Abstract
Introduction:
We tested the hypothesis that REM sleep contributes to core features of cognitive dysfunction of anxious depression including negative self-appraisals, biased memory processing and unpleasant dream content.
Methods:
After a habituation night in a sleep lab, a convenience sample of 35 healthy college students and 20 depressed/anxious students were awakened 10 minutes into a REM sleep episode and then 10 minutes into a NREM sleep episode. Awakenings were counterbalanced to control circadian effects. After each awakening participants reported a dream and then completed memory recall, mood and self-appraisal tasks.
Results:
Self-appraisals of depressed/anxious participants were significantly less positive and significantly more negative after awakenings from REM sleep vs NREM sleep. Appraisal of the REM sleep dream self was negative for depressed/anxious subjects only. Recall of negative memories was significantly more frequent after REM vs NREM sleep awakenings for both depress/anxious and healthy participants. REM sleep dreams were associated with greater frequencies of negative emotion, greater aggression and victimization rates than dreams in NREM sleep for depressed/anxious participants.
Limitations:
Depressed/anxious participants were classified as such on the basis of mood scales rather than clinical interview. All participants were drawn from a volunteer college student population and thus our results may not be applicable to some elderly clinical populations.
Conclusions:
REM appears to facilitate cognitive distortions of anxious depression.
Keywords: REM sleep, NREM sleep, depression, anxiety, dreams, autobiographical memory, mood
1. Introduction
Although REM sleep has long been known to be altered in depression, including anxious depression, its role in production of the symptomology of mood disorder has never been adequately clarified (for reviews see Armitage, 2007; Nutt et al., 2008; Tsuno et al., 2005). The replicated finding that both selective REM sleep and total sleep deprivation provide dramatic and immediate (though temporary) relief for some people with mood disorder (Giedke and Schwarzler, 2002; Vogel, 1975; Wu et al., 2008) supports the claim that REM does indeed play some role in production of at least some clinical symptoms in some people with mood disorder.
In this project, we explored the possibility that REM sleep physiology differentially impacts the neurocognitive symptoms of anxious depression including executive cognitive dysfunction, distorted evaluative appraisals of self, unpleasant dream content and biased emotional memory processing.
Most forms of depression, including anxious depression, are associated with a range of symptom clusters including the hallmark emotional problems of persistent sadness, anxiety or anxious feelings (American Psychiatric Association, 2000). Cognitive distortions of anxious depression feature a barrage of negative self-appraisals that lead to feelings of hopelessness and/or pessimism, guilt, worthlessness and/or thoughts of suicide, or even suicide attempts in major depressive disorder. There is often a variety of other cognitive distortions besides the major distortion regarding self-worth. These other cognitive distortions involve difficulty with concentration, planning or making decisions (executive dysfunction). Memory too is affected with negative and painful memories favored in recall and in acquisition and consolidation processes. Dreams become intensely unpleasant experiences with elevated levels of what used to be called ‘masochistic content’ or scenes of aggression by unknown strangers against the dreamer/self (McNamara, 2008). Nightmares become more frequent occurrences in the dream life of the anxious depressed individual and predict suicidal ideation (Agargun et al., 2007). Our hypothesis is that REM sleep physiology significantly contributes to the production of these cognitive distortions.
REM sleep mechanisms are prime candidate sources of neurocognitive dysfunction of anxiety and depression because they reproduce the known pathophysiology of anxious depressive disorder. Both lesion (Koenigs et al., 2008) and neuroimaging studies (e.g., Davidson, 2002; Drevets, 2007; Mayberg et al., 1999; Nofzinger, 2008) suggest that the pathogenesis of mood disorder involves abnormally high levels of activity in paralimbic structures and ventromedial prefrontal cortex (vmPFC), and abnormally low levels of activity in dorsolateral prefrontal cortex (dPFC). The person with mood disorder therefore cannot effectively recruit dPFC to regulate paralimbic and vmPFC-related negative emotional activity via reappraisal/suppression strategies (e.g., Ochsner et al., 2004). Attention in the field has therefore turned to the question of why paralimbic/vmPFC structures are chronically overactivated and dPFC structures underactivated in major mood disorders.
While a host of genetic, neurochemical, and psychological factors have been implicated in production of anxiety and depressive symptomology, none of these factors link up directly with the known pathophysiology of depression or anxiety. We suggest that one proximate mechanism that does directly yield the pathophysiologic pattern of hyperactive paralimbic/vmPFC systems and hypoactive dPFC systems in mood disorders is REM hyperactivation or dis-inhibition. Paralimbic/vmPFC hyperactivation and dPFC hypoactivation exactly characterizes ‘normal’ REM-related brain activation/deactivation patterns throughout the sleep cycle (e.g., Maquet et al., 1996, 1999; Nofzinger, 2005; and see review of REM neurophysiology below). Several times each night REM selectively and intensively activates key structures in paralimbic/vmPFC systems (e.g., amygdala, vmPFC itself etc) and down regulates dPFC systems (Muzur et al., 2002; Pace-Schott, 2007). To the best of our knowledge, this pattern of vmPFC overactivation and dPFC hypoactivation occurs naturally only in REM sleep. REM sleep, furthermore, is also associated with production of negative affect and selective consolidation of negative emotional memories (Hu et al., 2006; Rauchs et al, 2004; Walker and Stickgold, 2006). REM-related indices, such as REM density, are strongly correlated with neurocognitive distortions in depression such as self-attack, suicidal ideation, rumination and concentration difficulties (Agargun and Cartwright, 2003; Ellman et al., 1991; Giles et al., 1986). REM sleep deprivation in depressed patients is known to (temporarily) alleviate depression in some patients (Ellman et al., 1991; Giedke and Schwarzler, 2002; Vogel, 1975; Wu et al., 2008).
Despite these indirect but converging lines of evidence for a role for REM sleep in production of neurocognitive dysfunction in mood disorders, no direct test of the links between REM sleep and cognitive distortions of anxiety and depression has yet been conducted. We therefore constructed a set of experimental protocols to do so in the following set of pilot studies. Results supported the hypothesis that REM sleep differentially contributes to cognitive distortions in anxious depression.
