Abstract
Background & Aims
Cholecystokinin (CCK) acts on vagal afferent neurons to inhibit food intake and gastric emptying; it also increases expression of the neuropeptide cocaine- and amphetamine-regulated transcript (CART), but the significance of this is unknown. We investigated the role of CARTp in vagal afferent neurons.
Methods
Release of CART peptide (CARTp) from cultured vagal afferent neurons was determined by ELISA. The expression of receptors and neuropeptides in rat vagal afferent neurons in response to CARTp was studied using immunohistochemistry and luciferase promoter reporter constructs. The effects of CARTp and CCK were studied on food intake.
Results
CCK stimulated CARTp release from cultured nodose neurons. CARTp replicated the effect of CCK in stimulating expression of Y2R and of CART itself in these neurons in vivo and in vitro, but not in inhibiting cannabinoid (CB)1, melanin-concentrating hormone (MCH), and MCH1 receptor expression. The effects of CCK on Y2R and CART expression were reduced by CART siRNA or brefeldin A. Exposure of rats to CARTp increased the inhibitory action of CCK on food intake after short-, but not long-duration, fasting.
Conclusions
The actions of CCK in stimulating CART and Y2R expression in vagal afferent neurons and in inhibiting food intake are augmented by CARTp; CARTp is released by CCK from these neurons, indicating that it acts as an autocrine excitatory mediator.
Keywords: CART, CCK, vagus, MCH, CB1
Vagal afferent neurons play an important role in regulating the delivery of nutrient to the small intestine via control of both gastric emptying and rates of food intake.1 They respond to gastric distension and ingestion of food mediated by the action of a variety of regulatory peptides and lipid signalling molecules.1–3 For example, the work of several groups has established that cholecystokinin (CCK) inhibits food intake and gastric emptying via stimulation of these neurons.4–8 In contrast, factors such as the gastric orexigenic peptide, ghrelin, that stimulate food intake and gastric emptying are associated with inhibition of vagal afferent neurons.9–12 Accumulating evidence over a number of years has suggested the existence of extensive patterns of interactions between the regulatory peptides acting on these neurons but there remain doubts about the functional significance and cellular basis of these interactions.13–18
Recently, it has been shown that vagal afferent neurons exhibit profound changes in their neurochemical phenotype depending on food intake over the previous day.16, 19–21 Expression in these neurons of the cannabinoid (CB)-1 receptor is increased in fasted rats and is inhibited by endogenous CCK; the CB1 agonist anandamide increases in intestine with fasting and acts via vagal afferent neurons to stimulate food intake.19, 22 Conversely, expression of Y2 receptors (Y2R) is decreased by fasting and is stimulated by endogenous CCK; the intestinal satiety peptide PPY3-36, which is released after a meal, is a Y2R agonist and there is evidence that it inhibits food intake and gastric emptying via vagal afferent neurons.20, 23, 24 The same neurons also express the genes encoding two neuropeptide transmitters: cocaine and amphetamine regulated transcript (CART) and melanin concentrating hormone (MCH).21, 25 The expression of CART is increased in response to endogenous CCK and conversely MCH expression is inhibited by endogenous CCK.16, 21 Although MCH1 receptors are also expressed by vagal afferent neurons, suggesting an autocrine regulatory function, in general little is known of the role of these neuropeptides in vagal afferent signalling.
In the central nervous system there is evidence that CARTp is associated with inhibition of food intake and MCH with stimulation.26, 27 The inhibition of food intake by CARTp is reported after administration in the hypothalamus and also via the fourth ventricle suggesting actions in the brain stem as well.28, 29 In view of the evidence that CART is expressed in vagal afferent neurons in a CCK-dependent manner we hypothesised that it might play a role in influencing the actions of CCK on these cells.16, 25 In the present study we therefore examined the action of CARTp on vagal afferent neurons. We report here that CARTp exhibits some, but not all, of the actions of CCK on vagal afferent neurons and provide the first evidence indicating an autocrine stimulatory function triggered by CCK and augmenting the action of CCK on these neurons.
