Abstract
Ventral-visual activity in older adults has been characterized by dedifferentiation, or reduced distinctiveness, of responses to different categories of visual stimuli such as faces and houses, that typically elicit highly specialized responses in the fusiform and parahippocampal brain regions respectively in young adults (Park et al., 2004). In the present study, we demonstrate that age-related neural dedifferentiation applies to within-category stimuli (different types of faces) as well, such that older adults process less distinctive representations for individual faces than young adults. We performed a functional magnetic resonance imaging adaptation experiment while young and older participants made same-different judgments to serially presented face-pairs that were Identical, Moderate in similarity through morphing, or Different. As expected, older adults showed adaptation in the fusiform face area (FFA), during the Identical as well as the Moderate conditions relative to the Different condition. Young adults showed adaptation during the Identical condition, but minimal adaptation to the Moderate condition. These results indicate that older adults’ FFA treated the morphed faces as Identical faces, reflecting decreased fidelity of neural representation of faces with age.
Keywords: Aging, Dedifferentiation, Faces, Fusiform Area, Adaptation
INTRODUCTION
There is compelling evidence that with age, the neural function of highly specialized cortical areas become less selective or “dedifferentiated”. In particular, in the ventral-visual cortex of young adults, the fusiform region typically responds maximally to faces but is less responsive to other categories, such as houses (Kanwisher, McDermott, & Chun, 1997). Similarly, the parahippocampal region responds preferentially to houses but relatively little to faces or other categories (Epstein & Kanwisher, 1998). In older adults, however, this differentiation of responses to faces and houses in these regions is reduced, suggesting that the distinctiveness of underlying neural representations for these cross-category stimuli diminishes with age (Park et al., 2004).
Thus far, the evidence for dedifferentiation with age rests on how strongly cortical regions that respond preferentially to one category of visual stimuli (e.g. faces) responds to another category (e.g. houses), utilizing relatively crude measures of differences in mean activation of a population of voxels to these different visual categories (Park et al., 2004). Single-neuron recordings in primate aging studies on the primary visual cortex have shown, however, that there is an age-related reduction in selectivity of neural response tuning for specific exemplars within a single category of visual stimuli (Leventhal, Wang, Pu, Zhou, & Ma, 2003; Schmolesky, Want, Pu, & Leventhal, 2000; Wang, Zhou, Ma, & Leventhal, 2005; Yang, Liang, Li, Wang, Zhou, & Leventhal, 2008; Yu, Wang, Li, Zhou, & Leventhal, 2006) that may be the underlying mechanism for dedifferentiation of ventral visual activity. In these studies, visual neurons of older animals showed less inhibition with more spiking activity to a greater range of different line orientations whereas in young animals, neurons only responded to a narrow range of orientations. In other words, in older animals, the same neurons responded to various line orientations while in young animals, different neurons responded uniquely to specific orientations, even when the orientation differences were minimal. The net result of such reductions in neural selectivity in older animals is a loss of distinctiveness between functional responses to different types of stimuli – a dedifferentiation of neural responses to different stimuli with aging.
In the present study, we assessed such differences in neural selectivity within a single category of visual stimuli, faces, in young and older human adults using a functional magnetic resonance imaging (fMRI) adaptation paradigm (Grill-Spector et al., 1999; Grill-Spector & Malach, 2001; Grill-Spector, Henson, & Martin, 2006; Henson, 2003), which allowed us to approach the issue of within-category dedifferentiation with age much like the animal studies.
fMRI adaptation refers to a decrease in neural activation in response to a repetition of identical or similar stimuli relative to novel stimuli. Although the specific mechanism of fMRI adaptation is still not entirely clear, it is generally thought that this decrease in neural response may be due to the involvement of fewer neurons or lower activity of a neuronal population when processing stimuli that are repeated (Grill-Spector et al., 2006; Henson, 2003). Importantly, the degree to which a population of neurons processes two serially encountered stimuli as “same” will be reflected as a more attenuated response to the second stimulus. Moreover, if a brain region has high selectivity it would engage different neuronal populations when representing two slightly different stimuli, and thus show less attenuation. In contrast, a dedifferentiated brain region would use a similar population of neurons to represent two different stimuli, resulting in an adapted response to the second stimulus because the neurons are not able to process the difference. Essentially, the magnitude of an adaptation response in the ventral visual cortex measures whether a brain region processes differences among exemplars of related stimuli, making this paradigm ideally suited to the study of neural selectivity differences with age for within-category stimuli such as faces.
We reasoned that if dedifferentiation is a general phenomenon in older adults, we should be able to demonstrate reductions in neural selectivity for within-category exemplars that differ from each other by varying degrees (e.g. different facial identities), mirroring findings from the animal studies. We focused on the particular difficulty older adults have with face processing (Bartlett & Leslie, 1986; Bartlett, Leslie, Tubbs, & Fulton, 1989; Habak, Wilkinson, & Wilson, 2008; Owsley, Sekuler, & Boldt, 1981; Smith & Winograd, 1978) and examined the adaptation responses in the fusiform face area (FFA; Kanwisher et al., 1997) of young and older adults to face stimuli that differed slightly from earlier-presented faces. Note that while face-processing involves other areas apart from the FFA (Haxby et al., 2001; Kriegeskorte, Formisano, Sorger, & Goebel, 2007), we focused on this region due to its robust selectivity demonstrated across many studies (Kanwisher & Moscovitch, 2000; Kanwisher & Yovel, 2006). We hypothesized that young adults would show a differentiated and selective response to subtle face differences but older adults would not. Additionally, we hypothesized that reduced selectivity for faces in the FFA, as measured by the adaptation response, would also be associated with poorer behavioral ability to discriminate between faces; linking age-related brain changes with changes in behavior.
