Abstract
N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine (Ac-DCVC) and S-(1,2-dichlorovinyl)-L-cysteine (DCVC) are the glutathione conjugation pathway metabolites of a common industrial contaminant and potent nephrotoxicant trichloroethylene (TCE). Ac-DCVC and DCVC are accumulated in the renal proximal tubule where they may be secreted into the urine by an unknown apical transporter(s). In this study we explored the hypothesis that the apical transport of Ac-DCVC and/or DCVC may be mediated by the multidrug resistance associated protein 2 (Mrp2, ABCC2), which is known to mediate proximal tubular apical ATP-dependent transport of glutathione and numerous xenobiotics and endogenous substances conjugated with glutathione. Transport experiments using membrane vesicles prepared from mouse proximal tubule derived cells expressing mouse Mrp2 utilizing ATPase assay and direct measurements of Ac-DCVC/DCVC using liquid chromatography/tandem mass-spectrometry (LC/MS/MS) demonstrated that mouse Mrp2 mediates ATP-dependent transport of Ac-DCVC. Expression of mouse Mrp2 antisense mRNA significantly inhibited the vectorial basolateral to apical transport of Ac-DCVC but not DCVC in mouse proximal tubule derived cells endogenously expressing mouse Mrp2. The results suggest that Mrp2 may be involved in the renal secretion of Ac-DCVC.
Keywords: Mrp2, mercapturates, kidney, proximal tubule, xenobiotics
Introduction
Trichloroethylene (TCE)1 is an industrial solvent produced in the United States at a rate of ∼130,000 tons per year, and is the most commonly found chemical contaminant in ground water (De Rosa et al., 1996). TCE is especially toxic for kidney, liver, heart, skin, and the central nervous and gastrointestinal systems (Agency for Toxic Substances and Disease Registry, 1993). TCE causes acute renal failure in mammals by selective necrosis of the proximal straight tubule (Silber et al., 1986; Wolfgang et al., 1989; Wallin et al., 1992; Kays et al., 1993; Cooper, 1994; Dekant et al., 1994; Lash et al., 2001).
An important biochemical mechanism of the detoxification and bioactivation of TCE starts with conjugation with glutathione (GSH) (Inoue et al., 1984). A GSH conjugation pathway metabolite, S-1,2-dichlorovinyl-L-cysteine (DCVC), is involved in the mediation of TCE toxicity via the formation of toxic compounds in reactions catalyzed by cysteine conjugate β-lyases or/and flavin monooxygenases (Anders et al., 1994; Anders, Dekant, 1998; Lash et al., 2006). The GSH conjugation pathway occurs predominantly in the liver and results in the production of DCVC and further N-acetyl-DCVC (Ac-DCVC) that both accumulate in the renal proximal tubule. Ac-DCVC is not directly toxic because it is not a substrate of β-lyases and flavin monooxygenases. Given that Ac-DCVC in the proximal tubule may be deacetylated by aminoacylase 3 (AA3) (Pushkin et al., 2004), thereby providing the substrate for β-lyases and flavin monooxygenases to generate toxic compounds, intracellular levels of both DCVC and Ac-DCVC in the proximal tubule significantly determine the TCE induced toxicity. Therefore knowledge of the transporter(s) involved in the renal proximal tubule secretion of Ac-DCVC and DCVC is necessary to understand mechanisms involved in the TCE induced nephrotoxicity.
DCVC and Ac-DCVC accumulated in the kidney may be filtered through glomeruli or/and secreted through the renal proximal tubule by the consecutive action of the basolateral amino acid system(s) (Wolfgang et al., 1989) and the basolateral organic acid transporter 1 (Oat1) respectively and apical transporters. The nature of the apical transporter(s) mediating secretion of DCVC and Ac-DCVC in the proximal tubule is unknown although a Na+-independent transport of DCVC mediated by isolated rat renal proximal tubule cells has been described (Lash and Anders, 1989) that may be involved in the apical secretion of DCVC in the proximal tubule. The Na+-dependent transport of DCVC into the rabbit renal brush-border membrane vesicles was described by Wright et al., (1998), which might be responsible for reabsorption of DCVC from the lumen of the proximal straight tubule given the predominant right-side out orientation of the membrane vesicles prepared from renal brush-border membranes (Haase et al., 1978). There are no data on the transport processes involved in reabsorption of Ac-DCVC in renal proximal tubules.
The multidrug resistance associated protein 2 (Mrp2, ABCC2) is a candidate transporter to mediate Ac-DCVC or/and DCVC secretion in the renal proximal tubule because it has been shown to mediate the apical transport of a wide range of endo- and xenobiotics, including bilirubin, hormones, drugs, and carcinogens, primarily as their glucuronide, GSH, or sulfate conjugates (Van Aubel et al., 2000; Nies, Keppler, 2007). Mrp2 is highly expressed at the apical membrane of renal proximal tubules (Schaub et al., 1997), mammalian hepatocytes (Büchler et al., 1996; Paulusma et al., 1996), and small intestinal villi (Van Aubel et al., 2000) where it mediates the ATP-dependent transport efflux of amphiphilic anionic substrates. In the present study we determined whether Mrp2 mediates the ATP-dependent transport of Ac-DCVC and/or DCVC using 1) membrane vesicles prepared from mouse proximal tubule derived cells expressing mouse Mrp2, and 2) monolayers of polarized mouse proximal tubule derived cells expressing mouse Mrp2.
Membrane vesicles containing Mrp2 provide an opportunity to characterize its transport characteristics. Given the ATP-dependence of this system, the hydrolysis of ATP is often used as an indirect measure of the transport kinetics. The transport characteristics of Mrp2 have been investigated extensively using canalicular membrane vesicles, and hepatocytes isolated from wild type (wt) and Mrp2-deficient rats (Oude Elferink et al., 1995; Suzuki, Sugiyama, 1998). Unfortunately, the predominant right-side out orientation of the membrane vesicles preparations using the renal brush-border proximal tubule membranes (Haase et al., 1978) prevents their use to study the transport kinetics mediated by renal Mrp2. Therefore, membrane vesicles prepared from the animal cells expressing recombinant Mrp2 is an attractive alternative experimental approach that we used in the present study. In addition, we measured the vectorial basolateral to apical and apical to basolateral transport of Ac-DCVC and DCVC through monolayers of the mouse proximal tubule derived mPCT cells endogenously expressing Mrp2. The results indicate that mouse Mrp2 mediates the ATP-dependent transport of Ac-DCVC but not DCVC, and may be involved in renal secretion of Ac-DCVC.
