Abstract
LRRK2 mutations are recognized as the most frequent genetic cause of both familial and sporadic parkinsonism identified to date. A remarkable feature of this form of parkinsonism is the variable penetrance of symptom manifestation resulting in a wide range of age-at-onset in patients. Herein we use a functional approach to identify the Lrrk1 protein as a potential disease modifier demonstrating an interaction and heterodimer formation with Lrrk2. In addition, evaluation of LRRK1 variants in our large Lrrk2 p.G2019S-parkinsonism series from a Tunisian (n=145) identified a missense mutation (p.L416M) resulting in an average 6.2 years younger age at disease onset. In conclusion we show for the first time that the interaction of Lrrk1-Lrrk2 can form protein dimers and this interaction may influence the age of symptomatic manifestation in Lrrk2-parkinsonism patients.
Keywords: LRRK2, PD, Dimerization, Genetics, p.G2019S
Introduction
The discovery of mutations in the leucine-rich repeat kinase 2 gene (LRRK2), now the most frequent genetic cause of Parkinson disease (PD), has lead to the search for disease modifiers that explain the variable penetrance observed in mutation carriers1, 2. One approach to identify disease modifiers is via studies of protein interactors, as recently demonstrated for mutant huntingtin3. Although various methods including yeast two-hybrid screening, and immunoprecipitation followed by mass spectrometry analysis have been used to identify interactors of Lrrk24–9, only HSP90 and Lrrk2 dimerization have been confirmed5, 10–12. While the interaction with HSP90 could be related to the (over)-expression of a large (286KDa) and complex protein, dimerization is a recognized mechanism to regulate the activity of specific protein kinases and GTPases. Recent attempts to map the protein regions responsible for the Lrrk2/Lrrk2 interaction showed that several domains (leucine-rich repeat (LRR), Ras of complex (Roc), C-terminus of ROC (COR), and WD40) strengthen dimer formation11. The contribution of multiple domains (isolated or as part of full length Lrrk2 protein) support the hypothesis that other proteins possessing a similar structure could potentially interact with Lrrk2.
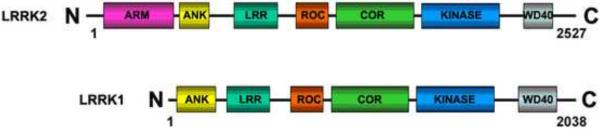
Lrrk2 has previously been classified as a member of the ROCO protein superfamily13, 14, and these proteins have the characteristic feature of a Ras-related GTPase or Roc domain followed by a COR motif, accompanied by a kinase domain. Three other proteins belonging to the ROCO family have been identified in humans; leucine-rich repeat kinase 1 (Lrrk1), death-associated protein kinase (Dapk1) and malignant fibrous histiocytoma-amplified sequence with leucine-rich tandem repeats-1 (Masl1). Lrrk1 and Lrrk2 share the highest degree of homology with both proteins possessing an almost identical domain structure (Figure 1)15, 16. Therefore, in the present study we examined the possible protein interaction between Lrrk1 and Lrrk2, and assessed the potential role of LRRK1 genetic variants acting as disease modifiers in PD patients carrying the pathogenic Lrrk2 p.G2019S substitution.
Figure 1.
Schematic of Lrrk1 and Lrrk2 domain structures: ARM= armadillo, ANK= ankyrin, LRR= leucine-rich, ROC= Ras of complex, COR= C-terminus of ROC.
Material and methods
Functional Analysis
Real time quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
For the absolute quantification of LRRK1 and LRRK2 mRNA expression, total RNA was isolated from human brain tissue using PureLink RNA mini kit (Invitrogen) according to the manufacturer's instructions. LRRK1/LRRK2 expression pattern across seven different brain regions was assessed in one male control (AOD 66 years), in addition expression in the substantia nigra was measured in a further four male controls (AOD 65–82 years). To ensure good quality template, the RNA integrity number (Agilent 2100, Agilent Technologies) was determined prior to further analysis. After cDNA was synthesized using High Capacity cDNA Reverse transcription Kit (Applied Biosystems), real time PCR assays were performed in triplicate on a 384 well plate using an ABI 7900 detection system to assess the level of LRRK2 (TaqMan® probe Hs00417273_mI) and LRRK1 (Hs00226465_mI) mRNA. A standard curve allowing for absolute quantification of the respective mRNA levels was generated using LRRK1 or LRRK2 expression constructs previously described.