Several studies have used the fMRI adaptation paradigm to evaluate face processing in young adults and have shown that neural adaptation in the FFA is generally sensitive to the degree of similarity or distinctiveness between different face stimuli (Gilaie-Dotan & Malach, 2007; Henson, Shallice, Gorno-Tempini, & Dolan, 2002; Jiang et al., 2006; Kadosh et al., 2009; Rotshtein et al., 2005; Winston et al., 2004). In particular, in Jiang et al.’s (2006) study, participants viewed serial pairs of face stimuli that were morphed to different levels of similarity and were required to detect a target face that intermittently appeared during the fMRI adaptation experiment, as an incidental task. The study showed that, in the FFA, moderately similar face pairs elicited intermediate adaptation magnitudes and completely identical face pairs resulted in greatest adaptation, relative to face pairs that were clearly distinct. In addition, during a separate behavioral face discrimination test in the same study, participants’ were less likely to detect differences between face pairs the more similar the faces were. In another similar adaptation experiment with morphed faces, Gilaie-Dotan & Malach (2007) also showed that adaptation magnitude was greater when face stimuli within a trial were more similar, while participants directly performed a face discrimination task in the scanner. Critically in this study, the authors showed that participants’ in-scanner face discrimination performance directly tracked FFA adaptation magnitude such that the probability of detecting face differences decreased with increasing adaptation magnitude.
Buttressing our argument is evidence from fMRI adaptation studies on a patient with acquired prosopagnosia. Schiltz et al. (2006) and Dricot et al. (2008) studied the same patient, PS, who had lesions in the left fusiform and right occipital regions but intact right fusiform region. In both studies, PS’s responses to novel and repeated faces were equally attenuated in the FFA whereas control participants showed attenuated responses only to repeated faces but significantly higher activity (recovery from adaptation) to novel faces. Importantly, PS could not discriminate individual faces despite preserved object discrimination, suggesting that the non-differentiated FFA response to novel and repeated faces in PS reflected less distinctive representations of face stimuli in the FFA. Taken together with the adaptation findings in normal young adults, these studies indicate that greater adaptation in the FFA is associated with less distinctive neural representations of faces that may in turn be associated with behavioral performance.
In this study, we expected that age-related dedifferentiation of neural responses in the FFA would be associated with less distinctive neural coding of faces in older adults relative to young. Hence, there would be greater FFA adaptation in older adults to face pairs that were somewhat different from one another, relative to clearly distinct face pairs. Using the fMRI adaptation paradigm, we presented young and older participants with pairs of unfamiliar faces that were either different, moderately different (40% morph difference), or identical, and measured the BOLD responses to these three face-pair conditions in the FFA while participants performed a same-different face discrimination task on the stimuli. We also obtained an individual measure of face discrimination thresholds in order to relate adaptation in the FFA during the moderate condition with a specific behavioral outcome. We hypothesized that individuals with greater adaptation would evidence higher discrimination thresholds related to their reduced ability to detect difference among faces. Finally, we also considered that older adults would engage greater neural resources than young adults in order to correctly perform the face discrimination judgments. Thus, we expected that older adults would show greater activity than younger adults in frontal brain regions sensitive to task difficulty (Barch et al., 1997; Jonides et al., 1997; Klingberg, O’Sullivan, & Roland, 1997; Rypma, Prabhakaran, Desmond, Glover, & Gabrieli, 1999), consistent with previous literature on increased frontal recruitment with aging (Cabeza et al., 1997; Cabeza, Anderson, Locantore, & McIntosh, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Grady et al., 1994; Madden et al., 1997, 1999; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Lustig, 2005; Reuter-Lorenz & Cappell, 2008).
METHODS
Participants
20 young (mean age 24.7 yrs, range 19 to 32 yrs; 9 males, 11 females) and 20 older adults (mean age 66 yrs, range 61 to 72 yrs; 10 males, 10 females) gave informed consent for participation in this study, which was approved by the University of Illinois at Urbana-Champaign institutional review board (IRB). Participants were healthy, right-handed individuals with no counter-indications for scanning, and were remunerated for their participation (performance in the Mini-Mental State Exam in older adults ≥ 27). Visual acuity in the scanner was corrected to 20/20 on the Snellen Scale and participants with cataracts and macular degeneration were not included in this experiment.
Procedural Overview
There were two components to the experimental procedure. The imaging component consisted of an fMRI adaptation session involving an in-scanner face discrimination task followed by a localizer session to locate the FFA in each participant. The out-of-scanner component occurred after the imaging component and was purely behavioral. This component involved another similar face discrimination task but with adaptive adjustments to the stimuli that allowed us to determine individual thresholds for detecting differences in faces with greater sensitivity.
fMRI Adaptation Session Stimuli and Procedure
For the adaptation session, photographs of 144 unique faces, balanced for age (young, old) and gender (male, female), were used from the Minear and Park (2004) face database to constitute the stimuli for the in-scanner face discrimination task. Face stimuli were prepared using Matlab scripts and Adobe Photoshop so that they were rendered in grayscale, equated for luminance, and sized to fit a fixed black oval frame in order to hide each person’s hair, ears, and neck (Figure 1). The 144 unique faces formed 72 pairs of matched faces with members of each pair from the same age group and gender. These face-pairs were then pseudo-randomly assigned to three conditions (24 per condition with age and gender equated across conditions): Identical Condition (0% difference between the two members of each pair), Moderate Condition (40% difference), and Different Condition (100% difference). To create the face-pairs for the Moderate condition, the two faces in the pair were morphed together using computer software (Sqirlz Morph: www.xiberpix.com) to produce a new face that consisted of 60% of the first face, and 40% of the other face. The original face always preceded the new morphed face in each Moderate condition trial.
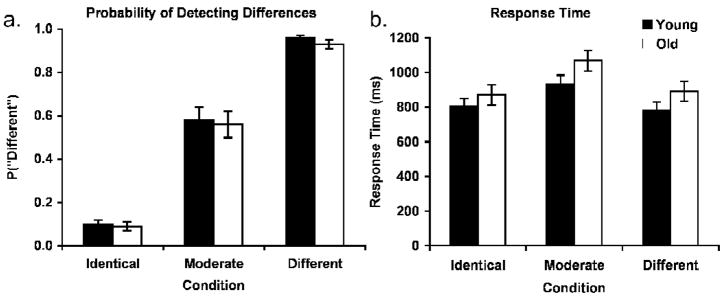
Figure 1.

fMR-A paradigm showing sample stimuli for the three face-pair conditions with the second face either Identical, Moderately different (morphed at 40% with another face), or a completely Different face. Inter-trial intervals (ITI) varied between 4, 6, and 10s.
