Abstract
Background
Hepatitis C virus (HCV) infection may be associated with thrombocytopenia and increased iron stores in patients receiving medical care. We aimed to determine how often changes in hematologic, iron metabolic and inflammatory markers occur in individuals with undiagnosed HCV in the community.
Methods
Inner-city African Americans (n=143) were recruited from the community according to reported ingestion of alcohol. They were divided broadly into those who drank more or less than 56 g alcohol/day as assessed by dietary questionnaire. HCV serology was determined and laboratory values were compared according to HCV seropositivity in analyses that adjusted for alcohol consumption.
Results
The prevalence of HCV seropositivity was 23% among men and 29% among women. Levels of hepatocellular enzymes were higher with HCV seropositivity (P <0.0001) but hemoglobin concentrations, white blood cell and platelet counts and serum ferritin concentrations did not differ. The globulin fraction of the serum protein concentration (P=0.002) was increased with HCV seropositivity as expected with chronic inflammation. However, erythrocyte sedimentation rate and serum iron and haptoglobin levels did not differ significantly according to HCV status. Furthermore, multivariate analysis revealed that C-reactive protein was decreased and transferrin concentration was increased with both HCV and alcohol consumption (P<0.014).
Conclusions
Previously undiagnosed HCV seropositivity has little effect on the complete blood count and body iron stores but appears to perturb the response to an inflammatory stimulus, causing reduced rather than increased circulating CRP concentrations and increased rather than decreased transferrin concentrations.
Keywords: HCV infection, African American, CRP, Iron metabolism
Introduction
Hepatitis C virus (HCV) infection is a public health problem for persons of all races and it has become the most common cause of death associated with liver disease in the U.S. [1]. African-American patients have a lower rate of viral clearance and, consequently, a higher rate of chronic hepatitis C compared to whites [2,3]. Hepatitis C is more prevalent among African Americans than among persons of any other racial group in the United States [2]. Although African Americans represent only 12% of the US population, they represent approximately 22% of the estimated Americans with chronic HCV infection [4].
Hemolytic anemia is a rare complication of untreated HCV infection [5]. HCV has been shown to induce cytopenias especially thrombocytopenia, which is thought to be an indicator of advanced liver disease. The cytopenias may be due to virus-induced bone marrow suppression as well as portal hypertension, hypersplenism, and decreased thrombopoietin production [6,7]. Patients being followed for chronic HCV infection frequently have increased serum iron levels and hepatic iron stores, which are thought to correlate with reduced expression of hepcidin, a peptide produced in the liver [8].
HCV infection may cause irreversible liver fibrosis. Viral proteins seem to modulate apoptosis and steatosis, ultimately leading to hepatic stellate cell activation, fibrosis and increased risk for hepatocellular carcinoma [9]. The net liver damage from HCV infection depends on the balance between the host’s antiviral mechanisms and the ability of the virus to subvert them. Alcohol consumption has been proposed as a risk factor for the progression of liver disease in chronic HCV infection [10]. There is alteration of cytokine secretion in HCV infection, which is especially marked among alcoholics [11]. One of these cytokines is interleukin 6, which induces the expression of C-reactive protein [12]. Consumption of alcohol has been associated with increased iron stores as assessed by serum ferritin concentration in several population studies [13–15].
Materials and Methods
Participants
The Howard University IRB approved the research and all participants gave written informed consent. The design of the study and the participants have previously been described [16]. Briefly, the participants were self-described African-American males and females >18 y who were recruited as one of the following 2 groups: i) self-reported average alcohol consumption of <2 drinks/week (n = 72); ii) self-reported average alcohol consumption of ≥4 drinks/day (n = 71). The participants were in their usual state of health at the time of the study. They were not presenting for acute or chronic health care but rather they were recruited from the community.
To quantify dietary iron content and alcohol consumption, participants filled out the University of Hawaii Multi-Ethnic Dietary Questionnaire with the help of the study research nurses. The test-retest reliability of this questionnaire has been validated [17]. The questionnaire asks about average eating habits over the past year. The questionnaire was analyzed at the University of Hawaii. Estimates for average daily intake of kilocalories, alcohol and dietary iron were provided. Any person with known diagnosis of HCV infection or history of treatment for HCV infection was excluded from the study.