Materials and Methods
Materials
CCK8s and ghrelin were obtained from Bachem (St. Helens, Merseyside, UK); leptin and phorbol 12-acetate-13-myristate ester (PMA) were obtained from Sigma (Poole, Dorset, UK). CARTp (55–102, human) was obtained from American Peptide Company (Sunnyvale, CA). Brefeldin-A (BFA) was obtained from Epicentre Biotechnologies (Madison, WI).
Animals
Adult male Wistar rats (225–300 g) were housed at 22°C under a 12 h light/dark cycle with ad libitum access to food and water, unless stated otherwise. Studies were conducted in compliance with the appropriate UK Home Office personal and project licences, and with the institutional ethical review processes of the University of Liverpool and the Institutional Animal Use and Care Committee, UC Davis.
Fasting-feeding experiments
Rats were fasted up to 24 h (water ad libitum) and subsequently refed for up to 2 h. Fasted rats received CARTp (2 nmol, i.p.), CCK8s (10 nmol i.p) or saline (i.p) and depending on the experiment were then killed at intervals after refeeding. For neurochemical studies, animals were killed by CO2 inhalation, and nodose ganglia were rapidly removed and immersed in 4% paraformaldehyde for 1 h at 22°C, followed by 25% sucrose in 1mol/L phosphate buffered saline (PBS) overnight at 4°C.
Nodose neuron cultures
Nodose ganglia were digested with 0.8–1 mg/ml collagenase type Ia (Roche Diagnostics, Indianapolis, IN) and cultured in HEPES-buffered DMEM (HDMEM) containing 10% fetal calf serum (FCS) (Hyclone/Perbio Science, Cramlington, Northumberland, UK) and 3% antibiotic/antimycotic solution as previously described.16 For many experiments, cells were transferred to serum-free medium for 2 h before treatment with CCK8s (10 nM), CARTp (up to 2 nM), PMA (100 nM), or leptin (up to 30 ng/ml); in some studies ghrelin (10 nM) or BFA (10 µg/ml) were added to the media 30 min before stimulation.
Secretion of CARTp
Vagal afferent neurons were cultured for 72 h, and transferred to serum-free medium overnight. Cells were treated with CCK, leptin or ghrelin alone or in combination for 2 h. Medium was collected for assay of CARTp secretion using a CART ELISA kit from Phoenix Pharmaceuticals Inc (Karlsruhe, Germany) according to the manufacturer’s instructions.
Immunohistochemistry
Cryostat sections of fixed nodose ganglia (5 µm) were mounted on polylysine-coated slides (Polysine; Menzel-Glaser, Braunschweig, Germany) and processed for immunohistochemistry. Cultured neurons were fixed in 4% paraformaldehyde in PBS. The following primary antibodies were used: affinity-purified rabbit polyclonal antibody to CARTp (Phoenix Laboratories, San Antonio, TX), affinity-purified goat polyclonal antibody to EGR1 (early growth response protein-1; R&D Systems, Minneapolis, MN), MCH (Santa Cruz Biotechnology, Wembley Middlesex, UK), MCH1R (Acris Antibodies; Hiddenhausen, Germany), CB1R (Santa Cruz Biotechnology), Y2R (Neuromics Antibodies), phosphoCREB (Cell Signaling Technology, Hitchin, Herts, UK) and TGN38 (Abcam, Cambridge, UK). Secondary antibodies were used as appropriate and included donkey anti-rabbit IgG conjugated to fluorescein isothiocyanate and donkey anti-goat IgG labelled with Texas Red (Jackson ImmunoResearch, West Grove, PA). Specificity of immunostaining was determined by omitting the primary antibody and by pre-incubation with an excess of appropriate peptide where available. Samples were mounted in Vectashield with 4’,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Peterborough, UK) for nuclear localization and examined using an Axioplan Universal microscope (Zeiss, Oberkochen, Germany), and images were processed using the Axio Vision 3.0 imaging system (Zeiss).
