Abstract
Voluntary exercise and endogenous cannabinoid activity have independently been shown to regulate hippocampal plasticity. The aim of the current study was to determine whether the endocannabinoid system is regulated by voluntary exercise and if these changes contribute to exercise-induced enhancement of cell proliferation. In Experiment 1, eight days of free access to a running wheel increased the agonist binding site density of the cannabinoid CB1 receptor; CB1 receptor-mediated GTPγS binding; and the tissue content of the endocannabinoid anandamide in the hippocampus but not in the prefrontal cortex. In Experiment 2, the CB1 receptor antagonist AM251 (1 mg/kg) was administered daily to animals given free access to a running wheel for 8 days, after which cell proliferation in the hippocampus was examined through immunohistochemical analysis of the cell cycle protein Ki-67. Voluntary exercise increased proliferation of progenitor cells, as evidenced by the increase in the number of Ki-67 positive cells in the granule cell layer of the dentate gyrus in the hippocampus. However, this effect was abrogated by concurrent treatment with AM251, indicating that the increase in endocannabinoid signaling in the hippocampus is required for the exercise-induced increase in cell proliferation. These data demonstrate that the endocannabinoid system in the hippocampus is sensitive to environmental change and suggest that it is a mediator of experience-induced plasticity.
Keywords: cell proliferation, antidepressant, running, 2-AG, FAAH, neuroprotection
Introduction
The development of new neurons, or neurogenesis, occurs when neural progenitor cells in the subgranular zone of the dentate gyrus (as well as in the olfactory bulb) undergo mitosis to produce a population of daughter cells, which primarily assume a neuronal phenotype, although some do become glia (Christie and Cameron, 2006; Zhao et al., 2008). As the new hippocampal neurons mature, to become functional they must extend axons and dendrites into the established cytoarchitecture of the existing neuronal networks, and also develop appropriate bioelectrical properties and connections for dentate granule cells (Lledo et al., 2006; Schmidt-Hieber et al., 2004; van Praag et al., 2002).
Cell proliferation and neurogenesis in the hippocampus are sensitive to regulation by a number of environmental factors, including stress, enrichment and exercise (Mirescu and Gould, 2006; Olson et al., 2006). Voluntary exercise (VEx) is a particularly robust way to enhance progenitor cell proliferation and neurogenesis, possibly because it also increases a myriad of physiological responses that include neurotrophin expression, dendritic length and complexity, spine density, angiogenesis and cerebral blood flow, within the dentate gyrus (Eadie et al., 2005; Farmer et al., 2004; Neeper et al., 1996; Pereira et al., 2007; Redila and Christie, 2006; Stranahan et al., 2007; van Praag et al., 1999). As a result of these changes, animals that exercise show enhanced long-term potentiation, as well as observable improvements in performance on cognitive tasks (van Praag et al., 1999; Vaynman et al., 2004, 2007).
Converging lines of evidence also support a role of the endocannabinoid system in neuroplastic phenomena, particularly within the hippocampus (Aguado et al., 2005, 2006; Hashimotodani et al., 2007; Zhu, 2006). The endocannabinoid system is a neuromodulatory system composed of two receptors (CB1 and CB2) and the arachidonate-derived endogenous ligands, N-arachidonylethanolamide (anandamide; AEA) and 2-arachidonoylglycerol (2-AG) (Hillard, 2000; Howlett et al., 2004). Both in vitro and in vivo studies have revealed that neural progenitor cells express both CB1 and CB2 receptors and synthesize AEA and 2-AG (Aguado et al., 2005, 2006; Jiang et al., 2005; Molina-Holgado et al., 2007; Palazuelos et al., 2006). Genetic deletion of the CB1 receptor suppresses progenitor cell proliferation (Aguado et al., 2005, 2006; Jin et al., 2004; Kim et al., 2006), while genetic deletion of the enzyme responsible for AEA hydrolysis (fatty acid amide hydrolase; FAAH) results in a profound increase in cell proliferation within the dentate gyrus (Aguado et al., 2005, 2006). In addition to these effects on progenitor cell proliferation, the endocannabinoid system interacts with several neurotrophic systems (Aso et al., 2008; Khaspekov et al., 2004; Williams et al., 2003), making this system an ideal candidate for mediating the effects of VEx on hippocampal cytogenesis. Consistently, in addition to the ability of VEx to increase circulating growth factors (Schwarz et al., 1996; Schobersberger et al., 2000), AEA increases in the circulation following a sustained period of physical exercise in humans (Sparling et al., 2003), demonstrating that exercise activates the endocannabinoid system. A recent report has also suggested that endocannabinoid signaling is involved in the rewarding properties of running (Keeney et al., 2008), indicating that functional associations may exist between VEx and the endocannabinoid system. Therefore, the current study sought to determine if VEx modulates hippocampal endocannabinoid signaling, and the extent to which these changes contribute to alterations in neuroplasticity following exercise.
Methods
Subjects
Seventy day old male Sprague-Dawley rats (300 g; Charles River Laboratories, Montreal, Canada) were housed in groups of three in triple mesh wire caging for a ten day acclimation period following arrival. After acclimation, half of the rats were randomly assigned to either standard caging (containing enrichment in the form of PVC tubing) or caging that contained a running wheel connected to a PC computer (Mini-Mitter Systems Inc., WA, USA). Animals in the VEx condition were housed in cages with running wheels for eight days while control animals were housed individually in equivalent caging without a running wheel for the same period of time. Colony rooms were maintained at 21 °C, and on a 12 h light/dark cycle, with lights on at 0900 h. All rats were given ad libitum access to Purina Rat Chow and tap water. All protocols were approved by the Canadian Council for Animal Care and the Animal Care Committee of the University of British Columbia.
Experiment 1: The Effects of VEx on the Endocannabinoid System in the Hippocampus and Prefrontal Cortex
Animals were housed and maintained as described above. VEx animals and sedentary control animals were sacrificed between 0900-1100 h on the morning following eight days of access to running wheels. A diagram of the treatment conditions can be seen in Fig. 1. The hippocampus and prefrontal cortex (consisting of medial prefrontal cortex and anterior cingulate cortex) were sectioned out as previously described (Hill et al., 2006a), frozen in liquid nitrogen within 5 min of decapitation and stored at -80 °C until analysis. Two cohorts of tissue were collected. One cohort of tissue (n = 7) was used for lipid extraction to determine endocannabinoid ligand content. The other cohort of tissue (n = 5) was used to create membrane fractions for subsequent determination of CB1 receptor binding, CB1 receptor-mediated GTPγS binding and FAAH activity.
