccoustic ganglion in Sox9-depleted tadpoles at stage 45.
(A) Representative cases of Bmp4 expression in the sensory organs of the inner ear of control and Sox9-depleted tadpoles (Sox9MO; 1 ng and 2.5 ng) at stage 45. The arrows indicate the position of the various sensory epithelium as they arise from anterior to posterior (left to right). AC, anterior crista; UM, utricular macula; HC, horizontal crista; SM, saccular macula; PC, posterior crista. The scale bar represents 100 μm. (B) Sensory epithelium of the saccular macula and associated hair cells and otoconia in control and Sox9-depleted embryos at stage 45. While hair cells and otoconia were missing in the inner ear of embryos injected with the higher dose of Sox9MO (2.5 ng), the statoaccoustic ganglion formed in its proper location in these embryos. The lower panels are higher magnification views of the boxed area shown in the upper panels. The scale bar represents 50 μm. MHC, hair cell; OTC, otoconia; SAG, statoaccoustic ganglion.
We also assessed the development of otoconia and mechanosensory hair cells in these embryos. Otoconia are small crystals of calcium carbonate that accumulate in the utricle and saccule of the inner ear. They facilitate vestibular and auditory function by transmitting accelerational forces and sound vibrations, respectively, to hair cells of the maculae. Around stage 45, a first otoconial mass is visible, associated with the saccular macula (Bever et al., 2003; Quick and Serrano, 2005; Fig 3B). The otoconia of the saccular macula, while normal in embryos injected with the lower dose of Sox9MO (n=5; Fig 3B, middle panels), was completely missing in the inner ear of embryos injected with the higher dose of Sox9MO (n=6; Fig 3B, right panels). At stage 47 the otoconia associated with the utricular macula becomes visible (Bever et al., 2003; Quick and Serrano, 2005), and this structure was also missing in Sox9-morphants (data not shown). The mechanosensory hair can be visualized in the whole embryo by phalloidin staining (Quick and Serrano, 2005), and on section after toluidine-blue (Baird et al. 1993) or hematoxylin and eosin staining (Fig 3B, lower panels). While hair cells were clearly visible in the sensory epithelia of the saccular and utricular maculae of control embryos and embryos injected with 1 ng of Sox9MO, mechanosensory hair cells were completely missing in the epithelia of embryos that received the higher dose of Sox9MO (n=6; Fig 3B, lower panels).
The statoacoustic ganglion that mediates the sensory function of the inner ear is derived from the otic vesicle. We next wished to determine whether this key component of the inner ear was also affected in Sox9-depleted embryos. Surprisingly, we found that the statoaccoustic ganglion formed in its proper location even for the higher dose of Sox9MO (Fig 3B, upper panels).
Taken together, these results indicate that the development of the sensory organs and associated structures (otoconia and mechanosensory hair cells) are also defective in Sox9-depleted tadpoles, suggesting that these embryos are very likely to have both vestibular and auditory deficiencies.
Maintenance of Wnt3a expression in the otic vesicle requires Sox9
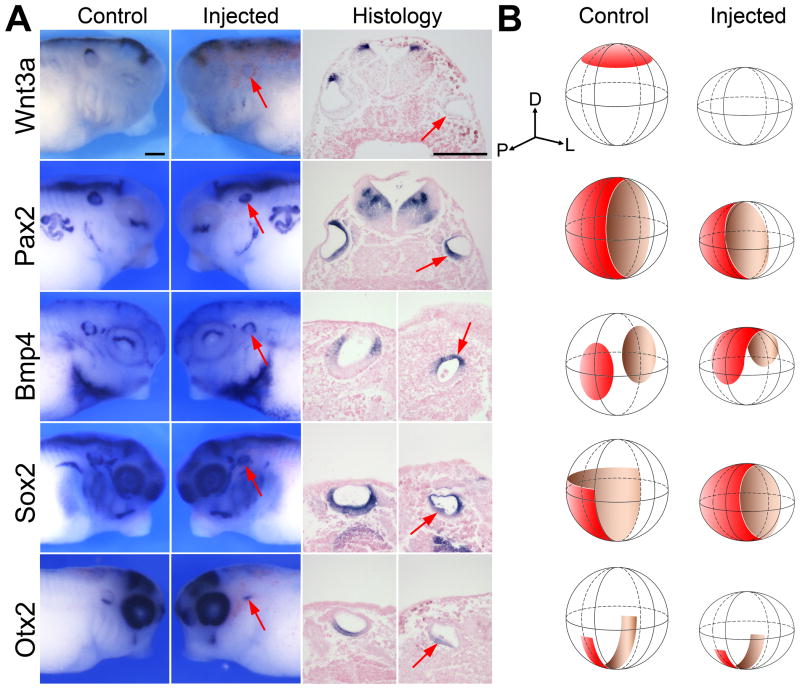
