Abstract
Francisella tularensis is a category A select agent. J5dLPS/OMP is a novel vaccine construct consisting of detoxified, O-polysaccharide side chain-deficient, lipopolysaccharide non-covalently complexed with the outer membrane protein of N. meningitidis group B. Immunization elicits hightiter polyclonal antibodies specific for the highly-conserved epitopes expressed within the glycolipid core that constitutes gram-negative bacteria (e.g., F. tularensis). Mice immunized intranasally with J5dLPS/OMP exhibited protective immunity to intratracheal challenge with the live vaccine strain, as well as the highly-virulent SchuS4 strain, of F. tularensis. The efficacy of J5dLPS/OMP vaccine suggests its potential utility in immunizing the general population against several different gram-negative select agents concurrently.
Keywords: tularemia, lungs, lipopolysaccharide
1. Introduction
Francisella tularensis is a highly pathogenic, gram-negative bacterium possessing attributes that favor its use as a weapon for bioterrorism [1]. F. tularensis is divided into two major subspecies: tularensis (type A), a highly virulent form most common in North America; and holarctica (type B), which is less virulent and responsible for most illnesses in Europe and Asia [2]. Inhalation of as few as 10 organisms can cause severe pneumonia and a high mortality rate [1]. Consequently, the CDC classifies F. tularensis as a category A select agent [1]. Immunization with the attenuated Live Vaccine Strain (LVS) derived from subspecies holarctica has been used for decades. While generally effective, vaccination provided human volunteers only partial protection against challenge with aerosolized type A F. tularensis [3]. Moreover, the need to vaccinate by scarification, an unclear understanding of the molecular basis for its attenuation, and a potential to revert to virulence negate its continued use [4].
Efforts to develop a safe and effective, alternative vaccine have focused on three approaches: heat- or chemically-inactivated whole cell, attenuated, and subunit vaccines. In the past, inactivated whole-cell preparations routinely failed to elicit protection in humans or animal models [5]. Recent studies, however, demonstrated varying degrees of protective immunity to respiratory infections in mice vaccinated with inactivated F. tularensis LVS that was: administered in conjunction with IL-12, targeted to Fc receptors via anti-F. tularensis LPS-specific monoclonal antibody, or adjuvanted with preformed immune-stimulating complexes suggesting promise of such a strategy [6-8]. Given the general effectiveness of F. tularensis LVS in preventing infections, attenuated microorganisms are a logical approach to vaccine development [9,10]. Despite their restricted ability to survive, replicate, and cause disease, however, attenuated microorganisms still represent considerable risk to immunocompromised individuals [11].
Subunit vaccines composed of a defined set of antigens offer a third approach to immunizing against F. tularensis. To date, only a limited number of subunit vaccines have proven effective; purified F. tularensis LPS is best described. Immunization with LPS provided protective immunity against systemic (not aerosol) challenge with type B Francisella, but negligible protection against challenge with type A strains regardless of route [12-15]. Immunity to infection appeared to derive from an elevated response to the O-polysaccharide component of LPS; free lipid A was an ineffective immunogen [14,16]. Besides LPS, a number of highly immunogenic outer membrane protein (OMPs) have been identified (e.g., FopA and TUL4); none of these has proven effective in eliciting protective immunity [12,17].
Both CD4+ and CD8+ T cells are essential to resolving primary and secondary tularemia infections [18,19]. The role of humoral immunity is also well-documented. Passive immunization with pooled human sera derived from vaccinated individuals protected mice against challenge with F. tularensis LVS [20]. Similarly, mice passively immunized with sera derived from animals previously infected with F. tularensis LVS resisted challenge with a lethal dose of LVS, but not type A F. tularensis (SchuS4) [13,21]. Notably, the protective antibodies comprising immune sera primarily recognized the LPS component of F. tularensis though antibodies reactive with a number of outer membrane and intracellular proteins are also present [20,22].
J5dLPS/OMP is a novel vaccine construct consisting of detoxified (de-O-acylated) LPS derived from Escherichia. coli 0111:B4, J5 (Rc chemotype), a mutant unable to attach the O-polysaccharide side chain to the outer core glycolipid [23-25]. The detoxified LPS (dLPS) core is complexed with the OMP of Neisseria meningitidis group B, a Toll-like receptor (TLR) 2 ligand that stabilizes the glycolipid structure [24,26,27]. In the absence of an immunodominant O side chain, immunization elicits a polyclonal antibody response to the highly-conserved epitopes expressed within the glycolipid core that constitutes gram-negative bacterial LPS [25,26]. Vaccine-induced antibody promotes polymicrobial clearance in rodents and protection against lethal infection in a number of experimental models of gram-negative sepsis [24-26,28]. Additionally, mice vaccinated i.n. with J5dLPS/OMP resist lethal respiratory challenge with Klebsiella pneumoniae [29]. Here, we report that J5dLPS/OMP vaccinated mice resist intratracheal (i.t.) challenge with type A F. tularensis SchuS4, as well as with type B F. tularensis LVS.