Figure 1. Flowchart of experimental procedures.
Schematic representation of the housing/exercise protocol employed to generate tissue for examining the effects of voluntary exercise on the endocannabinois system (Experiment 1) and the role of endocannabinoid signaling in the effects of exercise on cell proliferation in the hippocampus (Experiment 2).
Membrane Preparation
Dissected brain sections were homogenized in 10 volumes of 0.32 M sucrose containing 3 mM HEPES (pH 7.5) and 1 mM EDTA. The homogenates were centrifuged at 18,000 × g for 20 min after which the supernatant was rapidly decanted. The remaining pellet, which is the membrane fraction, was resuspended in 1-2 ml TME buffer (50 mM Tris HCl, pH 7.4; 1 mM EDTA and 3 mM MgCl2). Protein concentrations were determined by the Bradford method (Bio-Rad, Hercules, CA, USA).
CB1 Receptor Binding Assay
CB1 receptor binding assays were performed using a Multiscreen Filtration System with Durapore 1.2-μM filters (Millipore, Bedford, MA) as described previously (Hillard et al., 1995a). Incubations (total volume = 0.2 mL) were carried out using TME buffer containing 1 mg/mL bovine serum albumin (TME/BSA). Membranes (10 μg protein per incubate) were added to the wells containing 0.1, 0.25, 0.5, 1.0, 1.5 or 2.5 nM 3H-CP 55,940, a cannabinoid CB1 receptor agonist. Ten μM Δ9-tetrahydrocannabinol was used to determine non-specific binding. KD and Bmax values were determined by nonlinear curve fitting to the single site binding equation using GraphPad Prism (San Diego, CA, USA).
CB1 Receptor-mediated GTPγS Binding Assay
The assay for [35S]GTPγS binding was performed as previously described by Kearn et al. (1999). Briefly, membranes (final concentration, 5 μg of protein per incubation mixture) were added to TME buffer containing 0.1% fatty acid-free bovine serum albumin, 10 μmol/L GDP, and 150 mmol/L NaCl. [35S]GTPγS (final concentration, 0.65 nmol/L) was added, and the incubation was continued for 30 min at 37°C using the Multiscreen Filtration System with Durapore filters (pore size, 1.2 μm; Millipore, Bedford, MA, USA). Non-specific binding was determined in the presence of 10 μmol/L Gpp(NH)p and accounted for <15% of the total binding. Bound [35S]GTPγS was separated from free [35S]GTPγS by filtration followed by washing the filters four times with cold TME buffer containing NaCl and GDP. The cannabinoid CB1 receptor agonist WIN 55,212 was added in 1 μL of dimethyl sulfoxide at concentrations of 0, 0.1, 0.3, 0.6, 1, 2, 3, 6, 10, 20 and 30 μmol/L. In each experiment, the agonist-dependent [35S]GTPγS binding was divided by agonist-independent binding and multiplied by 100 to convert to a percentage. The EC50 values and maximal agonist-induced increase in binding (Emax) of [35S]GTPγS were determined by fitting the data to a sigmoidal concentration-response curve using nonlinear regression (Prism; GraphPad, San Diego, CA, USA).
Fatty Acid Amide Hydrolase Activity Assay
FAAH activity was measured as the conversion of AEA to arachidonic acid and ethanolamine by membrane preparations (Hillard et al., 1995b). AEA labeled with [3H] in the ethanolamine portion of the molecule ([3H]AEA; Omeir et al., 1995) was the radiolabeled substrate. Membranes were incubated in a final volume of 0.5 ml of TME buffer (50 mM Tris-HCl, 3.0 mM MgCl2, and 1.0 mM EDTA, pH 7.4) containing 1.0 mg/ml fatty acid-free bovine serum albumin and 0.2 nM [3H]AEA. Isotherms were constructed using eight concentrations of AEA at concentrations between 10 nM and 10 μM. Incubations were carried out at 37°C and were stopped with the addition of 2 ml of chloroform/methanol (1:2). After standing at ambient temperature for 30 min, 0.67 ml of chloroform and 0.6 ml of water were added. Aqueous and organic phases were separated by centrifugation at 1,000 rpm for 10 min. The amount of [3H] in 1 ml each of the aqueous and organic phases was determined by liquid scintillation counting and the conversion of [3H]AEA to [3H]ethanolamine was calculated. The KI and Vmax values for this conversion were determined by fitting the data to a single site competition equation using Prism. The r2 value for the goodness of fit of the data to the single site, hyperbolic equation was always greater than 0.9 and typically closer to 0.98.
Endocannabinoid Extraction and Analysis
For analysis of endocannabinoid content, brain regions were subjected to a lipid extraction process as described previously (Patel et al., 2003). Briefly, tissue samples were weighed and placed into borosilicate glass culture tubes containing 2 ml of acetonitrile with 84 pmol of [2H8]anandamide and 186 pmol of [2H8]2-AG for extraction. Tissue was homogenized with a glass rod and sonicated for 30 min. Samples were incubated overnight at -20°C to precipitate proteins then centrifuged at 1,500 × g to remove particulates. The supernatants were removed to a new glass tube and evaporated to dryness under N2 gas. The samples were resuspended in 300 μl of methanol to recapture any lipids adhering to the glass tube, and dried again under N2 gas. Finally, lipid extracts were suspended in 20 μl of methanol, and stored at −80°C until analysis. The contents of the two primary endocannabinoids AEA and 2-AG within lipid extracts in methanol from brain tissue and plasma were determined using isotope-dilution liquid chromatography–mass spectrometry as described previously (Patel et al., 2005).