Transfection of cultured neurons
Vagal afferent neurons were cultured for 48 h in 4-well plates. Medium was removed and cells were transfected with 0.25–1.0 µg of DNA per well using CombiMag (OZ Biosciences, Marseille, France) together with Transfast reagent (Promega, Madison, WI)on a magnetic plate according to the manufacturer’s instructions. Cells were further incubated for 24–48 h before stimulation.
Plasmids
A construct consisting of 3451 bp of the CART promoter linked to luciferase (CART-Luc) was a gift from Dr M. Kuhar.30, 31 A sequence corresponding to 3034 bp of the wild-type human Y2R promoter coupled to luciferase (Y2R-Luc) has previously been described.20 A fragment containing 1.6 kb of the rat MCH promoter sequence and 44 bp of exon 1 was generated by PCR from rat genomic DNA and inserted into the luciferase reporter vector pGL4 (Promega) to create MCH-Luc. A construct containing the cAMP-response element-binding protein (CREB) dominant-negative mutant, A-CREB, was a gift of Dr D. Ginty.32 A putative EGR1 response element 2380 bp upstream of the transcriptional start site of the CART promoter was mutated from gtgggg to acaaaa in 3451 bp CART-Luc (ie ΔEGR1-CART-Luc) using a QuikChange II XL site-directed mutagenesis kit (Stratagene Europe, Amsterdam, The Netherlands). A mutant of the cAMP response element site (Cre) in the CART promoter (ΔCre-CART-Luc) has previously been described.16
RNA interference
Expression of EGR1 or CART was knocked down in vagal afferent neurons using specific small interfering RNA (siRNA) (Supplementary Table 1) . Cells were transfected with 50 nM siRNA using 3 µl SilenceMag (OZ Biosciences) for each well and incubated for 15 min on a magnetic plate. Cells were incubated for 48 h at 37°C before stimulation.
Luciferase assays
Cells were transfected with firefly-luciferase constructs and a constitutively active Renilla luciferase plasmid as an internal control (Promega). Cells were lysed after overnight stimulation and the luciferase activity was measured by dual luciferase assay (Promega) according to the manufacturer’s instructions in a Lumat LB9507 luminometer (Berthold, Redbourne, Herts, UK). Results are presented as fold increase over unstimulated control, so 1.0 signifies no change in luciferase activity.
Food intake
For studies of food intake, animals were deprived of food 0.5–13 h into their dark cycle with water ad libitum. After either 13 h (long duration fast) or 30 min (short duration fast) they received CARTp (2 nmol, i.p.) or saline and 2 h later received either CCK8s (10 nmol, i.p.) or saline. Rats were then refed (Purina 5001 Rodent Chow) and food intake was monitored at 20 min intervals for 2 h. In some experiments using short duration fasting, animals receiving saline after 30 min subsequently received saline, CCK8s alone, CARTp alone or CARTp and CCK8s together 2 h later and food intake was then determined as before.
Statistical analysis
Results are expressed as mean ± SE. For multiple group designs, the data were analysed by one-way ANOVA with Bonferroni post hoc tests. For designs with only two groups, statistical validity was assessed with unpaired student’s t-test.