2. Materials and methods
2.1. Francisella tularensis
F. tularensis subspecies holarctica strain LVS was obtained, cultured, stored and used experimentally as we described previously [30]. F. tularensis subspecies tularensis strain SchuS4 was obtained from ATCC/BEI Resources (Manassas, VA). For experimentation, F. tularensis SchuS4 in stock vials stored at −80°C was thawed, grown and also used as previously reported [31]. Preliminary experiments established 1 LD50 equivalent to 1,280 CFUs F. tularensis LVS or 1-2 CFUs F. tularensis SchuS4 inoculated i.t. as described below.
The bacterial burden of the lungs, blood and livers of infected mice was calculated from the colonies that grew on supplemented Mueller-Hinton agar (F. tularensis LVS) or cysteine heart agar (F. tularensis SchuS4) plates in accordance with methods we described previously [30,31]. All experiments involving F. tularensis LVS were conducted within BSL2 facilities located at Rhode Island Hospital (Providence, RI) or The University of Maryland School of Medicine (Baltimore, MD); experiments with F. tularensis SchuS4 were performed within the confines of the BSL3 at the New England Regional Center of Excellence (NERCE; Boston, MA).
2.2. Animals
Specific pathogen-free female, BALB/c mice, purchased from The Jackson Laboratories, Bar Harbor, ME were treated in accordance with protocols approved by the Rhode Island Hospital Animal Care and Use Committee and Harvard Medical School’s (HMS) Standing Committee on Animals. Mice challenged i.t. with F. tularensis SchuS4 were housed and maintained in a specific pathogen-free facility utilizing TECHNIPLAST Sealsafe™ IVC cages (Exton, PA) at HMS.
2.3. Vaccination
Ten, 6- to 8-week-old mice were inoculated i.n. with 1 μg J5dLPS/OMP vaccine at 0, 2, and 4 weeks. Unmethylated cytidine-phosphate-guanosine (CpG) containing oligodinucleotide (ODN 10104; Coley Pharmaceutical Group, Wellesley, MA) was administered i.n. (25 μg/mouse) one hour prior to each vaccination. Control groups received CpG ODN only.
2.4. Antibody Responses
Sera and bronchoalveolar lavage (BAL) fluids were collected from non-infected mice on day 42 post-vaccination; core glycolipid-specific IgG and IgA antibodies were quantified by ELISA as previously described [25].
2.5. Respiratory challenge
Vaccinated mice were challenged i.t. on days 35 - 42 post-vaccination in accordance with methods previously described [30,32].
2.6. Myeloperoxidase
Myeloperoxidase activity in homogenates of the lungs was quantified spectrophotometrically by analyzing the H2O2-dependent oxidation of 3,3′5,5′-tetramethylbenzidine (Sigma-Aldrich) as we previously described [33].
2.7. RNA extraction, purification, and quantitative real-time RT-PCR
Total cellular RNA in representative tissue samples was extracted and purified using TRIzol (Invitrogen Corporation, Carlsbad, CA). Real-time RT-PCR was conducted using the methods and RNA primers we described previously [30].
2.8. Cytokine and chemokine analyses
The cytokines and chemokines present in representative tissue samples obtained from individual mice were quantified as we described previously using Bio-Plex cytokine assay kits and the Bio-Plex 200 system in accordance with the manufacturer’s instructions (Bio-Rad Laboratories GmbH, Munich, Germany) [30].
2.9. Statistical analysis
The data were analyzed using the SigmaStat statistics program (Jandel Scientific, San Rafael, CA). Individual means were compared using a non-paired Student’s t test, a Mann-Whitney Rank Sum test, or McNemar’s paired and ranked non-parametric test. Survival differences were determined using Kaplan-Meier survival plots and analyzed by a LogRank test. p< 0.05 was considered statistically different.
2.10. Abbreviations used
BAL, bronchoalveolar lavage; CpG ODN, unmethylated cytidine-phosphate-guanosine containing oligodinucleotide; i.t., intratracheal; i.n., intranasal; J5dLPS/OMP, detoxified O-polysaccharide side chain-deficient Escherichia coli J5 mutant lipopolysaccharide non-covalently complexed with the outer membrane protein of Neisseria meningitidis group B; KC, keratinocyte-derived chemokine; LVS, live vaccine strain; MCP-1, monocyte chemotactic protein-1; OMP, outer membrane protein; TLR, toll-like receptor.
3. Results
3.1. J5dLPS/OMP vaccine elicits anti-J5 core glycolipid antibody production
J5dLPS/OMP vaccine administered in combination with CpG ODN stimulated the production of anti-J5 core glycolipid antibody. Glycolipid-specific IgG was ~1,000-fold greater in the sera and ~100-fold greater the BAL fluids obtained from mice immunized i.n. with J5dLPS/OMP vaccine and CpG ODN than those obtained from control mice administered CpG ODN alone (Figure 1A). Relative to the controls, the same sera and BAL fluids obtained from vaccinated mice contained 5- to 10-fold more anti-J5 core glycolipid-specific IgA (Figure 1B).
Fig. 1.