Materials and methods
Cloning of mouse Mrp2
Mouse full-length Mrp2 cDNA was amplified by RT-PCR from a mouse kidney total RNA (Stratagene, La Jolla, CA) using following sense (CGGTACCTATGGACGAATTCTGCAACTCTAC) and antisense (TCTCGAGCTAGAGCTCCGTGTGGTTCACACT) primers. For expression in the mouse proximal tubule derived mPCT-A2 cells, it was inserted into the KpnI and XhoI site of the pcDNA3.1/His vector (Invitrogen, Carlsbad, CA).
Preparation of Mrp2-containing membrane vesicles
The mPCT-A2 cell line that does not endogenously express Mrp2 (Fig. 1A) was used in these experiments. Cells were grown on plastic dishes with mouse renal tubular epithelium medium, which contained 12 ml of a 1:1 mixture of Dulbecco's modified Eagle's medium and Ham's F12 medium, and 2 mM glutamine, 10 ng·ml-1 epidermal growth factor, 5 μg·ml-1 insulin, 5 μg·ml-1 transferrin, 4 μg·ml-1 dexamethasone, 10 units·ml-1 interferon-γ, and 5 % fetal bovine serum. This medium is identical to the medium used for growth of the mouse proximal tubule derived mPCT cell line (Gross et al., 2001). Cells were transfected with 3 μg·μl-1 of purified pcDNA3.1/His vector (Qiagen, Santa Clarita, CA) containing the coding sequence of mouse full-length Mrp2 using the Lipofectamine method (Invitrogen) as recommended by the manufacturer. The cells collected from 10 plates 48 h after transfecton were washed 2 times with phosphate buffered saline (PBS) and resuspended in 0.5 mM sodium phosphate, pH 7.4, containing 0.1 mM EDTA and tablet/20 ml of complete protease inhibitors cocktail and 1 μg·ml-1 pepstatin (both from Roche, Indianapolis, IN). After incubation at 4°C for 90 min, the suspension was centrifuged at 14,000 g for 15 min, and then the supernatant was centrifuged at 100,000 g for 40 min, and the pellet was homogenized in ice-cold 50 mM Tris-HCl, pH 7.5, containing 250 mM sucrose, in a tight-fitting Dounce homogenizer. After centrifugation at 500 g for 10 min at 4°C, the supernatant was centrifuged at 15,000 g for 10 min, and then the supernatant was centrifuged at 4°C for 40 min at 100,000 g. The pellet was resuspended in 50 mM Tris-HCl, pH 7.5, containing 250 mM sucrose, and passed 25 times through a 27-gauge needle. The vesicles were dispensed in aliquots, frozen in liquid nitrogen, and stored at −80°C until use but not for more than 3 months. The content of Mrp2 in the vesicles was analyzed by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting using our RP-4 antibody specific to mouse Mrp2.
Fig. 1.



(A) Quantification of mouse Mrp2 in the membrane vesicles prepared from the mPCT-A2 cells (mPCT-A2) transiently expressing mouse Mrp2 (Ex) and from control untransfected (Un) and mock-transfected (Mock) mPCT-A2 cells were resolved on 7.5% SDS-PAGE, transferred onto PDF membranes and probed with RP-4 mouse Mrp2 specific antibody (IgG) and with the same antibody preincubated with the peptide (10:1) used for immunization (IgG + P). Loading: 7 μg. mPCT cells (mPCT) endogenously express Mrp2. The membranes were isolated from mPCT cells using the technique described for mPCT-A2 cell membrane isolation, resolved on 7.5% SDS-PAGE and immunoblotted with RP-4 antibody (IgG) and with the same antibody preincubated with the peptide used for immunization (IgG + P). Loading: 50 μg.
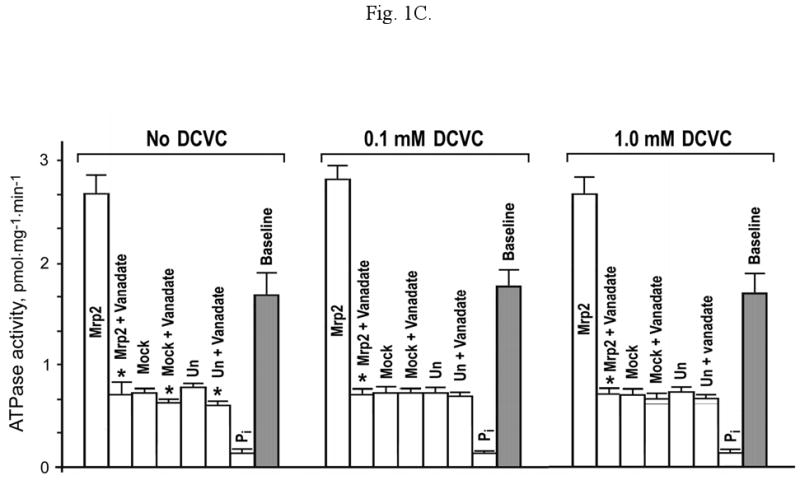
The effect of Ac-DCVC (B) and DCVC (C) on ATPase activity of the membrane vesicles prepared from mPCT-A2 cells expressing mouse Mrp2 (Mrp2), mock-transfected (Mock) or untransfected (Un) mPCT-A2 cells without and in the presence of 1.2 mM vanadate (Vanadate). ATPase activity was measured in an assay containing 5 mM ATP, 10 mM MgCl2, 50 mM KCl, 2 mM GSH, 2 mM dithiothreitol, and 50 mM HEPES, pH 7.5. The amount of inorganic phosphate formed nonenzymatically in the assay was determined in the same assay containing 2% SDS (Pi). The 0.1 mM or 1 mM compound was added into the ATPase assay without preincubation of vesicles. * Shows significant difference (p<0.05) of the total ATPase activity of the vesicles prepared from the cells expressing Mrp2 (or mock-transfected or untransfected cells) without vanadate to the same activity in the presence of 1.2 mM vanadate.