During a trial, the two faces in each pair were presented serially and in rapid sequence so that each face lasted for 800 ms, separated by a 300 ms fixation, a design used successfully to study adaptation in other studies (e.g. Epstein, Graham, & Downing, 2003; Jiang et al., 2006). In each of four functional runs (each 218 s long) of this session, trials were separated by fixation intervals that varied between 4, 6, and 10 s. Each run was preceded by a 30 s fixation interval and another 30 s fixation interval at the end of each run. There were 6 trials of each face-pair condition per run for a total of 18 pairs, with no face-pairs repeated across the experiment.
Face-pair stimuli in the scanner were presented using E-Prime software. Images were back-projected onto a screen behind the MRI scanner, and participants viewed the stimuli using a mirror mounted on the head coil and indicated their responses with a button press. Faces subtended a visual angle of approximately 5.2° × 4.3°. Participants were instructed to decide, for each face-pair, whether or not the second face was exactly the same as the first face, and indicate their judgment with button presses.
Localizer Scan Stimuli and Procedure
In order to localize the FFA for each individual participant, we also performed a separate blocked design functional localizer scan session, similar to those used in several other studies for localization purposes (Epstein & Kanwisher, 1998; Epstein et al., 2003; Grill-Spector, Knouf, & Kanwisher, 2004; Jiang et al., 2006; Kanwisher et al., 1997). Participants passively viewed 60 Faces, 60 Houses, and 60 Phase-Scrambled images in the scanner. All stimuli were presented in grayscale and equated for luminance. All stimuli occupied the same visual viewing angle, which was approximately 4.6° × 6.3°. There was one functional run (370 s long), with 4 blocks each of the Face, House, and Scrambled conditions. 15 pictures were presented per block with stimulus duration of 2 s, with the order of conditions randomized across participants, who were instructed to simply view the stimuli.
Imaging Protocol
All brain imaging data were acquired using a 3.0T Siemens Allegra scanner (Siemens, Erlangen, Germany) with a single-channel head coil. Functional scans were acquired with 32 axial slices oriented along the anterior and posterior commissural axis, slice thickness of 4 mm (0.4 mm gap), 3 × 3 mm in-plane voxel sizes, and 64 × 64 matrix, giving an in-plane FOV of 192 × 192 mm; TR was 2000 ms and echo-time 32 ms. For each participant, 109 functional scans were acquired for each of the 4 runs of the adaptation session and 185 functional scans were acquired for the localizer session. Co-planar structural T2 images were also acquired to register and overlay the functional images to a 3D-MPRAGE T1 structural image in Talairach space. Imaging data were processed using BrainVoyager QX version 2.0 (Brain Innovation, Maastricht, The Netherlands) and in-house Matlab scripts. Motion and slice-time corrections were applied to the functional data, along with a Gaussian smoothing kernel with full-width at half maximum of 8 mm. A temporal high-pass filter with cut-off at 3 cycles within the data time course for each run was also applied.
Localizing Individual FFAs
For each participant’s functional data from the localizer session, a general linear model (GLM) was applied to each voxel. The model consisted of a design matrix with Face, House, and Scrambled condition onset predictors convolved with the canonical hemodynamic response function (HRF) to obtain response estimates for each condition (note, the Scrambled condition is not utilized in this current experiment and is left out of subsequent analyses). To isolate individual FFA regions-of interest (ROIs) for each participant, we first identified the peak voxel that responded most significantly to Faces in contrast to Houses on the left and right fusiform regions in each participant. The identified voxels on each side had a minimum threshold of p < . 05 (uncorrected). This lenient threshold was used so that nearly all participants’ FFA could be identified and contributed to the later analyses of the fMR-A session data. Participants with no identifiable peak in the FFA even at this low threshold were excluded from the individual ROI based analyses for that hemisphere (right: 3 young and 2 older participants were excluded; left: 3 young and 1 older participants). Next, for each of the participants’ left and right FFA peaks, we selected the 10 most face selective voxels as defined by the Face > House contrast, within a 20 mm cube around each peak (Park et al., 2004; Spiridon & Kanwisher, 2002). These top 10 most face selective voxels in the left and right FFA constituted the FFA ROIs for each participant that were used to extract responses to the three face-pair conditions of the face discrimination task from the fMR-A session data. This method involved minimal assumptions about where the FFA is located in each individual, and allowed us to compare age differences in selectivity within brain regions that are already selective at the individual level. We performed the same analyses using the top 15 and 20 voxels and found similar results for significance tests, thus, only the analysis of the top 10 voxels are reported in this article.
fMRI Adaptation Data Analysis
For each participant’s functional data from the adaptation session, we modeled the hemodynamic response in each brain voxel using a GLM consisting of finite impulse response (FIR) functions. This FIR model consisted of nine time point predictors (delta-functions), convolved with the stimulus onsets for each of the three face-pair conditions of the face discrimination task (Identical, Moderate, Different), as well as an additional six predictors that further accounted for possible motion not captured during the motion correction step (33 predictors per participant in total). Note that in keeping with this rapid sequential adaptation design, the responses to the two faces in each trial were modeled as the linear summed response for that trial rather than as two separate events (Boynton et al., 1996; Dale & Buckner, 1997; Epstein et al., 2003; Goh et al., 2004; Jiang et al., 2006).
The FFA ROIs of each participant identified from the localizer session data were then applied to each participant’s estimated adaptation session GLM data. From these ROIs, we extracted the nine FIR estimates constituting the response time course for each of the three face-pair conditions in the left and right FFA of each participant. In each participant, the peak of the FIR time course typically occurred within 6 to 10 s from stimulus onset, corresponding to the 4th to 6th time points. The estimates from the maximum peak time point from each participant were then used to compute the adaptation magnitudes to each condition in the ROI. Adaptation magnitudes were defined as the amount of response reduction for both the Moderate condition (Different response – Moderate response) and the Identical condition (Different response – Identical response), relative to the Different condition, for each participant. Corresponding repeated-measures analysis of variance (ANOVA) with age (young, old) and condition (Identical, Moderate, Different) as factors, and planned comparisons were then performed on these FIR peak time points and computed adaptation magnitudes.