Results
CCK releases CARTp from nodose ganglion neurons
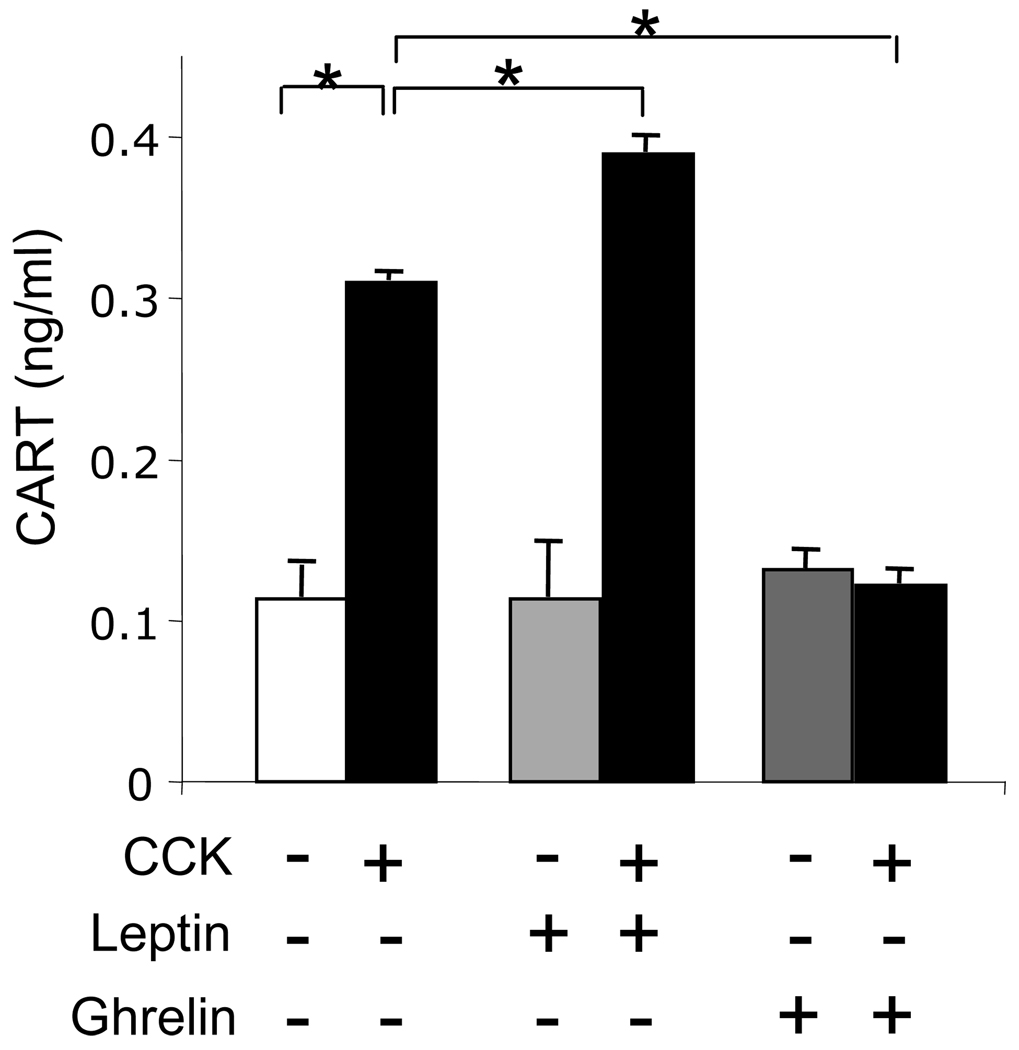
To determine whether CARTp is secreted from vagal afferent neurons in response to stimulation, we cultured neurons for 72 h and then transferred cells into serum-free medium overnight before treatment with CCK8s for 2 h. The concentration of CARTp in media from unstimulated cells was 113±20 pg/ml and in response to CCK8s was increased approximately 3-fold (p<0.001). Leptin augmented the action of CCK8s and ghrelin inhibited it (p<0.001 in both cases) but neither had any action alone (Figure 1).
Figure 1.
Increased CARTp secretion in vagal afferent neurons stimulated with CCK8s. In cultured vagal afferent neurons treated with CCK8s (10 nM, 2h) there was increased CARTp secretion that was enhanced by leptin (10 ng/ml) and inhibited by ghrelin (10 nM). Mean ± SE, n=3 independent experiments; * p<0.001.