(D) Effect of estradiol 17-β-D-glucuronide (E17bG), leukotriene C4 (LTC4), pronencid, Ac-DCVC, DCVC, N-acetyl-S-(2,2-dichlorovinyl)-L-cysteine (Ac-2,2-DCVC), N-acetyl-S-(4-chlorobenzyl)-L-cysteine (Ac-4-CBC), N-acetyl-S-(4-bromobenzyl)-L-cysteine (Ac-4-BBC), N-acetyl-S-(1,1-difluoro-2,2-dichloroethyl)-L-cysteine (Ac-1,1-DF-2,2-DCC), N-acetyl-S-(1,1-dichloro-2,2-difluoroethyl)-L-cysteine (Ac-1,1-DC-2,2-DFC), N-acetyl-L-cysteine (Ac-Cys) and N-acetyl-L-tyrosine (Ac-Tyr) of the baseline ATPase activity (None) of membrane vesicles prepared from the mPCT-A2 cells expressing Mrp2. The 0.1 mM or 1 mM compound (black and white bars, respectively) was added into the ATPase assay. LTC4 was used at a concentration of 4 μM. The ATPase activity, measured in the presence of compound, is shown as a ratio to the baseline activity without compound (None). * Significant difference (p<0.05) from the baseline activity.
(E) Inhibition of baseline ATPase activity of membrane vesicles prepared from untransfected and Mrp2 expressing mPCT-A2 cells by anti-Mrp2 antibody. Vesicles (100 μg) were pretreated at 37°C for 30 min with RP-4 antibody (100 μg) before ATPase activity was measured as described in Materials and methods. * Shows significant difference (p<0.05) between ATPase activity of untreated membrane vesicles in comparison with the vesicles incubated with RP-4 antibody.
Vesicles were also prepared using an identical procedure from mock-transfected and untransfected mPCT-A2 cells.
Mouse Mrp2-specfic antibody RP-4
The mouse Mrp2-specific antibody RP-4 was generated in rabbit using a specific peptide (KKSQQSPEGTSHGL, amino acids 261–274 in mouse Mrp2) coupled to keyhole lymphet hemocyanin (KLH). The antibody was affinity purified using the immunizing peptide coupled to Sepharose 6B beads (GE HealthCare, Piscataway, NJ). The antibody worked well on immunoblotting, imunohistochemistry of frozen mouse kidney and liver tissues, and on immunoelectron microscopy of mouse kidney.
Immunoblotting
Protein samples were resolved by SDS-PAGE using 7.5% polyacrylamide ready gels from Bio-Rad (Hercules, CA) and then electrotransferred onto PVDF membranes (GE HealthCare). Non-specific binding was blocked by incubation for 1 h in TBS (20 mM Tris-HCl, pH 7.5, 140 mM NaCl) containing 5% dry milk and 0.05% Tween 20 (Bio-Rad). The primary RP-4 antibody and secondary horseradish peroxidase-conjugated mouse anti-rabbit antibody (Jackson ImmunoResearch, West Grove, PA) were used at a dilution 1:1,000 and 1:20,000 respectively. Bands were visualized using an ECL kit and ECL Hyperfilm (both GE HealthCare).
Vesicular ATPase activity
Vesicle ATPase activity was measured in an assay of a total volume 200 μl containing 5 mM ATP, 10 mM MgCl2, 50 mM KCl, 2 mM GSH, 2 mM dithiothreitol, 50 mM HEPES, pH 7.5, 3 μl of membrane vesicles and up to 1 mM Ac-DCVC or other compound. After incubation at 37°C for 30 min, inorganic phosphate (Pi) was quantified with ammonium molybdate (Bencini et al., 1983) by measuring the absorbance at 350 nm on a VMax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). The amount of Pi was calculated using a standard curve generated with potassium phosphate. The assay was performed using vesicles from Mrp2 transfected, untransfected and mock-transfected cells, all in the presence or absence of 1.2 mM Na3VO4. Background Pi levels in the assay were measured in the presence of 2% SDS. The baseline ATPase activity of Mrp2 measured with GSH as Mrp2 substrate without Ac-DCVC was equal to the vanadate inhabitable ATPase activity of the vesicles from Mrp2 transfected cells minus the Pi formed nonenzymatically and generated by vesicles from mock-transfected (untransfected) cells.
The assay was also performed in the presence of the Mrp2-specific antibody RP-4. In this case the antibody added into the assay, what was omitted ATP, in an amount equal to the vesicle protein content. After incubation for 30 min at 37°C, ATP was added into the assay, and the content of Pi was measured after 30 and 75 min incubation as described above.
Quantification of the vesicular transport of Ac-DCVC and DCVC using mass-spectrometry
Direct measurements of the ATP-dependent transport of Ac-DCVC and DCVC into vesicles were performed using a reaction assay of a total volume 200 μl that contained 50 mM HEPES, pH 7.5, 5 mM ATP, 10 mM MgCl2, 50 mM KCl, 2 mM GSH, 2 mM dithiothreitol, 15 μl of membrane vesicles and 1– 1,000 μM Ac-DCVC or DCVC. After 60 min incubation at 37°C vesicles were precipitated by centrifugation for 5 min at 1,000 g, washed twice with 100 μl ice-cold reaction buffer without ATP and finally dissolved in 30 μl of 0.1% dodecyl β-D-maltopyranoside (DDM). In control experiments instead of ATP, an equimolar concentration of AMP was used.
