Abstract
Attention selects which sensory information is preferentially processed and ultimately reaches our awareness. Attention, however, is not a unitary process: It can be captured by unexpected or salient events (stimulus-driven) or it can be deployed under voluntary control (goal-directed), and these two forms of attention are implemented by largely distinct ventral and dorsal parieto-frontal networks. Yet, for coherent behavior and awareness to emerge, stimulus-driven and goal-directed behavior must ultimately interact. Here we show that the ventral, but not dorsal, network can account for stimulus-driven attentional limits to conscious perception, and that it is in the lateral prefrontal component of that network where stimulus-driven and goal-directed attention converge. Although these results do not rule out dorsal network involvement in awareness when goal-directed task demands are present, they point to a general role for the lateral prefrontal cortex in the control of attention and awareness.
Reading this manuscript requires attention to be voluntarily deployed, in a `top-down' fashion, to this task. As a consequence of selectively attending to the page, one may become oblivious to surrounding sounds and sights. If a fire alarm suddenly blares, however, this salient stimulus will likely capture attention in a `bottom-up' manner and interrupt the ongoing task so that an appropriate course of action can be initiated. This simple example illustrates a fundamental aspect of attention: What ultimately reaches our awareness and guides our behavior depends on the interaction between the two principal forms of attention, goal-directed and stimulus-driven1,2.
While much is known about the neural mechanisms supporting goal-directed1,3–7 and stimulus-driven attention1,5,8–12, how these two forms of attention are ultimately coordinated is not yet understood. The finding that these attentional forms are supported by largely distinct neural networks – with a dorsal network that includes the frontal eye field (FEF) and superior parietal cortex1,4–7,13 supporting goal-directed attention, and a ventral one that consists of the lateral and inferior frontal/prefrontal cortex and the temporo-parietal junction (TPJ) underlying stimulus-driven attention1,3,8,9,12,13 – has further complicated the issue. As a result, several hypotheses have been proposed to explain how these two forms of attention may be coordinated: through an interaction between the ventral and dorsal networks1, across the dorsal14,15 or ventral5 attention network, or in the anterior component of the ventral network16. Many of these proposals, however, are based on studies employing tasks that conflate stimulus-driven and goal-directed attention, thereby making it difficult to determine the relative contribution of bottom-up and top-down neural processes to task performance. For example, the brain mechanisms of stimulus-driven attention cannot easily be dissociated from those supporting goal-directed behavior if the stimulus-driven attention task involves spatial shifts of attention, goal-oriented processes, or motor responses, as none of these cognitive processes is necessary to capture attention exogenously but all are known to engage the dorsal attention network1,3–6.
The same concern also applies to our current understanding of how attention controls awareness. Both the ventral and dorsal attention networks have been associated with conscious perception5,17–23, lending support to theories of awareness that posit widespread changes in brain activity accompanying conscious perception17,24. To date, however, there has not been a specific attempt to assess the relative contribution of the dorsal and ventral networks to the neural basis of attentional limits to conscious perception by using tasks that dissociate between stimulus-driven and goal-directed attentional processes. Hence, the extent to which each of these attention networks may be necessary for awareness is currently unclear22,23,25.
We have recently developed an experimental procedure that reveals a profound but fleeting deficit in visual awareness resulting from the foveal presentation of an unexpected, task-irrelevant stimulus that involves neither an overt response nor a shift in spatial attention. The deficit, termed Surprise-induced Blindess (SiB), is triggered by an event absent from the observer's goal-directed attentional set and is not under the observer's initial control26. As such, the procedure represents a powerful way to assess whether stimulus-driven attentional limits to conscious perception can arise within the ventral attention network in the absence of dorsal network involvement. Moreover, because the unexpected event ultimately affects the goal-directed task of detecting a target, SiB experiments are also well suited to reveal the neural mechanisms by which stimulus-driven attention affects goal-directed behavior.
Results
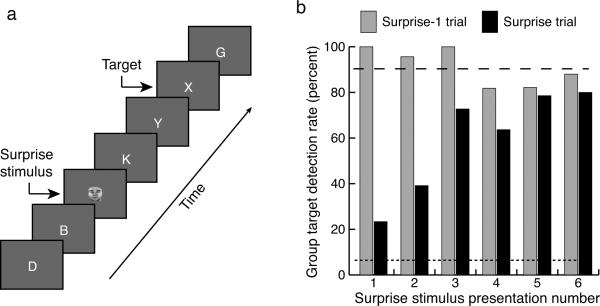
We scanned 30 participants while they were searching for a target in a rapid serial visual presentation (RSVP) stream of distractor items (Fig. 1a). Unannounced to the participants, a salient but task-irrelevant stimulus occurred 330 ms before the target in a small proportion of trials (Surprise trials), causing Surprise-induced Blindness (Fig. 1b). Specifically, group target detection performance differed across the Surprise stimuli presentations (Cochran's Q(4) = 30.3, p < 0.0001; See Methods), with target detection worse following the first Surprise trial (SS1) than SS3–SS6 (Sign tests, p's < 0.040; See Methods), and worse following SS2 compared to SS5 and SS6 (p's < 0.027). In the third through sixth Surprise trials, performance was comparable to Search trials (trials without Surprise stimuli, for which the target detection rate was 90.4%; Fig. 1b). Target-detection performance in the trials immediately preceding the first two Surprise trials was far better than for their respective Surprise trials (Fig. 1b, Sign tests, p's < 0.0001), indicating that SiB does not result from an initial difficulty with the target detection task. Rather, the finding that unexpected, task-irrelevant stimuli triggered a profound but short-lived impairment in target detection that was essentially dissipated by the third Surprise stimulus presentation is consistent with a stimulus-driven, attention-based origin for this deficit26.
Figure 1.