We also isolated frontal regions sensitive to task difficulty by performing a multi-subject whole-brain conjunction analysis on the FIR time course peaks from the adaptation data. Specifically, frontal group-level ROIs were obtained using a conjunction of two contrasts: the Moderate > Identical contrast, and the Moderate > Different contrast, at a threshold of p < .005 and cluster size > 10. This conjunction analysis identified voxels that showed significantly more activity during the Moderate condition (the most difficult condition for face discrimination) compared to both the Identical and Different conditions (the easier conditions). We defined the ROIs as contiguous significant voxels within a 30 mm cube around the peak voxels to capture all significant voxels resulting from this contrast. We then evaluated age differences in recruitment of these frontal ROIs using ANOVA and planned comparisons of the peak FIR responses to the face-pair conditions from each participant.
Face Discrimination Thresholds
We also conducted an out-of-scanner adaptive testing experiment to determine behavioral face discrimination thresholds for each participant, after the scanning sessions. Another 40 sets of faces were obtained and morphed as previously described for use in this behavioral component. Participants were presented with trials of face-pairs in succession using Psychtoolbox for Matlab. Stimulus presentation parameters and participants’ task were the same as those in the in-scanner face discrimination task except that the inter-trial interval was fixed at 3000 ms and the degree of face-pair morph difference in each trial was adaptively adjusted based on participants’ responses in the previous trial. For example, if the face-pair of a current trial was at 30% morph difference, and a participant responded “same”, the program would present a face-pair morphed at a higher level in the next trial (e.g. 45% morph difference). If the participant responded “different”, however, the program would present a more similar pair in the next trial (e.g. 15%). During a single adaptive testing session, the amount of morph adjustment became progressively smaller across trials to calibrate the discrimination threshold. The face discrimination threshold for a single test session was defined as the percentage morph level about which participants fluctuated between a “same” or “different” response the most (the mode) during that session. Participants underwent 6 sessions of 40 trials each, and the individual discrimination threshold was obtained as the mean mode of these sessions.
RESULTS
In-Scanner Face Discrimination Performance
Repeated measures ANOVA with age and condition as factors were computed for the in-scanner face discrimination task performance. The analysis showed a significant linear trend in the accuracy of detecting face-pair differences such that the probability of responding “different” increased with the level of morph difference of the face-pair stimuli from the Identical, to Moderate, to Different conditions [F(1, 38) = 1703.38, p < .01, η2 = .98; Figure 2a]. There was no effect of age and no interaction. This nearly identical behavioral performance across age groups in the scanner further excludes the possibility of age differences in neural response due to visual acuity. Analysis of response times showed a significant quadratic effect of condition [F(1, 38) = 73.75, p < .01, η2 = .66; Figure 2b] that confirmed the difficulty of the Moderate condition. Specifically, participants took significantly longer in the Moderate condition compared to the Identical [t(39) = 6.69, p < .01] and the Different [t(39) = 8.17, p < .01] conditions, with no difference between the Identical and Different conditions. Again, there was no effect of age and no interaction, with only a somewhat longer response time in older adults than young adults for the Moderate condition [t(38) = 1.73, p < .05].
Figure 2.
Behavioral performance during the face discrimination task in the scanner. a) Probability that participants respond “different” for each face-pair condition. Error bars denote s.e. for all graphs. b) Mean response times for each condition.
Greater FFA Adaptation to Moderately Different Face-Pairs in Older Adults
The right and left FFA peaks of individual young and older participants are displayed on axial slices in Figure 3. Mean Talairach locations of FFA peaks across all participants for the right FFA [Mean (s.d.) x, y, z coordinates in mm: 39 (3.4), −42 (6.1), −17 (4.9)] and left FFA [−39 (3.4), −45 (8.5), −17 (3.5)] were consistent with previous reports (Kanwisher et al., 1997).
Figure 3.
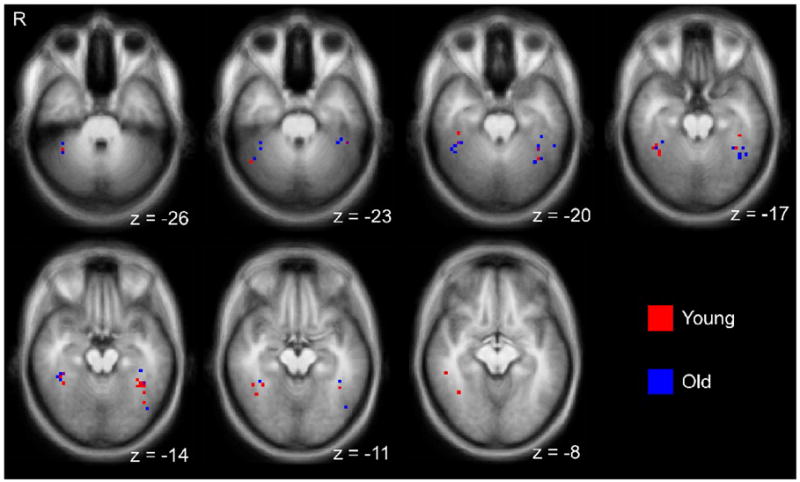
Face selective peak voxels in the left and right fusiform regions from individual participants, identified from the localizer scan. Voxels are overlaid on a group averaged anatomical brain image.