2. Methods
2.1 Participants
Sixty participants (30 males, 30 females; mean age = 21.20 years, SD=2.72) between the ages of 18 and 50 years were recruited from the Boston area and local universities. Of this sample, 38 were undergraduates, 5 were graduate or medical students, and 17 were not attending school. All were informed of the nature of the study and then completed a demographics questionnaire which recorded their age at the time of the study, gender, years of education, college major (if applicable), socio-economic factors (including self-rated social class and annual household income), handedness, ethnicity, and religious affiliation. Participants were required to not have a prior history of any neurological, psychiatric, sleep (particularly sleep apnea), drug or alcohol problems, or head injury. In addition, they were asked to refrain from using alcohol throughout the study period. Informed consent was obtained from all participants prior to entering the sleep lab or completing any measures. Two participants were excluded from the study for severe sleep apnea, as determined by Dr. Auerbach. Apneas were defined as cessation of airflow for 10 seconds or more and hypopneas were defined as a reduction in respiratory effort accompanied by a 4% drop in oxyhemoglobin saturation. The Apnea- Hypopnea index (AHI: number of apneas plus hypopneas per hour of total sleep time) was used in conjunction with clinical judgment to identify sleep apnea. Another participant was excluded from the study for abnormal sleep architecture; the participant never entered into REM sleep. A fourth was excluded because an error occurred in the procedure that made it unclear if the tasks were completed after REM or NREM awakenings, and a fifth withdrew from the study in the middle of the night. A total of 55 participants were included in analyses (26 males, 29 females; mean age = 21.16 years (SD=2.63), mean education = 14.55 years (SD=1.87); see Table 1).
Table 1.
Demographics and baseline mood measure for sleep study participants in each group
| Healthy (n=35) | Depressed/Anxious (n=20) | t-value | p-value | |
|---|---|---|---|---|
| Age (in years) | 20.83 (2.19) | 21.75 (3.24) | −1.26 | n.s. |
| Sex | 17 Male, 18 Female | 9 Male, 11 Female | n.s. | |
| Education (in years) | 14.40 (1.68) | 14.80 (2.17) | −0.76 | n.s. |
| WTAR (scaled score) | 121.89 (7.32) | 116.65 (8.25) | 2.44 | 0.018* |
| DASS Total | 6.23 (3.40) | 15.45 (4.93) | −8.24 | 0.000*** |
| DASS Stress | 3.54 (2.29) | 6.65 (2.74) | −4.50 | 0.000*** |
| DASS Anxiety | 1.17 (1.38) | 3.30 (2.54) | −4.04 | 0.000*** |
| DASS Depression | 1.46 (1.24) | 5.50 (3.10) | −6.84 | 0.000*** |
| POMS Total | 47.74 (14.04) | 76.10 (24.53) | −5.47 | 0.000*** |
| POMS Tension | 13.63 (3.80) | 18.95 (5.98) | −4.04 | 0.000*** |
| POMS Depression | 17.06 (2.25) | 25.85 (8.14) | −6.04 | 0.000*** |
| POMS Anger | 13.83 (2.54) | 17.15 (6.70) | −2.64 | 0.011* |
| POMS Vigor | 21.83 (6.03) | 17.35 (4.49) | 2.89 | 0.006** |
| POMS Fatigue | 13.29 (4.64) | 16.45 (5.69) | −2.24 | 0.029* |
| POMS Confusion | 11.77 (3.14) | 15.05 (4.41) | −3.21 | 0.002** |
Note: n.s. = not significnant
p<0.05
p<0.01
p<0.001
2.2. Materials
2.2.1. Wechsler Test of Adult Reading (WTAR)
This test yields a reliable measure of verbal IQ (Wechsler, 2001). The WTAR consists of a list of 50 words that are not easy to pronounce. Participants are instructed to pronounce each word aloud even if they are unsure of how to pronounce the word. A point is issued for each word correctly pronounced. The WTAR is scaled to age and is conormed with the WAIS-III and the WMS-III. Norms are available for persons aged 16-89. The minimum scaled score is 50 and the maximum scaled score is 134. Our participants achieved a mean WTAR score of 119.9 (SD=8.9) which is within the high normal range. This high verbal IQ was likely due to the large proportion of college students in the sample.
2.2.2. Profile of Mood States (POMS)
The Profile of Mood States (POMS) is a 65-item adjective rating scale designed to measure multiple dimensions of affect both economically and rapidly (McNair et al., 1971). Participants read these adjectives and rate how much that adjective applies to them at that time on a scale of 1 (not at all) to 5 (extremely). The minimum score is 10 and the maximum score is 242 with lower scores indicating people with more stable moods and higher scores indicating more mood disturbance. The POMS also consists of six subscales: Tension, Depression, Anger, Vigour, Fatigue, and Confusion. Our healthy participants scored within the normal range on all subscales for the POMS, which were significantly different from our depressed/anxious participants (see Table 1).
2.2.3. Depression Anxiety Stress Scale (DASS)
The Depression Anxiety Stress Scale (DASS) was developed by Lovibond and Lovibond (1995). The DASS-21 is a shortened version of the DASS questionnaire that contains 21-items that assesses depression, anxiety, and stress. There are seven questions within each subscale. Participants were asked to indicate on a scale of 0 (did not apply to me at all) to 3 (applied most of the time) how much each question applied to them over the past week. Crawford and Henry (2003) and Antony et al. (1998) have reported excellent reliability, validity, and other psychometric properties for the three subscales of the DASS. The DASS Manual (Lovibond and Lovibond, 1995) reports the following cutoff scores for depression (normal = 0-9, mild = 10-13, moderate = 14-20, severe = 21-27, and extremely severe = 28+); anxiety (normal = 0-7, mild = 8-9, moderate = 10-14, severe = 15-19, and extremely severe = 20+), and stress (normal = 0-14, mild = 15-18, moderate = 19-25, severe = 26-33, and extremely severe = 34+). The DASS manual indicates that the scores of the DASS-21 are comparable to the scores of the full length DASS, but the scores need to be doubled before compared to the cutoff scores as specified in the DASS Manual. In addition, the DASS-21 has independently been found to be reliable, valid, and accurately reflect the scores of the full length DASS (Henry and Crawford, 2005).
The score on the DASS-21was used to divide the participants into a healthy (n=35) and depressed/anxious (n=20) groups. Depressed/anxious was defined as having mild depression or higher as indicated by the DASS-21 (a depression subscale score of 5 or above), mild anxiety or higher (an anxiety sub-scale score above 8), or high stress (determined by a stress sub-scale score above 9). Our healthy participants scored within the normal range on all 3 subscales for the DASS and our depressed/anxious group scored at least mild or higher on one or more of the subscales (see Table 1).