Laboratory tests
Peripheral blood was collected in the morning. EDTA-anticoagulated blood was used for performing complete blood count, reticulocyte count (Coulter® LH750, Beckman Coulter, Inc., Fullerton, CA) and erythrocyte sedimentation rate (ESR) (Westergren method). Serum was used to determine hepatitis B surface antigen (Diagnostic Products Corporation, Los Angeles, CA), antibody to hepatitis C (Ortho® HCV Ver 3.0 ELISA Test System, Ortho-Clinical Diagnostics, Inc., Raritan, NH), aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, total protein, albumin, iron, transferrin (Unicel® DxC 600 Synchron® Clinical System, Beckman Coulter) and ferritin (Access® 2 Beckman Coulter). These tests were performed in the clinical laboratory of Howard University Hospital. Concentrations of C-reactive protein (CRP) were determined from serum samples that had been stored at −80°C by enzyme-linked immunosorbant assay (ALPCO Diagnostics, Windham, NH, USA) (expected plasma range provided by the manufacturer of 0.068–8.2 mg/l). Transferrin saturation was calculated by dividing the serum iron in µg/dl by 1.27 X transferrin concentration in mg/l and multiplying by 100 [18]. The ferritin to AST ratio was calculated by dividing the serum ferritin concentration by the serum AST activity; this index has been shown to reflect hepatic iron stores in the setting of acute alcohol consumption, shortly after stopping consumption and after prolonged abstention from alcohol [19]. The normal range for the ferritin to AST ratio is approximately 1–10 µg/U for men and 1–7 µg/U for women. Liver biopsies were not performed as a part of this study.
Statistical analysis
Continuous variables were tested for normality and the best transformation was used if the variable had a non-normal distribution. In Table 1, ANOVA was used to compare variables according to HCV status with adjustment for sex, age, and total alcohol intake/kg per day. In Table 2 and Table 3, multivariate linear regression with backwards and forwards modeling was used to develop models of the effect of HCV seropositivity on CRP concentration and transferring concentration that only included covariates having a significance level of <0.05. Analysis was performed using STATA 10.0 (StataCorp, College Station, TX).
Table 1.
Clinical characteristics according to hepatitis C status. Results are mean values (95% confidence interval)* unless otherwise specified1.
| HCV− (n=98) | HCV+ (n=36) | P value | |
|---|---|---|---|
| Age (y) | 47 (46–49) | 52 (50–54) | 0.002 |
| Male sex** | 68 (69%) | 27 (75%) | NS |
| Body mass index (kg/m2) | 29.3 (27.7–30.9) | 28.2 (25.8–30.6) | NS |
| Alcohol intake (g per kg/day) | 0.5 (0.3–0.7) | 1.0 (0.5–1.4) | NS |
| Iron intake (g per kg/day) | 0.3 (0.3–0.4) | 0.3 (0.2–0.4) | NS |
| Hemoglobin (g/dl) | 14.1 (13.9–14.3) | 13.9 (13.5–14.3) | NS* |
| MCV (fl) | 89 (87–91) | 90 (89–92) | NS* |
| Lymphocyte count (1000/µl) | 1.9 (1.8–2.1) | 2.2 (2.0–2.5) | 0.051* |
| White blood cell (1000/µl)1 | 5.7 (5.2–5.9) | 5.7 (4.9–6.3) | NS* |
| Granulocytes (1000/µl)1 | 3.0 (2.7–3.3) | 3.0 (2.5–3.5) | NS* |
| Basophils (1000/µl) | 0.04 (0.01–0.07) | 0.02 (0–0.07) | NS* |
| Monocytes (1000/µl) | 0.44 (0.41–0.48) | 0.43 (0.38–0.49) | NS* |
| Eosinophils (1000/µl) | 0.12 (0.10–0.15) | 0.08 (0.04–0.12) | NS* |