Immunoprecipitation studies
Full-length LRRK1 was reverse transcribed from human brain mRNA and Topo-cloned into the pcDNA3.1/V5-His TOPO TA® expression vector (Invitrogen). Positive clones were selected and sequence of the full-length LRRK1 insert was verified and compared with the Entrez database (mRNA; NM_024652) and SwissProt (ID# Q38SD2) entries for human LRRK1. Expression constructs coding for LRRK2 with a C-terminal V5 tag have been described previously. For the generation of FLAG-tagged LRRK1 and LRRK2 constructs stop codons preventing translation of the C-terminal V5/His tags were introduced by mutagenesis (Stratagene). The sequence encoding the triple FLAG-tag was amplified from the p3xFLAG-CMV-7.1® vector (SIGMA-Aldrich) and subcloned into the respective modified LRRK1/LRRK2 pcDNA3.1 vectors after the introduction of a unique restriction site immediately upstream of the ATG start codon. A matched pcDNA3.1/V5-His expression construct encoding LacZ-V5 was included as a negative control (Invitrogen).
HEK293T cells, maintained in Opti-mem 1+GlutaMAX1 with 10% of fetal calf serum and penicillin/streptomycin (all Gibco) at 37°C and 5% CO2, were transfected using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). Cells were harvested 48hrs post transfection and lysed in 50mM Tris/HCL, 150mM NaCl and 0.1% Triton-X-100. To avoid unspecific binding during the actual immunoprecipitation step, the crude lysates were precleared by rotating at 4°C for 1hr with Protein A/G ultralink resin (Thermo Scientific) followed by centrifugation at 13,000 x g for 10min. After performing a BCA Protein assay (Pierce) equal amounts of supernatant were combined with EZview Red ANTI-FLAG M2 Affinity Gel® (SIGMA-Aldrich) and rotated on a spinning wheel for 4hrs at 4°C. The resulting immunocomplex was stringently washed with IP buffer/PBS and eluted in SDS-Sample buffer (Invitrogen). Immunoprecipitated and co-precipitated proteins were analyzed with SDS-PAGE/Western Blot technologies. For protein detection monoclonal anti-V5 (Invitrogen) and both mono- as well as polyclonal anti-FLAG antibodies (SIGMA-Aldrich) were applied. For Lrrk1, in addition to the respective tagged antibodies, the identity of the over-expressed protein was confirmed with specific Lrrk1 antibodies (SIGMA-Aldrich) (supplementary data).
Genetic Analysis
Subjects
A total of 89 families, 231 sporadic PD patients from the Institut National de Neurologie, Tunis, and 352 control participants from the same geographic region were included in the study. The Institut National de Neurologie, Tunis provides a specialized neurological service to the entire country of Tunisia17. The site obtained local ethics committee approval before beginning recruitment and studies have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Subjects were informed of the study and gave either written or proxy consent. Individuals were diagnosed as “affected” if they satisfied the United Kingdom PD Society Brain Bank (UKPDS) criteria18 or “controls” if all signs of parkinsonism were absent and there was no family history of parkinsonism. Analysis of Lrrk2 p.G2019S PD patients (n=145; age, 69.1 ± 12.1 years; 73 male, 45%) from either the familial series (n=74) or patient-control series (n=71) was performed to assess the modifying effect of LRRK1 variants on age at onset (AAO; 58.3 ± 11.9 years). In addition the patient-control series of 512 Tunisian p.G2019S negative subjects (160 cases; age, 64.9 ± 12.0 years and 352 controls; age, 57.5 ± 11.0 years) was used to assess the association of LRRK1 variants on disease risk (Supplemental Table 1).
DNA sequencing and genotyping of LRRK1 Gene
Genomic DNA was extracted from peripheral blood lymphocytes using standard protocols. Primer pairs for LRRK1 were used to sequence all 34 exons by polymerase chain reaction (PCR) in all familial probands (n=89) and sequence analysis was performed as previously described16,19. All LRRK1 exonic variants detected through sequencing or those previously reported were screened through the Tunisian pedigrees (affected and unaffected members) and patient-control series employing the Sequenom MassArray iPLEX platform (San Diego, CA). Lrrk2 p.G2019S screening was previously performed and reported2. All primer sequences are available on request.
Statistical Analysis
Numerical variables were summarized with the sample mean, standard deviation (SD), and range. For p.G2019S positive PD cases from either the case/control series or the familial series (N=145), associations between AAO and SNPs with a minor allele frequency >2% were evaluated using linear mixed effects regression models. Models were adjusted for gender, and a random effect was included for each family. Regression coefficients and 95% CIs were estimated, and dominant models were considered in all linear regression analysis. For each family of statistical tests, a Bonferroni adjustment for multiple testing was used to control the family-wise error rate at 5%. Statistical analyses were performed using SPLUS (version 8.0.1; Insightful Corporation, Seattle, Washington).