SiB experiment (Experiment 1). A) Trial design. Participants searched for a target letter in a rapid serial visual presentation (RSVP) stream of distractor letters. In a small proportion of trials (Surprise trials), a Surprise face stimulus was shown before the target. B) Group target detection performance. Black bars represent accuracy in Surprise trials, and gray bars represent accuracy in trials immediately preceding the Surprise trials. Dashed line corresponds to the average target hit rate for Search trials (target only). Dotted line corresponds to the false alarm rate.
Neural correlates of SiB
To identify the neural substrates that underlie stimulus-driven attentional limits to conscious perception, we first isolated the brain regions sensitive to the Surprise stimuli, irrespective of presentation number (See Methods). We then examined the BOLD (blood-oxygen-level dependent) signal from these brain regions, testing whether the response pattern mirrored the behavioral performance. Specifically, because the presentations of rare, task-irrelevant stimuli are known to increase neural activity8–10,27 and because SiB was only observed for the first pair of Surprise stimuli (SS1+2), we predicted that this pair would cause a greater BOLD response than the two subsequent pairs (SS3+4 and SS5+6) of Surprise stimuli (the time courses from each consecutive pair of Surprise stimuli were averaged to improve statistical power; See Methods).
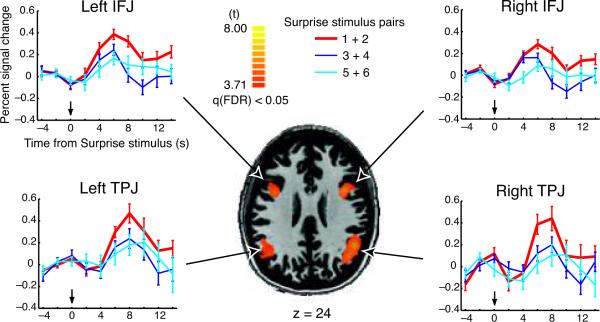
A statistical parametric map (SPM) revealed several areas that were recruited more during Surprise than during Search trials (Table 1; Fig. 2 and Supplementary Fig. 1). Several of these areas showed invariant BOLD responses across the six Surprise stimuli presentations, most notably the fusiform gyrus in visual cortex, suggesting that SiB may be a primarily central phenomemon that occurs at later stages than visual information processing. Correspondingly, the only two regions that demonstrated a BOLD response that quickly habituated after the first two Surprise stimulus presentations were in association cortex (Fig. 2): the inferior frontal junction (IFJ), located in the posterior aspect of the inferior frontal sulcus (parts of Brodmann areas 9, 44, 6), and the temporo-parietal junction (TPJ), at the intersection of the superior temporal gyrus, supramarginal gyrus, and superior temporal sulcus (parts of Brodmann areas 40, 22, 39). For these two brain regions, in both hemispheres, the peak response to SS1+2 was higher than the response to the two other SS pairs (two-tailed paired t-tests, t(29)'s > 2.05, p's < 0.049), while the peak responses to SS3+4 and SS5+6 were indistinguishable (t(29)'s < 1.30, p's > 0.20; Fig. 2). Thus, the IFJ and TPJ exhibited an activity pattern that mirrored the magnitude of SiB. This activity modulation was caused by the Surprise stimuli, not the perceived absence of a target—which co-varies with SiB—because the peak responses in target-absent trials and Search trials (See Methods) were indistinguishable (two-tailed paired t-tests, t(29)'s < 1.17, p's > 0.25). No other brain regions appeared to show an SiB-like pattern of activation, as an additional SPM that directly contrasted SS1+2 with SS3+4 and SS5+6 demonstrated.
Table 1.
Anatomical Location and Statistical Assessment of Activation for the ROIs isolated from Surprise trials in Experiment 1 (Surprise trial – Search trial contrast).
| Region | Hemi | Tal co-ords (x, y, z) | SS1+2 vs. SS3+4 (t) | SS3+4 vs. SS5+6 (t) | SS1+2 vs. SS5+6 (t) |
|---|---|---|---|---|---|
| TPJ | Right | 46, −56, 27 | 2.11* | 1.07 | 2.76* |
| TPJ | Left | −49, −56, 23 | 2.35* | 1.30 | 2.86* |
| IFJ | Right | 37, 5, 29 | 2.72* | −0.37 | 2.05* |
| IFJ | Left | −40, 8, 25 | 2.46* | 0.05 | 2.18* |
| FG | Right | 30, −44, −11 | 0.42 | −0.92 | −0.53 |
| FG | Left | −32, −51, −10 | 1.05 | −0.70 | 0.05 |
| IFG | Right | 40, 19, 13 | 0.75 | 0.30 | 1.09 |
| IFG | Left | −48, 19, 7 | −0.57 | 0.77 | 0.19 |
| OFC | Right | 34, 27, −10 | −0.22 | −0.19 | −0.53 |
| OFC | Left | −37, 26, −8 | 0.27 | 0.15 | 0.40 |
| Pulvinar | Bilateral | −7/9, −27, 1 | 1.46 | 0.71 | 1.83 |
| PG | Right | 32, −3, −13 | −0.09 | −0.70 | −0.80 |
| STG | Right | 33, 12, −27 | 0.95 | 0.07 | 1.26 |
| MTS | Left | −51, 2, −12 | −0.17 | −0.28 | −0.36 |
| SFG | Right | 13, 24, 49 | 0.49 | 0.74 | 0.90 |
| Amygdala / SLEA | Right | 16, −9, −8 | 1.58 | −0.17 | 1.26 |
The three rightmost columns list the t-values resulting from paired t-tests of the given Surprise Stimulus pairs. An asterisk (*) marks those comparisons significant at p < 0.05. Tal co-ords = Talairach coordinates45. TPJ = Temporo-Parietal Junction (Brodmann areas 39, 40, 22), IFJ = Inferior Frontal Junction (Brodmann areas 9, 44, 6), FG = Fusiform Gyrus (Brodmann area 37), IFG = Inferior Frontal Gyrus (Brodmann areas 44, 45), OFC = Orbitofrontal Cortex (Brodmann area 47), PG = Parahippocampal Gyrus, STG = Superior Temporal Gyrus (Brodmann area 38), MTS = Middle Temporal Sulcus (Brodmann area 21), SFG = Superior Frontal Gyrus (Brodmann area 8), Amygdala / SLEA = Amgydala and Sub-Lenticular Extended Amygdala. With the exception of the IFJ and TPJ, none of these brain regions showed activation differences between any of the three Surprise stimulus pairs (all p's > 0.1), although the amygdala/SLEA and pulvinar showed non-significant trends for greater SS1+2 activation relative to the two other SS pairs. Note that the TPJ foci are anatomically distinct from, and superior to, regions of the superior temporal sulcus involved in processing facial expressions and eye gaze46.