| Platelets (1000/µl) | 257 (242–273) | 248 (224–275) | NS* |
| Haptoglobin (mg/dl) | 141 (127–156) | 139 (113–165) | NS* |
| AST (units/l) | 24 (23–26) | 38 (33–45) | <0.0001* |
| ALT (units/l) | 22 (20–24) | 38 (32–46) | <0.0001* |
| GGT (U/l) | 30 (26–34) | 58 (43–83) | <0.0001* |
| Bilirubin (mg/dl) | 0.7 (0.7–0.8) | 0.7 (0.6–0.8) | NS* |
| Alakiline Phosphatase (units/l) | 69 (66–76) | 83 (69–89) | NS* |
| Total protein (g/dl) | 7.2 (7.1–7.3) | 7.5 (7.2–7.6) | 0.030* |
| Albumin (g/dl) | 4.0 (3.9–4.1) | 3.8 (3.7–4.0) | 0.043* |
| Globulin (g/dl) | 3.1 (3.0–3.2) | 3.5 (3.3–3.7) | 0.003* |
| Ferritin (µg/l) | 89 (74–108) | 108 (77–150) | NS* |
| Ferritin/AST ratio | 3.4 (2.8–4.1) | 2.5 (1.8–3.4) | NS* |
| Serum iron (µg/dl) | 79 (72–86) | 89 (76–105) | NS* |
| Transferrin (mg/dl) | 247 (240–255) | 262 (250–276) | 0.028* |
| Transferrin Saturation | 25 (23–28) | 25 (22–30) | NS* |
| C-reactive protein (mg/l) | 2.4 (1.9–3.2) | 1.3 (0.9–2.1) | 0.026* |
| Erythrocyte sedimentation rate (mm/hr) |
17 (14–21) | 19 (13–26) | NS* |
| Hepatitis B surface antigen, no.(%) | 1 (1%) | 0 (0%) | NS |
Mean (95% CI of mean) and p value were adjusted for sex, age and alcohol intake per kg/day.
Number (%)
Six cases with HIV/AIDS, of which three were HCV positive, one case with prior HCV treatment and two cases with outlier calorie intake were excluded from analysis. After adjusting for all other variables, alcohol intake correlated negatively with C-reactive protein (beta=−0.21, p = 0.015) and positively with transferrin (beta= 0.02, p = 0.022) and serum iron (beta = 0.06, p = 0.041).
Table 2.
Multivariate linear regression model of serum C-reactive protein concentration (natural log), N = 113.
| Beta | 95% confidence interval |
P | |
|---|---|---|---|
| Sedimentation rate (`root) | 0.21 | (0.11 – 0.32 | <0.0001 |
| Alcohol intake per kg/day (natural Log) | −0.21 | (−0.38 – −0.05) | 0.012 |
| Hepatitis C seropositivity | −0.78 | (−1.29 – −0.27) | 0.003 |
| Constant | −0.24 | (−0.77 – 0.28) | NS |
R2 = 0.23. Variables entered into model were: age, sex, BMI, ESR, HCV, alcohol and iron intake, 6 cases with HIV/AIDS, 1 case with prior HCV treatment and 2 cases with outlier calorie intake were excluded from analysis.
Table 3.
Multivariate linear regression model of serum transferrin concentration (natural log), N = 131.
| Beta | 95% confidence interval |
P | |
|---|---|---|---|
| Serum ferritin (square root) | −0.06 | −0.09– −0.04 | <0.0001 |
| Alcohol intake per kg/day (natural Log) | 0.02 | 0.01–0.04 | 0.005 |
| Hepatitis C seropositivity | 0.07 | 0.02–0.12 | 0.013 |
| Constant | 5.82 | 5.70–5.94 | <0.0001 |
R2= 0.21. Variables entered into model were: age, sex, BMI, ESR, HCV, alcohol and iron intake, 6 cases with HIV/AIDS, 1 case with prior HCV treatment and 2 cases with outlier calorie intake were excluded from analysis.
Results
The median age was 48 y and 71% of the participants were males. The prevalence (95% confidence interval) of hepatitis C seropositivity was 27% (20%–36%). This prevalence was 23% and 29% in males and females, respectively. One participant was positive for hepatitis B surface antigen, and this person was HCV negative. Median daily estimated dietary iron was 20 mg. The median hemoglobin level was 14.1 g/dl and the median CRP concentration was 2.3 mg/l.