Results
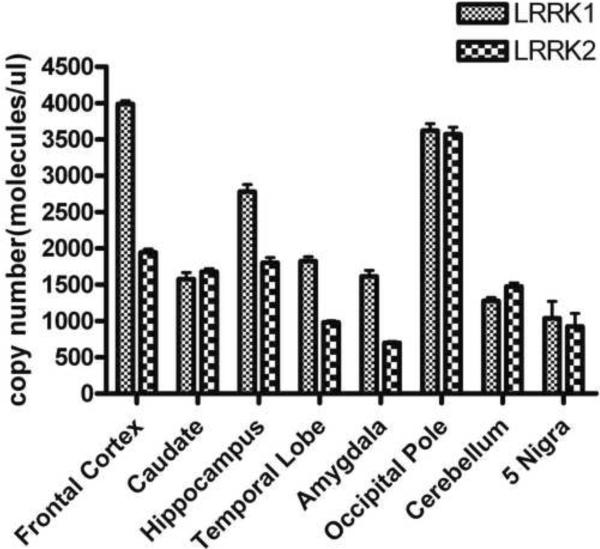
To evaluate our hypothesis of Lrrk1 as a potential Lrrk2 interacting protein, we first examined LRRK1 and LRRK2 mRNA expression in human control brain as an indirect way to assess the possibility of an interaction between the two proteins in vivo. The respective LRRK1 and LRRK2 mRNA copy numbers were determined by real-time PCR and subsequent absolute quantification. Both LRRK1 and LRRK2 are widely expressed throughout the brain (figure 2). Our results confirm elevated LRRK1 expression in cortical regions and the hippocampus as previously reported by Westerlund and colleagues20. In contrast to these authors who claim that there is very low or no LRRK1 gene transcription in the striatum, we could also detect significant levels of LRRK1 mRNA in the caudate nucleus. To assess the respective mRNA expression in the substantia nigra as the region predominantly affected in PD, we performed real-time PCR on five healthy controls. Absolute quantification revealed that LRRK1 and LRRK2 expression in this brain region are comparable to each other but generally low.
Figure 2.
Absolute LRRK1 and LRRK2 mRNA expression levels in human control brain. Copy numbers for LRRK1 and LRRK2 were calculated by comparison to a standard curve of co-amplified plasmids encoding full length Lrrk1 or Lrrk2 proteins.
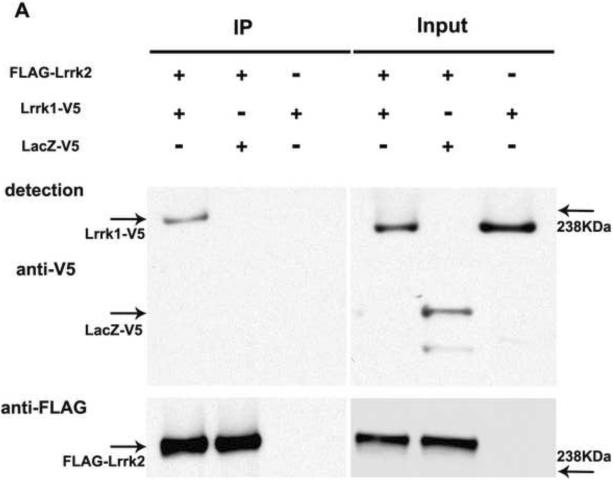
We performed co-immunoprecipitation assays with tagged full-length Lrrk1 and Lrrk2 proteins. When FLAG-Lrrk2 protein was immunoprecipitated using FLAG antibodies, V5-tagged Lrrk1 but not LacZ-V5 could be detected in the precipitate (Figure 3A). A specific interaction between Lrrk1 and Lrrk2 could also be confirmed in the opposite direction (Figure 3B). Interestingly, Lrrk2-V5 was consistently more difficult to detect in the FLAG-Lrrk1 immunoprecipitate than Lrrk1-V5 in the opposite experiment.
Figure 3.