2.2.4. Pittsburgh Sleep Quality Index (PSQI)
Participants answered questions based on their sleep behaviors for the past month such as normal bedtime, average number of hours of sleep, and night time disturbances. A Global Sleep Quality Index score was derived based on seven components of sleep behavior: Subjective Sleep Quality, Sleep Latency, Sleep Duration, Habitual Sleep Efficiency, Sleep Disturbances, Use of Sleep Medication, and Daytime Dysfunction (Buysse et al., 1989). Higher scores indicate greater sleep disturbance and lower sleep quality and generally a cut-off score of 5 is used to separate good sleepers from poor sleepers in the general population. Buysse et al. (1989) found the PSQI to have acceptable test-retest reliability, validity, and internal homogeneity. The PSQI was found to have a high internal consistency (Cronbach α = 0.83), high sensitivity (89.6%), and high specificity (86.5%; kappa = 0.75, p<0.001) in distinguishing good and poor sleepers (Buysse et al., 1989). The PSQI correlates better with measures of mood than with measures of sleep function (Grandner et al., 2006) so it is a measure of choice when investigating potential relationships between mood and sleep functions. In the adult population a score above 5 is considered poor sleep (Buysse et al., 1989). However, Pilcher (1998) found a mean of 5.27 (SD=1.84) when examining college students and Cacioppo et al. (2002) found an average of 5.2 (SD=0.4) for a non-lonely group of undergraduates. The means in our sample were 4.17 (SD=1.69) for the healthy group and 6.35 (SD=2.08) for the depressed/anxious group (p<0.05) (see Table 3). These data indicate that the PSQI is accurately distinguishing our depressed/anxious participants from healthy participants. Consistent with Buysse et al's findings, our healthy participants fall below the 5.0 cut-off criterion for poor adult sleep.
Table 3.
Average sleep characteristics from sleep diary and sleep questionnaire
| Healthy (n=35) | Depressed/Anxious (n=20) | p-value | |
|---|---|---|---|
| Pittsburg Sleep Quality Index | 4.17 (1.69) | 6.35 (2.08) | 0.000*** |
| Average sleep onset latency | 15.58 (11.52) | 20.69 (15.62) | 0.539 |
| Average number of awakenings per night |
1.19 (0.71) | 1.74 (0.67) | 0.106 |
| Average hours of sleep per night |
7.04 (0.79) | 7.34 (0.65) | 0.231 |
*Note: Sleep onset and hours slept per night are given for four days before entering the sleep lab, the habituation night, and the night of the study. Awakenings per night does not include the night of the study
p<0.001
2.2.5. Overnight polysomnography (PSG)
The polysomnographic recording included EEG activity recorded from the C3 and C4 electrodes (referenced to an average of A1 and A2), EOG activity, submental EMG activity, airflow, respiratory effort, EKG activity, oximetry and video monitoring. All methods and sleep scoring procedures followed the guidelines of the American Academy of Sleep Medicine (Iber et al., 2007). Sleep architecture measures included: total sleep time (TST; minutes), number of awakenings, wake time after sleep onset (WASO; minutes), sleep latency (minutes), sleep efficiency (%), number of minutes and percentage of total sleep time spent in Wakefulness, REM, Stage 1, Stage 2, and Stage 3, arousals and arousal index (see Table 2). Respiration was evaluated with EKG leads, airflow monitors, effort monitors and oximeters. Measures included: apnea (cessation of airflow for 10 s or more) and hypopnea (reduction in respiratory effort accompanied by a 4% drop in oxyhemoglobin saturation). Apnea- hypopnea index (AHI) was defined as the number of apneas plus hypopneas per hour of total sleep time.
Table 2.
Sleep Architecture Characteristics for Entire Night
| Healthy (n=35) | Depressed/Anxious (n=20) | p-value | |
|---|---|---|---|
| Mean total sleep time (in min) | 346.74 (34.49) | 333.58 (40.12) | 0.205 |
| Mean sleep onset latency (in min) | 19.94 (22.91) | 25.83 (22.40) | 0.360 |
| Sleep efficiency (%) | 0.82 (0.07) | 0.78 (0.08) | 0.052† |
| Number of arousals | 47.34 (15.46) | 45.26 (19.20) | 0.667 |
| Arousal Index | 8.14 (2.94) | 8.05 (3.22) | 0.923 |
| Stage 1 (in min) | 9.46 (3.78) | 11.62 (4.72) | 0.069† |
| Stage 2 (in min) | 178.38 (39.80) | 181.90 (30.37) | 0.478 |
| Stage 3 (in min) | 74.60 (23.49) | 69.69 (26.83) | 0.482 |
| Total NREM (in min) | 262.44 (47.59) | 263.20 (26.73) | 0.313 |
| Total REM (in min) | 76.14 (24.17) | 70.39 (26.40) | 0.901 |
| Average Latency to REM (in min) | 89.57 (31.23) | 86.98 (27.07) | 0.757 |
Note:
Trend towards significance
2.3 Procedures
2.3.1 Prior to entering sleep lab
Each participant completed a battery of tests measuring personality, mood, sleep, and behavior before coming into the sleep lab. Four days before coming into the lab, participants started to complete a sleep diary and activity log, which they continued until they completed the study on the morning after the awakenings night (see Table 3). We used the sleep diary to identify persons with aberrant sleep cycles or habits. None of the participants evidenced abnormal sleep cycles during the 4 days before entering the study.
2.3.2 Habituation night in sleep lab
The first night in the sleep lab was the habituation night. The participant entered the lab at 9pm and was attached to the polysomnograph to simulate the experience for the study night and to allow them to adjust to the sleep lab. They were allowed to sleep through the night. They were woken up at 6:30 in the morning, given breakfast then allowed to go home.
2.3.3. Awakenings night in sleep lab
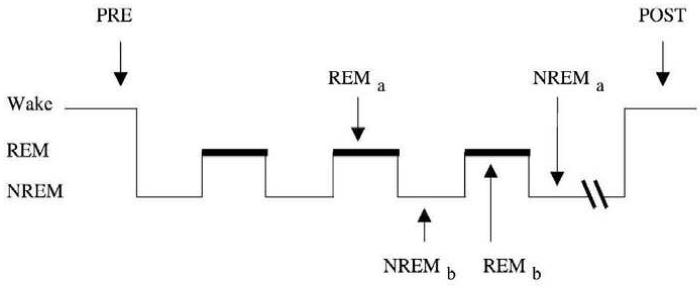
We used an ‘awakenings’ paradigm adapted from Walker et al. (2002). There is evidence that when a person is awakened from a particular sleep state, brain state activation patterns associated with that state persist for several minutes and influence cognitive performance and perhaps mood (Bertini and Violani, 1992; Reinsel and Antrobus, 1992; Balkin et al., 1999; Walker et al, 2002). Thus, we can to some extent probe the neurocognitive properties of that state via the awakenings procedure. Participants returned at 9:00pm on the second night and were attached to the polysomnogram again, but this time the recording was used. In addition, participants completed a task packet four different times during their second visit to the sleep lab: right before going to bed (PRE), when awoken from REM sleep, when awoken from Stage II NREM sleep, and in the morning before they left the sleep lab (POST). We used the PRE and POST test sessions as checks on participant's ability to perform the tests in question. The REM and NREM test sessions were the analytic focus of this project. When participants were woken up after entering a REM or NREM episode, they were asked to describe any dreams they were having right before waking up and then to complete the task packet. The task packet consisted of written and spoken tasks and took about 15 minutes to complete. The order of awakenings from REM and NREM sleep was counterbalanced as described by Walker et al. (2002) (see Figure 1). All but three participants returned to the sleep lab for the second night after completing the habituation night.