Comparison of HCV positive and HCV negative participants is presented in Table 1. HCV positive cases were significantly older than HCV negative study subjects. After adjusting for the effects of sex, age and alcohol intake, values for AST, ALT, GGT, total protein, globulin and transferrin were higher in HCV seropositive cases while albumin and CRP were lower. Hemoglobin concentration and other components of the complete blood count did not differ significantly according to HCV status or alcohol intake. After adjusting for the effects of age, sex and HCV infection, greater alcohol intake was associated with lower CRP concentration, higher serum iron concentration and higher transferrin (Table 1, see footnotes).
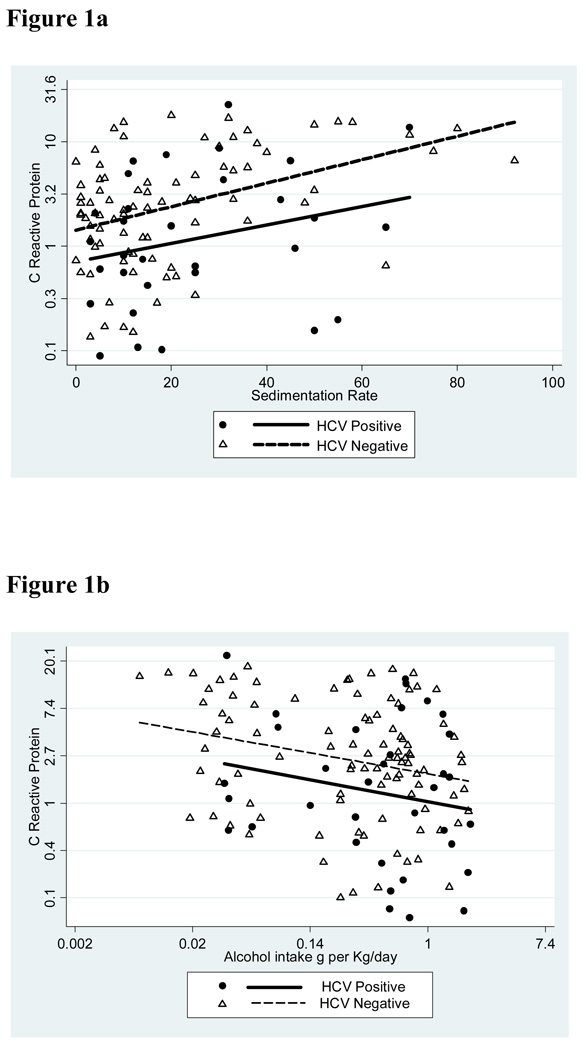
Further multivariate analysis revealed that serum CRP concentration increased significantly according to increasing erythrocyte sedimentation rate (P <0.0001) and decreased significantly according to increasing alcohol intake (P = 0.012) (Table 2 and Fig. 1). After adjustment for both of these factors, the trend to lower CRP concentration in HCV patients shown in Table 1 was strengthened. According to the analysis shown in Table 2 and with all other things being equal, an increased CRP of 10 mg/l in an HCV seronegative individual would translate to a normal CRP of 4.6 mg/l in an HCV seropositive individual (95% CI of 2.8–7.6) (P = 0.003).
Figure 1.
Relationships among C-reactive protein, HCV seropositivity, erythrocyte sedimentation rate and alcohol intake. a. Correlation between C-reactive protein concentration and erythrocyte sedimentation rate in HCV seronegative and seropositive cases. b. Correlation between C-reactive protein and alcohol intake in HCV seronegative and seropositive cases.
Multivariate analysis also revealed that the serum transferrin concentration decreased significantly according to increasing serum ferritin concentration (P <0.0001) and increased according to increasing alcohol intake (P = 0.003) (Table 3 and Figure 2). After adjustment for both of these factors, the trend to higher transferrin concentrations in HCV positive participants shown in Table 1 was strengthened. According to the analysis shown in Table 3 and with all other things being equal, a transferrin concentration of 247 mg/dl in an HCV negative individual would translate to a transferrin concentration of 262 mg/dl in an HCV seropositive individual (95% CI of 252–276) (P = 0.013).