Action of CARTp on vagal afferent neurons
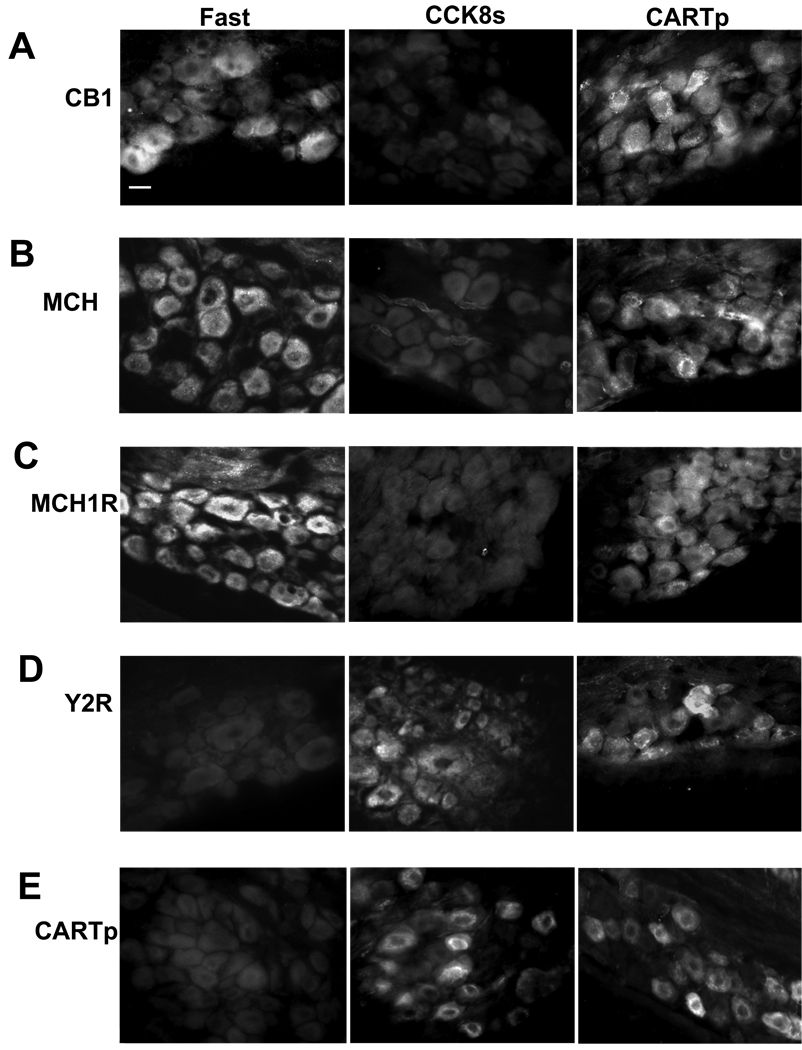
In rats fasted for 24 h there was increased expression in vagal afferent neurons of CB1R, MCH and MCH1R compared with rats fed ad libitum, but decreased expression of Y2R and CARTp (Figure 2). This pattern of expression was reversed by administration of CCK8s. Administration of CARTp to fasted rats for 2 h had no effect on CB1, MCH or MCH1R immunoreactivity but increased Y2R and CARTp immunoreactivity.
Figure 2.
CARTp increases Y2R- and CARTp- immunoreactivity in vagal afferent neurons in fasted rats but has no effect on CB1R, MCH1R and MCH abundance. (A) CB1R immunoreactivity is detected in vagal afferent neurons of rats fasted for 24 h and is decreased by CCK8s (10 nmol, ip, 1 h) but not by CARTp (2 nmol, ip, 1 h). (B) Similar data for MCH and (C) for MCH1R expression. (D) Expression of Y2R is barely detectable in nodose neurons of rats fasted 24 h but is increased by CCK8s (10 nmol, ip, 1 h) and by CARTp (2 nmol, ip, 1 h); (E) similar data for CARTp expression. Representative results from 4–5 rats in each group. Scale bar = 30 µm.