Figure 2.
SiB experiment (Experiment 1) SPM. Brain regions showing rapid attenuation of Surprise stimulus-related activation. The SPM highlights brain regions that responded to all six Surprise trials (See Methods), specifically the IFJs (Talairach coordinates45 37, 5, 29 and −40, 8, 25) and TPJs (Talairach coordinates 46, −56, 27 and −49, −56, 23). The time courses illustrate the brain regions from the SPM that showed greater activity in the first pair of Surprise trials compared to the two other pairs of Surprise trials. The Surprise stimulus appears at approximately time zero. Error bars represent standard errors of the mean.
Taken together, these results indicate that the presentation of unexpected, task-irrelevant stimuli activates a large network of cortical and subcortical regions, yet only a subset of this network in the frontal/prefrontal and temporo-parietal cortex show a rapid BOLD response adaptation commensurate with the behavioral performance. This subset of brain regions is anatomically consistent with areas previously implicated in novelty processing8,9,11,27–30, attentional orienting to sensory events31,32 and, most strikingly, to the core components of the ventral attention network1,13.
Late dorsal network activation
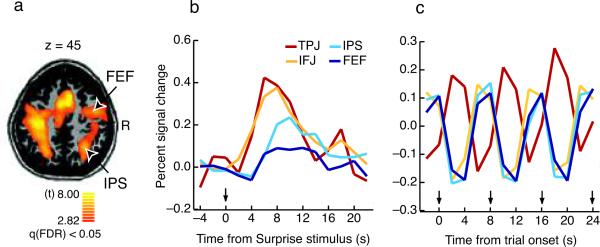
In contrast to the ventral network, the SPMs (even with a liberal threshold of p < 0.001, uncorrected) provided no evidence for Surprise stimulus-related activation in the core brain regions associated of the goal-directed attention network1,13, namely the frontal eye field (FEF) and the intraparietal sulcus (IPS). To analyze the dorsal network's association with SiB with greater sensitivity, for each participant we functionally defined regions of interest (ROIs) for the putative FEF and IPS based on their activation in Search trials, as these regions were strongly activated by the task of searching for and responding to targets (See Methods, Fig. 3a, Table 2). The anatomical locations of the resulting ROIs corresponded very well to the conventional positions of the FEF and IPS in goal-directed attention tasks1,3–6,12,13,16. When probed during the Surprise trials, activity in these dorsal regions was greater during SS1+2 compared to Search trials (t(29)'s > 2.50, p's < 0.018 save right FEF's marginal effect at t(29) = 1.79, p = 0.084), but not during subsequent pairs (Table 2). Thus, the more sensitive ROI analysis revealed that the FEF and IPS are also activated by the first two Surprise stimulus presentations. Strikingly, however, the time courses of activation specific to the first pair of Surprise stimuli, revealed by subtracting the underlying Search-related activity from the first two Surprise stimulus trials (See Methods), showed that the IPS and FEF responded significantly later than did the IFJ and TPJ (Fig. 3b; all pair-wise comparisons p < 0.048; See Methods), while activity within each of these two pairs did not differ (all p's > 0.55). These results were obtained regardless of whether the IFJ and TPJ ROIs were defined exactly as the IPS and FEF ROIs or defined based on the group-level Surprise trial ROIs (see Tables 1 and 2).
Figure 3.
Stimulus-driven and goal-directed attention activity in Experiment 1. A) Dorsal brain regions active during Search trials. B) Surprise stimulus-specific waveform in dorsal (FEF, IPS) and ventral (IFJ, TPJ) regions of interest (ROIs) defined in individual participants (See Methods). Each time course was constructed by subtracting the Search trial time course from the time course for the first two Surprise Stimulus trials. The Surprise stimulus appears at approximately time zero. C) Search trial time course over the same period of time for the same ROIs. Arrows mark each trial's onset. Note that the activation pattern is cyclical, mirroring the trial structure (one trial every eight seconds). The observed hemodynamic responses match the predicted responses for the hypothesized neural activity in each region (see Supplementary Fig. 3).
Table 2.
Average Anatomical Location and Statistical Assessment of Activation for the individually-defined ROIs from Search trials in Experiment 1 (open contrast SPM).
| Region | Hemi | Tal co-ords (x, y, z) ± SD | SS1+2 vs. Search (t) | SS3+4 vs. Search (t) | SS5+6 vs. Search (t) |
|---|---|---|---|---|---|
| IPS | Right | 26 ± 4, −65 ± 6, 36 ± 5 | 2.81* | 0.46 | 0.28 |
| IPS | Left | −22 ± 4, −66 ± 6, 39 ± 6 | 3.45* | 0.44 | 1.38 |
| FEF | Right | 34 ± 4, −7 ± 3, 51 ± 5 | 1.79 | 0.51 | 0.63 |
| FEF | Left | −32 ± 5, −8 ± 3, 51 ± 5 | 2.50* | 1.95 | 0.96 |
| TPJ | Right | 47 ± 5, −55 ± 5, 28 ± 5 | 4.52* | 3.21* | 1.65 |
| TPJ | Left | −49 ± 2, −58 ± 5, 24 ± 4 | 5.33* | 2.37* | 1.73 |
| IFJ | Right | 40 ± 4, 6 ± 3, 27 ± 3 | 4.99* | 1.67 | 1.06 |
| IFJ | Left | −42 ± 3, 8 ± 3, 25 ± 2 | 5.92* | 2.51* | 1.12 |
The three rightmost columns list the t-values resulting from paired t-tests of the given Surprise Stimulus pairs versus Target Only activity. See Table 1 for abbreviation key. An asterisk (*) marks those comparisons that are significant at p < 0.05. These ROI coordinates closely matched those isolated from the Surprise trials (see Table 1).