The S0.5 values for ATP-dependent transport of Ac-DCVC into membrane vesicles were determined as the substrate concentration at a half-maximal velocity of transport under the experimental conditions described above using the double-reciprocal plot (Lineweaver, Burk, 1934).
For the quantification of Ac-DCVC, aliquots (typically 2 μl) of the DDM-treated samples were diluted with water/acetonitrile (90/10, 100 μl) and N-acetyl-L-valine (50 pmol in 100 μl of water). Aliquots (typically 100 μl) of this mixture were injected onto a reverse phase HPLC column (PLRP-S, 300 Å, 5 μM; Varian, Palo Alto, CA) equilibrated in buffer A (aqueous 0.01% formic acid) and eluted (250 μl/min) with the increasing concentrations of buffer B (0.01% formic acid in acetonitrile). The effluent from the column was passed directly to an Ionspray® ion source connected to a triple quadrupole mass spectrometer (API III+; PerkinElmer Sciex Instruments, Boston, MA) operating in the negative ion MS/MS MRM mode.
Preliminary experiments revealed that DCVC was poorly retained on reverse phase HPLC columns. Therefore, the N-propionyl derivative (Pr-DCVC), which was formed within a few minutes of treating aqueous DCVC solutions with propionic anhydride, was used instead of DCVC. For quantification of both Ac-DCVC and DCVC, an aliquot (typically 2 μl) of the DDM-treated samples was diluted with water/acetonitrile (90/10, 100 μl) to which propionic anhydride (1 μl) was added. After incubation for 30 min at room temperature, 50 pmol of N-acetyl-L-valine in 100 μl water were added and the mixture was processed as described above.
The following m/z values of the specific transitions were recorded during LC-MS/MS-MRM: Ac-DCVC, 256.0→126.9 and 258.0→128.9; DCVC, 214.0→126.9 and 216.0→128.9; Pr-DCVC, 270.0→126.9 and 272.0→128.9; N-acetyl-L-valine, 158.0→116.0. To enhance the signal intensity for low-abundance samples, both Q1 and Q3 were detuned so that the isotope clusters for the polypropylene calibrant ions between m/z 100 and 500 were not separated from one another. This strategy resulted in a ∼5-fold signal enhancement for the DCVC and related compounds. The MacSpec version 3.3 software was used to calculate peak heights from the MRM traces. For quantification, the most intense of the parent→fragment transitions for each compound were used. These were 256.0→126.9 for Ac-DCVC, 270.0→126.9 for Pr-DCVC, and 158.0→116.0 for N-acetyl-L-valine. Injection of 0.125 pmol for each compound gave strong signals, with peak height/baseline noise ratios exceeding 50:1. MRM sensitivity was routinely checked with injections of 1.25, 6.25, and 12.5 pmol of each compound. At the maximum sensitivity (detuned mass spectrometric conditions), the lower limit of detection for all compounds was around 1.0 pmol injected on column (equivalent to 10 nM in the assay mixture). The retention times of DCVC, internal standard, Ac-DCVC, and Pr-DCVC were 6.2, 12.50, 16.70, and 17.8 min, respectively.
Transcellular transport of Ac-DCVC and DCVC
The vectorial basolateral to apical transport and apical to basolateral transport of Ac-DCVC and DCVC was measured through a monolayer of mPCT cells. These cells endogenously express Mrp2 (Fig. 1A). To suppress Mrp2 synthesis, the cells were transiently transfected with the anti-mouse Mrp2 SureSilencing™ (shRNA) plasmid (SuperArray Biosciences, Frederick, MD) using the Lipofectamine method (Invitrogen). The transfection efficiency of 63±11% was determined by measuring the green fluorescent protein coexpressed with the anti-mouse Mrp2 shRNA. In control experiments the non-specific anti-mouse AA3 shRNA plasmid (SuperArray Biosciences) was used. The transfection efficiency was 70±14%. Forty eight hours post transfection, cells were seeded on Millicell®-24 cell culture plates (Millipore) at the density of 100,000 cells.cm-2. Transport of Ac-DCVC and DCVC across the cell monolayer was measured from both the apical and basolateral directions after the cells formed a monolayer with the resistance of >700 ohm. At this point, cells were washed twice with PBS, and then 0.1 mM or 0.5 mM Ac-DCVC (or DCVC) in PBS was added to either the apical or basolateral compartment. Aliquots of 10 μl were taken from the opposite compartment after 15 and 60 min, and were frozen. The amount of Ac-DCVC and DCVC was measured using LC/MS/MS MRM as described above. The experiments were performed in triplicate.
Synthesis of mercapturic acids and DCVC
DCVC, Ac-DCVC, N-acetyl-S-(2,2-dichlorovinyl)-L-cysteine, N-acetyl-S-(4-chlorobenzyl)-L-cysteine, N-acetyl-S-(4-bromobenzyl)-L-cysteine, N-acetyl-S-(1,1-difluoro-2,2-dichloroethyl)-L-cysteine, N-acetyl-S-(1,1-dichloro-2,2-difluoroethyl)-L-cysteine were synthesized and characterized as described before (Birner et al., 1997; Werner et al., 1997; Scholz et al., 2005).
Data analysis
All values are means ±S.E. of measurements of at least three separate experiments unless specifically indicated. Statistical significance of differences was determined by the unpaired Student t test. A probability (p) level of < 0.05 was considered as significant.
Results and discussion
Vesicular ATPase activity
Mrp2 is known to transport a wide range of substrates including organic anions, and various toxins and carcinogens conjugated with glucuronide, GSH and sulfate (Van Aubel et al., 2000; Neis, Keppler, 2007). Mrp2 may cotransport GSH together with other substrates. Given the ATP-dependence of the transport mediated by Mrp2, in order to determine whether Mrp2 transports Ac-DCVC or/and DCVC, we studied ATP hydrolysis mediated by membrane vesicles prepared from the mouse proximal tubule derived mPCT-A2 cells exogenously expressing mouse Mrp2 with and without Ac-DCVC/DCVC. The mPCT-A2 cell line that does not endogenously express Mrp2 (Fig. 1A) was used in these experiments because it was significantly more efficiently transfected than the parent mPCT cell line.