J5 core glycolipid-specific antibodies are elevated in mice immunized with J5dLPS/OMP vaccine. Groups of mice were inoculated i.n. with J5dLPS/OMP and CpG ODN (squares); control mice received CpG ODN alone (circles). Anti-J5 core glycolipid-specific IgG (A) and IgA (B) antibodies in the sera (open symbols) and BAL fluids (closed symbols) obtained on day 28 following the last inoculation were quantified. The pooled data derived from 2 separate experiments are shown. IgG and IgA anti-J5 core glycolipid-specific antibody concentrations in the sera and BAL fluids obtained from the vaccinated animals are significantly greater (P<0.05; non-paired Student’s t test or Mann-Whitney Rank sum test).
3.2. Vaccinated mice exhibit protective immunity against respiratory F. tularensis LVS infections
Mice immunized i.n. with J5dLPS/OMP vaccine and CpG ODN were subsequently challenged i.t. with a lethal dose F. tularensis LVS. Sixty percent of the vaccinated mice survived compared to 10% of the control mice administered CpG ODN alone (Figure 2). The elevated survival rate observed among vaccinated mice correlated inversely with the bacterial burden of tissues derived from these same animals. Two- to five-fold fewer organisms were recovered from the lungs, blood and livers of mice immunized with the J5dLPS/OMP vaccine and CpG ODN relative to the controls (Figures 3A-C).
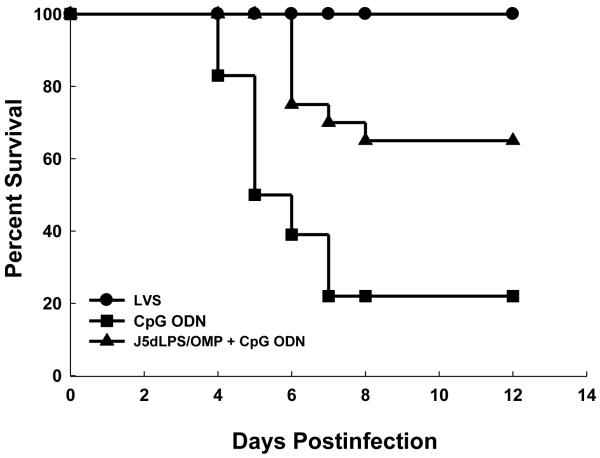
Fig. 2.
J5dLPS/OMP vaccine protects mice against F. tularensis LVS respiratory challenge. Groups of 10 control (CpG ODN alone) and 10 vaccinated (J5dLPS/OMP plus CpG ODN) mice were challenged i.t. with 9,800 CFUs F. tularensis LVS on day 28 post-vaccination. Data are derived from a single experiment representative of 4 separate experiments. J5dLPS/OMP vaccinated and CpG ODN control groups are significantly different; P = 0.0006 (LogRank test).
Fig. 3.
The bacterial burden is diminished in the tissues of vaccinated mice. Mice administered J5dLPS/OMP plus CpG ODN or CpG ODN alone were challenged i.t. with 6,800 CFUs (~5 LD50) F. tularensis LVS on day 35 following final vaccine delivery. The lungs (A), blood (B) and livers (C) were collected on day 4 postinfection and the bacterial burden was determined. The pooled data derived from 2 separate experiments and a total of 5-6 mice per group are shown; each symbol represents a single mouse. The bacterial burden was significantly lower in all tissues derived from vaccinated animals (p<0.05, Student’s t test).
3.3. Reduced cytokine/chemokine levels in the livers of vaccinated mice infected with F. tularensis LVS
The expression of proinflammatory cytokine messages, IL-12, IFN-γ and TNFα, was unchanged in the lungs of mice soon after respiratory challenge with F. tularensis LVS; at 4 hours post-infection, mice immunized with J5dLPS/OMP vaccine and CpG ODN were equivalent to those administered CpG ODN alone (Figure 4A-C). Additional analyses demonstrated no significant changes in the expression of IL-1β, IL-4, IL-6, IL-10, MIP-1α, MIP-1β, KC MIP-2 mRNAs and in the lungs of either the vaccinated or control group of mice at 4 hours postinfection (data not shown). These findings are supported by bead array analyses demonstrating equivalent protein concentrations of the same cytokines/chemokines in the lungs of both groups at 4 hours postinfection (Figure 4D). Notably, at 3 days post-challenge, the protein concentrations of these same cytokines/chemokines were elevated relative to those assessed in the lungs at 4 hours. There was no statistical difference, however, comparing levels in the lungs of vaccinated and control animals at this time (Figure 5A). In contrast, the concentrations of IL-12, TNF-α, IL-1β, IL-6, IL-10 and KC were significantly less in the livers of vaccinated, relative to control, mice at 3 days post-infection i.t. (Figure 5B).
Fig. 4.
Proinflammatory cytokine/chemokine message and protein concentrations in the lungs are unchanged shortly following i.t. inoculation of F. tularensis LVS. Groups of control and vaccinated mice were challenged i.t. with 7,200 CFUs F. tularensis LVS. The lungs were dissected at 4 hours post-infection, and the expression of IL-12 (A), IFN-γ (B) and TNF-α (C) mRNAs quantified. The protein concentrations of the cytokines and chemokines listed were quantified (D). Data are the means ± SD derived from 4 mice treated similarly in each group. Second experiments yielded comparable results. ND = not detected. All differences between vaccinated and control mice were statistically insignificant; P>0.05 (Student’s t test).