Figure 1.
Awakening protocol: all subjects were tested before lights out (PRE), REM and NREM awakening tests were performed across the night accompanied by a memory and word-stem task. Half of the subjects receive the tests 10 minutes into the second REM period (REMa) and forth NREM period (NREMa); half received the tests 10 minutes into the third NREM period (NREMb) and the third REM period (REMb). After awaking in the morning, all subjects were given the tasks again (POST) ((Reprinted from Cogn. Brain Res., 14, Walker, Liston, Hobson, and Stickgold, Cognitive flexibility across the sleep- wake cycle: REM-sleep enhancement of anagram problem solving, 317-324, Copyright (2002), with permission from Elsevier).
2.5. Awakenings tasks
Participants were given a task packet to be completed after an awakening. Each section of the packet contained the same tasks, but in randomized order.
2.5.1 Positive and Negative Affect Scale (PANAS)
The PANAS (Watson et al., 1988) was administered as the first measure in all 4 conditions: before bedtime, after REM and NREM awakenings, and in the morning. Participants were instructed to ‘indicate to what extent you feel this way right now’ in response to ten positive and ten negative affective words. Each word was evaluated on a five point scale, with 1 being ‘very slightly’ and 5 being ‘extremely’. The score is divided into positive affect (PA) and negative affect (NA). Scores for both PA and NA scores range from 10 – 50, with higher scores indicating more positive or negative affect being experienced. Using the PANAS to determine mood in the moment has been found to have an average PA score of 29.7 (SD=7.9) and an average NA score of 14.8 (SD=5.4) for a college student sample (Watson et al., 1988). Our participants indicated an average PA score of 22.77 (SD=7.29) in the PRE condition and 23.30 (SD=8.31) in the POST condition and an average NA score of 13.77 (SD=4.70) in the PRE and 12.63 (SD=3.47) in the POST condition indicating that our awakening procedures had no significant impact on morning mood. No participant left the sleep lab with significantly higher negative mood ratings than they had when they entered the lab. The PANAS has been found to have high internal consistency, high reliability coefficients (PA scale = 0.89, NA scale = 0.85), and has been found to be a valid measure of mood that is sensitive to mood fluctuation when the participant is instructed to rate their mood at the moment (Watson et al., 1988).
2.5.2. Autobiographical Memory Recall
For each of the 4 conditions, participants were asked to read a word and then recall a personal memory. The directions specified that the participant recall a time in which they felt a particular way and describe that memory into a tape recorder. In each condition a positive, negative, and neutral cue word were give (i.e. happy, lonely, and apathetic) and varied in the order they were presented. Latency to retrieval for each type of cued memory was recorded as indicated by the number of seconds between when the word was read aloud and when they actually began reporting the memory. After the memory was recalled, the subject wrote down the approximate date of the memory. Later analysis revealed that the bulk of the memories (>90%) were within a 5 year pre-test time window (i.e. all were relatively recent memories). All memories were later transcribed and assessed on a global scale as to whether the memory was predominantly a negative, positive or neutrally toned memory by blind independent raters. Two raters blinded to the major hypothesis in this study judged the memories as being either negative, positive, or neutral in affective tone and arrived at the same conclusion 90% of the time.
2.5.3. Self-Other Word Recall Task
Participants were presented with two lists of ten trait adjectives, each list containing five positive and five negative traits. Words were obtained from Mueller (1986), who developed a list of positive and negative words most commonly associated with younger people and older people. The words used to create our lists were those from the young-positive and young-negative lists that were able to be matched on frequency and length. Frequency was determined by using wordcount.org, which rates 86800 words in order of how frequently they occur in the English language. Several versions of the lists were then created and randomly used. For the first list the participant received, they were asked to write detailed sentences about how these words applied to themselves. For the second list, they were asked to describe in detail how these traits applied to a significant other. Both times they were awoken, they were asked to recall any of the words from the beginning of the night (regardless of which list the words had appeared on). In addition, they were given a stem completion task that consisted of the first three letters of every word they had seen and asked to form words from the stems. The number and type of words recalled after REM vs NREM awakenings were tabulated for each participant.
2.5.4. Ratings of the Self, Other, and the Dream Self
For each condition, participants were given a different list of twenty trait words (four versions were compiled consisting of ten positive traits and ten negative traits; see Appendix I), and were asked to rate how well these same traits applied to themselves (the self), another person (the other), and themselves in a dream (the dream self). When asked about the other, the same person was used for each condition and the relationship of that person to the participant recorded. Negative and positive trait adjectives in each list were equal in likableness and frequency. We used the Anderson (1968) ratings to assign each personality trait word a likableness rating. Words from the beginning of the list (high likableness) and end of the list (low likeableness) were used for the positive and negative trait words. In addition, all words were matched in frequency using wordcount.org. Positive words had an average likableness of 452.58 and an average frequency of 0.88. Negative words had an average likeableness of 119.3 and an average frequency of 0.76. Each participant received each of the four versions of the list, but the condition in which each version was received was randomized.
2.6. Scoring of dream content
We use the so-called Hall-Van de Castle content scoring system to score emotion content and social interactions in dreams. Schneider and Domhoff (http://www.dreamresearch.net) provide a spreadsheet program ‘DreamSat’, which allows for tabulation of dream content scores and automatic computation of derived scales and percents when using the Hall/Van de Castle scoring system. This spreadsheet program greatly increases the reliability of results obtained with use of the system. The Hall/Van de Castle system for scoring dream content (Domhoff, 1996; Hall and Van de Castle, 1966) is a standardized and reliable content scoring system which consists of up to 16 empirical scales and a number of derived scales useful for an analysis of social interactions in dream content. Three primary types of emotional interaction are scored: aggressive, friendly and sexual with the ability to score subtypes as well (e.g., physical vs. verbal aggression). Along with identification of the physical settings within which these interactions take place, the character that initiated the social interaction is identified as well as the target or recipient of the interaction. The ‘characters’ scale allows for classification of characters known to the dreamer (e.g., family members, friends, etc.) as well as those unknown to the dreamer.