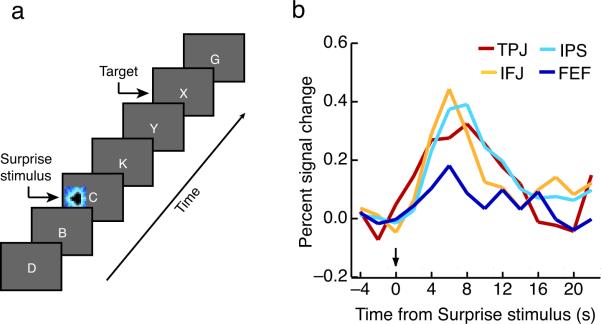
Thus, the ROI analysis revealed that the dorsal network is activated by the Surprise stimuli, but unlike the swift activation pattern in the ventral network following presentations of the first two Surprise stimuli, the dorsal network appears to respond too late (by about 3 seconds) for it to play a causal role in SiB. This conclusion, however, critically depends on the dorsal activation delay reflecting a genuine late neural response rather than inherent differences in the hemodynamic properties of the ventral and dorsal parieto-frontal networks. We therefore performed a follow-up `spatial SiB' experiment (Experiment 2) to distinguish between these two possibilities. Given the role of the dorsal network in the control of visuo-spatial attention1,4,6, we predicted that the sporadic presentations of highly salient, categorically distinct task-irrelevant stimuli in the periphery instead of in the center of the RSVP stream (Fig. 4a) would not only persist in capturing attention26, but also lead to shifts of visual-spatial attention or eye movements, thereby promptly recruiting the FEF and IPS in addition to the ventral network. Consistent with our hypothesis, we found a persistent SiB effect and robust activations in both the dorsal and ventral networks (Fig. 4b, Supplementary Tables 1, 2). Most importantly, there was no longer a delay in activation between the dorsal and ventral networks (p's > 0.36), in marked contrast with Experiment 1 (timing delay difference across experiments: one-tailed p = 0.038; See Methods). Taken together, these results suggest that the delay in activation of the dorsal brain regions during the Surprise trials of Experiment 1 has a neural—not hemodynamic—origin. Additional evidence suggests that this delayed activation may reflect the modulation of top-down attentional settings in anticipation of post-Surprise stimulus trials (see Supplementary SiB RT Experiment, Supplementary Fig. 2). Regardless of the function of this delayed dorsal response to Surprise stimulus presentations, it appears to play no part in Surprise-induced Blindness, for the FEF and IPS are likely activated after the events that trigger the perceptual deficit.
Figure 4.
Spatial SiB Experiment (Experiment 2). A) Trial design. The procedure was identical to that in Experiment 1 save that in a small proportion of trials, a colorful Surprise stimulus was shown before the target away from fixation (see Fig. 1a). B) Surprise stimulus-specific waveforms in dorsal and ventral attention network ROIs defined in individual participants. Time courses were constructed in the same fashion as those in Experiment 1 (see Fig. 3b, main text). Note that all four regions show an immediate response to the Surprise stimulus presentations.
Interaction of goal-directed and stimulus-driven attention
If the core components of the dorsal attentional network (the FEF and IPS) are not responsible for SiB, then how do the Surprise stimuli ultimately impair the goal-directed task of searching for and responding to a target? That is, how does stimulus-driven attention disrupt goal-directed behavior? The answer is provided by an examination of the temporal dynamics of activation in the two attention networks during the Search trials of Experiment 1. In these trials, the FEF and IPS showed the pattern expected of brain regions associated with goal-directed behavior, namely an activation profile that tightly correlated with performing the primary search task (Fig. 3c). By contrast, TPJ activity was out of phase with the dorsal brain regions, showing deactivation when the others were activated (Fig. 3c, onset shifts of approximately 4 seconds: p's < 0.0001), consistent with the finding that attention-demanding cognitive tasks are often accompanied by suppression of TPJ activity13,21,33–35. Strikingly, the time course of IFJ activity no longer closely followed that of its ventral network cohort, the TPJ (onset shifts of approximately 4 seconds: p's < 0.0001), but instead closely tracked the activation time course of the dorsal brain regions (Fig. 3c). This IFJ activity in the Search trials does not simply reflect target detection, for the same activity pattern was found in target-absent trials. These findings suggest that the IFJ may be not only a core member of the ventral attention network supporting stimulus-driven attention, but also functionally integrated with the dorsal network during goal-directed behavior.