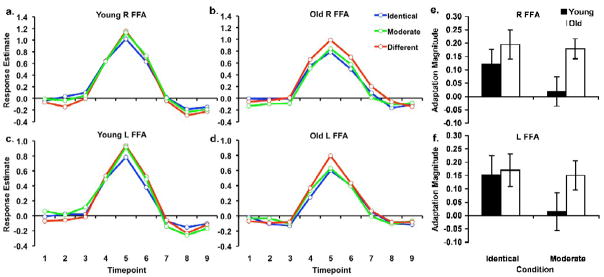
The mean FIR time course responses in the right and left FFA ROIs individually defined from the peaks (see Methods) are shown in Figure 4(a–d) for the Identical, Moderate and Different face-pair conditions, for young and older participants. Because different numbers of participants showed peaks in the right and left FFA, each hemisphere was analyzed separately. Analysis of the time course peak responses in the right FFA revealed a significant linear effect of Condition [F(1, 33) = 15.86, p < .01, η2 = .33], no main effect of Age, and, critically, an Age × ConditionQuadratic Interaction [F(1, 33) = 4.43, p < .05, η2 = .12; Figure 4a, b]. Specifically, whereas young adults only showed significantly lower response to the Identical relative to the Different Condition [t(16) = 2.10, p < .05], older adults showed significantly reduced responses during both the Identical [t(17) = 3.57, p < .01] and the Moderate [t(17) = 4.79, p < .01] Conditions relative to the Different Condition. Both young and older adults responded equivalently during the Different Condition [t(33) = .65, n.s.]. Similarly, in the left FFA, there was also a linear effect of Condition [F(1, 34) = 11.69, p < .01, η2 = .26], no main effect of age, and an Age × ConditionQuadratic interaction that approached significance [F(1, 34) = 2.79, p = .10, η2 = .08; Figure 4c, d]. Young adults only showed significantly lower response to the Identical relative to the Different Condition [t(16) = 2.11, p < .05], older adults showed significantly reduced responses during both the Identical [t(18) = 2.77, p < .01] and the Moderate [t(18) = 2.74, p < .01] Conditions relative to the Different Condition, and both young and older adults responded equivalently during the Different Condition [t(34) = 1.08, n.s.].
Figure 4.
Responses to the face-pair conditions in the right and left FFA ROIs defined from peak voxels identified in each individual. Mean FIR time courses to the three conditions for young and older adults are shown for the right (a, b) and left (c, d) FFAs. Adaptation magnitudes during the Identical and Moderate conditions are also shown for the right (e) and left (f) FFAs. Error bars denote s.e.
Follow-up direct comparisons of the adaptation magnitudes in these FFA ROIs corroborated the peak response analysis. There was significantly greater adaptation in older adults than young adults during the Moderate Condition in the right FFA [t(33) = 2.43, p < .01, Figure 3e] with a marginal effect of age in the left [t(34) = 1.54, p < .08, Figure 3f] but no age differences in adaptation responses during the Identical condition [right: t(33) = .95, n.s; left: t(34) = .18, n.s.].
These results are consistent with reduced selectivity in the FFA of older adults for subtle face-pair differences during the Moderate condition. The FFA in older adults reflected less distinctive face representations by responding with greater adaptation to the moderately different face-pairs, thereby treating them more like Identical face-pairs compared to young adults.
Behavioral Face Discrimination Thresholds and FFA Adaptation
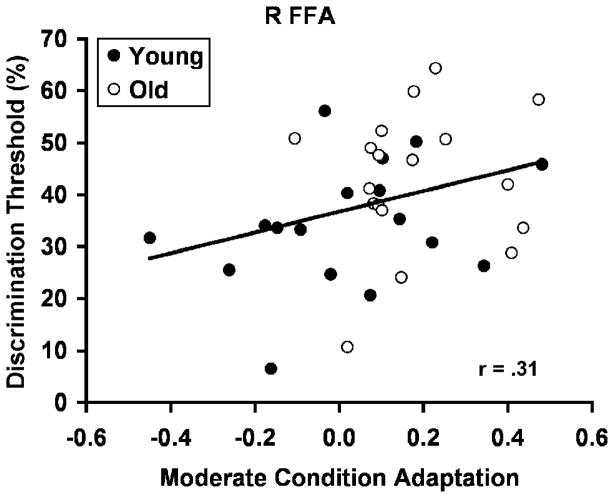
Analysis of the individual face discrimination thresholds (out of the scanner) showed that older adults had a higher discrimination threshold than young [mean threshold in old: 41.6 % (s.d. = 13.3) vs. young: 34.2% (s.d. = 12.0); t(38) = 1.87, p < .05], a finding congruent with the notion that older adults require greater face-pair differences before they can discriminate them. Nevertheless, we note that we did not find behavioral differences in the in-scanner performance of face discrimination, probably reflecting the greater sensitivity of the thresholding procedure. The behavioral differences are consistent with the literature on face memory and discrimination. To understand the relationship between behavioral and neural response, we conducted a correlation of the FFA adaptation magnitude during the Moderate Condition to the behavioral discrimination threshold in the whole sample (Figure 5). There was a significant positive correlation in the right FFA [r = .31, p < .05] but not in the left [r = .16], indicating that participants who showed greater right FFA adaptation also had higher discrimination thresholds. The within-group correlations were not significant for either age group alone [young: r = .31; old: r = .10], but the correlations were in the expected direction and the correlation approached significance in young. The failure to find within-group correlations is not surprising given that we had a small sample size and necessarily used difference scores as a measure of adaptation. In addition, the range between the minimum and maximum FFA adaptation in older adults was narrower than in younger adults (.58 vs. .93). Thus, range restriction of adaptation responses in older adults may have also limited the correlation with discrimination threshold. Importantly, as a group, older adults had greater FFA adaptation as well as higher discrimination thresholds than young adults.
Figure 5.
Correlation between right FFA adaptation during the Moderate condition and participants’ face discrimination thresholds. Regression line (and r value) is shown for the entire sample of both young and older participants together.