Lrrk1 and Lrrk2 proteins interact in vitro: A. Co-immunoprecipitation of full length Lrrk1-V5 with FLAG-Lrrk2 in HEK293T cells. The left panel shows the presence of Lrrk1-V5 but not LacZ-V5 in the FLAG-Lrrk2 immunoprecipitate (IP). The inputs on the right panel represent the amounts of transfected proteins introduced in the immunoprecipitation assay.B. Co-precipitation of Lrrk2-V5 with FLAG-Lrrk1. Successful co-pull-down of Lrrk2-V5 occurs only in the presence of FLAG-Lrrk1 (IP left; Inputs right). A stronger detection signal was observed when Lrrk2 was used to pull-down Lrrk1 than vice versa, which may indicate a higher affinity of Lrrk2 for Lrrk1, whereas Lrrk1 might preferentially engage in interactions with other proteins. However as co-immunoprecipitation assays do not provide a reliable quantitative assessment of binding affinities this warrants further investigation.
All results presented so far were obtained with lysates from HEK293T cells transfected with LRRK1, LRRK2, LacZ or a combination. To confirm our results in an environment that would be more apt to mimic in vivo stoichiometry, we expanded our studies to the endogenous proteins in human cell lines or brain. Although we could clearly detect over-expressed Lrrk1 protein with the human Lrrk1 specific antibody developed by Atlas Antibodies (commercially available through SIGMA-Aldrich, supplementary figure 1) a specific band of endogenous Lrrk1 could not be detected in HEK293T cells, human lymphoblastoid cell lines (known for their high Lrrk2 content) nor human brain (data not shown). Using over-expressed Lrrk1 protein as a positive control, we have also screened several other commercially available Lrrk1 antibodies. However, no antibody proved sensitive or specific enough to be applied in co-precipitation studies with the endogenous proteins.
LRRK1: a genetic modifier of LRRK2-parkinsonism?
To assess the potential influence of LRRK1 variation on LRRK2-parkinsonism we employed our familial and sporadic patient-control PD series from Tunisia. Given the high frequency of the pathogenic substitution Lrrk2 p.G2019S in this population, our series provides a unique opportunity to assess the role of genetic modifiers on one genetically-defined form of PD in a single population from an isolated geographic location. To determine the variation in LRRK1 in the Tunisian PD population we sequenced all coding exons and exon-intron boundaries in 89 familial PD probands. Through this sequencing study we identified ten novel exonic LRRK1 variants including three non-synonymous (p.A1350T, p.G1702S and p.R1726W). Segregation analysis of these novel variants within the families harboring them was equivocal. To further assess pathogenicity of LRRK1 genetic variation all reported exonic variants and the ten novel identified in this study were assessed for disease association in our Tunisian PD patient-control series (Supplemental Tables 1–3). Prior to correction, evidence of a possible association with protection from PD was observed for the minor alleles of rs4965778 p.T1149T (OR: 0.68, 95% CI: 0.46 – 1.02, P=0.061) and rs55739947 p.L416M (OR: 0.21, 95% CI: 0.05 – 0.94, P=0.04).
Interestingly, when we examined the LRRK1 variants as age-at-onset modifiers of LRRK2-parkinsonism SNP rs55739947 (p.L416M) appeared to increase the disease penetrance (Table 1). Age-at-onset was a mean of 6.2 years younger (95% CI: −14.4 to 2.0) in the nine patients with a copy of the “A” allele for rs55739947 (p.L416M) compared to patients with no copies of the “A” allele (P=0.13).
Table 1.