The dorsalmost cells of the otic vesicle fated to form the vestibular apparatus express the secreted factor Wnt3a (Wolda et al., 1993; Saint-Germain et al., 2004). To determine whether Sox9 is required in this region of the otic vesicle we analyzed the expression of Wnt3a in Sox9MO-injected embryos at stage 35. Wnt3a expression was only marginally affected in a small proportion of embryos that received 1 ng of Sox9MO (not shown). Upon injection of 2.5 ng of Sox9MO, in embryos that still formed an otic vesicle-like structure, Wnt3a expression domain was either lost or substantially reduced (51% and 42%, respectively; n=45; Fig 4A, B). Similarly, Pax2, a factor normally expressed in the medial half of the otocyst along the dorso-ventral axis (Heller and Brandli, 1999), while unaffected for 1ng of Sox9MO (n=27), was reduced (47%) or lost (53%) for a dose of 2.5ng of Sox9MO (n=30; Fig 4A, B). Interestingly, at the same dose of Sox9MO (2.5 ng) Bmp4 and Sox2 expression domains, two genes that are normally restricted along the antero-posterior and medio-ventral aspect of the otocyst, respectively, were often expanded dorsally in these embryos (Fig 4A, B). The transcription factor Otx2 which is restricted to the ventral aspect of the otic vesicle (Pannese et al., 1995), was also reduced along the antero-posterior axis in embryos that received 2.5ng of Sox9MO (69% of embryos; n=45) but to a lesser extent as compared to what was seen for Wnt3a or Pax2 (Fig 4A, B). The expression domains of Bmp4, Otx2, and Sox2 were unaffected at the lower dose of Sox9MO (1 ng). These results suggest that Sox9 is required for Wnt3a and Pax2 expression in the dorsalmost cells of the otic vesicle, and that upon Sox9 attenuation there is a dorsal shift in the positioning of Bmp4 and Sox2 expression, consistent with the loss of structures of the pars superior observed at stage 48 and stage 52.
Figure 4. Sox9 depletion results in the loss of gene expression in the dorsal aspect of the otic vesicle.
(A) The otic vesicle of embryos injected in one blastomere at the 8-cell stage with 2.5 ng of Sox9MO exhibited reduced Wnt3a and Pax2 expression associated with a dorsal expansion of Bmp4 and Sox2 at stage 35. The expression of Otx2 in the ventral aspect of the otic vesicle was also reduced but primarily along the antero-posterior axis. For whole-mount in situ hybridization, lateral views, dorsal to top. Anterior is to the right (control) or to the left (injected). Histology panels (dorsal to top) show transverse sections of Wnt3a- and Pax2-stained embryos. In the case of Bmp4-, Otx2- and Sox2-stained embryos longitudinal sections were performed. The arrows point to the otic vesicle on the injected side The scale bars represent 200 μm. (B) A three dimensional representation of the otic vesicle at stage 35 illustrates the changes in expression pattern of Wnt3a, Pax2, Bmp4, Sox2 and Otx2 in injected as compared to controls otocysts. The position of the dorso-ventral, antero-posterior and medio-lateral axis is indicated. D, dorsal; L, lateral; P, posterior.
Sox9 is expressed throughout the otic vesicle
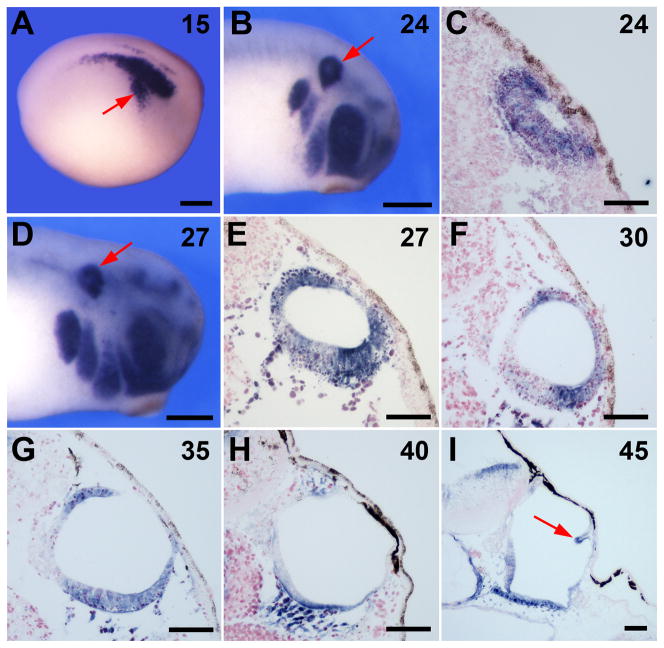
Sox9 expression has been reported at early stage of otic placode development in Xenopus (Saint-Germain et al., 2004). Sox9 is detected from early neurula stage (Fig 5A), in the pre-placodal ectoderm and persists in the otic epithelium at least until stage 45 (Fig 5B–I). Interestingly, around stage 27, Sox9 shows graded expression along the dorso-ventral axis of the otic vesicle, with the strongest expression at the dorsal and ventral aspects of the developing otocyst (Fig 5E–H). As the protrusions of the semicircular canals are emerging, Sox9 is also detected in the developing semicircular canals (Fig 5I). The expression pattern of Sox9 in the dorsal aspect of the developing otic vesicle and its maintenance in the semicircular canal protrusions is consistent with the inner ear phenotype of Sox9 morphants.