Experiment 2: The Role of the Endocannabinoid System in Exercise-induced Increase in Cell Proliferation in the Dentate Gyrus
Subjects were acclimated and assigned to groups as described above. Once in their respective housing conditions, half of the VEx and sedentary control received daily injections of the cannabinoid CB1 receptor antagonist AM251 (1 mg/kg; Tocris Biosciences, Ellisville, MO, USA), while the other half received daily injections of vehicle (1:1:8 solution of DMSO: Tween 80: 0.9% saline). Injections began the morning following the initial transfer to the new cages and were performed using 26 gauge 1/2″ needles at a volume of 1 ml/kg. To minimize any disruption in activity levels, daily injections were given at the onset of the light cycle (between 0900-1100h), when the animals were the least active. A diagram of the treatment conditions can be seen in Fig. 1.
Following the morning of the eighth consecutive day of VEx, all subjects were overdosed with sodium pentobarbital (120 mg/kg) and perfused transcardially with 60 ml of 0.9% saline, followed by 60 ml of 4% paraformaldehyde. Brains were removed and stored in paraformaldehyde for 24 hours before being transferred to 30% sucrose until saturated. Coronal sections (40 μm) were obtained throughout the extent of the hippocampus with a Leica VT1000 vibratome.
Ki-67 Immunohistochemistry and Volumetric Analysis of the Dentate Gyrus
For Ki-67 immunohistochemistry, the tissue was rinsed in 0.1 M TBS, followed by 0.3% H2O2 and then transferred to the primary antibody solution containing 0.5% Triton X, 1% normal horse serum and 1:1000 rabbit anti-Ki-67 (Vector, Burlington ON, Canada) in 0.1 M TBS. Tissue was incubated in the primary solution for 20 h at room temperature (approximately 21 °C) and then rinsed in 0.1 M TBS. Tissue was then incubated in Biotinylated goat anti-rabbit IgG (Vector, Burlington ON, Canada) diluted 1:1000 in 0.1 M TBS for 1 h at room temperature and was then washed in 0.1 M TBS. An avidin-biotin peroxidase was applied for 60 min (ABC elite kit; Vector, Burlington ON, Canada). Labeling was visualized by incubating tissue in 5 mg/ml 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, Oakville ON, Canada). Finally, the tissue was mounted on glass slides.
We utilized a modified stereological approach to determine the expression of Ki-67-labeled cells across the rostral-caudal extent of the hippocampus. Ki-67-labeled cells were counted in every 10th section (400 μm apart) throughout the neurogenic region of the granule cell layer (which is the subgranular zone and is defined as zero to two cell bodies from the inner edge of the molecular layer) and the hilus to obtain an estimate of the total number of labeled cells in each region. Counting was performed using a 100× oil immersion objective and a Nikon E600 light microscope. Corresponding area measurements were made of the dentate gyrus (granule cell layer and hilus calculated separately) using the software program Image J. Volume estimations of the dentate gyrus were then calculated using Cavalieri's principle (Gundersen and Jensen, 1987) by multiplying the aggregated areas by the distance between sections (400 μm). Total cell counts were calculated by multiplying the number of Ki-67-labeled cells per animal by 10. Densities of Ki-67-labeled cells per cubic millimeter in each region (granule cell layer or hilus) were also calculated by dividing the total number of Ki-67-labeled cells by the volume of the region (granule cell layer or hilus).
Statistical Analysis
For Experiment 1, the effects of VEx on the endocannabinoid system were analyzed by comparing VEx and sedentary control animals using an independent t-test. For Experiment 2, a univariate analysis of variance (ANOVA) was used with exercise and AM251 as fixed factors. Post hoc analysis was performed using a Tukey's test. Significance was established against an alpha level equal to 0.05.
Results
Exercise Increases Endocannabinoid Signaling in the Hippocampus
Animals with free access to a running wheel for eight days exhibited a significant increase in the maximal binding (Bmax) of [3H]CP55940 to the CB1 receptor in the hippocampus [t (8) = 5.29, p < 0.005; Fig. 2 and Table 1]. This increase in the Bmax of the CB1 receptor was accompanied by a significant reduction in the affinity (Kd) of [3H] CP55940 for the CB1 receptor [t (8) = 2.84, p < 0.05; Fig. 2 and Table 1]. There was no effect of VEx on the Bmax [t (8) = 0.48, p > 0.05; Table 1] or the Kd [t (8) = 0.43, p > 0.05; Table 1] of [3H]CP55940 to bind to the CB1 receptor in the prefrontal cortex.
Figure 2. Voluntary exercise augments hippocampal endocannabinoid signaling.
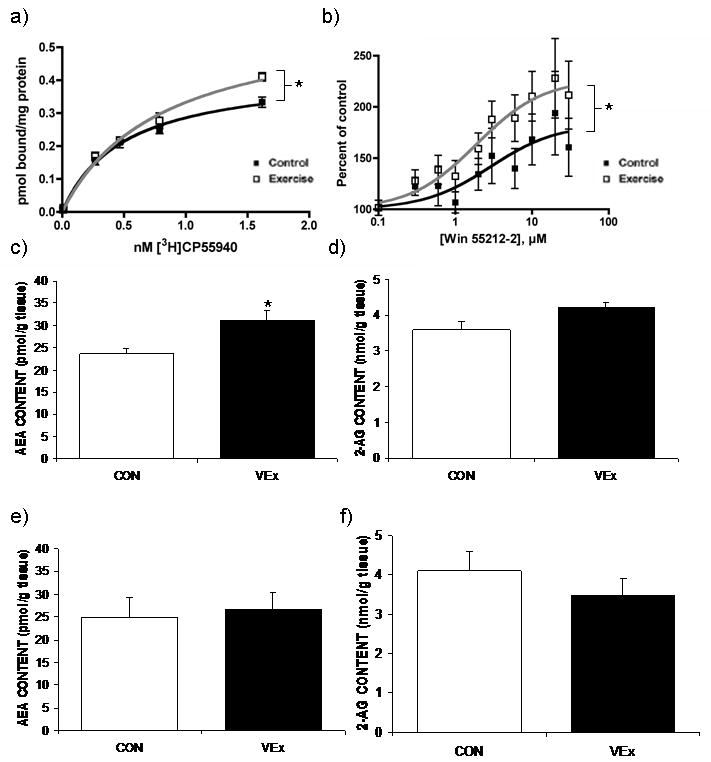
Eight days of voluntary exercise resulted in a significant increase in (a) CB1 receptor binding site density in the hippocampus (n = 4-5 / condition), (b) CB1 receptor mediated 35S- GTPγS binding within the hippocampus (n = 4-5 / condition), and (c) tissue content of the endocannabinoid ligand anandamide (AEA) within the hippocampus (n = 7 / condition). There was no effect of voluntary exercise on (d) tissue content of the endocannabinoid ligand 2-arachidonoylglycerol (2-AG) in the hippocampus (n = 7 / condition), or (e) AEA content (n = 7 / condition) or (f) 2-AG content in the prefrontal cortex (n = 7 / condition). Values denoted are means ± SEM. * denotes significant differences (p < .05) between VEx animals and the control group (CON).