Single SNP associations with AAO for SNPs with a minor allele frequency >1% in p.G2019S PD cases
| SNP rs# | Minor allele | Protein | Minor allele frequency (Fraction, %) | Estimated regression coefficient (95% CI) | P-value |
|---|---|---|---|---|---|
| rs1163091 | C | R26R | 109/288 (38%) | 1.74 (−2.30, 5.79) | 0.39 |
| rs2412000 | A | P251P | 44/290 (15%) | −1.65 (−6.23, 2.94) | 0.47 |
| rs55739947 | A | L416M | 9/290 (3%) | −6.21 (−14.44, 2.02) | 0.13 |
| rs56215707 | T | N556N | 20/288 (7%) | 1.07 (−4.89, 7.02) | 0.72 |
| rs41381646 | G | K632K | 35/290 (12%) | 0.67 (−4.14, 5.48) | 0.78 |
| rsll247253 | G | S686S | 71/290 (25%) | −0.25 (−4.30, 3.80) | 0.90 |
| rs41319544 | C | V829V | 42/290 (15%) | −1.01 (−5.63, 3.60) | 0.66 |
| rs41339845 | A | I1141I | 39/290 (13%) | −0.16 (−4.80, 4.49) | 0.95 |
| rs4965778 | G | T1149T | 104/290 (36%) | 0.91 (−3.12, 4.95) | 0.65 |
| rs41372244 | A | L1387L | 20/290 (7%) | 2.69 (−3.37, 8.74) | 0.37 |
| rsll857803 | A | P1511P | 126/290 (43%) | 0.46 (−3.84, 4.76) | 0.83 |
| rsll853661 | T | T1549T | 116/290 (40%) | 1.05 (−3.14, 5.24) | 0.61 |
| rs3764739 | T | T1873T | 19/286 (7%) | 1.19 (−5.19, 7.57) | 0.71 |
| rs17161161 | T | P1884P | 11/288 (4%) | 2.16 (−5.78, 10.10) | 0.58 |
| rs2924835 | A | G1938D | 80/290 (28%) | −0.69 (−4.70, 3.32) | 0.73 |
- Estimated regression coefficients and p-values result from linear mixed effects regression models adjusted for gender and including a random effect for family. Estimated regression coefficients are interpreted as the increase in the mean AAO corresponding to the presence of the minor allele. Highlighted in bold rs55739947 (pL416M) showed a suggestive decrease in PD age-at-onset (Suppl. Table 3). P-values ≤ 0.0033 are considered statistically significant after a Bonferroni adjustment for multiple testing.
Discussion
Even for the so-called monogenic forms of PD age remains the strongest risk factor for development of clinical symptoms. This is highlighted in the most common genetic form, LRRK2-associated PD, with the identification of numerous sporadic patients1, 2, 21. These observations demonstrate the importance of disease modifiers, which may provide novel therapeutic targets by simply delaying symptomatic onset. LRRK2 encodes a multifunctional protein with a reported toxic gain of kinase activity, therefore attempts have been made to identify both Lrrk2 protein substrates and interactors.
Lrrk2 dimerization has been shown to modulate the kinase activity and therefore we hypothesized that given the high degree of homology and domain structure of Lrrk1 (Lrrk2 paralog) it may act in a similar mechanism to Lrrk2 homo-dimerization. To address the question of whether the interaction could occur in vivo we investigated LRRK1 and LRRK2 mRNA expression profiles in human brain. While previous results by Westerlund et al. indicate that LRRK2 mRNA but little or no LRRK1 mRNA is expressed in human striatum20, our results reveal similar copy numbers for LRRK1 and LRRK2 in the caudate. Based on our expression patterns of LRRK1 and LRRK2 mRNA, for the brain regions assayed including striatum and substantia nigra, a potential protein interaction in vivo is supported.
Through co-immunoprecipitation studies in cells over-expressing both proteins we observed a heterodimeric interaction between Lrrk1 and Lrrk2. In the absence of purified, full length recombinant Lrrk1 and Lrrk2 proteins it remains difficult however to predict whether this interaction is direct or mediated by other binding partners. Sen and colleagues22 recently reported the presence of oligomeric Lrrk2 species in high-molecular weight complexes in transfected HEK293FT and human lymphoblast cell lines. Several proteins belonging to the heat shock family and the cellular trafficking machinery were associated with the Lrrk2 protein. As a similar scenario is also likely for Lrrk1 therefore it can not be excluded that our observed interaction is mediated by chaperones. While Lrrk2 kinase activity is directly linked to its presence as a dimer11, oligomeric Lrrk2 species are thought to be enzymatically inactive. It is now important to determine whether the presence of Lrrk1 in high molecular Lrrk2 complexes influences the equilibrium between dimeric and oligomeric Lrrk2 species and secondarily affects kinase activity. In the case of a Lrrk1/Lrrk2 heterodimer (direct interaction), a scenario is conceivable where association of Lrrk1 and Lrrk1 could alter phosphorylation of substrates (potentially Lrrk2 itself23) or render binding of a yet to be identified Lrrk2 regulator impossible. Unfortunately, efforts to extend our studies to the endogenous proteins and in vivo environment were compromised by poor antibody specificity and/or sensitivity. However, even at low levels of the Lrrk1/Lrrk2 dimer under normal physiological conditions, Lrrk1 expression might still present an important therapeutic target in the future. Toxic effects resulting from increased kinase activity of mutant Lrrk2 could potentially be inhibited by the over-expression of Lrrk1.