Figure 2.
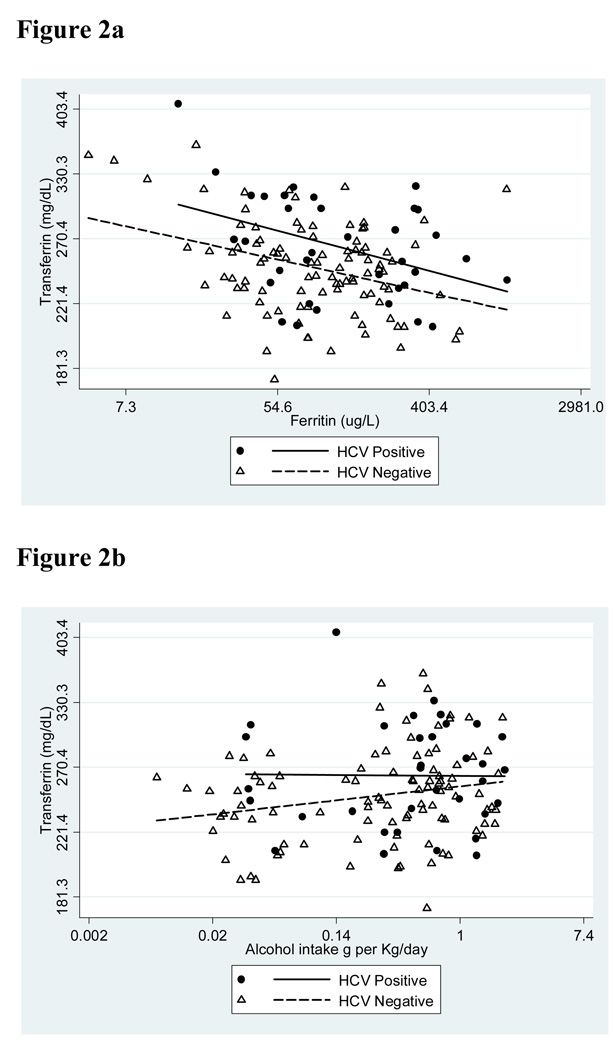
Relationships among serum transferrin concentration, HCV seropositivity, serum ferritin concentration and alcohol intake. a. Correlation between serum concentrations of transferrin and ferritin in HCV seronegative and seropositive cases. b. Correlation between serum transferrin concentration and estimated alcohol intake in HCV seronegative and seropositive cases.
Discussion
The prevalence of previously undiagnosed and untreated HCV infection in inner city African Americans, discovered through screening individuals from the community in their usual state of health, was 27%. This was a cross-sectional study of persons without a past history of HCV infection, and so we cannot comment on the chronicity of the infection.
HCV seropositivity in this study was not associated with anemia, thrombocytopenia or other changes in the complete blood count except for a trend to higher lymphocyte counts. Nor were the serum ferritin concentration or transferrin saturation influenced by HCV seropositivity. These observations are in contrast to reports of hemolytic anemia, thrombocytopenia and other cytopenias, and increased iron stores in association with untreated HCV infection [5–8], and they indicate that the hematologic manifestations of HCV infection in previously undiagnosed individuals from the community may differ from those of symptomatic individuals presenting for medical care. Alcohol consumption estimated by dietary questionnaire also did not have a discernable effect on the complete blood count.
HCV seropositivity was associated with significant increases in AST, ALT and GGT, and with changes in serum proteins consistent with a chronic inflammatory process, i.e., increase in the globulin fraction and decrease in albumin concentration. Other aspects of the inflammatory response were lacking. In particular, the erythrocyte sedimentation was not increased, the serum iron was not decreased, the transferrin concentration was increased rather than decreased, and the CRP concentration was decreased rather than increased.