3. Data/statistical analysis
Analyses were carried out on a Windows PC machine using systat and SAS. Comparisons on continuous measures between the depressed/anxious and healthy control groups were computed with Bonferroni – corrected t-tests for independent groups. With respect to dream content, because the Hall/Van de Castle categories and content indicators are based on nominal rating scales we used tests for the significance of differences between two proportions as well as chi square analyses to compare REM -NREM differences. We used the DreamSat program http://www.dreamresearch.net) to compute all of the scales, percent differences and certain p values we report. The program also produces Cohen's h statistic which is an effect size value for samples involving nominal measurement scales.
4. Results
4.1. Mood scores across sleep states
There were few significant differences on the PANAS scales for positive or negative mood states between REM and NREM sleep states within each group. Mean positive mood scores for healthy participants after REM sleep was 15.17 (SD=5.96) and after NREM sleep was 14.69 (SD=5.59) (n.s.). However there was a significant difference in the mean negative mood score after REM sleep (14.14 (SD=4.32)) compared to NREM sleep (12.69 (SD=2.86), p<0.05). Mean positive mood scores for the depressed/anxious group after REM sleep was 14.00 (SD=4.44) and NREM sleep was 14.11 (SD=5.30) (n.s.). Similarly, mean negative mood score for REM sleep was 15.55 (SD=5.09) and for NREM sleep 14.89 (SD=4.42) (n.s.).
4.2. Self-other Word Recall task
In this sample, recall of ‘other’-encoded words was significantly reduced relative to ‘self’ encoded words but only after awakenings from REM sleep (mean=2.2 (SD=1.3)) vs NREM sleep (mean=4.0 (SD=1.2)) (p<0.05). No group differences were noted.
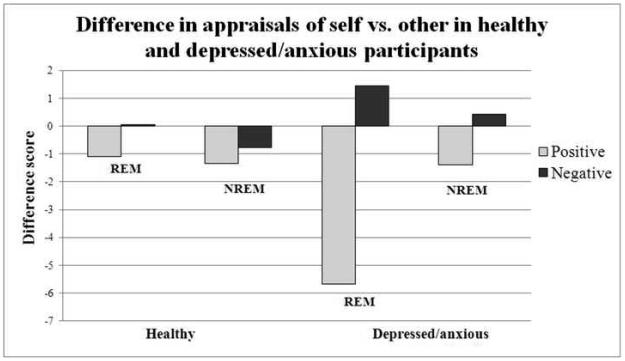
4.3. Trait adjective ratings on self vs other (see Figure 2)
Figure 2.
This figure represents the difference in appraisals such that positive scores indicate the self has more of that trait whereas negative scores indicate the other possesses more of that trait.
Ratings of self vs a significant other were significantly less positive after awakenings from REM sleep than NREM sleep in the depressed/anxious group, but not the healthy group. For healthy participants, mean REM sleep awakening self-other difference score= −1.09 (SD=3.34), while NREM sleep awakening self-other difference score =−1.33 (SD=4.14), n.s.). For depressed/anxious participants, mean REM sleep awakening self-other difference score= −5.69 (SD=9.52), while NREM sleep awakening self-other difference score =−1.38 (SD=5.46) (p<0.05). Conversely ratings of self vs other were significantly more negative after awakenings from REM sleep for depressed/anxious (mean self-other difference score for healthy = +0.06 (SD=2.30), mean self-other difference score for depressed = +1.44(SD=4.34)) (p<0.05) than after awakenings from NREM sleep (mean self-other difference score for healthy = −0.76 (SD=4.04), mean self-other difference score for depressed/anxious = +0.44 (SD=5.48)) (n.s.). There were no significant differences between wake conditions and awakening conditions on self – other scores. When comparing the groups, the depressed/anxious group rated themselves significantly less positive compared to the other after REM sleep awakenings than the healthy group (t(47)=−2.50, p<0.05).
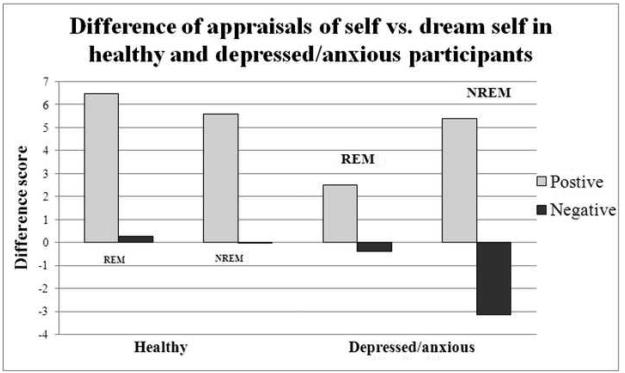
4.4. Trait adjective ratings on self vs dream self (see Figure 3)
Figure 3.
This figure represents the difference in appraisals such that positive scores indicate the self has more of that trait whereas negative scores indicate the dream self possesses more of that trait.
Ratings of self vs the dream self were significantly different after awakening from REM sleep in the depressed/anxious participants compared to NREM sleep awakening ratings, but were not significantly different in healthy participants. Depressed/anxious participants had mean REM sleep awakening self-dream self difference score= 2.50 (SD=7.08) and a NREM sleep awakening mean self-dream self difference score=5.38 (SD=9.14), p<.05), while healthy participants did not have significantly different positive ratings after REM sleep awakenings (mean self-dream self difference score= 6.47 (SD=6.53)) compared to NREM sleep awakenings (mean self-dream self difference scores= 5.58 (SD=6.13), n.s.). In both healthy and depressed/anxious participants, negative ratings after REM sleep awakenings (mean self-dream self difference scores: healthy= +0.28 (SD=4.16), depressed/anxious=−0.03 (SD=3.01)) were not significantly different than after awakenings from NREM sleep (mean self-dream self difference scores: healthy=−0.38 (SD=3.67), n.s., depressed/anxious= −3.13 (SD=5.62), n.s.). There were no significant differences between wake conditions and awakening conditions on self – dream self scores. When comparing the groups, the depressed/anxious group rated the dream self significantly less negatively than the self after NREM sleep awakenings than the healthy group (t(47)=−2.50, p<0.05)
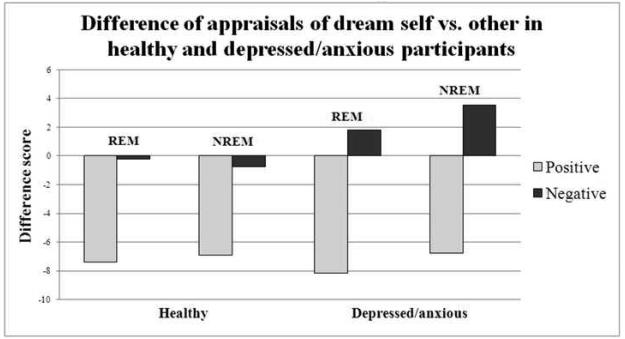
4.5. Trait adjective ratings on dream self vs other (see Figure 4)
Figure 4.