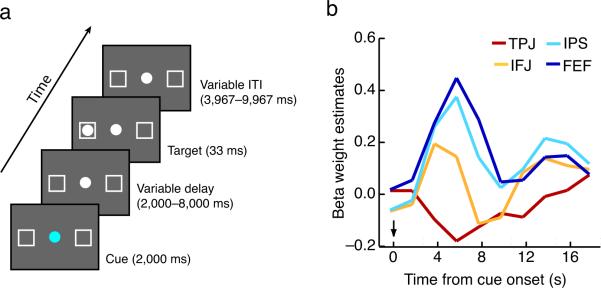
To test the hypothesis that the same IFJ ROIs identified in Experiment 2 play a key function in goal-directed behavior, we carried out an additional experiment (Experiment 3) that assessed whether the IFJ is activated, along with the FEF and IPS, in a prototypical goal-directed attention task, an endogenous Posner cueing task3,36 (see Fig. 5a, Methods). In this task, participants made a speeded response to a target presented at a location that was cued by the color of a central fixation point, with the cue validly predicting the location of the target on 80% of the trials. The task was successful in engaging goal-directed attention, evidenced by the fact that participants were faster at detecting the target at validly cued than at invalidly cued positions (RT ± SD: 317 ± 36 ms versus 396 ± 64 ms, t(5) = 4.53, p = 0.0062). Furthermore, the dorsal brain regions were activated during the cue-related period (t(5)'s > 3.53, p's < 0.017; See Methods), as expected of brain regions involved in goal-directed attention1,3,6 (Fig. 5b). Most importantly, the IFJ was also activated by the cue (t(5) = 2.74, p = 0.041), and at the same time as the FEF and IPS. These results were obtained regardless of whether the ROIs were defined in the cueing task or in the Search trials of the RSVP task, attesting to the fact that the brain regions exhibiting goal-directed activity in the Search trials are also involved in visuo-spatial shifts of attention. We therefore conclude that IFJ supports goal-directed behavior, as it is activated along with core members of the dorsal network during the cue period of a classic goal-directed attention task. These conclusions are consistent with previous reports suggesting that similar brain regions are activated in other cued attention shift tasks37,38.
Figure 5.
Endogenous Cueing Task Experiment (Experiment 3). A) Trial design. A color cue predicted the location of an upcoming target, to which the participant then responded in a speeded manner. B) Cue-related activity in dorsal and ventral attention network ROIs isolated from Experiment 2 (See Methods). The arrow marks cue onset.
Taken together, the results of our experiments indicate that the IFJ participates in both stimulus-driven and goal-directed attention. While the pattern of IFJ activity is consistent with brain regions involved in stimulus-driven attention during the presentation of a Surprise stimulus, its activity profile is instead more consistent with those of goal-directed brain regions during the Search task. It follows from these results that the IFJ should be more functionally integrated with core members of the dorsal network during goal-directed attention, but with core members of the ventral network during stimulus-driven attention. These predictions are borne out by a functional connectivity analysis (See Methods), which showed that IFJ activity was correlated positively with FEF and IPS (IFJ-FEF: t(29) = 9.13, p < 0.0001; IFJ-IPS: t(29) = 12.19, p < 0.0001; FEF-IPS: t(29) = 6.87, p < 0.0001)—but negatively with TPJ (t(29) = −4.27, p = 0.00019)—during the Search trials. Conversely, following the presentation of a Surprise stimulus, the IFJ-FEF and IFJ-IPS correlations decreased (paired t-test of the changes: t(29) = −2.12, p = 0.043; t(29) = −2.36, p = 0.025), while those between IFJ and TPJ increased (t(29) = 2.07, p = 0.047) and those between FEF and IPS did not change (t(29) = −1.07, p = 0.29). These connectivity results provide additional evidence that the IFJ acts as a neural site underlying stimulus-driven and goal-directed attention, with its response profile and network allegiance dependent on task demands. In that context, SiB would result from the `bottom-up' engagement of IFJ by the presentation of a Surprise stimulus, thereby transiently disrupting or altering this brain region's control of the target detection task. With behavioral and neuronal habituation to the repeated Surprise stimulus presentations, the IFJ may be able to maintain its goal-oriented activity even in the face of task-irrelevant stimulus presentations.
Discussion
Our study reveals that both functional divergence and convergence of the dorsal and ventral attentional networks underlie attention and awareness.
Divergence of function between these two networks is clearly evidenced by the SiB procedure, as it shows that stimulus-driven attentional limits to conscious perception can arise from the ventral attention network in the absence of dorsal network or visual cortex modulation. In contrast to these results, previous neurobiological investigations17–22,39,40 have frequently implicated regions of both the ventral and dorsal networks in awareness. Such large-scale activation patterns are consistent with `global workspace' models that posit that awareness emerges from the reverberating activity of a widely distributed cortical network17,22,24. The difference in activation patterns between these previous studies and the present one are likely a result of differences in task design. Whereas previous tasks have included spatial shifts of attention3,5,14,15,18,39 or covert or overt responses3,5,11,12,18–21,39,40 to the critical attention-capturing stimulus, our task was specifically designed to exclude these components as they are unnecessary for exogenous attentional capture but can activate the dorsal attention network1,3–6. As such, our task demonstrates that the dorsal network's contribution to conscious perception may be negligible under these controlled circumstances. This conclusion that is consistent with the suggestion that awareness is not necessarily an emergent property of the dorsal network25 but poses a challenge to global network theories of awareness.
These conclusions, however, do not imply that the dorsal network never plays a role in attentional limits to explicit perception. Dorsal structures may contribute to, and be essential for, conscious perception during tasks that involve top-down or goal-oriented processing, such as change detection or binocular rivalry17,18,22,40. Indeed, just as deficits of awareness in different visual domains often have dissociable neural origins (e.g. prosopagnosia versus achromatopsia)22, awareness may also be fractionated at central, attentional stages of information processing. Additional research, aided by better delineation of the topographically distinct sub-regions of the IPS, will be necessary to assess the specific contributions that the dorsal network may play in attentional limits to conscious perception and awareness in general.