Fig. 5.
Proinflammatory cytokine/chemokine protein levels are diminished in the livers of vaccinated mice on day 3 post-challenged with F. tularensis LVS. Control (CpG ODN) and vaccinated (J5dLPS/OMP plus CpG ODN) mice were challenged i.t. with ~5 LD50 F. tularensis LVS. On day 3 post-infection, the lungs (top panel) and livers (bottom panel) were dissected; the cytokines and chemokines listed were quantified. Data are the means ± SD derived from 4 mice treated comparably. A second experiment yielded similar results. *Vaccinated group is significantly less than control: P<0.05 (Student’s t test). ND = not detected.
3.4. Fewer neutrophils accumulate in the lungs of vaccinated mice infected with F. tularensis
A general reduction in cytokine/chemokine concentrations in the tissues of vaccinated mice at 3 days post-infection suggests a diminished inflammatory response. This suggestion is supported by a significant difference in myeloperoxidase activity (a correlate of neutrophil numbers) assessed in the lungs of vaccinated and control mice at 3 days (Figure 6).
Fig. 6.
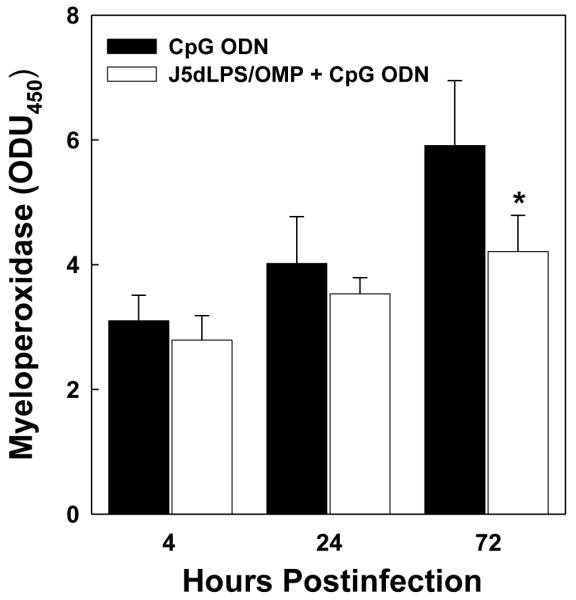
Vaccination suppresses the accumulation of neutrophils in the lungs subsequent to infection. Control and vaccinated groups of mice were challenged i.t. with 7,200 CFUs F. tularensis LVS. At 4, 24 and 72 hours post-infection, the lungs were dissected from four mice in each group and myeloperoxidase activity was quantified as a measure of neutrophil accumulation. *Significantly less than 72-hour group administered CpG ODN alone; P<0.01 (McNemar’s test).
3.5. J5dLPS/OMP vaccine protects mice against respiratory, F. tularensis SchuS4 challenge
Mice vaccinated with J5dLPS/OMP and CpG ODN resisted infection by type A F. tularensis. Sixty-five percent of vaccinated animals survived challenge i.t. with 10 LD50 F. tularensis SchuS4 (Figure 7). Notably, 100% of the mice vaccinated i.t. with a sublethal dose of F. tularensis LVS (positive control group) and 20% of mice that received CpG ODN alone (negative control), survived the same challenge. Inasmuch as the lethal dose of SchuS4 inoculated i.t. is only 1-2 CFUs, it seems unlikely that the surviving negative controls received a challenge dose.
Fig. 7.
Vaccination with J5dLPS/OMP and CpG ODN protects mice against respiratory, F. tularensis SchuS4 challenge. Groups of mice were vaccinated with J5dLPS/OMP and CpG ODN; negative control mice received CpG ODN alone; positive control mice were immunized by infection i.t. with 100 CFUs F. tularensis LVS on day 0. All three groups were challenged i.t. with 10 CFUs F. tularensis SchuS4 on day 37 post-vaccination. Data are combined from 2 separate experiments and a total of 18-20 mice per group. J5dLPS/OMP vaccinated and CpG ODN control groups are significantly different; P = 0.0013 (LogRank test).
4. Discussion
The majority of humans who survive primary exposure to F. tularensis possess specific serum antibodies at 8 years post-infection; LPS is the principal antigen recognized though its role in infection is unknown [20,34]. Similarly, LPS is the predominant antigen recognized by serum antibodies derived from mice infected sublethally with F. tularensis LVS [35]. Since it is intrinsically less toxic than LPS derived from enteric gram-negative bacteria [36-38], F. tularensis LPS represents a critical target for vaccine development. Indeed, the intact molecule and O-polysaccharide side chain have been screened as candidates [13-16]. While LPS-based vaccines demonstrate efficacy in mice challenged with type B strains of F. tularensis, immunity is incomplete; mice challenged with aerosolized type A F. tularensis succumb to infection. Mice vaccinated with J5dLPS/OMP, on the other hand, resisted respiratory challenge with both LVS and SchuS4 strains of F. tularensis.