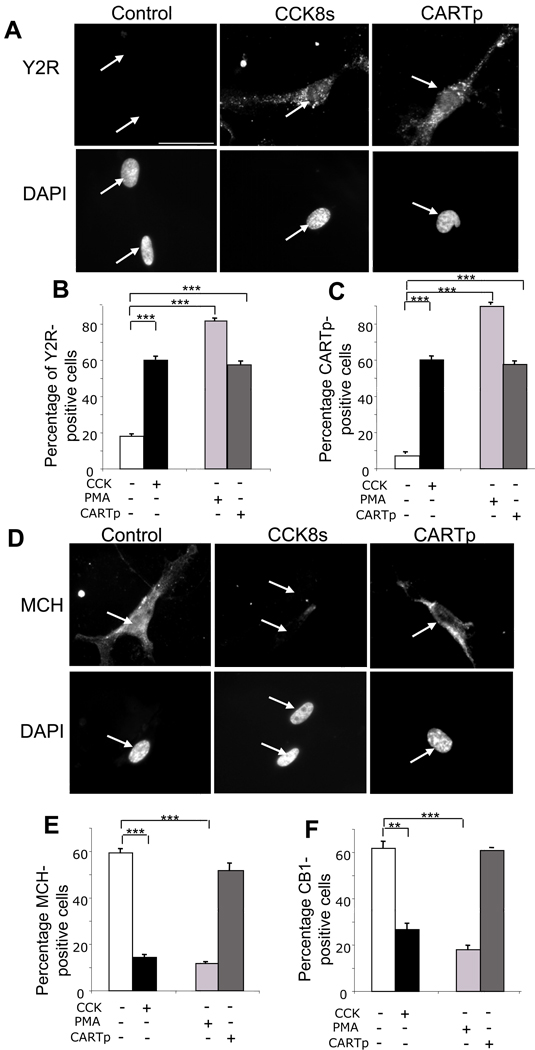
The neurochemical phenotype of vagal afferent neurons in fasted rats can, at least in some respects, be replicated in cultured neurons exposed to serum-free medium.16, 20 We examined, therefore, the action of CARTp on cultured neurons in serum-free medium. Like CCK8s and PMA, CARTp increased the proportion of neurons exhibiting Y2R and CARTp immunoreactivity (p<0.001) (Figure 3A–C; Supplementary Figure 1). However, while CCK8s and PMA both depressed the expression of MCH (p<0.001) and of CB1R (p<0.01), CARTp had no effect on MCH and CB1R expression (Figure 3D–F; Supplementary Figure 1). Thus both in vivo and in vitro, CARTp mimicked the excitatory but not the inhibitory effects of CCK8s.
Figure 3.
CARTp stimulates expression of Y2R and CARTp but has no effect on CB1R and MCH in cultured vagal afferent neurons. (A) Y2R immunoreactivity and corresponding DAPI staining in neurons (arrow) in serum-free medium (2 h; control), or treated with 10 nM CCK8s (2 h) or CARTp (2 nM) in serum-free medium. (B) Quantification of neurons exhibiting Y2R immunoreactivity after treatment with CCK8s, PMA (100 nM) or CARTp based on visual identification of positively stained neurons in counts of at least 1000 cells in each independent experiment(C) Similar data to panel B for CARTp-immunoreactive neurons. (D) MCH immunoreactivity and corresponding DAPI staining in neurons in serum-free medium (2 h control), or treated with CCK8s (10 nM) or CARTp (2 nM) in serum-free media; (E) Quantification of neurons exhibiting MCH immunoreactivity after treatment with CCK8s, PMA (100 nM) or CARTp. (F) Similar data to panel E for CB1R-immunoreactive neurons. Scale bar, 50 µm. Mean ± SE, n= 6 independent experiments; **, p<0.01; ***, p<0.001.