Having demonstrated an interaction between Lrrk1-Lrrk2 in vitro we examined the possible influence genetic variants in LRRK1 may have on the clinical presentation of PD and Lrrk2 p.G2019S-parkinsonism. Our comprehensive sequencing of the LRRK1 gene in 89 familial PD probands from Tunisia identified three novel non-synonymous changes. Our association study examining both novel and all known exonic variants in LRRK1 showed limited of association with susceptibility to PD (see supplemental Table 3 and 4)16, 24. Of note, it has also been reported that artificial LRRK1 variants corresponding to known pathogenic LRRK2 mutations appear to be less toxic25.
Our series of Lrrk2 p.G2019S carriers (n=145) PD patients give us a unique opportunity to examine the effect of LRRK1 variants on the age at onset in Lrrk2 p.G2019S carriers. We observed the effect of the LRRK1 SNP rs55739947 (p.L416M) lowering the age-at-onset within our Lrrk2 p.G2019S patients; the same allele however appeared protective in non- Lrrk2 p.G2019S carriers suggesting different factors may modulate disease susceptibility or penetrance. These findings show LRRK1 genetic variation may have a limited role in LRRK2-parkinsonism susceptibility and warrants further studies in larger series of LRRK2 carriers.
In conclusion, we demonstrate that the Lrrk1 and Lrrk2 proteins can form heterodimers in vitro. The role of genetic variants in LRRK1, such as rs55739947 (p.L416M), in PD risk or an earlier age at onset in Lrrk2 p.G2019S carriers remains to be clearly resolved; however given the overlapping mRNA expression patterns and the protein interaction further functional studies evaluating the properties of the Lrrk1/Lrrk2 heterodimer are warranted.
Supplementary Material
In the case/control series considering only p.G2019S negative patients, associations between PD and SNPs with a minor allele frequency > 1% were measured using odds ratios (ORs) and 95% confidence intervals (CIs) obtained from logistic regression models adjusted for age and gender. Dominant models were considered in all logistic regression analysis due to the low minor allele frequencies observed for many of the SNPs. Fisher's exact test was used to evaluate associations with PD for SNPs with a minor allele frequency ≤ 1% in an exploratory analysis. We also collapsed information across SNPs with minor allele frequencies ≤ 1% and ≤ 2% to create binary categorical variables that represent presence of the minor allele for any of these SNPs 26; associations between PD and these binary categorical variables were assessed using logistic regression models adjusted for age and gender. For SNPs with a minor allele frequency >1%, haplotype analysis was performed using SPLUS score tests for association 27, with adjustments made for age and gender; p-values were obtained from the asymptotic distribution of the score statistic and haplotypes of less than 1% were not considered. Linkage disequilibrium between markers in study controls was measured by pair-wise r2 values.
Supplementary Figure 1: FLAG-Lrrk1 transiently transfected into HEK293T cells can be detected by SDSPAGE/Western blot and subsequent detection with the Lrrk1 specific antibody from SIGMA-Aldrich. High specificity but limited sensitivity is indicated by the absence of the respective bands for over-expressed Lrrk2 or endogenous Lrrk1.
Acknowledgements
The authors wish to thank the patients and families who participated in the study. Special thanks to Drs Jina Swartz, Ray Watts, and David Burns for the neurological expertise provided during study design and for their clinical input. We are indebted to the contributions of Lefkos T. Middleton, MD, Mounir Kefi, MD, Lianna Ishihara-Paul, PhD, Rim Amouri, PhD, Samia Ben Yahmed, MD, Samia Ben Sassi, MD, Mourad Zouari, MD, Ghada El Euch, MD. GlaxoSmithKline financially supported the patient recruitment and clinical data collection. Statistical analysis was supported by the Neurogenetic Core of a Morris K. Udall Center, National Institute of Neurological Disorders and Stroke P50 NS40256. MJF and FH were supported in part by NIH grant R21 NS64885. KN was supported by an Eli-Lilly scholarship and Herb Geist gift for Lewy body research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haugarvoll K, Rademakers R, Kachergus JM, Nuytemans K, Ross OA, Gibson JM, Tan EK, Gaig C, Tolosa E, Goldwurm S, Guidi M, Riboldazzi G, Brown L, Walter U, Benecke R, Berg D, Gasser T, Theuns J, Pals P, Cras P, De Deyn PP, Engelborghs S, Pickut B, Uitti RJ, Foroud T, Nichols WC, Hagenah J, Klein C, Samii A, Zabetian CP, Bonifati V, Van Broeckhoven C, Farrer MJ, Wszolek ZK. Lrrk2 R1441C parkinsonism is clinically similar to sporadic Parkinson disease. Neurology. 