Table 1.
The effects of voluntary exercise (VEx) on the maximal binding (Bmax) and dissociation constant (Kd) of 3H-CP55940, a cannabinoid CB1 receptor agonist, from the CB1 receptor and CB1 receptor-mediated 35S- GTPγS binding evoked by the CB1 receptor agonist WIN 55,212-2.
Animals which engaged in voluntary exercise (VEx) exhibited a significant increase in the Bmax and Kd of the CB1 receptor in the hippocampus, that was accompanied by a significant reduction in the EC50 and a tendency for increased maximal stimulation (Emax) of CB1 receptor-mediated 35S- GTPγS binding in the hippocampus. There was no effect of VEx on CB1 binding parameters within the prefrontal cortex. CB1 receptor binding assays were performed in triplicate for each concentration of 3H-CP55940 within each sample; GTPγS binding assays were performed in quadruplicate for each concentration of WIN 55,212-2 within each sample. Values denoted are means ± SEM. * denotes significant differences (p < 0.05) between VEx animals (n = 4-5) and the control group (CON; n = 4-5).
| CON | VEx | |
|---|---|---|
| Hippocampus | ||
| Bmax (pmol/mg protein) | 0.44 +/- 0.03 | 0.60 +/- 0.02* |
| Kd (nM) | 0.54 +/- 0.08 | 0.82 +/- 0.06* |
| Emax (% baseline) | 152.0 +/- 4.5 | 232.3 +/- 31.0 |
| EC50 (nM) | 4708.3 +/- 586.7 | 2240.6 +/- 396.1* |
| Prefrontal Cortex | ||
| Bmax (pmol/mg protein) | 0.38 +/- 0.06 | 0.34 +/- 0.03 |
| Kd (nM) | 0.51 +/- 0.06 | 0.60 +/- 0.09 |
VEx also increased CB1 receptor mediated GTPγS binding [t (8) = 3.63, p < 0.02; Fig. 2 and Table 1]. The EC50 for the CB1 receptor agonist, WIN55,212-2 was dramatically reduced in animals allowed VEx. VEx evoked a trend toward an increase in the maximal stimulation of GTPγS binding (Emax) elicited by the CB1 receptor agonist WIN-55212,2 in the hippocampus [t (8) = 1.94, p = 0.10; Fig. 2 and Table 1].
The tissue content of AEA was significantly increased in the hippocampus following VEx [t (12) = 2.93, p < 0.02; Fig. 2]. This change was not associated with a decrease in the hydrolysis of AEA by FAAH because VEx had no effect on the maximal hydrolytic activity (Vmax) of FAAH [t (8) = 0.55, p > 0.05; Table 2] or the binding affinity (Km) of AEA for FAAH [t (8) = 0.66, p > 0.05; Table 2]. There was also a trend toward an increase in 2-AG content within the hippocampus [t (12) = 1.91, p = 0.08; Fig. 2]. Neither AEA [t (12) = 0.31, p > 0.05; Fig. 2] nor 2-AG [t (12) = 0.90, p > 0.05; Fig. 2] content was altered in samples from prefrontal cortex.
Table 2.
The effects of voluntary exercise (VEx) on the maximal hydrolytic activity (Vmax) and the binding affinity (Km) of fatty acid amide hydrolase for anandamide. There was no effect of VEx on either the Vmax or the Km of FAAH in the hippocampus. For both treatment conditions, n = 5. Data are presented as means +/- SEM.
| CON | VEx | |
|---|---|---|
| Hippocampus | ||
| Vmax (pmol/mg protein) | 619.4 +/- 56.9 | 556.7 +/- 99.4 |
| Km (nM) | 0.53 +/- 0.09 | 0.61 +/- 0.09 |
Exercise-induced Increase of Cell Proliferation in the Denate Gyrus requires Cannabinoid CB1 Receptor Activity
Since VEx increases endocannabinoid signaling in the hippocampus and both endocannabinoid and physical activity can promote cell proliferation (Aguado et al., 2005, 2006; Eadie et al., 2005; Pereira et al., 2007), we examined if the exercise-induced increase in hippocampal endocannabinoid signaling contributes to enhanced cell proliferation in the dentate gyrus following VEx. Using the expression of an endogenous cell cycle protein, Ki-67, as a marker of neural progenitor cell proliferation, we found that there was a significant interaction between voluntary exercise and administration of AM251 on the total number of Ki-67 positive (Ki-67+) cells in the granule cell layer of the dentate gyrus [F (1, 19) = 7.40, p < 0.02; Fig. 3]. Post hoc analysis revealed that this interaction was due to an increase in Ki-67+ cells in the granule cell layer of animals that had engaged in VEx (p < 0.04); animals administered AM251, alone or in conjunction with VEx, did not exhibit any significant changes in Ki-67 expression in the dentate gyrus relative to vehicle treated controls (p > 0.05). This increased expression of Ki-67+ cells following VEx was not an artifact of structural changes within the dentate gyrus as there was neither a significant interaction [F (1, 19) = 0.25, p > 0.05; Table 3] nor main effect of either VEx [F (1, 19) = 0.35, p > 0.05] or AM251 administration [F (1, 19) = 1.90, p > 0.05] on the volume of the granule cell layer of the dentate gyrus.
Figure 3. Antagonism of the cannabinoid CB1 receptor attenuates the increase in cell proliferation in the dentate gyrus following voluntary exercise.