In addition to revealing a functional dissociation between the ventral and dorsal attentional networks in awareness, the very nature of SiB—a profound deficit in the detection of a goal-relevant target as a result of the presentation of an unexpected and task-irrelevant stimulus—underscores that stimulus-driven and goal-directed attention must ultimately interact1,2,5,13–16. The present study suggests that the ventral attention network's lateral prefrontal component, the inferior frontal junction, is the site of convergence for stimulus-driven and goal-directed attention, a finding that is consistent with recent resting state functional connectivity data suggesting that this brain region functionally interacts with both ventral and dorsal brain structures16. The IFJ has also been implicated in task-switching and cognitive control42,43 more generally. This brain region is therefore ideally suited to act as the neural site of coordination for stimulus-driven and goal-directed attention. Moreover, the IFJ's involvement in both the non-spatial and spatial SiB tasks (see Experiments 1 and 2) indicates that this brain region's function generalizes across both spatial and non-spatial forms of attention. While it remains to be seen whether all these attentional processes are mediated by the same or different sub-populations of IFJ neurons, a central role for this brain region in the coordination of stimulus-driven and goal-directed attention across both spatial and non-spatial domains resonates very well with the proposal that the IFJ is a critical neural substrate underlying our severely limited attentional capacities44.
Methods
Participants in all experiments had normal or corrected-to-normal vision and received monetary compensation. The Vanderbilt University Institutional Review Board approved the experimental protocol and written informed consent was obtained from each participant.
SiB Experiment (Experiment 1)
Thirty-one right-handed individuals (12 females) participated. One individual's data were excluded due to technical problems.
Trial design
Participants searched for a target letter (`X') in a rapid serial visual presentation (RSVP) stream of distractor letters (white Helvetica font, 1.8° × 1.8°, presented on a dark gray background). Each 8 s trial began with a 3.4 s RSVP of 31 letters randomly chosen from a set of 20 (vowels were excluded), with no letter presented twice in a row. Each stimulus was presented at fixation for 100 ms followed by a 10 ms inter-stimulus interval (ISI). Following the RSVP, a screen appeared for 2 s prompting the participants to respond with an appropriate key press (right index finger for `target present' and right middle finger for `target absent'). The response period was followed by a 2.6 s inter-trial interval (ITI) consisting of a white fixation cross.
Each participant completed a single fMRI run of 40 trials. The target (present on 77.5% of trials) appeared between frames 25–29. In six of the trials, a Surprise stimulus (grayscale face, 1.8° × 1.8°, distinct for each trial) appeared between frames 22–26 of the RSVP, 330 ms before the target (5 trials) or in a trial with no target (1 trial). Surprise trials occurred between trials 2 and 38 and were separated by a minimum of two Search trials (trials with a target but no Surprise stimulus). Participants practiced Search trials exclusively prior to the fMRI session. Feedback was given only during practice, and participants were required to reach target accuracy above 80% before scanning. At no time were participants informed about the Surprise stimuli.
Behavioral analysis
To assess the effect of repeated Surprise stimulus presentations, we used Cochran Q tests for categorical data of dependent samples47. We then applied Sign tests to determine the significance of the relevant pair-wise comparisons. These and all subsequently described tests were two-tailed with alpha at 0.05 unless otherwise noted.
fMRI procedure
Anatomical 3D high-resolution images were acquired using conventional parameters on a 3T GE MRI system (Milwaukee, WI). Nineteen 7 mm thick axial slices (0 mm skip; 3.75 × 3.75 mm in-plane) were taken parallel to the AC-PC line. T2*-weighted image parameters: 25 ms echo time, 70° flip angle, 240 mm FOV, 64 × 64 matrix, 2000 ms repetition time. The functional scan included 166 brain volumes, with the first 6 volumes discarded for signal stabilization. Trials were presented using Psychophysics ToolBox48,49 for Matlab on an Apple G4 Macintosh. Stimuli were back-projected from an LCD projector onto a screen viewed through a prism mirror by the supine participant.
Data Analysis
Data analysis was performed using BrainVoyager 4.9.1, BrainVoyager QX 1.7.9 (Brain Innovation, Maastricht, The Netherlands), and custom Matlab software. Data preprocessing included image realignment, 3D motion correction, linear trend removal, and correction for slice acquisition timing. Statistical Parametric Maps (SPMs) of BOLD activation were created using a multiple regression analysis, with regressors defined for the six Surprise stimuli, Search trials, and No-target trials; boxcar functions for each trial type were convolved with a canonical double © hemodynamic function (SPM2, http://www.fil.ion.ucl.ac.uk/spm) to generate each regressor. The resulting maps from all participants were spatially smoothed with a 6 mm Gaussian kernel (FWHM), standardized to Talairach space45, and superimposed to create composite maps. The model fit was assessed using t statistics, with significance determined by the false discovery rate (FDR) threshold at q < 0.05 (random-effects analysis).
For the group region of interest (ROI) analysis, the center of mass and surrounding activated voxels for each activated focus were selected, up to 1 cm3. The time-course for each Surprise trial was extracted from each ROI for each participant and then converted to percent signal change (baseline from the time point of Surprise stimulus (SS) onset and two preceding points). The average time courses for pairs of SS (SS1+SS2, SS3+SS4, SS5+SS6) were next computed for each participant. For statistical tests of amplitude, we identified the time point with the largest percent signal change between 6 and 8 seconds following Surprise stimulus presentation for each SS pair for each participant, and then used paired t-tests for the appropriate comparisons.
For the individually-defined ROI analyses, we identified ROIs whose activity correlated with performing the primary target-detection task (SPM of open contrast of the predictor for Search trials). Positive β weights for the predictor were associated with FEF, IPS, and IFJ, whereas negative ones were associated with TPJ. Each ROI in each participant was defined as the peak voxel and significantly activated surrounding area up to 1 cm3. Anatomical landmarks (FEF at the junction of the superior frontal sulcus and precentral sulcus; IPS in the intraparietal sulcus between y = −50 and y = −70; IFJ at the junction of the inferior frontal sulcus and precentral sulcus; TPJ around the posterior Sylvian fissure) were used to identify each region, consistent with earlier work3,5,13,44. We next extracted time courses for the Surprise and Search trials, creating baselines and averages as above. For statistical tests of amplitude, we compared the corresponding time points across a given pair of time courses using paired t-tests.