CARTp stimulation of CART and Y2R, but not MCH, promoters
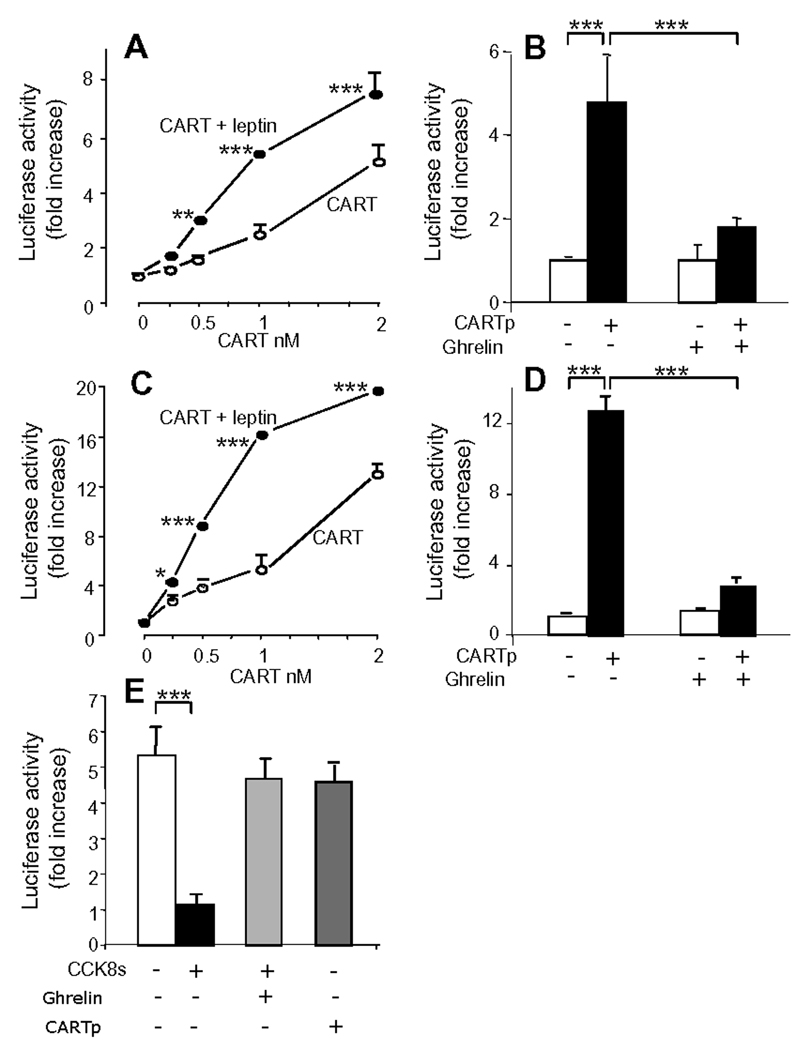
To determine whether the changes described above reflected promoter activity we used 3.45 kb of CART promoter sequence coupled to luciferase and demonstrated that over a concentration range of 0.2–2.0 nM, CARTp increased expression of CART-Luc in a concentration-dependent manner (Figure 4A). Since previous work has established that the excitatory effects of CCK on vagal afferent neurons are potentiated by leptin and inhibited by ghrelin,12, 15, 33 we examined the effect of these peptides on CART-Luc responses. The action of CARTp on CART-Luc was potentiated by leptin and inhibited by ghrelin (Figure 4A,B). In parallel experiments, CARTp also stimulated Y2R-Luc expression and this was significantly potentiated by leptin, and inhibited by ghrelin (Figure 4C,D). We then examined expression of an MCH promoter-reporter construct. In serum-free media there was expression of MCH-Luc that was depressed by CCK8s. However, CARTp had no effect on MCH-Luc expression (Figure 4E).
Figure 4.