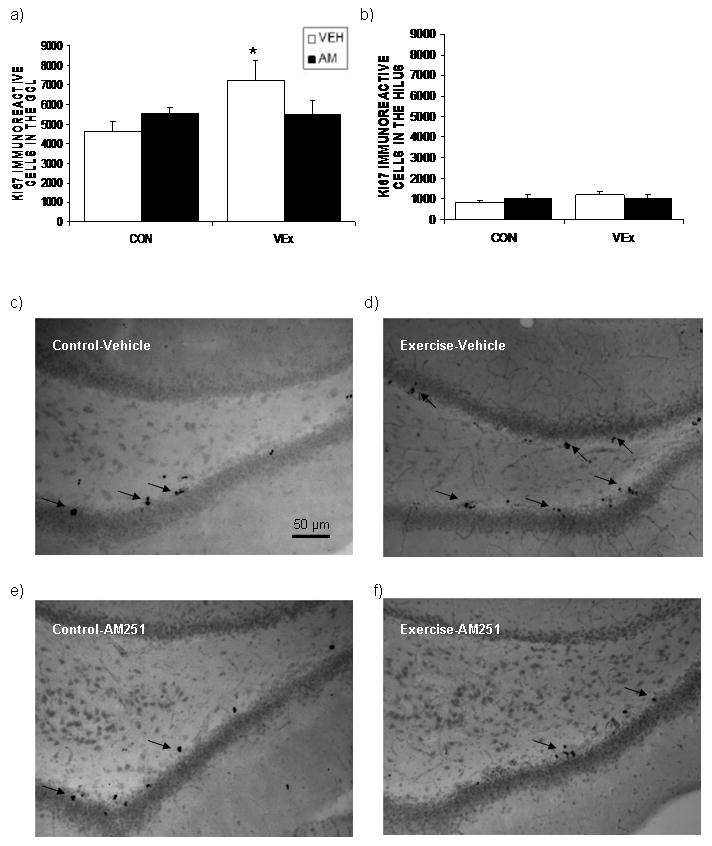
(a) Animals which engaged in voluntary exercise (VEx) exhibited a significant increase in the total estimated number of proliferating cells expressing the endogenous cell cycle protein Ki-67 within the neurogenic region of the granule cell layer (GCL) of the dentate gyrus. This phenomenon was not seen following concurrent administration of the cannabinoid CB1 receptor antagonist AM251 (AM; 1 mg/kg), which alone had no effect on cell proliferation. (b) Neither treatment, alone nor in combination, had any effect on the cellular expression of Ki-67 within the hilus of the dentate gyrus. Values denoted are means ± SEM; n = 5-6 / condition. * denotes significant differences (p < .05) between a treatment condition and the control group (CON) receiving vehicle injections (VEH). Representative photomicrographs (c, d, e, f) of the effects of voluntary exercise, AM251 administration (1 mg/kg), or both treatments combined on the immunoreactivity of Ki-67 (denoted by arrows) in the dentate gyrus at 10× magnification.
Table 3.
The effects of voluntary exercise (VEx) and administration of the cannabinoid CB1 receptor antagonist AM251 (1 mg/kg) on the volume of the granule cell layer (GCL) and hilus of the dentate gyrus.
There was no effect of VEx or AM251 on the volume of the GCL or the hilus of the dentate gyrus. For all treatment conditions, n = 5-6. Data are presented as means +/- SEM.
| CON | VEx | |
|---|---|---|
| GCL (mm3) | ||
| Vehicle | 3.08 +/- 0.12 | 3.19 +/- 0.13 |
| AM251 | 3.14 +/- 0.18 | 3.28 +/- 0.29 |
| Hilus (mm3) | ||
| Vehicle | 7.16 +/- 0.29 | 7.27 +/- 0.58 |
| AM251 | 7.81 +/- 0.57 | 7.12 +/- 0.12 |
When the expression of Ki-67+ cells was examined as a density measurement of the number of Ki-67+cells/mm3 of the granule cell layer, an interaction was found between VEx and AM251 administration [F (1, 19) = 6.90, p < 0.02; data not shown]. Post hoc analyses revealed that VEx increased the density of Ki-67+ cells within the dentate gyrus (p < 0.04), which was abolished by administration of AM251. The effect of VEx and CB1 receptor antagonism on cell proliferation was specific to the granule cell layer of the dentate gyrus as there was no significant interaction between exercise and AM251 [F (1, 19) = 1.39, p > 0.05; Fig. 3] nor main effects of either exercise [F (1, 19) = 1.14, p > 0.05] or AM251 administration [F (1, 19) = 0.00, p > 0.05] on the total number of Ki-67+ cells in the hilus of the dentate gyrus. Similarly, there was no effect of exercise and AM251 administration on the volume of the hilus of the dentate gyrus [F (1, 19) = 3.23, p > 0.05; Table 3] or the density of Ki-67+ cells within the hilus [F (1, 19) = 0.40, p > 0.05; data not shown]. Representative photomicrographs of the expression of Ki-67+ proliferating cells in the dentate gyrus following VEx, AM251 administration, or both treatments combined can be seen in Fig. 3.
It is important to note that there was no effect of drug treatment on the total distance run during the eight day running period [t (10) = 1.41, p > 0.05; data not shown] indicating that the reduction of proliferation following AM251 administration was not due to a reduction in VEx.
Discussion
These data demonstrate that VEx significantly increases endocannabinoid signaling within the hippocampus, and endocannabinoid signaling is required for VEx to increase proliferation of progenitor cells within the dentate gyrus. It is our hypothesis that these events are linked such that the effects of VEx on hippocampal endocannabinoid signaling drive the increase in progenitor cell proliferation. While the fate of these cells was not characterized in the present study, increased CB1 receptor signaling has been found to promote both gliogenesis (Aguado et al., 2006) and neurogenesis (Jiang et al., 2005). However, an abundance of research has demonstrated that voluntary exercise promotes neurogenesis, with no changes in gliogenesis (Fabel et al. 2003; Olson et al., 2006; Pereira et al., 2007; van Praag et al., 1999), suggesting that the increase in cell proliferation documented in this study (and others) results in an increase in the production of new neurons. Consistent with this suggestion, it has been hypothesized that the mechanism by which VEx increases neurogenesis is through an up-regulation of cell proliferation in the dentate gyrus, thus producing a larger population of progenitor cells which can subsequently mature into neurons (Olson et al., 2006).