In the absence of Ac-DCVC/DCVC, ∼2.7 pmol Pi.mg-1.min-1 were formed in the assay, of which less than 5% were attributed to the background nonenzymatic Pi formation (Fig. 1B,C). The vanadate sensitive activity was ∼75% of the total Pi liberated in the assay. Pi formation in assays conducted with vesicles prepared from mock-transfected and untransfected cells was the same as that from the “Mrp2 vesicles + vanadate” assay. Inclusion of vanadate in the assay using mock-transfected and untranfected cell vesicles resulted in only a small insignificant decrease in the rate of Pi formation. Therefore the baseline ATPase activity of Mrp2 was equal to the vanadate inhabitable activity of Mrp2 minus the Pi formed nonenzymatically and generated by vesicles from mock-transfected cells. The baseline ATPase activity of Mrp2 was reduced by ∼60% in the presence of both 0.1 mM and 1.0 mM Ac-DCVC (Fig. 1B) whereas this mercapturic acid had no effect on the baseline ATPase activity of vesicles from mock-transfected and untransfected cells. In contrast, DCVC had no significant effect on the baseline activity of Mrp2 (Fig. 1C).
Given the indirect character of ATPase assay, the experiments were performed utilizing in this assay typical substrates of Mrp2; estradiol 17-β-D-glucuronide (E17bG) and leukotriene C4 (LTC4). Both E17bG and LTC4 inhibited the baseline ATPase activity of Mrp2 (Fig. 1D) and the rates of inhibition by 1 mM E17bG and Ac-DCVC were similar.
LTC4 was a more potent inhibitor of the baseline ATPase activity of Mrp2 than E17bG. Human and rat Mrp2 have high affinities for LTC4 (Km ∼1 μM) than E17bG (Km ∼7 μM) (Cui et al., 1999; Ishikawa et al., 1990). The higher affinity of human and rat Mrp2 for LTC4 than E17bG correlates with the higher inhibition effects of LTC4 than E17bG on the baseline ATPase activity of mouse Mrp2 in the present study. Therefore our data suggested that Ac-DCVC might be a substrate of mouse Mrp2.
In addition to Ac-DCVC, several mercapturic acids, namely N-acetyl-S-(2,2-dichlorovinyl)-L-cysteine, N-acetyl-S-(4-chlorobenzyl)-L-cysteine, N-acetyl-S-(4-bromobenzyl)-L-cysteine, and N-acetyl-S-(1,1-difluoro-2,2-dichloroethyl)-L-cysteine also inhibited the baseline ATPase activity of Mrp2 (Fig. 1D) suggesting that mouse Mrp2 can also mediate the ATP-dependent transport of these mercapturic acids. In contrast to the strong inhibiting effect of N-acetyl-S-(1,1-difluoro-2,2-dichloroethyl)-L-cysteine, its isomer N-acetyl-S-(1,1-dichloro-2,2-difluoroethyl)-L-cysteine had no significant effect on the baseline ATPase activity of Mrp2 suggesting that structure of the conjugated group may be important for transport activity. N-acetylated amino acids did not affect the baseline ATPase activity of Mrp2.
The results suggested that mouse Mrp2 may transport Ac-DCVC and several halogenated mercapturic acids but does not mediate transport of DCVC.
Inhibition of ATPase activity of membrane vesicles by anti-Mrp2 antibody
To further explore the hypothesis that Mrp2 mediates transport of Ac-DCVC, we determined whether this process is affected by our Mrp2-specific antibody RP-4. Based on the generally accepted topology model of Mrp2 (Borst et al., 1999; Farrel et al., 2005; Ito et al., 2001; Leslie et al., 2005), the sequence used for rabbit immunization (aa. 261–274) is located in the third intracellular loop of Mrp2. This sequence is likely to be located on the outside of the vesicles and will be accessible for RP-4 antibody. Therefore binding of the antibody to Mrp2 could potentially decrease Mrp2 transport activity. In the ATPase assay utilizing GSH as substrate, preincubation of Mrp2 containing vesicles with RP-4 antibody for 30 min at 37°C decreased baseline ATPase activity by ∼50% and had no effect on ATPase activity of the vesicles prepared from untransfected cells (Fig. 1E). The inhibition was not complete probably because the antibody only partially blocked the access of substrates to the substrate binding site of Mrp2. The results supported our hypothesis that Ac-DCVC is a substrate of mouse Mrp2.
Quantification of vesicular transport using mass-spectrometry
Direct measurements of the import of Ac-DCVC and DCVC into vesicles were made using LC/MS/MS-MRM. The conditions under which the vesicles were incubated were essentially identical to those used for the ATPase assay with the additional control in which ATP was substituted with an equimolar concentration of AMP. At 0.1 mM concentration of Ac-DCVC, the ATP-dependent transport of Ac-DCVC was ∼6 times of the transport measured in the presence of AMP (Fig. 2A). In contrast, the ATP-dependent transport of DCVC mediated by membrane vesicles prepared from untransfected cells was not significantly different from the transport mediated by membrane vesicles prepared from Mrp2 expressing cells measured in the presence of AMP. This value was not significantly changed when AMP was used instead of ATP, suggesting that there is no ATP-dependent transport of Ac-DCVC mediated by the transporters other than Mrp2. The results supported the hypothesis that mouse Mrp2 mediates ATP-dependent transport of Ac-DCVC.
Fig. 2.



(A) The ATP-dependent transport of Ac-DCVC (black bars) and DCVC (grey bars) into membrane vesicles prepared from the mPCT cells expressing mouse Mrp2 (Ex) measured by LC/MS/MS. ATP was replaced with an equimolar concentration of AMP in control experiments (white bars). * Significant difference (p<0.05) from control with AMP instead of ATP. (B) A representative curve of ATP-dependent Ac-DCVC transport inside Mrp2 vesicles. The data are means ± S.D. of two measurements. (C) The Lineweaver-Burk plot of the data shown in (B). S0.5 values were determined as the substrate concentration at half-maximal velocity of transport under the experimental conditions described above using the double-reciprocal plot (Lineweaver, Burk, 1934).