The anemia of chronic inflammation [20] was noticeably absent in the patients with untreated HCV in this study, and this observation along with a lack of inflammatory changes in erythrocyte sedimentation rate, iron, transferrin and CRP raises the possibility that HCV may have certain properties in suppressing certain aspects of the systemic inflammatory response. Our finding of reduced rather than increased CRP concentrations in the present cohort parallels recent reports that co-infection with HCV is associated with reduced CRP concentrations in HIV infected patients [21,22].
C-reactive protein is an acute phase plasma protein produced by hepatocytes under the transcriptional control of the pro-inflammatory cytokine, interleukin 6 [12]. Some investigators have reported that HCV infection is associated with increased expression of interleukin-6 [23–26], while others have not observed increased expression [27,28]. One study indicated that the interleukin-6 response to stimulation of toll like receptors 3 and 4 is compromised in HCV infection [29]. Lack of increased CRP expression in association with HCV seropositivity is not likely to be due simply to poor functioning of hepatocytes, for there was increased expression of transferrin. Transferrin is ordinarily a negative acute phase reactant, but in the present study serum levels were increased in association with HCV in tandem with decreased serum CRP concentration.
Interleukin 6 not only promotes the transcription of CRP by hepatocytes but also that of of hepcidin [30]. Hepcidin is a peptide hormone that regulates iron absorption by enterocytes and iron release by macrophages; decreased hepcidin levels lead to increased iron absorption [31]. Ferroportin is an iron exporter present on the surface of absorptive enterocytes, macrophages, hepatocytes and placental cells that also serves as a receptor for hepcidin. The binding of hepcidin leads to internalization and degradation of ferroportin, thereby decreasing export of cellular iron [31]. Relatively low hepcidin expression has been reported in HCV infection [8], and this might help to explain a failure to observe hypoferremia in association with HCV infection in the present study.
Acknowledgment
This work was supported in part by grant nos. P20 AA014643-03 and P50 AA11199 from NIAAA, grant no. 2 R25 HL003679-08 from NHLBI, and Howard University GCRC grant no 2MOI RR10284-10 from NCRR, NIH, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30–S34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 2.Pearlman BL. Hepatitis C virus infection in African Americans. Clin Infect Dis. 2006;42:82–91. doi: 10.1086/498512. [DOI] [PubMed] [Google Scholar]
- 3.Pyrsopoulos N, Jeffers L. Hepatitis C in African Americans. J Clin Gastroenterol. 2007;41:185–193. doi: 10.1097/01.mcg.0000225689.60335.44. [DOI] [PubMed] [Google Scholar]
- 4.Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31 Suppl 1:88–91. doi: 10.1016/s0168-8278(99)80381-x. [DOI] [PubMed] [Google Scholar]
- 5.Etienne A, Gayet S, Vidal F, et al. Severe hemolytic anemia due to cold agglutinin complicating untreated chronic hepatitis C: efficacy and safety of anti-CD20 (rituximab) treatment. Am J Hematol. 2004;75:243–245. doi: 10.1002/ajh.20004. [DOI] [PubMed] [Google Scholar]
- 6.McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227–2236. doi: 10.1056/NEJMoa073255. [DOI] [PubMed] [Google Scholar]
- 7.Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol. 2000;95:2936–2939. doi: 10.1111/j.1572-0241.2000.02325.x. [DOI] [PubMed] [Google Scholar]
- 8.Fujita N, Sugimoto R, Takeo M, et al. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97–104. doi: 10.2119/2006-00057.Fujita. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mengshol JA, Golden-Mason L, Rosen HR. Mechanisms of Disease: HCV-induced liver injury. Nat Clin Pract Gastroenterol Hepatol. 2007;4:622–634. doi: 10.1038/ncpgasthep0961. [DOI] [PubMed] [Google Scholar]
- 10.Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27:1730–1735. doi: 10.1002/hep.510270637. [DOI] [PubMed] [Google Scholar]