Rats with free access to a running wheel for eight days exhibited a significant increase in the agonist binding site density of the cannabinoid CB1 receptor as revealed by the significant increase in the Bmax of the CB1 receptor; a significant increase in CB1 receptor agonist potency to induce GTPγS binding as revealed by the significant reduction in the EC50 of WIN 55,212-2 to stimulate CB1 receptor mediate GTPγS binding; and a significant increase in the hippocampal total tissue concentration of the endocannabinoid AEA. None of these effects were observed in the prefrontal cortex, indicating some degree of specificity to this phenomenon; however, it remains to be determined why these effects occurred within the hippocampus but not the prefrontal cortex. VEx increased the number of Ki-67+ cells in the granule cell layer of the dentate gyrus of the hippocampus, consistent with previous reports (Eadie et al., 2005; Fabel et al., 2003; Pereira et al., 2007; van Praag et al., 1999). However, the VEx-induced increase in cell proliferation did not occur in rats receiving daily administration of the cannabinoid CB1 receptor antagonist, AM251 (1 mg/kg) throughout the eight day exercise period. Taken together, these data suggest a role for increased endocannabinoid signaling within the hippocampus by VEx in increasing mitotic activity within the granule cell layer of the dentate gyrus. It should be noted that the CB2 receptor has also been identified to contribute to cell proliferation in the dentate gyrus (Palazuelos et al., 2006). While the current data do argue for an integral role of endocannabinoid signaling via the CB1 receptor in the ability of exercise to augment cell proliferation in the hippocampus, it is also possible that endocannabinoid signaling via the CB2 receptor may contribute to this phenomenon; however, further research is required to examine this question.
We recently reported that administration of the endocannabinoid uptake inhibitor/FAAH inhibitor, AM404, prevented stress-induced suppression of cell proliferation in the dentate gyrus (Hill et al., 2006b). Given that acute exposure to stress reduces AEA content within the hippocampus (Gorzalka et al., 2008), these data suggest that reductions in AEA/CB1 receptor signaling contribute to the potential of stress to suppress cell proliferation. In light of the current study, these data, collectively, indicate that the endocannabinoid system, and particularly AEA/CB1 receptor signaling, is an important mediator of experience-induced plasticity within the hippocampus, being both sensitive to environmental stimuli and a potent regulator of neuroplastic processes.
The mechanism(s) by which VEx increases endocannabinoid signaling remain to be determined. It is possible that the changes in receptor binding may be a result of changes in ligand availability. Previous studies have indicated that the regulation of the cannabinoid CB1 receptor and its endogenous ligands are not coupled in the typical negative regulation relationship, in which the ligand down-regulates its own receptor (Hill et al., 2005). In fact, direct infusion of 2-AG into the brain up-regulates CB1 receptor mRNA transcription (Kola et al., 2005) and 2-AG stimulates membrane expression of CB1 receptors in striatal tissue slices (Maccarrone et al., 2008), indicating that increased endocannabinoid signaling can promote both the genetic expression and surface recycling of the CB1 receptor. Therefore, the VEx-induced increase of endocannabinoid content in the hippocampus observed in the current study could precede, and drive, the increase in the CB1 receptor pool.
The increase in AEA content within the hippocampus, but not the prefrontal cortex, observed here was not due to a reduction in FAAH activity. Accordingly, it is not likely that the mechanism of action of this phenomenon involves decreased catabolism but rather is due to an enhancement in biosynthesis. Currently, there are three biochemical pathways that have been defined through which AEA synthesis can occur (Liu et al., 2006; Simon and Cravatt, 2006; Sun et al., 2004) and it is not known which of these is the predominant pathway in determining neuronal AEA synthesis, making it difficult to ascertain the enzymatic cascade responsible for mediating the increase in AEA following VEx. However, the synthesis of AEA is tightly coupled to calcium signaling and neuronal activation and is positively regulated by excitatory neurotransmission in the hippocampus (Jung et al., 2005; Marsicano et al., 2003; Ohno-Shosaku et al., 2002). Any type of movement, particularly running, would be expected to enhance network activity (i.e. theta activity) in the hippocampus (e.g., Keleman et al., 2005; Vanderwolf, 1969) and this may further enhance endocannabinoid synthesis and transmission. Alternately, cAMP-protein kinase signaling has been found to promote AEA synthesis in neuronal cultures and slice preparations (Azad et al., 2004; Cadas et al., 1996; Malcher-Lopes et al., 2006; Vellani et al., 2008) and voluntary exercise is known to increase hippocampal cAMP signaling (Shen et al., 2001); thus, it is also possible that changes in intracellular cAMP signaling, and subsequent protein kinase activity, could drive the up-regulation of hippocampal AEA content.
Despite the fact that both AEA and 2-AG are cognate ligands to the CB1 receptor, it is not surprising that the two ligands responded differentially to VEx. AEA and 2-AG do not share common pathways of synthesis and metabolism, with 2-AG being largely produced by phospholipase C-mediated generation of diacylglycerol, which is subsequently converted to 2-AG by the actions of diacylglycerol lipase (Bisogno et al., 2005). Additionally, 2-AG is primarily metabolized by monacylglycerol lipase, while AEA is primarily metabolized by FAAH (Bisogno et al., 2005). As the pathways of synthesis and metabolism of these ligands are dissociable, it is not unexpected that VEx would evoke changes in one of these ligands and not the other.
Neural progenitor cells in the hippocampus express CB1 and CB2 receptors and synthesize both AEA and 2-AG (Aguado et al., 2005, 2006; Jiang et al., 2005; Molina-Holgado et al., 2007; Palazuelos et al., 2006). Activation of these receptors, presumably on the progenitor cells themselves, pushes these precursor cells into a mitogenic state to produce a progeny population. Several in vitro and in vivo studies have demonstrated that the CB1 receptor can activate the phosphatidylinositol-3 kinase (PI3K)/Akt pathway (Galve-Roperh et al., 2002; Gomez del Pulgar et al., 2000; Molina-Holgado et al., 2002, 2005, 2007; Ozaita et al., 2007) and indicate that this pathway is instrumental for cannabinoid-induced proliferation (Molina-Holgado et al., 2007). Activation of the PI3K/Akt pathway within progenitor cells is sufficient to induce proliferation and is a common pathway through which many growth factors also induce proliferation (Aberg et al., 2003; Jin et al., 2005; Peltier et al., 2007). VEx can activate the PI3K/Akt pathway (Chen and Russo-Neustadt, 2005) although it remains to be determined if this signaling pathway is required for VEx-induced proliferation. It should also be noted that endocannabinoids are likely not the sole mediators of the proliferatve effects of VEx; in particular, trophic factors, such as VEGF and IGF-1, have also been found to be essential for this phenomenon (Fabel et al., 2003; Trejo et al., 2001). Thus, the most parsimonious explanation is that endocannabinoids and neurotrophic factors act in concert to regulate VEx-induced cell proliferation; a hypothesis that seems quite feasible given the convergence in signal transduction pathways that are utilized by these systems (Aberg et al., 2003; Bouaboula et al., 1997; Galve-Roperh et al., 2002; Gomez del Pulgar et al., 2000; Jin et al., 2005; Molina-Holgado et al., 2002, 2005, 2007; Ozaita et al., 2007; Peltier et al., 2007).