This figure represents the difference in appraisals such that positive scores indicate the dream self has more of that trait whereas negative scores indicate the other possesses more of that trait.
Positive ratings of dream self vs a significant other did not significantly differ after awakenings from REM (mean dream self-other difference score: healthy= −7.38 (SD=7.32), depressed/anxious=−8.19 (SD=9.14)) than after awakenings from NREM (mean dream self-other difference score: healthy =−6.91 (SD=7.38), depressed/anxious=−6.75 (SD=7.86), n.s.). Negative ratings of dream self vs other did not significantly differ after awakenings from REM (mean dream self-other difference score: healthy =−0.22 (SD=4.03), depressed/anxious=1.81(SD=4.43)) than after awakenings from NREM (mean dream self-other difference score: healthy=−0.73 (SD=3.23), depressed/anxious=3.56 (SD=5.28), n.s.). There were no significant differences between wake conditions and awakening conditions on dream self – other scores. When comparing the groups, the depressed/anxious group rated the dream self significantly more negative than the other after NREM sleep awakenings than the healthy group (t(47)=3.52, p<0.001).
4.6. Retrieval of negative vs positive vs neutral autobiographical memories
Using retrieval time of an emotionally ‘neutral memory’ after a REM sleep awakening as a baseline, it took healthy participants about 2.63 seconds longer to retrieve a positive memory and about 0.19 seconds longer to retrieve a negative memory after REM sleep awakenings. Similarly, it took depressed/anxious participants about 2.71 seconds longer to retrieve a positive memory and they were about 3.06 seconds faster to retrieve a negative memory after REM sleep awakenings.
Across the sample, negative memories were significantly more likely to be retrieved in response to a neutral cue word after awakenings from REM vs NREM sleep. Nearly half (49%) of memories retrieved in response to a neutral cue word were negative in tone/affect (by blinded judges) after awakenings from REM sleep while only about 1/3rd (30%) of memories retrieved in response to a neutral cue were negative in tone after awakenings from NREM.
In depressed participants, two-thirds (67%) of memories retrieved in response to a neutral cue word were negative in tone/affect (as judged by blinded raters) after awakenings from REM while only about one-third (35%) of memories retrieved in response to a neutral cue were negative in tone after awakenings from NREM.
4.7. Dream content
Depressed/anxious participants had more aggressive social interactions compared to friendly social interactions (69% in depressed/anxious compared to 40% in healthy, p=<0.05), more negative emotions (100% in depressed/anxious compared to 82% in healthy, p<0.05), and a larger number of dreams with at least one aggressive social interaction (44% in depressed/anxious compared to 13% in healthy, p<0.001). REM-related (dream) representation of self was decidedly more negative after REM vs NREM sleep awakenings in the depressed/anxious group. When comparing the REM dreams of healthy and depressed/anxious participants, depressed/anxious participants had significantly higher percentage of dead and imaginary characters present (h=0.43, p=0.03) and more dreams where there was at least one aggressive act (h= 0.78, p=0.01) relative to the healthy participants.
5. Discussion
We found a significant reduction in positive ratings of the self vs a significant other and a significant increase in negative ratings of the self vs a significant other after awakenings from REM sleep but not NREM sleep in depressed/anxious persons. To our knowledge, our study is the first to experimentally demonstrate an impact of REM sleep physiology on the negative self-concept of depressed/anxious persons. The dream self was also consistently rated as negative relative to both a significant other and relative to the daytime self after REM sleep awakenings for depressed/anxious subjects. These are important clinical findings as both REM-related indices and poor self-concept predict mood dysfunction and suicidal ideation and attempts (Agargun and Cartwright, 2003). We also found a greater production of emotionally negative memories with neutral cue words after REM sleep awakenings in both the depressed/anxious and healthy subjects. There was also some indication that negative memories were retrieved more quickly after REM sleep awakenings vs NREM sleep awakenings or wake conditions. Dreams from REM sleep contained greater amounts of negative emotion and aggression than did NREM sleep dreams. All of these REM sleep related findings were present in and more severe in participants who scored in depressive and anxious range on our mood scales.
Taken together, these results suggest to us that REM sleep may significantly contribute to cognitive distortions of mood disorder. REM sleep mechanisms apparently differentially impact the content or cognitions that occur in anxious depression. Our results are consistent with a body of research demonstrating negative mood and cognitive effects of REM sleep.
5.1. REM facilitates acquisition and recall of negative emotional memories
A prominent symptom of depression is a mood-related bias toward negatively valenced memories (Bradley et al., 1995; Watkins et al., 1996). Aspects of REM and NREM sleep neurobiology have been implicated in facilitation of various types of memory consolidation (Born and Wagner, 2004; Stickgold et al., 2001; but see Vertes and Eastman, 2000). REM sleep, in particular, appears to facilitate consolidation of negatively valenced memories (Hu et al., 2006; Nishida et al., 2009) and this consolidation effect is correlated with prefrontal theta activity (Nishida et al., 2009).
5.2. Dreams from REM in both healthy and depressed persons are associated with negative emotion and unpleasant content
When healthy subjects are awakened from REM sleep, they generally report a narrative involving the dreamer, with vivid visual detail, and more unpleasant than pleasant emotions (Domhoff, 2003; Hobson and Pace-Schott, 2002; Nielsen et al., 2001; Strauch and Meier, 1996). Revonsuo (2000) presented a large amount of evidence which suggests that the majority of REM dreams involve unpleasant emotional states wherein the dreamer experiences some sort of physical or emotional threat in the dream. Smith et al. (2004) scored both REM and NREM sleep dream reports for emotion content from 25 dreamers. They identified eight emotions that they then divided into positive and negative categories. They found that negative emotions were significantly more intense in REM sleep dream than NREM sleep dreams. Nightmares that occur in REM sleep are unequivocally unpleasant and predict suicidal ideation in some depressed patients (Agargun and Cartwright, 2003; McNamara, 2008).
Despite these normative findings regarding REM sleep dreams, it is important to recall that depressed people may use their dreams to work through unpleasant affect (Cartwright, 1992; Cartwright et al., 1998, Hartmann, 1998). Whether REM sleep dreaming is depressogenic in itself or can be used to respond adaptively to depressogenic crises in a person's life, REM sleep dreams are likely in either case to contain more negative affect than NREM sleep dreams or waking cognitions.