2008;70:1456–1460. doi: 10.1212/01.wnl.0000304044.22253.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hulihan MM, Ishihara-Paul L, Kachergus J, Warren L, Amouri R, Elango R, Prinjha RK, Upmanyu R, Kefi M, Zouari M, Sassi SB, Yahmed SB, El Euch-Fayeche G, Matthews PM, Middleton LT, Gibson RA, Hentati F, Farrer MJ. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a casecontrol genetic study. Lancet Neurol. 2008;7:591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- 3.Kaltenbach LS, Romero E, Becklin RR, Chettier R, Bell R, Phansalkar A, Strand A, Torcassi C, Savage J, Hurlburt A, Cha GH, Ukani L, Chepanoske CL, Zhen Y, Sahasrabudhe S, Olson J, Kurschner C, Ellerby LM, Peltier JM, Botas J, Hughes RE. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genet. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dachsel JC, Taylor JP, Mok SS, Ross OA, Hinkle KM, Bailey RM, Hines JH, Szutu J, Madden B, Petrucelli L, Farrer MJ. Identification of potential protein interactors of Lrrk2. Parkinsonism Relat Disord. 2007;13:382–385. doi: 10.1016/j.parkreldis.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gloeckner CJ, Kinkl N, Schumacher A, Braun RJ, O'Neill E, Meitinger T, Kolch W, Prokisch H, Ueffing M. The Parkinson disease causing LRRK2 mutation I2020T is associated with increased kinase activity. Hum Mol Genet. 2006;15:223–232. doi: 10.1093/hmg/ddi439. [DOI] [PubMed] [Google Scholar]
- 6.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, Kim CH, Han BS, Tong Y, Shen J, Hatano T, Hattori N, Kim KS, Chang S, Seol W. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Zheng XY, Yang M, Tan JQ, Pan Q, Long ZG, Dai HP, Xia K, Xia JH, Zhang ZH. Screening of LRRK2 interactants by yeast 2-hybrid analysis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:883–891. [PubMed] [Google Scholar]
- 8.Gillardon F. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability - a point of convergence in Parkinsonian neurodegeneration? J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06235.x. [DOI] [PubMed] [Google Scholar]
- 9.Gillardon F. Interaction of elongation factor 1-alpha with leucine-rich repeat kinase 2 impairs kinase activity and microtubule bundling in vitro. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 10.Dachsel JC, Mata IF, Ross OA, Taylor JP, Lincoln SJ, Hinkle KM, Huerta C, Ribacoba R, Blazquez M, Alvarez V, Farrer MJ. Digenic parkinsonism: investigation of the synergistic effects of PRKN and LRRK2. Neurosci Lett. 2006;410:80–84. doi: 10.1016/j.neulet.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Greggio E, Zambrano I, Kaganovich A, Beilina A, Taymans JM, Daniels V, Lewis P, Jain S, Ding J, Syed A, Thomas KJ, Baekelandt V, Cookson MR. The Parkinson disease-associated leucine-rich repeat kinase 2 (LRRK2) is a dimer that undergoes intramolecular autophosphorylation. J Biol Chem. 2008;283:16906–16914. doi: 10.1074/jbc.M708718200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Xie C, Greggio E, Parisiadou L, Shim H, Sun L, Chandran J, Lin X, Lai C, Yang WJ, Moore DJ, Dawson TM, Dawson VL, Chiosis G, Cookson MR, Cai H. The chaperone activity of heat shock protein 90 is critical for maintaining the stability of leucine-rich repeat kinase 2. J Neurosci. 2008;28:3384–3391. doi: 10.1523/JNEUROSCI.0185-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosgraaf L, Van Haastert PJ. Roc, a Ras/GTPase domain in complex proteins. Biochim Biophys Acta. 2003;1643:5–10. doi: 10.1016/j.bbamcr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Marin I, van Egmond WN, van Haastert PJ. The Roco protein family: a functional perspective. Faseb J. 2008;22:3103–3110. doi: 10.1096/fj.08-111310. [DOI] [PubMed] [Google Scholar]