At the clinical level, exercise has demonstrated therapeutic benefit for an array of psychiatric and neurological conditions, such as Alzheimer's disease and depressive illness (Cotman et al., 2007; Dunn et al., 2005; Ernst et al., 2006; Stevens and Killeen, 2006). We have previously demonstrated that endocannabinoid signaling is dampened in the hippocampus in an animal model of depression and increased following treatment with a conventional antidepressant (Hill et al., 2005, 2006a), whereas local infusions of a CB1 receptor agonist directly into the dentate gyrus evoked an antidepressant-like response (McLaughlin et al., 2007). It has been suggested that induction of neural progenitor proliferation contributes to the antidepressant effects of exercise (Bjornebekk et al., 2005; Ernst et al., 2006). Therefore, the VEx-induced increase in hippocampal endocannabinoid activity could contribute to the antidepressant effects of this regimen, potentially via its contribution to changes in cell proliferation. Additionally, the neuroprotective effects of exercise might be afforded by increased endocannabinoid signaling. Both exercise and endocannabinoid signaling can dampen excitotoxic damage within the hippocampus and improve long-term outcomes following a neurological insult, indicating the possibility of functional overlap (Gobbo and O'Mara, 2005; Griesbach et al., 2004; Marsicano et al., 2003; Panikashvili et al., 2001). Future research should examine the extent to which endocannabinoid signaling contributes to the neuroprotective and antidepressant effects of exercise.
In conclusion, these data demonstrate that VEx enhances endocannabinoid signaling in the hippocampus through both effects on ligand availability and receptor sensitivity to agonist. Furthermore, this enhanced endocannabinoid signaling appears to contribute to the VEx-induced increase in cell proliferation in the dentate gyrus, as treatment with a CB1 receptor antagonist attenuated this effect. These data add to an increasing body of evidence supporting the hypothesis that the endocannabinoid system contributes to experience-induced alterations in plasticity within the hippocampus.
Acknowledgments
The authors would like to thank L. Galea and S. Lieblich for technical assistance.
Grant Information:
MNH: Grant Sponsor-MSFHR and NSERC (salary)
AKT: Grant Sponsor-Pacifica Family Addictions Foundation, Pacifica Century Graduate Scholarship (salary)
JGM: Grant Sponsor-Portugese FCT (salary)
BBG: Grant Sponsor-CIHR and NSERC (operating)
CJH: Grant Sponsor-NIH; Grant Number- R21DA022439 (operating)
Grant Sponsor- Research for a Healthier Tomorrow, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin (operating)
BRC: Grant Sponsor-CIHR and NSERC (operating); MSFHR (salary)
References
- Aberg ND, Johansson UE, Aberg MA, Hellstrom NA, Lind J, Bull C, Isgaard J, Anderson MF, Oscarsson J, Eriksson PS. IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–30. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, Marsicano G, Kokaia Z, Guzman M, Galve-Roperh I. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso E, Ozaita A, Valdizan EM, Ledent C, Pazos A, Maldonado R, Valverde O. BDNF impairment in the hippocampus is related to enhanced despair behavior in CB(1) knockout mice. J Neurochem. 2008;105:565–572. doi: 10.1111/j.1471-4159.2007.05149.x. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Ligresti A, Di Marzo V. The endocannabinoid signaling system: Biochemical aspects. Pharmacol Biochem Behav. 2005;81:224–238. doi: 10.1016/j.pbb.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. Int J Neuropsychopharmacol. 2005;8:357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Russo-Neustadt AA. Exercise activates the phosphatidylinositol 3-kinase pathway. Mol Brain Res. 2005;135:181–193. doi: 10.1016/j.molbrainres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: efficacy and dose response. Am J Prev Med. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486:39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- Ernst C, Olson AK, Pinel JP, Lam RW, Christie BR. Antidepressant effects of exercise: evidence for an adult-neurogenesis hypothesis? J Psychiatry Neurosci. 2006;31:84–92. [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Rueda D, Gomez del Pulgar T, Velasco G, Guzman M. Mechanism of extracellular signal-regulated kinase activation by the CB(1) cannabinoid receptor. Mol Pharmacol. 2002;62:1385–1392. doi: 10.1124/mol.62.6.1385. [DOI] [PubMed] [Google Scholar]
- Gobbo OL, O'Mara SM. Exercise, but not environmental enrichment, improves learning after kainic acid-induced hippocampal neurodegeneration in association with an increase in brain-derived neurotrophic factor. Behav Brain Res. 2005;159:21–26. doi: 10.1016/j.bbr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Gomez del Pulgar T, Velasco G, Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci Biobehav Rev. 2008;32:1152–1160. doi: 10.1016/j.neubiorev.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinella F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, Gorzalka BB. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology. 2005;30:508–515. doi: 10.1038/sj.npp.1300601. [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB. Involvement of the endocannabinoid system in the ability of long-term tricyclic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2006a;31:2591–2599. doi: 10.1038/sj.npp.1301092. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JS, Galea LA, Gorzalka BB. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006b;24:1845–1849. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000;61:3–18. doi: 10.1016/s0090-6980(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J Neurochem. 1995a;64:677–683. doi: 10.1046/j.1471-4159.1995.64020677.x. [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta. 1995b;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childer SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47 1:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji SP, Bai G, Zhang X. Cannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic-and antidepressant-like effects. J Clin Invest. 2005;115:3104–3116. doi: 10.1172/JCI25509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J, Greenberg DA. Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol. 2004;66:204–208. doi: 10.1124/mol.66.2.204. [DOI] [PubMed] [Google Scholar]