J5dLPS/OMP vaccine stimulates the production of high antibody titers specific for the conserved glycolipid core of bacterial endotoxin [26,28,29]. These polyclonal antibodies recognize a broad range of LPS structures comprising heterologous gram-negative (but not gram–positive) bacteria. Previously, we reported that mice vaccinated i.n. with J5dLPS/OMP survived a lethal intratracheal challenge with K. pneumoniae [29]. Survival correlated with elevated core glycolipid-specific IgG and IgA class antibody titers in the sera and BAL fluids obtained from immunized mice, results comparable to those presented herein. The relevance of these antibodies to host resistance is supported by their ability to confer protection in a number of experimental models of sepsis; passive immunization with J5dLPS/OMP-specific antibody protected animals in a dose dependent manner [26,28]. Other investigators reported similar findings in mice passively immunized with serum antibodies that primarily recognize the LPS component of F. tularensis, i.e., enhanced resistance to challenge with a lethal dose of F. tularensis LVS [13,20-22]. While the role of humoral immunity in host defenses to F. tularensis is well documented, the specific antibody function remains unclear. Previously we reported that core glycolipid-specific IgA and IgG class antibodies in sera and BAL fluids obtained from mice vaccinated with J5dLPS/OMP promoted the ingestion and killing of bacteria by alveolar macrophages [29]. In this regard, it is relevant to note that mice vaccinated with inactivated F. tularensis LVS coated with anti-LPS-specific mAb and targeted to Fc receptors expressed by alveolar macrophages exhibited IgA-dependent resistance to respiratory F. tularensis challenge [7].
The OMP component of the J5dLPS/OMP vaccine is a TLR2 ligand [26,28]. The literature suggests it plays a key role in the elevated resistance of J5dLPS/OMP-vaccinated mice challenged i.t. with F. tularensis SchuS4. TLRs expressed by antigen presenting cells are principally involved in innate host resistance [39]. Ligation up-regulates the production of proinflammatory cytokines/chemokines and the expression of cell-surface co-stimulatory molecules that foster the development of acquired immunity. The adjuvant-like activity of TLR ligands has lead investigators to suggest incorporating them into subunit vaccines in order to boost efficacy [40].
TLR2, heterodimerized with TLR1 or TLR6, recognizes a wide variety of microbial ligands [41]. While F. tularensis LPS is incapable of binding TLR2, two lipoproteins that can bind (TUL4 and FTT1103) are thought to initiate proinflammatory cytokine production during tularemia infections [42,43]. TLR2 expression is required for optimal host defenses against F. tularensis; TLR2-deficient mice exhibit increases in the bacterial burden of the lungs, spleen and liver, and a high rate of mortality [44]. These findings substantiate our conjecture that N. meningitidis OMP is a key factor contributing to the efficacy of J5dLPS/OMP vaccine. This conjecture is supported by a recent report demonstrating higher LPS-specific serum antibody titers and enhanced resistance to intranasal F. tularensis LVS challenge in mice immunized with purified F. tularensis LPS in combination with N. meningitidis Porin B (a TLR2 ligand) relative to mice immunized with LPS alone [45]. Similarly, we reported that both OMP and TLR2 were essential for an optimal response to J5dLPS/OMP vaccination. J5dLPS administered in the absence of OMP was poorly immunogenic [26]; serum glycolipid-specific IgG levels were significantly less in TLR2-deficient mice vaccinated with J5dLPS/OMP than in their vaccinated wild-type counterparts [46]. Notably, the serum antibody titers were higher in mice vaccinated i.n. with J5dLPS/OMP administered in conjunction with unmethylated CpG ODN (a TLR9 agonist with adjuvant-like properties) than in animals vaccinated with J5dLPS/OMP vaccine alone [29]. Other investigators reported similar findings: co-administration of CpG ODNs and vaccines by a respiratory route promoted a vigorous polyclonal B cell response, the production of antigen-specific IgA antibodies, and mucosal immunity [47].
CpG ODN administered alone exerts only a transient beneficial effect on innate host defenses to microbial infections [25]. Thus, it is unlikely the survival of control mice challenged with F. tularensis SchuS4 on day 35 following administration (shown in Figure 7) reflects the residual effects of CpG ODN. Rather, it seems more plausible that their survival represents a failure in protocol and our varied ability to challenge i.t. each animal uniformly with the exceedingly small number of organisms that constitutes a lethal dose. Assuming the same 22% rate of failure in challenging both the vaccinated and control groups, the actual survival rate of vaccinated mice challenged with a lethal F. tularensis dose would be 60% (9 out of 15), rather than the 65% shown in Figure 7.