5.3. Limitations of our study
To our knowledge, our study is the first to experimentally demonstrate an impact of REM on the self-concept. As noted above, that impact appears to be largely negative, particularly in depressed/anxious persons. Caution is in order, however, because our study had several limitations. First, we tested only 20 individuals who reported symptoms of depression but none of them carried clinical diagnoses of depression. Therefore, our results should not be incautiously extrapolated to all depressed persons. Second, our overall N was relatively small for some tests. So statistical power was correspondingly reduced for these tests. Third, we focused only on potential depressogenic effects of REM sleep in this study. NREM sleep, however, is also known to be affected in depression. Specific components of NREM sleep, especially slow wave activity (SWA) likely contribute to various symptom complexes of depression (e.g. melancholic disturbance in men; somatic disturbance in women, see Armitage, 2007). NREM sleep will be a crucial component of any complete account of sleep-related contributions to depression (Borbély and Wirz-Justice, 1982). Fourth, we studied only a convenience sample of volunteer participants, most of whom were college students and thus our sample may not be representative of older depressed clinical populations.
Despite these important limitations of our study, our results document a remarkably potent depressogenic effect of REM sleep. This is an extremely important clinical finding. Identifying specific depressogenic effects of REM sleep will clarify the role of sleep dysregulation in production of depressive symptomology and thus allow for better and more targeted therapeutic interventions for depression. REM-related measures, such as REM density and REM sleep dreams and nightmares, are significant predictors of suicidal ideation in depressed individuals (Agargun et al., 1998; McNamara, 2008). If a clinician monitored such REM sleep and dream-related indicators in individuals at risk for suicide, he or she could potentially identify early warning signs of new ideation around suicide and could therefore act to prevent a new suicide attempt. In addition, REM-related indices of persons with post-traumatic stress disorder (PTSD) predict severity of PTSD (Germain and Nielsen, 2003). Indeed incorporation of trauma-related memories into REM sleep dreams is one of the DSM-IV criteria for the disorder. Nevertheless, it should be noted that the dreams and nightmares of PTSD may not be solely dependent on REM-related mechanisms. REM sleep atonia may be decreased in PTSD, noradrenergic modulation is impaired, and as many as 25% of these nightmares may appear outside of REM sleep (Woodward et al., 2000). Our findings speak directly to the ways in which REM sleep favors negatively valenced mnemonic content thus illuminating distorted memory functions in both PTSD and depression. Independent studies of links between sleep architecture and health have also shown that abnormal amounts of REM sleep predict not only PTSD and depression but also poor general health and decreased longevity (Brabbins et al., 1993; Dew et al., 2003; Kripke, 2003). However, as Kripke (2003) notes, these studies need to be replicated before any firm conclusions regarding REM sleep's effect on health can be drawn.
In sum, REM sleep appears to ‘normally’ exhibit a preference for negative emotional and cognitive processing. In depressed persons, this processing preference of REM sleep may significantly contribute to cognitive distortions of anxiety and depression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119:33–39. doi: 10.1016/s0165-1781(03)00111-2. [DOI] [PubMed] [Google Scholar]
- Agargun MY, Cilli AS, Kara H, Tarhan N, Kincir F, Oz H. Repetitive frightening dreams and suicidal behavior in patients with major depression. Compr. Psychiatry. 1998;39:198–202. doi: 10.1016/s0010-440x(98)90060-8. [DOI] [PubMed] [Google Scholar]
- Agargun MY, Besiroglu L, Cilli AS, Gulec M, Aydin A, Inci R, Selvi Y. Nightmares, suicide attempts, and melancholic features in patients with unipolar major depression. J. Affect. Disord. 2007;98:267–270. doi: 10.1016/j.jad.2006.08.005. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association Press; Washington, DC: 2000. [Google Scholar]
- Anderson NH. Likeableness ratings of 555 personality-trait words. J. Pers. Soc. Psychol. 1968;9:272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales (DASS) in clinical groups and a community sample. Psych. Assess. 1998;10:176–181. [Google Scholar]
- Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. 2007;115:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- Balkin TJ, Braun AR, Wesensten NJ, Varga PB, Carson RE, Belenky G, Herscovitch P. Bi-directional change in regional cerebral blood flow across the first 20 min. of wakefulness. Sleep Res. Online. 1999;2:6. [Google Scholar]
- Bertini M, Violani C. The postawakening testing technique in the investigation of cognitive asymmetries during sleep. In: Antrobus JS, Bertini M, editors. The Neuropsychology of Sleep and Dreaming. Erlbaum; Hillsdale, NJ: 1992. pp. 47–62. [Google Scholar]
- Borbély AA, Wirz-Justice A. Sleep, sleep deprivation and depression, a hypothesis derived from model of sleep regulation. Hum. Neurobiol. 1982;1:205–210. [PubMed] [Google Scholar]
- Born J, Wagner U. Memory consolidation during sleep: role of cortisol feedback. Ann. N. Y. Acad. Sci. 2004;1032:198–201. doi: 10.1196/annals.1314.020. [DOI] [PubMed] [Google Scholar]
- Brabbins CJ, Dewey ME, Copeland JRM, Davidson IA, McWilliam C, Saunders P, Sharma VK, Sullivan C. Insomnia in the elderly: prevalence, gender differences and relationships with morbidity and mortality. Int. J. Geriatr. Psychiatry. 1993;8:473–480. [Google Scholar]
- Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in depression and anxiety. Behav. Res. Ther. 1995;33:755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: Potential mechanisms. Psychosom. Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Cartwright R. Masochism in dreaming and its relation to depression. Dreaming. 1992;2:79–84. [Google Scholar]
- Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: a study of normal volunteers. Psychiatry Res. 1998;81:1–8. doi: 10.1016/s0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br. J Clin. Psychol. 2003;42:111–131. doi: 10.1348/014466503321903544. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol. Psychiatry. 2002;51:29–37. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF., III Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom. Medicine. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Domhoff GW. Finding Meaning in Dreams: A Quantitative Approach. Plenum; New York, NY: 1996. [Google Scholar]
- Domhoff GW. The scientific study of dreams: neural networks, cognitive development, and content analysis. American Psychological Association; Washington, DC: 2003. [Google Scholar]