Frontal Activation and Task Difficulty
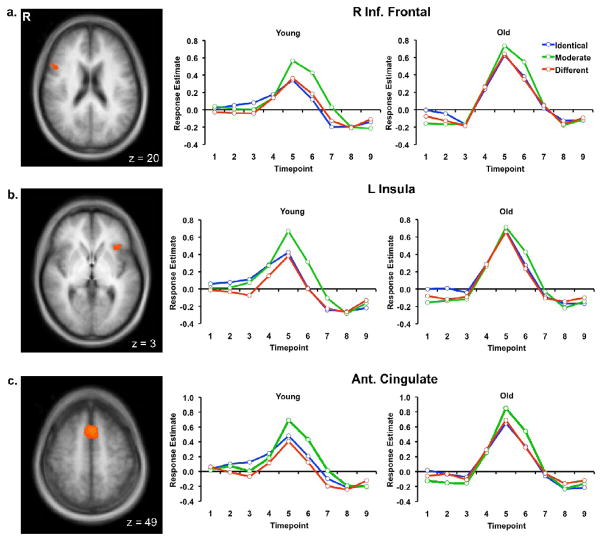
The whole-brain conjunction analysis of regions sensitive to task difficulty revealed frontal areas (Figure 6) that included the right inferior frontal gyrus (Talairach peak: 48, 14, 22), the left insula (Talairach peak: −30, 23, 10), and the anterior cingulate (Talairach peak: −3, 14, 49). Within these frontal regions, we examined age differences in time course peak activity for each of the three task conditions. Generally, while younger adults effectively modulated frontal activity in response to task difficulty (with the Moderate Condition being the most difficult), older adults engaged greater frontal activity across the three conditions and were less sensitive to different levels of difficulty. In the right inferior frontal region (Figure 6a), there was a quadratic effect of Condition [F(1, 38) = 21.07, p < .01, η2 = .36] and a main effect of Age [F(1, 38) = 5.87, p < .05, η2 = .13] that was due to older adults engaging greater levels of activity than young adults in this frontal region. In the left insula (Figure 6b), there was a marginal effect of Age [F(1, 38) = 2.85, p = .10, η2 = .07] and a quadratic effect of Condition [F(1, 38) = 8.43, p < .01, η2 = .18]. These two variables interacted [F(1, 38) = 5.14, p < .05, η2 = .12] due to older adults engaging greater activity than young adults in the Identical [t(38) = 1.70, p < .05] and Different [t(38) = 2.85, p < .01] Conditions, but the two age groups had equivalently high levels of activity in the Moderate Condition [t(38) = .38, n.s.]. In the anterior cingulate (Figure 6c), there was a quadratic effect of Condition [F(1, 38) = 19.57, p < .01, η2 = .34] and a marginal effect of Age [F(1, 38) = 3.23, p < .10, η2 = .08] with older adults engaging more than younger adults. Overall, these frontal findings are consistent with the notion that older adults required more neural activity than young adults to make discrimination judgments during the easier conditions.
Figure 6.
Axial slices showing frontal ROIs in the a) right inferior frontal, b) left insula, and c) anterior cingulate regions, sensitive to task difficulty along with their FIR time courses for the three face-pair conditions for both young and older adults.
Interestingly, we also found significant correlations between frontal responses during the Moderate Conditions and right FFA adaptation magnitudes (Supplementary Figure 1). Greater right FFA adaptation, but not left, was associated with greater activity during the Moderate Conditions in the left insula [r = .32, p < .05] and anterior cingulate [r = .43, p < .01] in the whole sample, with no significant correlations with the right inferior frontal region. In addition, when the age groups were analyzed separately, young adults showed significant positive correlations [left insula: r = .53, p < .05; anterior cingulate: r = .42, p < . 05] whereas the correlations were marginal or not significant in older adults [left insula: r = .03, n.s.; anterior cingulate: r = .37, p = .06]. Unlike in the right FFA, we did not find significant correlations between activity in these frontal regions and discrimination thresholds. Taken together, these findings are consistent with the notion that reduced selectivity in the right FFA is associated with increases in activity in the frontal regions in younger adults. Moreover, older adults may have poorer functional correlation between the frontal and posterior regions than young adults during the face discrimination task.
DISCUSSION
The results of this study indicate that dedifferentiation of neural responses in older adults is associated with a reduction of the distinctiveness of within-category representations in ventral visual cortex. In support of this point, we reported that older adults showed greater adaptation in the FFA when faces in a pair were moderately similar, indexing less neuronal selectivity and less distinctive face representations, whereas younger adults showed minimal adaptation. Older adults also required higher levels of differences between face-pairs in order to discriminate the faces outside the scanner, and these discrimination levels were associated with the level of adaptation in the right FFA across the entire sample. Additionally, in the frontal regions, we found greater levels of activity in older adults compared to young adults. Whereas young adults were able to modulate activity in response to task difficulty in frontal regions, older adults did not modulate neural response to task demands, evidencing equivalently large amounts of neural activity in frontal regions when making both easy and difficult judgments, particularly in the left insula. Finally, greater adaptation in the right FFA was associated with greater frontal activity in the anterior cingulate and left insula in young adults, whereas older adults showed a lower correlation between the two regions.
The reduced selectivity we observed in older adult ventral visual areas for within-category stimuli shows similarities to primate studies on loss of selectivity with aging at the single-neuron level. As previously mentioned, Schmolesky et al. (2000) and others showed that V1 neurons in old rhesus monkeys responded more non-selectively to varying line orientations, whereas neurons in young monkeys responded only to a specific preferred orientation. Importantly, this pattern of reduced selectivity with aging is also propagated and accentuated in V2 and middle temporal areas later on in the visual processing stream (Wang et al., 2005; Yang et al., 2008; Yu et al., 2006), and has also been demonstrated in cats (Hua et al., 2006). This ubiquity of age-related reduction of selectivity at the single-neuron level across regions of the ventral visual cortex observed in different species of older animals, combined with other demonstrations of dedifferentiation in a number of human neural and behavioral studies (Baltes & Lindenberger, 1997; Goh & Park, 2009; Li, Lindenberger, & Sikström, 2001; Lindenberger, Scherer, & Baltes, 2001; Park & Goh, 2009; Park et al., 2004; Park & Reuter-Lorenz, 2009) supports the notion that dedifferentiation is a central biological process that occurs in many species under a broad range of conditions. This decreased neural selectivity results from a neural infrastructure that is less able to support the processing of subtle differences among stimuli. Conceivably, the consequence of this will be that cognitive representations are less distinctive with aging, affecting many cognitive operations, including encoding and retrieval. Indeed, older adults have particular difficulty in visually discriminating and remembering faces (Bartlett & Leslie, 1986; Bartlett et al., 1989; Habak et al., 2008; Owsley et al., 1981; Smith & Winograd, 1978), as well as discriminating other types of stimuli other than faces (Baracat & Marquie, 1992; Betts, Sekular, & Bennett, 2007; Spear, 1993). In addition, Wang et al.’s (2005) study showed that along with reduced neural selectivity in older primate V1 neurons, transfer of information from one neural region to the next was also delayed, possibly because of the increased time required to resolve non-selective signals in each region. Thus, dedifferentiation is potentially also a source of age-related slowing observed in behavioral studies (Ratcliff, Thapar, & McKoon, 2003; Salthouse, 1996). Rousselet et al. (2009) also demonstrated an age-related delay in humans in the onset of electrophysiological signals known to reflect face processing (P1 and N170 components). These delays in neural signal propagation in early visual processing may cumulatively result in older adults taking longer times for cognitive processing further downstream.