Common mouse strains vary widely in their susceptibility to tularemia infections [48]. Relative to BALB/c, for example, C57BL/6 mice characteristically exhibit diminished resistance. In particular, only BALB/c mice vaccinated intradermally with F. tularensis LVS were protected against an aerosol challenge with virulent F. tularensis Type B [49]. Similarly, in experiments undertaken in our laboratories, C57BL/6 mice vaccinated with J5dLPS/OMP and CpG ODN in accordance with the methods outlined here remained fully susceptible to intratracheal challenge with F. tularensis LVS (unpublished observation). Notably, C57BL/6 mice vaccinated i.p. with J5dLPS/OMP and CpG ODN exhibited marked increases in anti-core glycolipid-specific serum antibody [46]. Consequently, the failure of vaccinated C57BL/6 mice to resist intratracheal tularemia infection does not appear due to the absence of antibody production albeit the response to J5dLPS/OMP and CpG ODN administered mucosally remains to be determined.
Protective immunity and the decreased number of organisms found in the liver of J5dLPS/OMP vaccinated mice collected on day 3 post-infection i.t. with F. tularensis LVS correlated with a reduction in the cytokine/chemokine levels assessed. Other investigators reported similar findings, i.e., diminished levels of IL-1β, IL-6, IL-12p40, G-CSF, IFN-γ, TNF-α, MCP-1, and/or KC in the tissues of vaccinated, compared to non-vaccinated, mice challenged i.n. with F. tularensis [7,15,45]. Indeed, in a separate study, less MIP-2 (a neutrophil chemokine) and the accumulation of fewer neutrophils in the tissues of infected animals correlated directly with long-term survival [50]. Notably, the accumulation of neutrophils in the lungs of J5dLPS/OMP vaccinated, F. tularensis LVS-infected mice was reduced in the experiments reported here.
The findings presented herein suggest the potential use of J5dLPS/OMP vaccine to induce broad humoral immunity to a number of gram-negative select agents (e.g., F. tularensis, Yersinia pestis, Burkholderia mallei and B. pseudomallei) in the general population when the specific organism(s) speculated in an act of bioterrorism remains to be identified. The ability of J5dLPS/OMP vaccination to elicit protective immunity to other select agents including those listed immediately above is a matter of ongoing investigation in our laboratories.
Acknowledgments
We are pleased to acknowledge Jennifer Phung (Brown University) and Sean Fitzgerald (NERCE/Harvard Medical School) for their fine technical assistance, and Dr. Jason T Machan (Rhode Island Hospital) for his help in statistical analyses of the data. This study was supported by National Institutes of Health Research Grant R21AI055657 (S.H. Gregory), NCRR K12-RR-023250 (W.H. Chen), and Regional Centers of Excellence for Biodefense and Emerging Infectious Diseases Research grants, U54 AI057159 (A.S. Cross) and U54 AI057168 (S.M. Opal and S.H. Gregory). Drs. Cross and Opal are named on a patent issued for the J5dLPS/OMP vaccine. They attest that the work contained in this article is free of bias.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the other authors have conflicts to disclose.
References
- [1].Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285(21):2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- [2].Johansson A, Ibrahim A, Goransson I, Eriksson U, Gurycova D, Clarridge JE, III, et al. Evaluation of PCR-based methods for discrimination of Francisella species and subspecies and development of a specific PCR that distinguishes the two major subspecies of Francisella tularensis. J.Clin.Microbiol. 2000;38(11):4180–5. doi: 10.1128/jcm.38.11.4180-4185.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study, II: respiratory challenge. Arch.Int.Med. 1961;107:134–46. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- [4].Oyston PC, Sjostedt A, Titball RW. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat.Rev.Microbiol. 2004;2(12):967–78. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- [5].Eigelsbach HT, DOWNS CM. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J.Immunol. 1961;87:415–25. [PubMed] [Google Scholar]
- [6].Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect.Immun. 2007;75(5):2152–62. doi: 10.1128/IAI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J.Immunol. 2008;180(8):5548–57. doi: 10.4049/jimmunol.180.8.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Eyles JE, Hartley MG, Laws TR, Oyston PC, Griffin KF, Titball RW. Protection afforded against aerosol challenge by systemic immunisation with inactivated Francisella tularensis live vaccine strain (LVS) Microb.Pathog. 2008;44(2):164–8. doi: 10.1016/j.micpath.2007.08.009. [DOI] [PubMed] [Google Scholar]
- [9].Wayne CJ, Oyston PC. Vaccines against Francisella tularensis. Ann.N.Y.Acad.Sci. 2007;1105:325–50. doi: 10.1196/annals.1409.012. [DOI] [PubMed] [Google Scholar]