Figure 5. Sox9 is expressed throughout the otic vesicle and in the developing semicircular canals.
Developmental expression of Sox9 in the otocyst of wild type embryos by whole-mount in situ hybridization (A, B, D) and by in situ hybridization on section (C, E–I). The embryonic stage (Nieuwkoop and Faber, 1967) is indicated in the upper right corner of each panel. Sox9 is initially expressed throughout the entire otic epithelium (A–C) and then become progressively restricted to the dorsal and ventral aspects of the otic vesicle (D–H). At stage 45 (I) Sox9 is also detected in the protrusions of the developing semicircular canals (arrow). In all panels dorsal to top. For whole embryos staining anterior is to the right. In panels (A, B, D) the scale bar represents 300 μm. In panels (C, E–I) the scale bar represents 50 μm.
Sox9 is required for the survival of otic progenitors
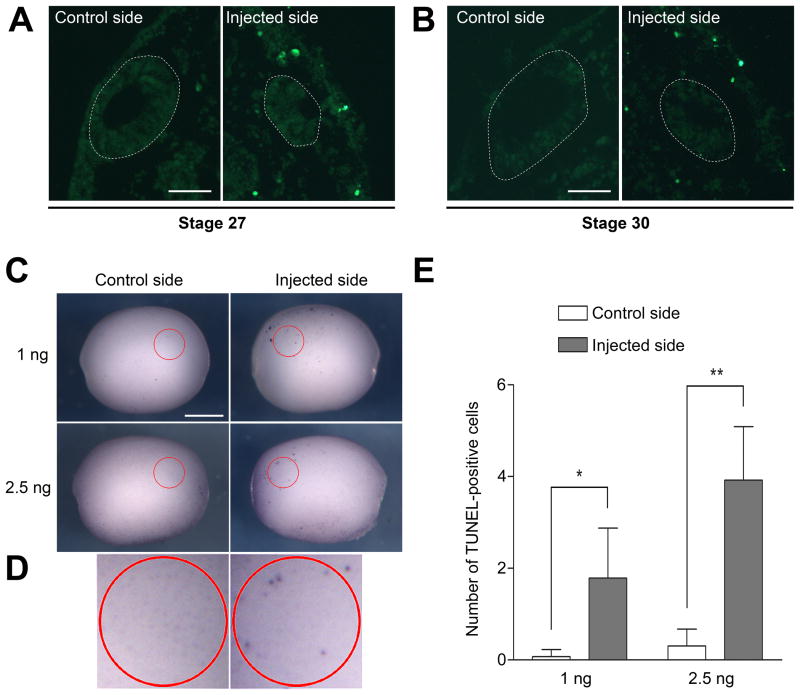
In the otic vesicle of Sox9-depleted embryos the loss of Wnt3a expression, associated with a dorsal expansion of Bmp4/Sox2 expression domains suggested that Sox9 might be required for the maintenance of dorsal otic progenitors. To evaluate this possibility, we analyzed the patterns of cell death in the otic vesicle of Sox9-depleted embryos at different stages. While the otocyst of these embryos were reduced in size as compared to the otic vesicle on the uninjected side, we did not observe any significant increase in TUNEL-positive cells within the otic epithelium of stage 27 or stage 30 embryos (Fig 6A–B). However, we noticed an increase in TUNEL-positive cells in the tissues surrounding the otocyst, suggesting that dorsal otic progenitors may have been excluded from the otic vesicle at an earlier time point. To test this possibility, we performed TUNEL staining at an earlier stage, when Sox9 expression is initiated in the presumptive otic epithelium (Saint-Germain et al., 2004; Fig 5A). At the neurula stage we observed an increase in the number of TUNEL-positive cells in the region of the presumptive otic epithelium on the injected side as compared to the uninjected side (Fig 6C). The increase in TUNEL-positive cells was more pronounced for the higher dose of Sox9MO (2.5 ng; Fig 6D). This result indicates that Sox9 is required for the survival of a population of progenitors in the otic epithelium.
Figure 6. Sox9 is required for the survival of otic precursors.
(A, B) Histological sections of embryos showing TUNEL staining at stage 27 (A) and stage 30 (B). The outlined areas demarcate the position of the otic vesicle, which is reduced in size on the injected side. Fluorescein-labeled TUNEL-positive cells are primarily found in tissues surrounding the otic vesicle. (C) Whole-mount TUNEL staining at stage 15. Lateral views, dorsal to top, anterior to the right (control side) or to the left (injected side). The circled areas (red) outline the approximate position of the prospective otic epithelium in which TUNEL-positive cells were counted. (D) Higher magnification views of the circled areas of the embryos injected with 2.5 ng Sox9MO shown in panel (C). In panels (A, B) the scale bars represent 50 μm. In panel (C) the scale bar represents 500 μm. (E) Graph illustrating the quantification of TUNEL-positive cells on control and injected sides in embryos that received unilateral injection of Sox9MO (1 ng, n=14; and 2.5 ng, n=13). (*) P-value is <0.003; (**) P-value is <0.001.