We note that the above arguments suggest neural dedifferentiation should be related to behavioral function. We did find evidence that adaptation differences correlated with behavioral face discrimination thresholds, but these results were only significant when we examined the entire sample. We recognize it to be critically important that neural measures of dedifferentiation show reliable correlations with a range of cognitive behaviors. fMRI adaptation within the FFA measures very small differences in neural responses that may have limited power to detect linkages between neural selectivity and behavior. Moreover, it is possible that the task demands in this study may have affected adaptation responses (Henson et al., 2002; Murray & Wojciulik, 2004; Yi, Kelley, Marois, & Chun, 2006) and masked out the neural and behavioral relationships. Future studies comparing the effect of task demands and attention, as well as more sensitive functional techniques (e.g. whole-brain multivariate pattern classification), are necessary to further elucidate the relationship between reduced neural selectivity and behavior in older adults.
Our finding of greater frontal recruitment in older adults is consonant with previous studies (Cabeza et al., 1997, 2002; Davis et al., 2008; Grady et al., 1994; Madden et al., 1997, 1999; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Lustig, 2005; Reuter-Lorenz & Cappell, 2008) and reflects either compensatory engagement for task performance or less efficient processing. In the present study, older participants in our sample performed equivalently as young participants during the in-scanner face discrimination task. Given the lack of age differences in the in-scanner behavioral accuracy as well as the reduced FFA selectivity in older adults that was associated with increased frontal activity, we suggest that the increased frontal engagement observed in this experiment played a compensatory role for declines in FFA processing, resulting in on par performance between young and old. Alternatively, older adults may also have been less efficient at engaging frontal processing to modulate FFA processing, as seen in the lack of a frontal-FFA correlation in older adults. Thus, older adults may have required more resources than young adults to arrive at an accurate discrimination. In all cases, however, it is clear that the same task decision which young adults make using relatively little resources requires greater frontal processing in older adults. When the task becomes more demanding, as is the case for the individual discrimination threshold task, the compensatory mechanisms may be insufficient, and older adults evince poorer performance than young adults.
In closing although previous studies have shown age-related reductions in specificity of ventral visual responses to faces and other categories of stimuli in older adults (Davis et al., 2008; Grady et al., 2000; Park et al., 2004), we have demonstrated age-related dedifferentiation more specifically by showing a within-category reduction of neural selectivity with aging. Moreover this decreased selectivity with age is associated with frontal changes and is broadly related to decreased ability to discriminate faces. While the findings in this present study pertain to face processing in the FFA, we submit that similar mechanisms are operating in other ventral visual regions of older adults as well. Moreover, age-related performance declines in other cognitive domains may stem from a similar lack of representational contrast due to loss of effective neural selectivity in other non-ventral visual cortical systems (Li et al., 2001). Possible subsequent directions for research include relating discrimination thresholds in other cognitive domains to neural selectivity as well as evaluating the effects of subjective perception (Fox, Moon, Iaria, & Barton, 2009; Rotshtein et al., 2005), attention and behavioral discrimination training in improving representational contrasts in ventral visual cortex of older adults.
Supplementary Material
Supplementary Figure 1. Correlations between right FFA adaptation during the Moderate condition and responses during the Moderate condition in the a) anterior cingulate and b) left insula. Regression lines are shown for the entire sample.
Acknowledgments
This work was supported by NIH grant number R-37-AG006265-26 awarded to Denise Park. Marion Reeds assisted in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baltes P, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Baracat B, Marquie JC. Age differences in sensitivity, response bias, and reaction time on a visual discrimination task. Experimental Aging Research. 1992;18(1–2):59–66. doi: 10.1080/03610739208253912. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TD, Nystrom LE, Forman SD, Noll DC, Cohen JD. Dissociating working memory from task difficulty in human prefrontal cortex. Neuropsychologica. 1996;35(10):1373–1380. doi: 10.1016/s0028-3932(97)00072-9. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE. Aging and memory for faces versus single views of faces. Memory & Cognition. 1986;14(5):371–381. doi: 10.3758/bf03197012. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE, Tubbs A, Fulton A. Aging and memory for pictures of faces. Psychology and Aging. 1989;4(3):276–283. doi: 10.1037//0882-7974.4.3.276. [DOI] [PubMed] [Google Scholar]
- Betts LR, Sekuler AB, Bennett PJ. The effects of aging on orientation discrimination. Vision Research. 2007;47(13):1769–1780. doi: 10.1016/j.visres.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17(3):1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Human Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? the posterior anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dricot L, Sorger B, Schiltz C, Goebel R, Rossion B. The roles of “face” and “non-face” areas during individual face perception: Evidence by fMRI adaptation in a brain-damaged prosopagnosic patient. NeuroImage. 2008;40:318–332. doi: 10.1016/j.neuroimage.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37:865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Moon SY, Iaria G, Barton JJS. The correlates of subjective perception of identity and expression in the face network: An fMRI adaptation study. NeuroImage. 2009;44:569–580. doi: 10.1016/j.neuroimage.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilaie-Dotan S, Malach R. Sub-exemplar shape tuning in human face-related areas. Cerebral Cortex. 2007;17(2):325–338. doi: 10.1093/cercor/bhj150. [DOI] [PubMed] [Google Scholar]