- [10].Bakshi CS, Malik M, Mahawar M, Kirimanjeswara GS, Hazlett KR, Palmer LE, et al. An improved vaccine for prevention of respiratory tularemia caused by Francisella tularensis SchuS4 strain. Vaccine. 2008;26(41):5276–88. doi: 10.1016/j.vaccine.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Richard JL, Grimes DE. Bioterrorism: class A agents and their potential presentations in immunocompromised patients. Clin.J.Oncol.Nurs. 2008;12(2):295–302. doi: 10.1188/08.CJON.295-302. [DOI] [PubMed] [Google Scholar]
- [12].Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13(13):1220–5. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- [13].Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19(31):4465–72. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- [14].Conlan JW, Vinogradov E, Monteiro MA, Perry MB. Mice intradermally-inoculated with the intact lipopolysaccharide, but not the lipid A or O-chain, from Francisella tularensis LVS rapidly acquire varying degrees of enhanced resistance against systemic or aerogenic challenge with virulent strains of the pathogen. Microb.Pathog. 2003;34(1):39–45. doi: 10.1016/s0882-4010(02)00194-8. [DOI] [PubMed] [Google Scholar]
- [15].Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. Native outer membrane proteins protect mice against pulmonary challenge with virulent Type A Francisella tularensis. Infect.Immun. 2008 doi: 10.1128/IAI.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Conlan JW, Shen H, Webb A, Perry MB. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine. 2002;20(2930):3465–71. doi: 10.1016/s0264-410x(02)00345-6. [DOI] [PubMed] [Google Scholar]
- [17].Fulop M, Manchee R, Titball R. Role of two outer membrane antigens in the induction of protective immunity against Francisella tularensis strains of different virulence. FEMS Immunol.Med.Microbiol. 1996;13(3):245–7. doi: 10.1111/j.1574-695X.1996.tb00245.x. [DOI] [PubMed] [Google Scholar]
- [18].Tarnvik A. Nature of protective immunity to Francisella tularensis. Rev.Infect.Dis. 1989;11(3):440–51. [PubMed] [Google Scholar]
- [19].Conlan JW, Sjostedt A, North RJ. CD4+ and CD8+ T-cell-dependent and -independent host defense mechanisms can operate to control and resolve primary and secondary Francisella tularensis LVS infection in mice. Infect.Immun. 1994;62:5603–7. doi: 10.1128/iai.62.12.5603-5607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am.J.Med.Sci. 1994;308(2):83–7. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- [21].Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J.Immunol. 2007;179(1):532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- [22].Janovska S, Pavkova I, Hubalek M, Lenco J, Macela A, Stulik J. Identification of immunoreactive antigens in membrane proteins enriched fraction from Francisella tularensis LVS. Immunol.Lett. 2007;108(2):151–9. doi: 10.1016/j.imlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [23].Cross AS, Opal SM, Palardy JE, Drabick JJ, Warren HS, Huber C, et al. Phase I study of detoxified Escherichia coli J5 lipopolysaccharide (J5dLPS)/group B meningococcal outer membrane protein (OMP) complex vaccine in human subjects. Vaccine. 2003;21(31):4576–87. doi: 10.1016/s0264-410x(03)00483-3. [DOI] [PubMed] [Google Scholar]
- [24].Cross AS, Opal S, Cook P, Drabick J, Bhattacharjee A. Development of an anti-core lipopolysaccharide vaccine for the prevention and treatment of sepsis. Vaccine. 2004;22(7):812–7. doi: 10.1016/j.vaccine.2003.11.025. [DOI] [PubMed] [Google Scholar]
- [25].Opal SM, Palardy JE, Chen WH, Parejo NA, Bhattacharjee AK, Cross AS. Active immunization with a detoxified endotoxin vaccine protects against lethal polymicrobial sepsis: its use with CpG adjuvant and potential mechanisms. J.Infect.Dis. 2005;192(12):2074–80. doi: 10.1086/498167. [DOI] [PubMed] [Google Scholar]
- [26].Bhattacharjee AK, Opal SM, Taylor R, Naso R, Semenuk M, Zollinger WD, et al. A noncovalent complex vaccine prepared with detoxified Escherichia coli J5 (Rc chemotype) lipopolysaccharide and Neisseria meningitidis Group B outer membrane protein produces protective antibodies against gram-negative bacteremia. J.Infect.Dis. 1996;173(5):1157–63. doi: 10.1093/infdis/173.5.1157. [DOI] [PubMed] [Google Scholar]
- [27].Zollinger WD, Mandrell RE, Griffiss JM, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J.Clin.Invest. 1979;63(5):836–48. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cross AS, Opal SM, Warren HS, Palardy JE, Glaser K, Parejo NA, et al. Active immunization with a detoxified Escherichia coli J5 lipopolysaccharide group B meningococcal outer membrane protein complex vaccine protects animals from experimental sepsis. J.Infect.Dis. 2001;183(7):1079–86. doi: 10.1086/319297. [DOI] [PubMed] [Google Scholar]
- [29].Chen WH, Kang TJ, Bhattacharjee AK, Cross AS. Intranasal administration of a detoxified endotoxin vaccine protects mice against heterologous Gram-negative bacterial pneumonia. Innate.Immun. 2008;14(5):269–78. doi: 10.1177/1753425908095959. [DOI] [PubMed] [Google Scholar]