Kinetics of vesicular transport of Ac-DCVC
Fig. 2B,C illustrate the kinetics of the ATP-dependent transport of Ac-DCVC mediated by Mrp2. The S0.5 value for Ac-DCVC was 36.6±21.1 μM. This value is higher than the Km value of rat and human Mrp2 determined for LTC4 and E17bG (Nies, Keppler, 2007). The S0.5 value of mouse Mrp2 for Ac-DCVC determined in the present study is lower than the Ki value of Ac-DCVC for the p-aminohippurate transport through the basolateral membrane of rabbit proximal tubule (Dantzler et al., 1995, 1998). Assuming that the Ki value of Ac-DCVC for p-aminohippurate transport mediated by OAT1 is similar to the Km value of this transporter for Ac-DCVC, despite potential differences in the kinetic properties between mouse and rabbit transporters, the very similar affinities of Ac-DCVC for OAT1 and Mrp2 suggest that these two transporters can mediate a consecutive secretion of Ac-DCVC through renal proximal tubule cells monolayers.
Transcellular transport of Ac-DCVC and DCVC
If Mrp2 is involved in secretion of Ac-DCVC in renal proximal tubules, it should mediate the basolateral to apical transport of Ac-DCVC through polarized proximal tubule cell monolayers. The mPCT-A2 cell line did not grow well on permeable filters; therefore to perform transport experiments we used the mouse proximal tubule derived mPCT cell line, which expresses Mrp2 endogenously (Fig. 1A). In addition, mPCT cells do not express AA3 (Newman et al., 2007) that otherwise has confounded the results. In order to inhibit the expression of Mrp2, mPCT cells were transfected with the plasmid expressing antisense mouse Mrp2 RNA. The basolateral to apical transport of Ac-DCVC was higher than the apical to basolateral transport of Ac-DCVC at the initial concentration of Ac-DCVC of 0.5 mM. The expression of antisense Mrp2 RNA did not significantly affect the rate of apical to basolateral transport of Ac-DCVC at both the 0.1 and 0.5 mM initial concentrations of Ac-DCVC (Fig. 3A). In contrast, the expression of the anti-Mrp2 shRNA significantly decreased the basolateral to apical transport of Ac-DCVC at both 0.1 and 0.5 mM initial basolateral concentrations of Ac-DCVC (Fig. 3B). In control experiments a nonspecific antisense mRNA (anti-AA3 shRNA) did not significantly affect the basolateral to apical, and apical to basolateral transport of Ac-DCVC (Fig. 3C). In correlation with the results of transport experiments shown in Fig. 3A-C, the expression of anti-Mrp2 shRNA decreased the level of Mrp2 protein by ∼79±9% whereas anti-AA3 shRNA did not (Fig. 3D). Because the transfection efficiency of mPCT cells with the both shRNA plasmids was near identical (∼60-70%), the results suggested that Mrp2 is involved in the vectorial basolateral to apical transport of Ac-DCVC. Given that the efficiency of transfection of mPCT cells with the plasmid coding for anti-mouse Mrp2 shRNA was 63±11%, it may be predicted that Mrp2 is responsible for >90% of the total basolateral to apical transport of Ac-DCVC in a 15 min interval, and for ∼2/3 of the total basolateral to apical transport of Ac-DCVC in a 60 min interval. Decrease of the percentage of Mrp2-dependent transport in the total basolateral to apical transport of Ac-DCVC with the increase of incubation time from 15 to 60 min may reflect the depletion of ATP or/and metabolism of Ac-DCVC that does not involve its deacetylation given that mPCT cells do not express AA3 (Newman et al., 2007). The non-Mrp2 dependent transport of Ac-DCVC could be mediated by other transport mechanisms including paracellular transport of this compound.
Fig. 3.





(A) Apical to basolateral and (B) basolateral to apical transport of Ac-DCVC through the monolayer of mPCT cells (white bars) or the mPCT cells transfected with anti-mouse Mrp2 shRNA plasmid (black bars). In control experiments (C) mPCT cells were transfected with a nonspecific anti-AA3 shRNA plasmid (Fig. 3C). Note that mPCT cells do not express AA3 (Newman et al., 2007). (D) Anti-Mrp2 shRNA significantly decreased the expression of Mrp2 protein in mPCT cells in comparison with untransfected cells (control) whereas anti-AA3 shRNA did not. A representative immunoblot is shown on the left. The membranes were isolated from untransfected (Control) and transfected with AA3 and Mrp2 shRNA plasmids mPCT cells using the technique described for mPCT-A2 cell membrane isolation, resolved on 7.5% SDS-PAGE and immunoblotted with RP-4 antibody. Loading: 50 μg. Graphic illustration of Mrp2 quantification in 3 separate experiments is shown on the right. The bands were scanned and quantified in Adobe Photoshop (Adobe Instruments). * Shows significant difference (p<0.05) between transfected and untransfected (control) cells. (E) Apical to basolateral and basolateral to apical transport of DCVC through the monolayer of mPCT cells (white bars) and the mPCT cells transfected with anti-mouse Mrp2 shRNA plasmid (black bars). * Shows significant difference (p<0.05) between transfected and untransfected (control) cells.
DCVC was transported from monolayers of mPCT cells in both the apical to basolateral and basolateral to apical transport directions (Fig. 3E). In contrast to Ac-DCVC, the expression of anti-Mrp2 shRNA did not inhibit the vectorial basolateral to apical transport of DCVC. These results correlated with the DCVC transport data obtained using membrane vesicles (Figs. 1C and 2A) and indicated that DCVC is not a substrate of Mrp2. Given that the magnitude of the vectorial transport of DCVC through the mPCT cell monolayer was very similar in both directions, it is likely to be mediated in our experiments by paracellular transport.