Sox9 is required for the proliferation of otic progenitors
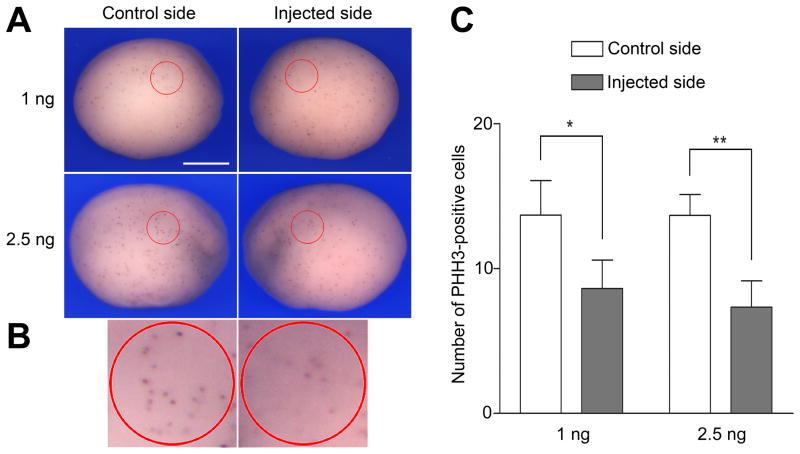
The relatively mild but significant increase in the number of apoptotic cells observed upon Sox9MO injection, cannot explain by itself the inner ear phenotype of Sox9-depleted embryos (Fig 1 and Fig 2). This led us to investigate whether Sox9 was also regulating cell proliferation in the otic epithelium. Using an antibody directed against phosphohistone H3 we found that the rate of cell proliferation in the region of the presumptive otic epithelium of neurula stage embryos was significantly reduced by approximately 50% on the injected side as compared to the uninjected side (Fig. 7A–B). These data strongly suggest that in addition to cell survival one of the functions of Sox9 is to maintain progenitor proliferation in the otic epithelium.
Figure 7. In the absence of Sox9 function otic precursors showed reduced proliferation.
(A) Whole-mount phosphohistone H3 (PHH3) staining at stage 15. Lateral views, dorsal to top, anterior is to the right (control side) or to the left (injected side). The circled areas (red) outline the approximate position of the prospective otic epithelium in which PHH3-positive cells were counted. The scale bar represents 500 μm. (B) Higher magnification views of the circled areas of the embryos injected with 2.5 ng Sox9MO shown in panel (A). (C) Graph illustrating the quantification of PHH3-positive cells on control and injected sides in embryos that received unilateral injection of Sox9MO (1 ng, n=16; and 2.5 ng, n=15). (*) P-value is <0.0008; (**) P-value is <0.0003.
Discussion
The fully developed amphibian inner ear has two major subdivisions, dorsally the pars superior formed by a utricle and three semicircular canals, and ventrally the pars inferior consisting of the saccule, basilar papilla and amphibian papilla (Paterson, 1948; Bever et al., 2003). As in most vertebrates, the sensory epithelia of the semicircular canals and utricle mediate the vestibular function of the inner ear (Lambert et al., 2008). However, unlike mammals, amphibians do not form a cochlea, it is the saccule, basilar papilla and amphibian papilla that will perform the auditory function (Smotherman and Narins, 2000; Riley and Phillips, 2003). In the present study we show that the development of the pars superior strongly depends on Sox9 function, while the pars inferior shows a lesser dependence on Sox9. In tadpoles lacking Sox9, otic progenitors show impaired proliferation and increase apoptosis leading to a dramatic loss of vestibular structures, semicircular canals and utricle, suggesting an important role for Sox9 in the maintenance of these progenitors. While the saccule was present, the development of the saccular macula was defective (missing otoconia and hair cells) in Sox9-depleted tadpoles, suggesting that these embryos may have both vestibular and auditory defects.
While our previous analysis of Sox9-depleted embryos revealed a requirement for Sox9 in otic progenitors specification as evidenced by the loss of early otic placode marker genes such as Pax8 and Tbx2 (Saint-Germain et al., 2004), the rather severe early defects in these embryos prohibited an analysis of Sox9 role in later stages of inner ear development. Activation of an inhibitory mutant of Sox9 at the neurula stage had little effect on otic development at later stages, indicating that Sox9 may not be required for the subsequent stages of otic development (Saint-Germain et al., 2004). This conclusion was based on the observation that the expression of regional marker genes, namely Otx2, Bmp4 and Wnt3a, appeared unaffected at stage 35. However in these experiments we could not exclude the possibility that subtler later defects in the architecture of the inner ear may still be occurring in these embryos. Moreover, it is also possible that the inhibitory mutant resulted only in an incomplete inhibition of Sox9 function. Here, by injection of increasing doses of Sox9 morpholino, we generated embryos with attenuated Sox9 function, and showed that dorsal otic structures are more sensitive to Sox9 depletion than the ventral structures, as these embryos show more pronounced defects in the pars superior.