- Drevets WC. Orbitofrontal cortex function and structure in depression. Ann. N. Y. Acad. Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- Ellman SJ, Spielman AJ, Luck D, Steiner SS, Halperin R. REM deprivation: a review. In: Ellman SL, Antrobus JS, editors. The Mind in Sleep: Psychology and Psychophysiology. John Wiley; NY: 1991. pp. 327–376. [Google Scholar]
- Germain A, Nielsen T. Impact of imagery rehearsal treatment on distressing dreams, psychological distress, and sleep parameters in nightmare patients. Behav. Sleep Med. 2003;1:140–154. doi: 10.1207/S15402010BSM0103_2. [DOI] [PubMed] [Google Scholar]
- Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep Med. Rev. 2002;6:361–377. [PubMed] [Google Scholar]
- Giles DE, Roffwarg HP, Schlesser MA, Rush AJ. Which endogenous depressive symptoms relate to REM latency reduction? Biol. Psychiatry. 1986;21:473–482. doi: 10.1016/0006-3223(86)90189-7. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol. Rhythms. 2006;4:129–136. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS, Van de Castle RL. The Content Analysis of Dreams. Appleton-Century-Crofts; New York, NY: 1966. [Google Scholar]
- Hartmann E. Nightmare after trauma as paradigm for all dreams: a new approach to the nature and function of dreaming. Psychiatry. 1998;61:233–238. doi: 10.1080/00332747.1998.11024834. [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat. Rev. Neurosci. 2002;3:679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotionally arousing declarative memory. Psychol. Sci. 2006;10:891–898. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events. American Academy of Sleep Medicine; Westchester, Il: 2007. [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat. Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kripke DF. Sleep and mortality. Psychosom. Med. 2003;65:74. doi: 10.1097/01.psy.0000039752.23250.69. [DOI] [PubMed] [Google Scholar]
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. The Psychology Foundation of Australia; Sydney, Australia: 1995. [Google Scholar]
- Maquet P, Peters JM, Aerts J, Delfiore G, Degueldre C, Luxen A, Franck G. Functional neuroanatomy of human rapid-eye-movement sleep and dreaming. Nature. 1996;383:163–166. doi: 10.1038/383163a0. [DOI] [PubMed] [Google Scholar]
- Maquet P, Phillips C. Rapid eye movement sleep: cerebral metabolism to functional brain mapping. In: Inoue S, editor. Rapid Eye Movement Sleep. Marcel Dekker; NY: 1999. pp. 276–285. [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva A, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual for the profile of mood states. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- McNamara P. Nightmares: the science and solution of those frightening visions during sleep. Praeger; Westport, CT: 2008. [Google Scholar]
- Mueller JH, Wonderlich S, Dugan K. Self-referent processing of age-specific material. Psychol. Aging. 1986;1(4):293–299. doi: 10.1037//0882-7974.1.4.293. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends Cogn. Sci. 2002;6:475–481. doi: 10.1016/s1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nielsen TA, Kuiken D, Hoffman R, Moffitt A. REM and NREM sleep mentation differences: a questions of story structure? Sleep & Hypnosis. 2001;3:9–17. [Google Scholar]
- Nishida M, Pearsall J, Buckner RL, Walker MP. REM sleep, prefrontal theta, and the consolidation of human emotional memory. Cereb. Cortex. 2009;19(5):1158–1166. doi: 10.1093/cercor/bhn155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofzinger EA. Neuroimaging and sleep medicine. Sleep Med. Rev. 2005;9:157–172. doi: 10.1016/j.smrv.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA. Functional neuroimaging of sleep disorders. Curr. Pharm. Des. 2008;14:3417–3429. doi: 10.2174/138161208786549371. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Wilson SJ, Paterson LM. Sleep disorders as core symptoms of depression. Dialogues in Clin. Neurosci. 2008;10:329–335. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF. The frontal lobes and dreaming. In: Barrett D, McNamara P, editors. The New Science of Dreaming. Praeger; CT: 2007. pp. 115–154. [Google Scholar]
- Pilcher JJ. Affective and daily event predictors of life satisfaction in college students. Soc. Indic. Res. 1998;43:291–306. [Google Scholar]
- Rauchs G, Bertran F, Guillery-Girard B, Desgranges B, Kerrouche N, Denise P, Fpret J, Eustache F. Consolidation of strictly episodic memories mainly requires Rapid Eye Movement sleep. Sleep. 2004;27:395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- Reinsel RA, Antrobus J. Lateralizing task performance after awakening from sleep. In: Antrobus JS, Bertini M, editors. The Neuropsychology of Sleep and Dreaming. Erlbaum; Hillsdale, NJ: 1992. pp. 63–85. [Google Scholar]
- Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav. Brain. Sci. 2000;23:877–901. doi: 10.1017/s0140525x00004015. [DOI] [PubMed] [Google Scholar]
- Schneider A, Domhoff GW. DreamSAT. 2009 Retrieved from http://www.dreamresearch.net/ on June 4, 2009.
- Smith MR, Antrobus JS, Gordon E, Tucker MA, Hirota Y, Wamsley EJ, Ross L, Doan T, Chaklader A, Emery RN. Motivation and affect in REM sleep and the mentation reporting process. Conscious Cogn. 2004;13:501–511. doi: 10.1016/j.concog.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Scott L, Fosse R, Hobson JA. Brain-mind states: I. Longitudinal field study of wake-sleep factors influencing mentation report length. Sleep. 2001;24:171–179. doi: 10.1093/sleep/24.2.171. [DOI] [PubMed] [Google Scholar]
- Strauch I, Meier B. search of dreams: results of experimental dream research. State University of New York Press; Albany, NY: 1996. [Google Scholar]
- Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin. Psychiatry. 2005;66:1254–1269. doi: 10.4088/jcp.v66n1008. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behav. Brain Sci. 2000;23(6):867–876. doi: 10.1017/s0140525x00004003. [DOI] [PubMed] [Google Scholar]
- Vogel GW. A review of REM sleep deprivation. Arch. Gen. Psychiatry. 1975;32:749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep- wake cycle: REM-sleep enhancement of anagram problem solving. Cogn. Brain Res. 2002;14:314–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep, memory and plasticity. Annu. Rev. Psychol. 2006;10:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- Watkins PC, Vache K, Verney SP, Muller S, Mathews A. Unconscious mood-congruent memory bias in depression. J. Abnorm. Psychol. 1996;105:34–41. doi: 10.1037//0021-843x.105.1.34. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. Psychological Corporation; San Antonio, CA: 2001. [Google Scholar]
- Woodward SH, Arsenault NJ, Bliwise DL. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol. Psychiatry. 2000;48(11):1081–1087. doi: 10.1016/s0006-3223(00)00917-3. [DOI] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, Fallon JH, Bunney WE. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J. Affect Disord. 2008;107:181–186. doi: 10.1016/j.jad.2007.07.030. [DOI] [PubMed] [Google Scholar]