In conclusion, our paper demonstrates for the first time that Mrp2 mediates the ATP-dependent transport of Ac-DCVC, a mercapturic acid derived from the metabolism of TCE, and may be involved in the secretion of DCVC in the renal proximal tubule. An important finding is that DCVC, the product of Ac-DCVC deacetylation, is not a substrate of mouse Mrp2. Therefore the AA3 mediated deacetylation of Ac-DCVC in S2 and S3 proximal tubule should prevent it from being secreted by Mrp2. Identification and characterization of the transporter(s) involved in the apical secretion of DCVC in the proximal tubule should clarify whether Ac-DCVC deacetylation decreases the total secretion rate of Ac-DCVC plus DCVC in the renal proximal tubule.
Acknowledgments
This work has been supported in part by the National Institutes of Health Grants ES012935 (A.P.), DK077162 (I.K.), DK058563 (I.K.) and DK063125 (I.K.).
Footnotes
Abbreviations: AA3, aminoacylase 3; DCVC, S-(1,2-dichlorovinyl)-L-cysteine; Ac-DCVC, N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine, Ac-DCVC; DDM, dodecyl β-D-maltopyranoside; E17bG, estradiol 17-β-D-glucuronide; HPLC, high performance liquid chromatography; GSH, reduced glutathione; KLH, keyhole lymphet hemocyanin; LC/MS/MS, liquid chromatography/tandem mass-spectrometry; LTC4, leukotriene C4; mPCT cells, mouse proximal tubule derived cells; Mrp2, multidrug resistance associated protein 2; PBS, phosphate buffered saline; Pi, inorganic phosphate; RT-PCR, reverse transcription polymerase chain reaction; Km, Michaelis constant; SDS-PAGE, sodium dodecylsulfate polyacrylamide gel electrophoresis; TBS, 20 mM Tris-HCl, pH 7.5, containing 140 mM NaCl; TCE, trichloroethylene; Vmax, maximum reaction rate.
Conflict of interests statement: The authors have no conflict of interests to state.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Trichloroethylene. U.S. Public Health Service, U.S. Department of Health and Human Services; Atlanta, GA: 1993. [PubMed] [Google Scholar]
- Anders MW, Dekant W. Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol. 1998;38:501–537. doi: 10.1146/annurev.pharmtox.38.1.501. [DOI] [PubMed] [Google Scholar]
- Anders MW, Dekant W, Vamvakas S. Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: cysteine conjugate beta-lyase pathway. Adv Pharmacol. 1994;27:115–162. doi: 10.1016/s1054-3589(08)61031-5. [DOI] [PubMed] [Google Scholar]
- Bencini DA, Wild JR, O'Donovan GA. Linear one-step assay for the determination of orthophosphate. Anal Biochem. 1983;132:254–258. doi: 10.1016/0003-2697(83)90004-0. [DOI] [PubMed] [Google Scholar]
- Birner G, Bernauer U, Werner M, Dekant W. Biotransformation, excretion, and nephrotoxicity of haloalkene-derived cysteine S-conjugates. Arch Toxicol. 1997;72:1–8. doi: 10.1007/s002040050461. [DOI] [PubMed] [Google Scholar]
- Borst P, Evers R, Kool M, Wijnholds J. The multidrug resistance protein family. Biochim Biophys Acta. 1999;1461:347–357. doi: 10.1016/s0005-2736(99)00167-4. [DOI] [PubMed] [Google Scholar]
- Büchler M, König J, Brom M, Kartenbeck J, Spring H, Horie T, Keppler D. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMRP, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradion sulfate be the mutidrug resistance proteins MRP1, MRP2, and MRP3. J Pharmacol Exp Ther. 2004;309:156–164. doi: 10.1124/jpet.103.062091. [DOI] [PubMed] [Google Scholar]
- Cooper AJL. Enzymology of cysteine S-conjugate β-lyases. Adv Pharmacol. 1994;27:71–113. [PubMed] [Google Scholar]
- Cui Y, König J, Buchholz U, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- Dantzler WH, Evans KK, Groves CE, Welborn JR, North J, Stevens JL, Wright SH. Relation of cysteine conjugate nephrotoxicity to transport by the basolateral organic anion transport system in isolated S2 segments of rabbit proximal renal tubules. J Pharmacol Exp Ther. 1998;286:52–60. [PubMed] [Google Scholar]
- Dantzler WH, Metzler M, Henschler D. Kinetics of interactions of para-aminohippurate, probenecid, cysteine conjugates and N-acetyl cysteine conjugates with basolateral organic anion transporter in isolated rabbit proximal renal tubules. J Pharmacol Exp Ther. 1995;272:663–672. [PubMed] [Google Scholar]
- De Rosa CT, Johnson BL, Fay M, Hansen H, Mumtaz MM. Public health implications of hazardous waste sites: finding, assessment and research. Food Chem Toxicol. 1996;34:1131–1138. doi: 10.1016/s0278-6915(97)00084-7. [DOI] [PubMed] [Google Scholar]
- Dekant W, Vamvakas S, Anders MW. Formation and fate of nephrotoxic and cytotoxic glutathione S-conjugates: cysteine conjugate β-lyase pathway. Adv Pharmacol. 1994;27:114–162. doi: 10.1016/s1054-3589(08)61031-5. [DOI] [PubMed] [Google Scholar]
- Farrel O, Jigorel E, Le Vee M, Payen L. Pharmacological and clinical features of the multidgug resistance protein 2. Biomed Pharmacother. 2005;59:104–114. doi: 10.1016/j.biopha.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Gross E, Hawkins K, Abuladze N, Pushkin A, Cotton CU, Hopfer U, Kurtz I. The stoichiometry of the electrogenic sodium bicarbonate cotransporter NBC1 is cell-type dependent. J Physiol. 2001;531:597–603. doi: 10.1111/j.1469-7793.2001.0597h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Okajima K, Morino Y. Hepato-renal cooperation in biotransformation, membrane transport, and elimination of cysteine S-conjugates of xenobiotics. J Biochem. 1984;95:247–254. doi: 10.1093/oxfordjournals.jbchem.a134591. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki H, Sugiyama Y. Charged amino acids in the transmembrane domains are involved in the determination of the substrate specificity of rat Mrp2. Mol Pharmacol. 2001;59:1077–1085. doi: 10.1124/mol.59.5.1077. [DOI] [PubMed] [Google Scholar]