- [30].Gregory SH, Mott S, Phung J, Lee J, Moise L, McMurry JA, et al. Epitope-based vaccination against pneumonic tularemia. Vaccine. 2009;27:5299–306. doi: 10.1016/j.vaccine.2009.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sebastian S, Pinkham JT, Lynch JG, Ross RA, Reinap B, Blalock LT, et al. Cellular and humoral immunity are synergistic in protection against types A and B Francisella tularensis. Vaccine. 2009;27(4):597–605. doi: 10.1016/j.vaccine.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kim J, Merry AC, Nemzek JA, Bolgos GL, Siddiqui J, Remick DG. Eotaxin represents the principal eosinophil chemoattractant in a novel murine asthma model induced by house dust containing cockroach allergens. J.Immunol. 2001;167(5):2808–15. doi: 10.4049/jimmunol.167.5.2808. [DOI] [PubMed] [Google Scholar]
- [33].Gregory SH, Cousens LP, van Rooijen N, Döpp EA, Carlos TM, Wing EJ. Complemetary adhesion molecules promote neutrophil-Kupffer cell interaction and the elimination of bacteria taken up by the liver. J.Immunol. 2002;168:308–15. doi: 10.4049/jimmunol.168.1.308. [DOI] [PubMed] [Google Scholar]
- [34].Bevanger L, Maeland JA, Kvan AI. Comparative analysis of antibodies to Francisella tularensis antigens during the acute phase of tularemia and eight years later. Clin.Diagn.Lab Immunol. 1994;1(2):238–40. doi: 10.1128/cdli.1.2.238-240.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Twine SM, Petit MD, Shen H, Mykytczuk NC, Kelly JF, Conlan JW. Immunoproteomic analysis of the murine antibody response to successful and failed immunization with live anti-Francisella vaccines. Biochem.Biophys.Res.Commun. 2006;346(3):999–1008. doi: 10.1016/j.bbrc.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [36].Vinogradov E, Perry MB, Conlan JW. Structural analysis of Francisella tularensis lipopolysaccharide. Eur.J.Biochem. 2002;269(24):6112–8. doi: 10.1046/j.1432-1033.2002.03321.x. [DOI] [PubMed] [Google Scholar]
- [37].Gunn JS, Ernst RK. The structure and function of Francisella lipopolysaccharide. Ann.N.Y.Acad.Sci. 2007;1105:202–18. doi: 10.1196/annals.1409.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458(7242):1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- [39].Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim.Biophys.Acta. 2002;1589(1):1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- [40].Guy B. The perfect mix: recent progress in adjuvant research. Nat.Rev.Microbiol. 2007;5(7):505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- [41].Wetzler LM. The role of Toll-like receptor 2 in microbial disease and immunity. Vaccine. 2003;21(Suppl 2):S55–S60. doi: 10.1016/s0264-410x(03)00201-9. [DOI] [PubMed] [Google Scholar]
- [42].Hajjar AM, Harvey MD, Shaffer SA, Goodlett DR, Sjostedt A, Edebro H, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect.Immun. 2006;74(12):6730–8. doi: 10.1128/IAI.00934-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thakran S, Li H, Lavine CL, Miller MA, Bina JE, Bina XR, et al. Identification of Francisella tularensis lipoproteins that stimulate the toll-like receptor (TLR) 2/TLR1 heterodimer. J.Biol.Chem. 2008;283(7):3751–60. doi: 10.1074/jbc.M706854200. [DOI] [PubMed] [Google Scholar]
- [44].Malik M, Bakshi CS, Sahay B, Shah A, Lotz SA, Sellati TJ. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect.Immun. 2006;74(6):3657–62. doi: 10.1128/IAI.02030-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chiavolini D, Weir S, Murphy JR, Wetzler LM. Neisseria meningitidis PorB, a Toll-like receptor 2 ligand, improves the capacity of Francisella tularensis lipopolysaccharide to protect mice against experimental tularemia. Clin.Vaccine Immunol. 2008;15(9):1322–9. doi: 10.1128/CVI.00125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chen WH, Basu S, Bhattacharjee AK, Cross AS. Enhanced antibody responses to a detoxified lipopolysaccharide-group B meningococcal outer membrane protein vaccine are due to synergistic engagement of Toll-like receptors. Innate.Immun. 2009 doi: 10.1177/1753425909346973. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Klinman DM, Currie D, Gursel I, Verthelyi D. Use of CpG oligodeoxynucleotides as immune adjuvants. Immunol.Rev. 2004;199:201–16. doi: 10.1111/j.0105-2896.2004.00148.x. [DOI] [PubMed] [Google Scholar]
- [48].Shen H, Chen W, Conlan JW. Susceptibility of various mouse strains to systemically- or aerosol-initiated tularemia by virulent type A Francisella tularensis before and after immunization with the attenuated live vaccine strain of the pathogen. Vaccine. 2004;22(1718):2116–21. doi: 10.1016/j.vaccine.2003.12.003. [DOI] [PubMed] [Google Scholar]
- [49].Chen W, Shen H, Webb A, KuoLee R, Conlan JW. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally, or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine. 2003;21(2526):3690–700. doi: 10.1016/s0264-410x(03)00386-4. [DOI] [PubMed] [Google Scholar]
- [50].Chiavolini D, Alroy J, King CA, Jorth P, Weir S, Madico G, et al. Identification of immunologic and pathologic parameters of death versus survival in respiratory tularemia. Infect.Immun. 2008;76(2):486–96. doi: 10.1128/IAI.00862-07. [DOI] [PMC free article] [PubMed] [Google Scholar]