The failure to form semicircular canals and utricle in Sox9-depleted embryos was associated with a reduction or loss of Wnt3a- and Pax2-expressing cells in the dorsal aspect of the otocyst, concomitant to a dorsal expansion of Bmp4 and Sox2 expression domains. In an attempt to visualize the loss in otic progenitors we performed TUNEL staining on morphant embryos at different stages. Surprisingly we found that these cells were excluded from the otic epithelium prior to the otic vesicle stage, strongly arguing for an early function of Sox9 in otic progenitors survival. The increase in apoptotic cells in Sox9 morphant embryos was also associated with a significant decrease in cell proliferation in the region of the otic epithelium. This is not the first example of a role for Sox9 in cell survival. In mouse embryos lacking Sox9 function prospective neural crest cells in the dorsal neuro-epithelium undergo programmed cell death shortly after migration is initiated (Cheung et al., 2005). Similarly the non-invaginating placodal cells in Sox9 mutant mouse embryos are eliminated by apoptosis (Barrionuevo et al., 2008).
Interestingly, Sox9 is expressed in both the dorsal and ventral aspect of the otic vesicle (Saint-Germain et al., 2004; Fig 5), however we observed the most dramatic defects in the dorsally-derived structures, semicircular canals and utricle in morphant tadpoles. The saccule was also affected in these embryos but to a lesser extent. This could be due to the fact that the formation of semicircular canals involves more intricate morphogenetic processes highly susceptible to alterations. Alternatively this difference could be explained by the fact that dorsal progenitors are more sensitive to Sox9 dosage than ventral progenitors.
Mutations in one Sox9 allele result in Campomelic Dysplasia (CD), a lethal human disorder characterized by autosomal XY sex reversal and severe skeletal malformations (Foster et al., 1994; Wagner et al., 1994; McDowall et al., 1999; Wunderle et al., 1998; Olney et al., 1999; Preiss et al., 2001). Patients affected by this condition usually die after birth due to respiratory distress. However, patients that survived through adolescence and adulthood are often affected by sensorineural deafness, and in some rare cases exhibited malformations of the cochlear duct (Tokita et al., 1979; Houston et al., 1981; Savarirayan et al., 2003). Our finding using Xenopus are consistent with a critical role of Sox9 in inner ear formation, but also highlights some differences across species in the relative importance of Sox9 in the development of specific components of the inner ear. These differences may reflect the evolutionary transformation of the inner ear associated with the changes from an aquatic to a terrestrial mode of life.
Our results suggest the necessity for a tight regulation of Sox9 activity during inner ear development. While we were not able to directly evaluate changes in endogenous protein levels in morphant embryos due to the lack of appropriate antibodies, we like to propose that the level of Sox9 proteins in the otic epithelium is essential to regulate multiple aspects of inner ear development. Consistent with this view, the severity of the phenotype of patients affected by CD has been shown to be highly sensitive to dosage and expression levels of SOX9 (Olney et al., 1999). Organ sensitivity to Sox9 gene dosage appears to differ for specific organs and between species. For example, while the skeletal defects characteristic of CD patients are also seen in Sox9 heterozygous mutant mice (Bi et al., 2001), complete sex reversal in mice is only observed upon inactivation of both Sox9 alleles (Barrionuevo et al., 2006). Along the same lines, Sox9 deficient mice demonstrate that Sox9 is required in the maintenance of early pancreatic progenitor cells (Seymour et al., 2007), while mice with a 50% reduction in Sox9 protein levels show that Sox9 is critical for the development of the endocrine lineage (Seymour et al., 2008). It is also important to mention that Sox9 activity is not only controlled by gene dosage, but also through post-translational modifications either by phosphorylation (Huang et al., 2000) or SUMOylation (Taylor et al., 2005).
How to explain mechanistically the dosage effect of Sox9? It has been reported that depending on the context Sox9 may function either as a dimer or as a monomer. For example, Sox9 dimerization is required for target gene activation during chondrogenesis, while during sex determination Sox9 is believed to act as a monomer (Bernard et al., 2003). Interestingly Sox9 monomers and dimers form at distinct protein thresholds (Sock et al., 2003), therefore a reduction in protein levels may differentially impact the activation of individual target genes. This is a mechanism, which together with the functional redundancy of other SoxE proteins (Sox8 and Sox10), may contribute to the variability of the phenotype observed in CD patients.