- 11.Castellano-Higuera A, Gonzalez-Reimers E, Aleman-Valls MR, et al. Cytokines and lipid peroxidation in alcoholics with chronic hepatitis C virus infection. Alcohol Alcohol. 2008;43:137–142. doi: 10.1093/alcalc/agm171. [DOI] [PubMed] [Google Scholar]
- 12.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming DJ, Jacques PF, Dallal GE, Tucker KL, Wilson PW, Wood RJ. Dietary determinants of iron stores in a free-living elderly population: The Framingham Heart Study. Am J Clin Nutr. 1998;67:722–733. doi: 10.1093/ajcn/67.4.722. [DOI] [PubMed] [Google Scholar]
- 14.Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160–1167. doi: 10.1093/ajcn/78.6.1160. [DOI] [PubMed] [Google Scholar]
- 15.Milman N, Kirchhoff M. Relationship between serum ferritin, alcohol intake, and social status in 2235 Danish men and women. Ann Hematol. 1996;72:145–151. doi: 10.1007/s002770050153. [DOI] [PubMed] [Google Scholar]
- 16.Gordeuk VR, Diaz SF, Onojobi GO, et al. Ferroportin Q248h, dietary iron, and serum ferritin in community African-Americans with low to high alcohol consumption. Alcohol Clin Exp Res. 2008;32:1947–1953. doi: 10.1111/j.1530-0277.2008.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stram DO, Hankin JH, Wilkens LR, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol. 2000;151:358–370. doi: 10.1093/oxfordjournals.aje.a010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk R, Wigand R, Dietrich CF, et al. Total iron-binding capacity and serum transferrin determination under the influence of several clinical conditions. Clin Chim Acta. 2000;293:127–138. doi: 10.1016/s0009-8981(99)00242-9. [DOI] [PubMed] [Google Scholar]
- 19.Ford C, Wells FE, Rogers JN. Assessment of iron status in association with excess alcohol consumption. Ann Clin Biochem. 1995;32(Pt 6):527–531. doi: 10.1177/000456329503200602. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 21.Floris-Moore M, Howard AA, Lo Y, Schoenbaum EE, Arnsten JH, Klein RS. Hepatitis C infection is associated with lower lipids and high-sensitivity C-reactive protein in HIV-infected men. AIDS Patient Care STDS. 2007;21:479–491. doi: 10.1089/apc.2006.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reingold J, Wanke C, Kotler D, et al. Association of HIV infection and HIV/HCV coinfection with C-reactive protein levels: the fat redistribution and metabolic change in HIV infection (FRAM) study. J Acquir Immune Defic Syndr. 2008;48:142–148. doi: 10.1097/QAI.0b013e3181685727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malaguarnera M, Di Fazio I, Laurino A, Ferlito L, Romano M, Trovato BA. Serum interleukin 6 concentrations in chronic hepatitis C patients before and after interferon-alpha treatment. Int J Clin Pharmacol Ther. 1997;35:385–388. [PubMed] [Google Scholar]
- 24.Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211–215. doi: 10.1007/BF02936370. [DOI] [PubMed] [Google Scholar]
- 25.Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–874. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migita K, Abiru S, Maeda Y, et al. Serum levels of interleukin-6 and its soluble receptors in patients with hepatitis C virus infection. Hum Immunol. 2006;67:27–32. doi: 10.1016/j.humimm.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 27.Gelderblom HC, Nijhuis LE, de Jong EC, et al. Monocyte-derived dendritic cells from chronic HCV patients are not infected but show an immature phenotype and aberrant cytokine profile. Liver Int. 2007;27:944–953. doi: 10.1111/j.1478-3231.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 28.Zumrutdal A, Ozer B, Singan M, et al. Effect of anti-HCV positivity on markers of malnutrition and inflammation in hemodialysis patients. Ren Fail. 2007;29:85–90. doi: 10.1080/08860220601039098. [DOI] [PubMed] [Google Scholar]
- 29.Villacres MC, Literat O, DeGiacomo M, Du W, Frederick T, Kovacs A. Defective response to Toll-like receptor 3 and 4 ligands by activated monocytes in chronic hepatitis C virus infection. J Viral Hepat. 2008;15:137–144. doi: 10.1111/j.1365-2893.2007.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews NC. Anemia of inflammation: the cytokine-hepcidin link. J Clin Invest. 2004;113:1251–1253. doi: 10.1172/JCI21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]