For statistical tests of activity onset timing, we first subtracted each participant's Search trial activity from their Surprise stimulus trial activity for the first pair (SS1+SS2), leaving activation specific to deficit-causing Surprise stimuli. To estimate the hemodynamic response's onset time for these subtracted time courses, we employed a bootstrap approach50 owing to the difficulty of acquiring reliable onset measures from each participant's pairs of Surprise stimulus trials. Using linear interpolation, each participant's time courses—for both Search-related (Search trials) and Surprise-related (Surprise trials – Search trials) activity—from each ROI were upsampled to 1 ms resolution and then smoothed with a Gaussian kernel (FWHM = 2 seconds). Thirty samples for each ROI were selected with replacement and averaged, a process that was repeated 10,000 times. For each of the resulting averaged samples, we computed the onset as the time when the time course had achieved 20% of its peak amplitude (results were similar for 10%). Finally, these onset values (10,000 per ROI) were compared across ROIs. For example, right IFJ onsets occurred before right IPS onsets for 9,977 of the samples. From this count, we computed a p-value, which in the example would be 0.0066, two-tailed. Interactions were computed by first subtracting the onset values for Surprise-related activity from those for Search-related activity and then comparing these differences across regions.
As no hemispheric differences were found in any of the above analyses and to increase statistical power, we collapsed the data across hemispheres for all subsequent analyses.
The correlation analyses were performed on the data derived from the GLM analyses after further processing steps had been applied. Global signal fluctuations in this data set were removed by regressing out the time courses from a ventricular region of interest, a white matter region of interest, and the average signal across the entire brain. Second, the data were filtered using a zero-phase forward and reverse band-pass filter (0.01 < f < 0.2 Hz). Next, we segmented the data from each individually-defined ROI by trial, performing a percent signal change transform on each trial as described above. Trials were then concatenated by condition, yielding 28 points associated with Surprise trials (SS1+2) and 28 points with Search trials (two randomly selected trials from about 10 that were at least three trials away from any Surprise trials). Time courses for each ROI (collapsed across hemispheres) pair of interest in each participant were then correlated by condition and the resulting values converted using Fisher's z transformation. The correlations between regions were then tested for significance across participants with one-sample t-tests, and the change in correlations between Search and Surprise trials compared across participants with paired t-tests.
Spatial SiB Experiment (Experiment 2)
Six right-handed individuals (3 females) participated.
Trial design
The timing of each trial was as in Experiment 1 except that 29 stimuli were shown on each trial and the ISI was 17 ms. There were 40 trials during each of six fMRI runs. The target (present on 80% of trials) appeared between frames 23–27. In four of the trials per run (3 target-present and 1 target-absent), a Surprise stimulus appeared 350 ms before the target at one of four spatial locations centered 4.2° from fixation. The Surprise stimuli consisted of distinct, large (6.5° × 6.5°), colorful items, each shown only once. Surprise trials occurred between trials 2 and 38, and were separated by a minimum of three Search trials. Participants practiced the Search task prior to the fMRI session. Participants were not informed about the Surprise stimuli.
fMRI procedure
Anatomical 3D high-resolution images were acquired using conventional parameters on a 3T Philips MRI system. Thirty-three 3.5 mm thick axial slices (0.5 mm skip; 1.875 × 1.875 mm in-plane) were taken parallel to the AC-PC line. T2*-weighted image parameters: 35 ms echo time, 79° flip angle, 240 mm FOV, 128 × 128 matrix, 2000 ms repetition time. There were 161 brain volumes per functional scan. Trials were presented using Psychophysics ToolBox48,49 for Matlab on an Apple MacBook Pro, and stimuli back-projected to the participant as explained for Experiment 1.
Data Analysis
Data analysis was performed using BrainVoyager QX 1.11.4 (Brain Innovation, Maastricht, The Netherlands), and custom Matlab software. Data preprocessing, SPM generation, ROI definition, and event-related average construction were identical to the methods in Experiment 1.
We employed a bootstrap analysis to test the hypothesis that the delay in dorsal network activity (relative to the ventral network) was significantly greater in Experiment 1 than in Experiment 2. After constructing bootstrap samples, we obtained the onset measures for the Search-related and Surprise-related activity for each ROI in each experiment. To increase power, we collapsed these measures across hemispheres and network nodes (dorsal or ventral). We then compared the onset measures for Experiment 1 ((Search – Surprise)Dorsal – (Search – Surprise)Ventral) with those for Experiment 2 (same subtractions). As the 90% confidence intervals for the resulting metrics did not overlap, the comparison was significant one-tailed at p < 0.05.
Endogenous Cueing Task Experiment (Experiment 3)
After completing Experiment 2, the same six individuals participated in Experiment 3 during the same scan session.
Trial design
Each trial began with a central dot (0.25° in diameter) changing from white to either green or blue for 2000 ms, which indicated in which of two squares (1.0° across, located 3.4° right or left of fixation) an upcoming target was likely to appear (81% validity). The cue's color-location mapping was counterbalanced across participants. The target, a white dot (0.25°) that appeared inside one of the boxes for 100 ms, was presented 4, 6, 8, or 10 seconds after the cue onset. The frequency of each delay period was exponentially distributed to maximize deconvolution efficiency. Participants responded to the target with a speeded button press. The next trial commenced after an ITI of 4–10 seconds (exponentially distributed). Participants completed 32 trials during each of 3 runs.
One participant's behavioral responses were not collected due to a technical error, and another participant performed the task incorrectly by withholding responses to invalidly-cued targets. Behavioral data for these two individuals were collected during a separate session outside the scanner.
fMRI procedure
The imaging procedure was identical to that used for Experiment 2.