Experimental Procedures
Xenopus embryo manipulations and injections
Embryos were staged according to Nieuwkoop and Faber (1967) and raised in 0.1X NAM (Normal Amphibian Medium; Slack and Forman, 1980). Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, under animal protocol # 880275. A translation blocking Sox9 morpholino antisense oligonucleotide (Sox9MO) was purchased from Gene-Tools LLC (Philomath, OR). The specificity of this morpholino has been previously described (Spokony et al., 2002; Saint-Germain et al., 2004). Sox9MO (1 ng, 2.5 ng or 5 ng) was injected in one animal ventral blastomere at the 8-cell stage to target the otocyst, as shown in several fate map studies (Moody, 1987; Dale and Slack, 1987; Takasaki, 1987; Huang et al., 1998). In most experiments, embryos were co-injected with β-galactosidase mRNA (β-gal, 1 ng) to identify the injected side.
In situ hybridization and histology
Embryos were fixed in MEMFA (Harland, 1991) and successively processed for Red-Gal (Research Organics) staining and in situ hybridization. Antisense DIG-labeled probes (Genius kit, Roche) were synthesized using template cDNA encoding Sox9 (Saint-Germain et al., 2004), Otx2 (Pannese et al., 1995), Xwnt3a (Wolda et al., 1993), Bmp4 (Kil and Collazo, 2001), Pax2 (Heller and Brandli, 1999) and Sox2 (Mizuseki et al., 1998). Whole-mount in situ hybridization was performed as previously described (Harland, 1991). In some cases stained embryos were embedded into Paraplast+, 12 μm sections cut on a rotary microtome and counterstained with eosin. For in situ hybridization on section, embryos were fixed in MEMFA for 1 hour, embedded into Paraplast+, and 12 μm sections hybridized with Sox9 or Bmp4 probes according to the procedure described by Henry et al. (1996). Sections were then briefly counter stained with eosin. To visualize otoconia and hair cells at stage 45, embryos were fixed in MEMFA, embedded into Paraplast+, cut on a rotary microtome (5 μm sections) and stained with a combination of hematoxylin and eosin (Sigma).
Paint-filling procedure and phenotype analysis
The three-dimensional architecture of the inner ear was analyzed by paint-filling of the endolymphatic spaces as described (Bever and Fekete, 2002; Bever et al., 2003). Embryos at stage 48 or 52 were fixed in Bodian’s fixative (71.2% ethanol, 5% glacial acetic acid, 5% formalin in water), dehydrated in a graded ethanol series, and cleared in several changes of methyl salicylate. Inner ears were injected with Benjamin Moore white paint (1% in methyl salicylate) using an infusion pump (Stoelting Co., IL) under a dissecting microscope. The architecture of the paint-filled ears was visualized by illumination of the specimen with an obliquely applied fiber optic light source using a Olympus SZX9 stereoscope. In a few specimens where paint leaked into surrounding tissue, digital images were processed with Adobe Photoshop software to clean up the image so that the endolymphatic spaces could be easily compared. The inner ear phenotypes of stage 52 and 48 embryos were divided into 4 classes. Class I: normal inner ear structure. Class II: abnormal semicircular canals and utricle. Class III: semicircular canals and utricle absent; saccule reduced in size. Class IV: no inner ear.
Proliferation assay and TUNEL staining
For phosphohistone H3 detection (Saka and Smith, 2001), Sox9MO-injected albinos embryos were fixed in MEMFA. Embryos were incubated successively in α-phosphohistone H3 antibody (Upstate Biotechnology; 1μg/ml) and anti-rabbit IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch; 1:1000). Alkaline phosphatase activity was revealed using NBT/BCIP (Roche). Whole-mount TUNEL staining was performed as previously described (Hensey and Gautier, 1998; O’Donnell et al., 2006). End labeling was carried out overnight at room temperature in TdT buffer containing 0.5 μM DIG-dUTP and 150 U/ml TdT (Roche). DIG was detected by an anti-DIG antibody conjugated to alkaline phosphatase (Roche; 1:2000) and the chromogenic reaction performed using NBT/BCIP (Roche). For proliferation assay and TUNEL staining fluorescein lysine dextran (FLDX; MW 10,000, Molecular Probes) was used as a lineage tracer to identify the injected side. In some cases (stage 27 and stage 30 embryos) TUNEL-positive cells were detected on histological sections using an in situ Cell Death Detection Kit (Roche Diagnostics), following the manufacturer instructions. Fluorescein-labeled TUNEL-positive cells then were observed using an epifluorescence microscope (Nikkon, Eclipse E800).
In-vitro transcription/translation
The in vitro transcription/translation coupled rabbit reticulocyte lysate system was used according to the manufacturer recommendations (Promega) in the presence of [35S] methionine and resolved on a NuPAGE BIS-Tris gel (Invitrogen). The activity of Sox9MO was determined by adding increasing amount of morpholino (1 ng, 10 ng and 50 ng) to the in vitro transcription/translation reaction directed by a Sox9 cDNA containing the morpholino target sequence (Sox9+5′UTR).