- Goh JOS, Park DC. Neuroplasticity and cognitive aging: The scaffolding theory of aging and cognition. Restorative Neurology and Neuroscience. 2009;27:1–13. doi: 10.3233/RNN-2009-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JOS, Soon CS, Park D, Gutchess A, Hebrank A, Chee MWL. Cortical areas involved in object, background, and object-background processing revealed with functional magnetic resonance adaptation. Journal of Neuroscience. 2004;24(45):10223–10228. doi: 10.1523/JNEUROSCI.3373-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14(3):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Rapoport SI. Age-related changes in the neural correlates of degraded and nondegraded face processing. Cognitive Neuropsychology. 2000;17:165–186. doi: 10.1080/026432900380553. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: A tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107(1–3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7(5):555–561. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A. Repetition and the brain: Neural models of stimulus-specific effects. Trends in Cognitive Sciences. 2006;10(1):14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Habak C, Wilkinson F, Wilson HR. Aging disrupts the neural transformations that link facial identity across views. Vision Research. 2008;48(1):9–15. doi: 10.1016/j.visres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Progress in Neurobiology. 2003;70(1):53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Gorno-Tempini ML, Dolan RJ. Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cerebral Cortex. 2002;12:178–186. doi: 10.1093/cercor/12.2.178. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiology of Aging. 2006;27(1):155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rosen E, Zeffiro T, Vanmeter J, Blanz V, Riesenhuber M. Evaluation of a shape-based model of human face discrimination using FMRI and behavioral techniques. Neuron. 2006;50(1):159–172. doi: 10.1016/j.neuron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber EJ, Awh E, Minoshima S, et al. Verbal working memory load affects regional brain activation as measured by PET. Journal of Cognitive Neuroscience. 1997;9(4):462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Kadosh KC, Henson RNA, Kadosh RC, Johnson MH, Dick F. Task-dependent activation of face-sensitive cortex: An fMRI adaptation study. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21224. in press. Epub ahead of print retrieved September 14 2009, from http://www.ncbi.nlm.nih.gov/pubmed/19320548. [DOI] [PubMed]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Moscovitch M. The cognitive neuroscience of face processing: An introduction. Cognitive Neuropsychology. 2000;17(123):1–11. doi: 10.1080/026432900380454. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, O’Sullivan BT, Roland PE. Bilateral activation of fronto-parietal networks by incrementing demand in a working memory task. Cerebral Cortex. 1997;7:465–471. doi: 10.1093/cercor/7.5.465. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proceedings of the National Academy of Sciences. 2007;104(51):20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5(11):479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Scherer H, Baltes PB. The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychology and Aging. 2001;16(2):196–205. doi: 10.1037//0882-7974.16.2.196. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Human Brain Mapping. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Hawk TC, Hoffman JM, Coleman RE. Selective and divided visual attention: Age-related changes in regional cerebral blood flow measured by H215O PET. Human Brain Mapping. 1997;5:389–409. doi: 10.1002/(SICI)1097-0193(1997)5:6<389::AID-HBM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behavior Research Methods, Instruments, & Computers. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nature Neuroscience. 2004;7(1):70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sekuler R, Boldt C. Aging and low-contrast vision: Face perception. Investigative Ophthalmology & Visual Science. 1981;21(2):362–365. [PubMed] [Google Scholar]
- Park DC, Goh JOS. Successful aging. In: Cacioppo J, Berntson G, editors. Handbook of Neuroscience for the Behavioral Sciences. Hoboken, NJ: Wiley; 2009. pp. 1203–1219. [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences. 2004;101(35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: Aging and neurocognitive scaffolding. Annual Review of Psychology. 2009;60(1):173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Thapar A, McKoon G. A diffusion model analysis of the effects of aging on brightness discrimination. Perception & Psychophysics. 2003;65(4):523–535. doi: 10.3758/bf03194580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: Reorganizing discoveries about the aging mind. Current Opinion in Neurobiology. 2005;15(2):245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science. 2008;17(3):177–182. [Google Scholar]
- Rotshtein P, Henson RNA, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neuroscience. 2005;8(1):107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Pernet CR, Gaspar CM, Bennett PJ, Sekuler AB. Age-related delay in information accrual for faces: Evidence from a parametric, single-trial EEG approach. BioMed Central Neuroscience. 2009;10:114. doi: 10.1186/1471-2202-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Glover GH, Gabrieli JDE. Load-dependent roles of frontal brain regions in the maintenance of working memory. NeuroImage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schiltz C, Bettina S, Caldara R, Ahmed F, Mayer E, Goebel R, Rossion B. Impaired face discrimination in acquired prosopagnosia is associated with abnormal response to individual faces in the right middle fusiform gyrus. Cerebral Cortex. 2006;16:574–586. doi: 10.1093/cercor/bhj005. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience. 2000;3(4):384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Smith AD, Winograd E. Adult age differences in remembering faces. Developmental Psychology. 1978;14(4):443–444. [Google Scholar]
- Spear PD. Neural bases of visual deficits during aging. Vision Research. 1993;33(18):2589–2609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Kanwisher N. How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron. 2002;35:1157–1165. doi: 10.1016/s0896-6273(02)00877-2. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhou Y, Ma Y, Leventhal AG. Degradation of signal timing in cortical areas V1 and V2 of senescent monkeys. Cerebral Cortex. 2005;15(4):403–408. doi: 10.1093/cercor/bhh143. [DOI] [PubMed] [Google Scholar]
- Winston JS, Henson RNA, Fine-Goulden MR, Dolan RJ. fMRI-Adaptation reveals dissociable neural representations of identity and expression in face perception. Journal of Neurophysiology. 2004;92:1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Li G, Wang Y, Zhou Y, Leventhal AG. Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience. 2008;156:748–757. doi: 10.1016/j.neuroscience.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Yi D, Kelley TA, Marois R, Chun MM. Attentional modulation of repetition attenuation is anatomically dissociable for scenes and faces. Brain Research. 2006;1080:53–62. doi: 10.1016/j.brainres.2006.01.090. [DOI] [PubMed] [Google Scholar]
- Yu S, Wang Y, Li X, Zhou Y, Leventhal AG. Functional degradation of extrastriate visual cortex in senescent rhesus monkeys. Neuroscience. 2006;140(3):1023–1029. doi: 10.1016/j.neuroscience.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Correlations between right FFA adaptation during the Moderate condition and responses during the Moderate condition in the a) anterior cingulate and b) left insula. Regression lines are shown for the entire sample.