Data Analysis
After employing the same preprocessing steps used for Experiment 2, time courses from Experiment 2's Search task ROIs were constructed using a deconvolution analysis. Z-transformed β estimates, corrected for serial auto-correlations, were derived for the 10 volumes following the cue onset and for the 10 volumes following target onset, and individual time courses were averaged across participants and hemispheres (Fig. 5b). To test for significant activation in each region, one-sample t-tests were performed on the average of the 3rd and 4th volumes after cue onset.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation grant 0094992 and NIMH grant R01 MH70776, both to R.M. Special thanks to Baxter Rogers, Jascha Swisher, and Ellie Conser.
References
- 1.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 2.Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annual Review of Psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nature Neuroscience. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 4.Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- 5.Serences JT, Shomstein S, Leber AB, Golay X, et al. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 6.Yantis S, Schwarzbach J, Serences JT, Carlson RL, et al. Transient neural activity in human parietal cortex during spatial attention shifts. Nature Neuroscience. 2002;5:995–1002. doi: 10.1038/nn921. [DOI] [PubMed] [Google Scholar]
- 7.Chiu Y-C, Yantis S. A domain-independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. Journal of Neuroscience. 2009;29:3930–3938. doi: 10.1523/JNEUROSCI.5737-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 9.Downar J, Crawley AP, Mikulis DJ, Davis KD. A cortical network sensitive to stimulus salience in a neutral behavioral context across multiple sensory modalities. Journal of Neurophysiology. 2002;87:615–620. doi: 10.1152/jn.00636.2001. [DOI] [PubMed] [Google Scholar]
- 10.Horovitz SG, Skudlarski P, Gore JC. Correlations and dissociations between BOLD signal and P300 amplitude in an auditory oddball task: a parametric approach to combining fMRI and ERP. Magnetic Resonance Imaging. 2002;20:319–325. doi: 10.1016/s0730-725x(02)00496-4. [DOI] [PubMed] [Google Scholar]
- 11.Linden DE, Prvulovic D, Formisano E, Völlinger M, et al. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cerebral Cortex. 1999;9:815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- 12.Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 13.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb J. From thought to action: The parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 16.He BJ, Snyder AZ, Vincent JL, Epstein A, et al. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends in Cognitive Sciences. 2006;10:204–211. doi: 10.1016/j.tics.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Beck DM, Rees G, Frith CD, Lavie N. Neural correlates of change detection and change blindness. Nature Neuroscience. 2001;4:645–650. doi: 10.1038/88477. [DOI] [PubMed] [Google Scholar]
- 19.Rees G, Russell C, Frith CD, Driver J. Inattentional blindness versus inattentional amnesia for fixated but ignored words. Science. 1999;286:2504–2507. doi: 10.1126/science.286.5449.2504. [DOI] [PubMed] [Google Scholar]
- 20.Marois R, Chun MM, Gore JC. Neural Correlates of the Attentional Blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 21.Marois R, Yi DJ, Chun MM. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- 22.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nature Reviews Neuroscience. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 23.Husain M, Nachev P. Space and the parietal cortex. Trends in Cognitive Sciences. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baars BJ. In the Theatre of Consciousness: Global Workspace Theory, A Rigorous Scientific Theory of Consciousness. Journal of Consciousness Studies. 1997:292–309. [Google Scholar]
- 25.Milner AD, Goodale MA. Separate visual pathways for perception and action. Trends in Neuroscience. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 26.Asplund CL, Todd JJ, Snyder AP, Gilbert CM, Marois R. Surprise-induced Blindness: A stimulus-driven attentional limit to conscious perception. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0020551. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi S, Hale LA, D'Esposito M, Knight RT. Rapid prefrontalhippocampal habituation to novel events. Journal of Neuroscience. 2004;24:5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- 29.Opitz B, Mecklinger A, Friederici AD, von Cramon DY. The functional neuroanatomy of novelty processing: integrating ERP and fMRI results. Cerebral Cortex. 1999;9:379–391. doi: 10.1093/cercor/9.4.379. [DOI] [PubMed] [Google Scholar]
- 30.Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- 31.Heilman KM, Watson RT. Mechanisms underlying the unilateral neglect syndrome. Advances in Neurology. 1977;18:93–106. [PubMed] [Google Scholar]
- 32.Karnath HO, Milner AD, Vallar G. The cognitive and neural bases of spatial neglect. Oxford University Press; 2002. [Google Scholar]
- 33.Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychological Science. 2005;16:965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- 34.Shulman GL, Astafiev SV, McAvoy MP, d'Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cerebral Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- 35.Fox MD, Snyder AZ, Vincent JL, Corbetta M, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology: General. 1980;109:160–174. [PubMed] [Google Scholar]
- 37.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 38.Kastner S, DeSimone K, Konen CS, Szczepanski SM, Weiner KS, Schneider KA. Topographic maps in human frontal cortex revealed in memory-guided saccade and spatial working-memory tasks. Journal of Neurophysiology. 2007;97:3494–3507. doi: 10.1152/jn.00010.2007. [DOI] [PubMed] [Google Scholar]
- 39.Huettel SA, Güzeldere G, McCarthy G. Dissociating the neural mechanisms of visual attention in change detection using functional MRI. Journal of Cognitive Neuroscience. 2001;13:1006–1018. doi: 10.1162/089892901753165908. [DOI] [PubMed] [Google Scholar]
- 40.Lumer ED, Friston KJ, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- 41.Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human PPC. Journal of Neuroscience. 2008;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction in cognitive control. Trends in Cognitive Sciences. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302:1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 44.Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends in Cognitive Sciences. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- 46.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 47.Sheskin DJ. Handbook of Parametric and Nonparametric statistical procedures. 2nd Ed. CRC Press; Boca Raton, FL: 2000. [Google Scholar]
- 48.Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- 49.Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- 50.Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge University Press; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.