Supplementary Material
Acknowledgments
We are extremely grateful to Dr. Donna Fekete (Purdue University) for invaluable help with the paint-filling technique. We also thank Drs. Randy Moon, Yoishiki Sasai, Andre Brandli and Thomas Sargent for reagents. This work was supported by a grant from the National Institutes of Health to J-P S-J (RO1-DC07175).
References
- Bagheri-Fam S, Barrionuevo F, Dohrmann U, Günther T, Schüle R, Kemler R, Mallo M, Kanzler B, Scherer G. Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev Biol. 2006;291:382–397. doi: 10.1016/j.ydbio.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Baird RA, Torres MA, Schuff NR. Hair cell regeneration in bullfrog vestibular otolith organs following aminoglycoside toxicity. Hear Res. 1993;65:164–174. doi: 10.1016/0378-5955(93)90211-i. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Naumann A, Bagheri-Fam S, Speth V, Taketo MM, Scherer G, Neubüser A. Sox9 is required for invagination of the otic placode in mice. Dev Biol. 2008;317:213–224. doi: 10.1016/j.ydbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Bernard P, Tang P, Liu S, Dewing P, Harley VR, Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- Bever MM, Fekete DM. Atlas of the developing inner ear in zebrafish. Dev Dyn. 2002;223:536–543. doi: 10.1002/dvdy.10062. [DOI] [PubMed] [Google Scholar]
- Bever MM, Jean YY, Fekete DM. Three-dimensional morphology of inner ear development in Xenopus laevis. Dev Dyn. 2003;227:422–430. doi: 10.1002/dvdy.10316. [DOI] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective precartilaginous mesenchymal condensations and premature skeletal mineralization. Proc Natl Acad Sci (USA) 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisonnette JP, Fekete DM. Standard atlas of the gross anatomyof the developing inner ear of the chicken. J Comp Neurol. 1996;368:620–630. doi: 10.1002/(SICI)1096-9861(19960513)368:4<620::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev Cell. 2005;8:179–92. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JMW. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:141–152. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Fekete DM. Cell fate specification in the inner ear. Curr Opin Neurobiol. 1996;6:533–541. doi: 10.1016/s0959-4388(96)80061-4. [DOI] [PubMed] [Google Scholar]
- Fekete DM. Development of the vertebrate ear: insights from knockouts and mutants. Trends Neurosci. 1999;22:263–269. doi: 10.1016/s0166-2236(98)01366-6. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kowk G, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–156. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon CM, Lewis JH. Hyaluronan as a propellant for epithelial movement: the development of semicircular canals in the inner ear of Xenopus. Development. 1991;112:541–50. doi: 10.1242/dev.112.2.541. [DOI] [PubMed] [Google Scholar]
- Hadrys T, Braun T, Rinkwitz-Brandt S, Arnold HH, Bober E. Nkx5.1 controls semicircular canals formation in the mouse inner ear. Development. 1998;125:33–39. doi: 10.1242/dev.125.1.33. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Heller N, Brandli AW. Xenopus Pax-2/5/8 orthologues: novel insights into Pax gene evolution and identification of Pax-8 as the earliest known marker for otic and pronephric cell lineages. Dev Genet. 1999;24:208–219. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<208::AID-DVG4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Henry GL, Brivanlou IH, Kessler DS, Hemmati-Brivanlou A, Melton DA. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. doi: 10.1242/dev.122.3.1007. [DOI] [PubMed] [Google Scholar]
- Hensey C, Gautier J. Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev Biol. 1998;203:36–48. doi: 10.1006/dbio.1998.9028. [DOI] [PubMed] [Google Scholar]
- Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, Gilbert EF, Herrmann J, Schinzel A. The Campomelic Syndrome: Review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Amer J Med Genet. 1981;15:3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- Huang S, Johnson KE, Wang HZP. Blastomeres show differential fate changes in 8-cell Xenopus laevis embryos that are rotated 90o before first cleavage. Develop Growth Differ. 1998;40:189–198. doi: 10.1046/j.1440-169x.1998.00008.x. [DOI] [PubMed] [Google Scholar]
- Huang W, Whou X, Lefebvre V, de Crombrugghe B. Phosphorylation of Sox9 by cAMP-dependent protein kinase A enhances Sox9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil SH, Collazo A. Origins of the inner ear sensory organs revealed by fate map and time-lapse analyses. Dev Biol. 2001;233:365–379. doi: 10.1006/dbio.2001.0211. [DOI] [PubMed] [Google Scholar]