- 15.Korr D, Toschi L, Donner P, Pohlenz HD, Kreft B, Weiss B. LRRK1 protein kinase activity is stimulated upon binding of GTP to its Roc domain. Cell Signal. 2006;18:910–920. doi: 10.1016/j.cellsig.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Taylor JP, Hulihan MM, Kachergus JM, Melrose HL, Lincoln SJ, Hinkle KM, Stone JT, Ross OA, Hauser R, Aasly J, Gasser T, Payami H, Wszolek ZK, Farrer MJ. Leucine-rich repeat kinase 1: a paralog of LRRK2 and a candidate gene for Parkinson's disease. Neurogenetics. 2007;8:95–102. doi: 10.1007/s10048-006-0075-8. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara L, Gibson RA, Warren L, Amouri R, Lyons K, Wielinski C, Hunter C, Swartz JE, Elango R, Akkari PA, Leppert D, Surh L, Reeves KH, Thomas S, Ragone L, Hattori N, Pahwa R, Jankovic J, Nance M, Freeman A, Gouider-Khouja N, Kefi M, Zouari M, Ben Sassi S, Ben Yahmed S, El Euch-Fayeche G, Middleton L, Burn DJ, Watts RL, Hentati F. Screening for Lrrk2 G2019S and clinical comparison of Tunisian and North American Caucasian Parkinson's disease families. Mov Disord. 2007;22:55–61. doi: 10.1002/mds.21180. [DOI] [PubMed] [Google Scholar]
- 18.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 19.Mata IF, Kachergus JM, Taylor JP, Lincoln S, Aasly J, Lynch T, Hulihan MM, Cobb SA, Wu RM, Lu CS, Lahoz C, Wszolek ZK, Farrer MJ. Lrrk2 pathogenic substitutions in Parkinson's disease. Neurogenetics. 2005;6:171–177. doi: 10.1007/s10048-005-0005-1. [DOI] [PubMed] [Google Scholar]
- 20.Westerlund M, Belin AC, Anvret A, Bickford P, Olson L, Galter D. Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: Implications for Parkinson's disease. Neuroscience. 2008;152:429–436. doi: 10.1016/j.neuroscience.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Healy DG, Falchi M, O'Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a casecontrol study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen S, Webber PJ, West AB. Leucine-Rich Repeat Kinase 2 (LRRK2) Kinase Activity: Dependence on Dimerization. J Biol Chem. 2009 doi: 10.1074/jbc.M109.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greggio E, Taymans JM, Zhen EY, Ryder J, Vancraenenbroeck R, Beilina A, Sun P, Deng J, Jaffe H, Baekelandt V, Merchant K, Cookson MR. The Parkinson's disease kinase LRRK2 autophosphorylates its GTPase domain at multiple sites. Biochem Biophys Res Commun. 2009;389:449–454. doi: 10.1016/j.bbrc.2009.08.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haugarvoll K, Toft M, Ross OA, White LR, Aasly JO, Farrer MJ. Variants in the LRRK1 gene and susceptibility to Parkinson's disease in Norway. Neurosci Lett. 2007;416:299–301. doi: 10.1016/j.neulet.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 25.Greggio E, Lewis PA, van der Brug MP, Ahmad R, Kaganovich A, Ding J, Beilina A, Baker AK, Cookson MR. Mutations in LRRK2/dardarin associated with Parkinson disease are more toxic than equivalent mutations in the homologous kinase LRRK1. J Neurochem. 2007;102:93–102. doi: 10.1111/j.1471-4159.2007.04523.x. [DOI] [PubMed] [Google Scholar]
- 26.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002;70:425–434. doi: 10.1086/338688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In the case/control series considering only p.G2019S negative patients, associations between PD and SNPs with a minor allele frequency > 1% were measured using odds ratios (ORs) and 95% confidence intervals (CIs) obtained from logistic regression models adjusted for age and gender. Dominant models were considered in all logistic regression analysis due to the low minor allele frequencies observed for many of the SNPs. Fisher's exact test was used to evaluate associations with PD for SNPs with a minor allele frequency ≤ 1% in an exploratory analysis. We also collapsed information across SNPs with minor allele frequencies ≤ 1% and ≤ 2% to create binary categorical variables that represent presence of the minor allele for any of these SNPs 26; associations between PD and these binary categorical variables were assessed using logistic regression models adjusted for age and gender. For SNPs with a minor allele frequency >1%, haplotype analysis was performed using SPLUS score tests for association 27, with adjustments made for age and gender; p-values were obtained from the asymptotic distribution of the score statistic and haplotypes of less than 1% were not considered. Linkage disequilibrium between markers in study controls was measured by pair-wise r2 values.
Supplementary Figure 1: FLAG-Lrrk1 transiently transfected into HEK293T cells can be detected by SDSPAGE/Western blot and subsequent detection with the Lrrk1 specific antibody from SIGMA-Aldrich. High specificity but limited sensitivity is indicated by the absence of the respective bands for over-expressed Lrrk2 or endogenous Lrrk1.