- Kays SE, Berdanier CD, Swagler AR, Lock EA, Schnellmann RG. An in vitro model of renal proximal tubule cell regeneration. J Pharmacol Toxicol Meth. 1993;29:211–215. doi: 10.1016/1056-8719(93)90027-c. [DOI] [PubMed] [Google Scholar]
- Lash LH, Anders MW. Uptake of nephrotoxic S-conjugates by isolated rat renal proximal tubular cells. J Pharmacol Exp Ther. 1989;248:531–537. [PubMed] [Google Scholar]
- Lash LH, Hueni SE, Putt DA. Apoptosis, necrosis, and cell proliferation induced by S-(1,2-dichlorovinyl)-L-cysteine in primary cultures of human proximal tubule cells. Toxicol Appl Pharmacol. 2001;177:1–16. doi: 10.1006/taap.2001.9295. [DOI] [PubMed] [Google Scholar]
- Lash LH, Putt DA, Parker JC. Metabolism and tissue distribution of orally administered trichloroethylene in male and female rats: identification of glutathione- and cytochrome P-450-derived metabolites in liver, kidney, blood, and urine. J Toxicol Environ Health A. 2006;69:1285–1309. doi: 10.1080/15287390500360133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EM, Deeley RG, Cole SPC. Multidrug resistance protein: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666. [Google Scholar]
- Newman D, Abuladze N, Scholz K, Dekant W, Tsuprunm V, Ryazantsev S, Bondar G, Sassani P, Kurtz I, Pushkin A. Specificity of aminoacylase III-mediated deacetylation of mercapturic acids. Drug Metabol Disposit. 2007;35:43–50. doi: 10.1124/dmd.106.012062. [DOI] [PubMed] [Google Scholar]
- Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (Mrp2) Pflugers Arch – Eur J Physiol. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- Oude Elferink RPJ, Meijer DKF, Kuipers F, Jansen PLM, Groen AK, Groothuis GMM. Hepatobiliary secretion of organic compounds: molecular mechanisms of membrane transport. Biochim Biophys Acta. 1995;1241:215–268. doi: 10.1016/0304-4157(95)00006-d. [DOI] [PubMed] [Google Scholar]
- Paulusma CC, Bosma PJ, Zaman GJR, Bakker CTM, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RPJ. Congenital jaundice in rats with a mutation in a multidrug resistance associated-protein gene. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- Pedersen JM, Matsson P, Bergström CA, Norinder U, Hoogstraate J, Artursson P. Prediction and identification of drug interactions with the human ATP-binding cassette transporter multidrug-resistance associated protein 2 (MRP2; ABCC2) J Med Chem. 2008;51:3275–3587. doi: 10.1021/jm7015683. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Carpenito G, Abuladze N, Newman D, Tsuprun V, Ryazantsev S, Motemoturu S, Sassani P, Solovieva N, Dukkipati R, Kurtz I. Structural characterization, tissue distribution, and functional expression of murine aminoacylase III. Am J Physiol. 2004;286:C848–C856. doi: 10.1152/ajpcell.00192.2003. [DOI] [PubMed] [Google Scholar]
- Schaub TP, Kartenbeck J, König J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- Scholz K, Dekant W, Völkel W, Pähler A. Rapid detection and identification of N-acetyl-L-cysteine thioethers using constant neutral loss and theoretical multiple reaction monitoring combined with enhanced product-ion scans on a linear ion trap mass spectrometer. J Am Soc Mass Spectrom. 2005;16:1976–1984. doi: 10.1016/j.jasms.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Silber PM, Gandolfi AJ, Brendel K. Early biological indicators of S-(1,2-dichlorovinyl)-L-cysteine nephrotoxicity in the rabbit. Drug Chem Toxicol. 1986;9:285–303. doi: 10.3109/01480548608998281. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Semin Liver Dis. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- Van Aubel RAMH, Masereeuw R, Russel FGM. Molecular pharmacology of renal organic transporters. Am J Physiol. 2000;279:F216–F232. doi: 10.1152/ajprenal.2000.279.2.F216. [DOI] [PubMed] [Google Scholar]
- Wallin A, Zhang G, Jones JW, Jaken S, Stevens JL. Mechanism of nephrogenic repair response. Studies on proliferation and vimentin expression after 35S-1,2-dichlorovinyl-L-cysteine nephrotoxicity in vivo and in cultured proximal tubule epithelial cells. Lab Invest. 1992;66:474–484. [PubMed] [Google Scholar]
- Werner M, Birner G, Dekant W. Sulfoxidation of mercapturic acids derived from tri- and tetrachloroethylene by cytochromes P450 3A: A bioactivation reaction in addition to deacetylation and cysteine conjugate β-lyase mediated cleavage. Arch Toxicol. 1997;9:41–49. doi: 10.1021/tx950075u. [DOI] [PubMed] [Google Scholar]
- Wolfgang GH, Gandolfi AJ, Stevens JL, Brendel K. N-acetyl S-(1,2-dichlorovinyl)-L-cysteine produces a similar toxicity to S-(1,2-dichlorovinyl)-L-cysteine in rabbit renal slices: differential transport and metabolism. Toxicol Appl Pharmacol. 1989;101:205–219. doi: 10.1016/0041-008x(89)90270-6. [DOI] [PubMed] [Google Scholar]
- Wright SH, Wunz TM, North J, Stevens JL. Na-dependent transport of S-(1,2-dichlorovinyl)-L-cysteine by renal brush-border membrane vesicles. J Pharmacol Exp Ther. 1998;285:162–169. [PubMed] [Google Scholar]