- Lambert FM, Beck JC, Baker R, Straka H. Semicircular canal size determines the developmental onset of angular vestibuloocular reflexes in larval Xenopus. J Neurosci. 2008;28:8086–8095. doi: 10.1523/JNEUROSCI.1288-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodelings that control semicircular canal formation in the developing mouse inner ear. Dev Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- McDowall S, Argentaro A, Ranganathan S, Weller P, Mertin S, Mansour S, Tolmie J, Harley V. Functional and structural studies of wild type SOX9 and mutations causing campomelic dysplasia. J Biol Chem. 1999;274:24023–24030. doi: 10.1074/jbc.274.34.24023. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam, The Netherlands: North Holland Publishing Company; 1967. [Google Scholar]
- O’Donnell M, Hong CS, Huang X, Delnicki RJ, Saint-Jeannet JP. Functional analysis of Sox8 during neural crest development in Xenopus. Development. 2006;133:3817–3826. doi: 10.1242/dev.02558. [DOI] [PubMed] [Google Scholar]
- Olney PN, Kean LS, Graham D, Elsas LJ, May KM. Campomelic syndrome and deletion of Sox9. Am J Med Genet. 1999;84:20–24. [PubMed] [Google Scholar]
- Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development. 1995;121:707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- Paterson NL. The development of the inner ear of Xenopus laevis. Proc Zool Soc Lond. 1948;119:269–291. [Google Scholar]
- Preiss S, Argentaro A, Clayton A, John A, Jans DA, Ogata T, Nagai T, Barrosso I, Schafer AJ, Harley VR. Compound effects of point mutations causing campomelic dysplasia/autosomal sex reversal upon Sox Structure, nuclear transport, DNA binding, and transcriptional activation. J Biol Chem. 2001;276:27864–27872. doi: 10.1074/jbc.M101278200. [DOI] [PubMed] [Google Scholar]
- Quick QA, Serrano EE. Inner ear formation during the early larval development of Xenopus laevis. Dev Dyn. 2005;234:791–801. doi: 10.1002/dvdy.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BB, Phillips BT. Ringing in the new ear: resolution of cell interactions in otic development. Dev Biol. 2003;261:289–312. doi: 10.1016/s0012-1606(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Saint-Germain N, Lee YH, Zhang Y, Sargent TD, Saint-Jeannet J-P. Specification of the otic placode depends on Sox9 function in Xenopus. Development. 2004;131:1755–1763. doi: 10.1242/dev.01066. [DOI] [PubMed] [Google Scholar]
- Savarirayan R, Robertson SP, Bankier A, Rogers JG. Variable expression of campomelic dysplasia in a father and his 46, XY daughter. Ped Pat Mol Med. 2003;22:37–46. doi: 10.1080/pdp.22.1.37.46. [DOI] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci (USA) 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour PA, Freude KK, Dubois CL, Shih H-P, Patel NA, Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack JM, Forman D. An interaction between dorsal and ventral regions of the marginal zone in early amphibian embryos. J Embryol Exp Morphol. 1980;56:283–299. [PubMed] [Google Scholar]
- Sock E, Pagon RA, Keymolen K, Lissens W, Wegner M, Scherer G. Loss of DNA-dependent dimerization of the transcription factor SOX9 as a cause for campomelic dysplasia. Hum Mol Genet. 2003;12:1439–1447. doi: 10.1093/hmg/ddg158. [DOI] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. The electrical properties of auditory hair cells in the frog amphibian papilla. J Neurosci. 1999;19:5275–5292. doi: 10.1523/JNEUROSCI.19-13-05275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotherman MS, Narins PM. Hair cells, hearing and hopping: a field guide to hair cell physiology in the frog. J Exp Biol. 2000;203:2237–2246. doi: 10.1242/jeb.203.15.2237. [DOI] [PubMed] [Google Scholar]
- Spokony RF, Aoki Y, Saint-Germain N, Magner-Fink EK, Saint-Jeannet J-P. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development. 2002;129:421–432. doi: 10.1242/dev.129.2.421. [DOI] [PubMed] [Google Scholar]
- Takasaki T. Fates and roles of the presumptive organizer region in the 32-cell embryo in normal development of Xenopus laevis. Develop Growth Differ. 1987;29:141–152. doi: 10.1111/j.1440-169X.1987.00141.x. [DOI] [PubMed] [Google Scholar]
- Taylor KM, LaBonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Tokita N, Chandra-Sekhar HK, Daly JF, Becker MH, Aleksic S. The Campomelic syndrome. Temporal bone histopathologic features and otolaryngologic manifestations. Arch Otolaryngol. 1979;105:449–54. [Google Scholar]
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;121:4057–4065. doi: 10.1242/dev.122.11.3381. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Dagna Bricarelli F, Keutel J, Heustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and Campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wang W, Van de Water T, Lufkin T. Inner ear and maternal reproductive defects in mice lacking Hmx3 homeobox gene. Development. 1998;125:621–634. doi: 10.1242/dev.125.4.621. [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3a and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Wunderle VM, Critcher R, Hastie N, Goodfellow PN, Schedl A. Deletion of long-range regulatory elements upstream of SOX9 causes campomelic dysplasia. Proc Natl Acad Sci (USA) 1998;95:10649–10652. doi: 10.1073/pnas.95.18.10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YL, Willoughby J, Liu D, Crump JG, Wilson C, Miller CT, Singer A, Kimmel C, Westerfield M, Postlethwait JH. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.