- Jin L, Hu X, Feng L. NT3 inhibits FGF2-induced neural progenitor cell proliferation via the PI3K/GSK3 pathway. J Neurochem. 2005;93:1251–1261. doi: 10.1111/j.1471-4159.2005.03118.x. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kearn CS, Greenberg MJ, DiCamelli R, Kurzawa K, Hillard CJ. Relationships between ligand affinities for the cerebellar cannabinoid receptor CB1 and the induction of GDP/GTP exchange. J Neurochem. 1999;72:2379–2387. doi: 10.1046/j.1471-4159.1999.0722379.x. [DOI] [PubMed] [Google Scholar]
- Keeney BK, Raichlen BA, Meek TH, Wijeratne AS, Middleton KM, Gerdeman GL, Garland T., Jr Differential response to a selective cannabinoid receptor antagonist (SR141716: rimonabant) in female mice from lines selectively bred for high voluntary wheel-running behaviour. Behav Pharmacol. 2008;19:812–820. doi: 10.1097/FBP.0b013e32831c3b6b. [DOI] [PubMed] [Google Scholar]
- Keleman E, Moron I, Fenton AA. Is the hippocampal theta rhythm related to cognition in a non-locomotor place recognition task? Hippocampus. 2005;15:472–479. doi: 10.1002/hipo.20071. [DOI] [PubMed] [Google Scholar]
- Khaspekov LG, Brenz Verca MS, Frumkina LE, Hermann H, Marsicano G, Lutz B. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur J Neurosci. 2004;19:1691–1698. doi: 10.1111/j.1460-9568.2004.03285.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Ledent C, Jin K, Greenberg DA. Role for neuronal nitric-oxide synthase in cannabinoid-induced neurogenesis. J Pharmacol Exp Ther. 2006;319:150–154. doi: 10.1124/jpet.106.107698. [DOI] [PubMed] [Google Scholar]
- Kola B, Hurbina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, Tasker JG. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Ziegelgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Morrish AC, Gorzalka BB. Local enhancement of cannabinoid CB1 receptor signalling in the dorsal hippocampus elicits an antidepressant-like effect. Behav Pharmacol. 2007;18:431–438. doi: 10.1097/FBP.0b013e3282ee7b44. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado E, Vela JM, Arevalo-Martin A, Almazan G, Molina-Holgado F, Guaza C. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Holgado F, Pinteaux E, Heenan L, Moore JD, Rothwell NJ, Gibson RM. Neuroprotective effects of the synthetic cannabinoid HU-210 in primary cortical neurons are mediated by phosphatidylinositol 3-kinase/AKT signaling. Mol Cell Neurosci. 2005;28:189–194. doi: 10.1016/j.mcn.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Rubio-Araiz A, Garcia-Ovejero D, Williams RJ, Moore JD, Arevalo-Martin A, Gomez-Torres O, Molina-Holgado E. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur J Neurosci. 2007;25:629–634. doi: 10.1111/j.1460-9568.2007.05322.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinella F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci. 2002;15:953–961. doi: 10.1046/j.1460-9568.2002.01929.x. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–260. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Omeir RL, Chin S, Hong Y, Ahern DG, Deutsch DG. Arachidonoyl ethanolamide-[1,2-14C] as a substrate for anandamide amidase. Life Sci. 1995;56:1999–2005. doi: 10.1016/0024-3205(95)00181-5. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Puighermanal E, Maldonado R. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. 2007;102:1105–1114. doi: 10.1111/j.1471-4159.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzman M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20:2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, Mechoulam R, Shohami E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–531. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Patel S, Rademacher DJ, Hillard CJ. Differential regulation of the endocannabinoids anandamide and 2-arachidonylglycerol within the limbic forebrain by dopamine receptor activity. J Pharmacol Exp Ther. 2003;360:880–888. doi: 10.1124/jpet.103.054270. [DOI] [PubMed] [Google Scholar]
- Patel S, Carrier EJ, Ho WS, Rademacher DJ, Cunningham S, Reddy DS, Falck JR, Cravatt BF, Hillard CJ. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46:342–349. doi: 10.1194/jlr.M400377-JLR200. [DOI] [PubMed] [Google Scholar]
- Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–1307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schobersberger W, Hobisch-Hagen P, Fries D, Wiedermann F, Rieder-Scharinger J, Villiger B, Frey W, Herold M, Fuchs D, Jelkmann W. Increase in immune activation, vascular endothelial growth factor and erythropoietin after an ultramarathon run at moderate altitude. Immunobiology. 2000;201:611–620. doi: 10.1016/S0171-2985(00)80078-9. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Brasel JA, Hintz RL, Mohan S, Cooper DM. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- Shen H, Tong L, Balazs R, Cotman CW. Physical activity elicits sustained activation of the cyclic AMP response element-binding protein and mitogen-activated protein kinase in the rat hippocampus. Neuroscience. 2001;107:219–229. doi: 10.1016/s0306-4522(01)00315-3. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endocannabinoid system. Neuroreport. 2003;14:2209–2211. doi: 10.1097/00001756-200312020-00015. [DOI] [PubMed] [Google Scholar]
- Stevens J, Killeen M. A randomised controlled trial testing the impact of exercise on cognitive symptoms and disability of residents with dementia. Contemp Nurse. 2006;21:32–40. doi: 10.5172/conu.2006.21.1.32. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto K, Tonai T, Murakami M, Kudo I, Ueda N. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–756. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinella F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinella F. The select action of hippocampal calcium calmodulin protein kinase II in mediating exercise-enhanced cognitive function. Neuroscience. 2007;144:825–833. doi: 10.1016/j.neuroscience.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellani V, Petrosino S, De Petrocellis L, Valenti M, Prandini M, Magherini PC, McNaughton PA, Di Marzo V. Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacology. 2008;55:1274–1279. doi: 10.1016/j.neuropharm.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Williams EJ, Walsh FS, Doherty P. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhu PJ. Endocannabinoid signaling and synaptic plasticity in the brain. Crit Rev Neurobiol. 2006;18:113–124. doi: 10.1615/critrevneurobiol.v18.i1-2.120. [DOI] [PubMed] [Google Scholar]