CARTp stimulation of CART-Luc and Y2R-Luc in vagal afferent neurons: potentiation by leptin and inhibition by ghrelin. (A) Graded concentrations of CARTp stimulated expression of 3.45 kb CART-Luc, and in the presence of leptin (10 ng/ml) the curve was moved to the left. (B) Pre-incubation (30 min) with 10 nM ghrelin inhibited CART-Luc responses to 2 nM CARTp. (C) Graded concentrations of CARTp stimulated expression of 3.034 kb Y2R-Luc and leptin (10 ng/ml) moved the curve to the left. (D) Pre-incubation (30 min) with 10 nM ghrelin inhibited Y2R-Luc responses to 2 nM CARTp. (E) CCK8s (10 nM) inhibited expression of 1.6 kb of MCH-Luc promoter-reporter construct, and this was reversed by ghrelin (10 nM), but CARTp (2 nM) had no effect. Mean ± SE., n=3 independent experiments; * p<0.05, ** p<0.01, *** p<0.001.
CARTp mediation of CCK effects
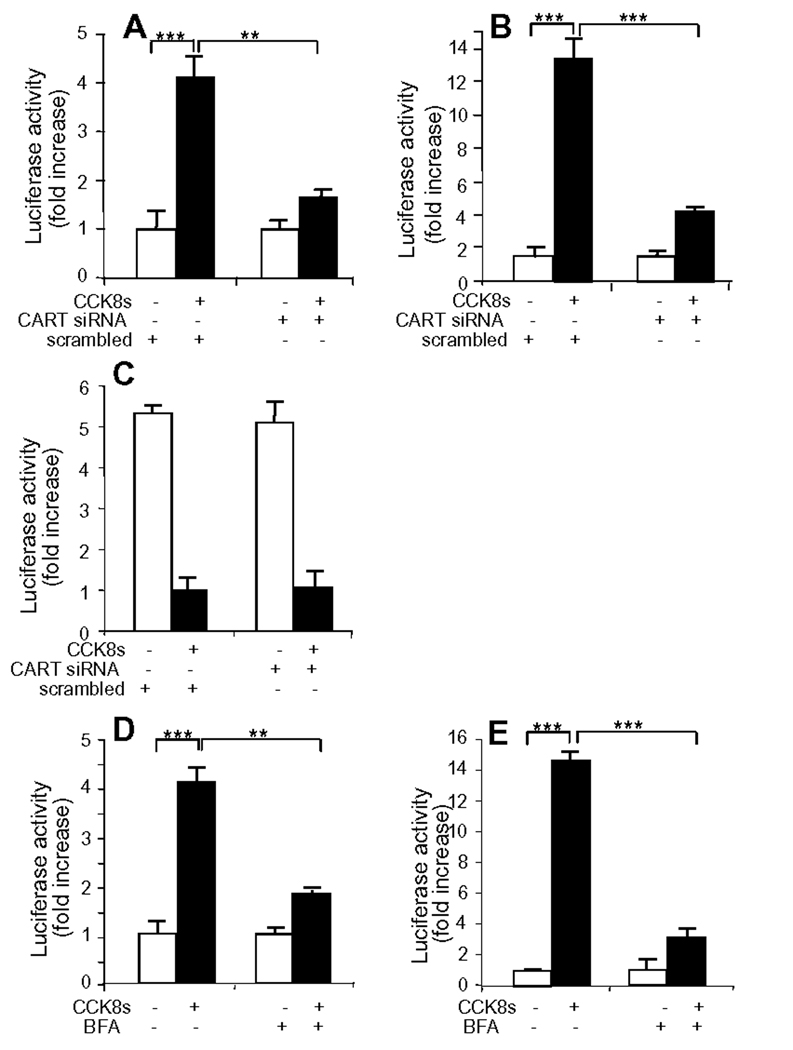
The data presented above led us to hypothesize that CCK released CARTp from vagal afferent neurons which then acted in an autocrine positive feedback loop to stimulate CART and Y2R expression. To test this we first suppressed CART expression using siRNA. Treatment of cultured vagal afferent neurons with CART siRNA significantly reduced the CART-Luc and Y2R-Luc responses (Figure 5A,B) to CCK8s. However, there was no difference in the response of MCH-Luc to CCK8s (Figure 5C). Since release of CARTp depends on secretion of the peptide from secretory vesicles we then examined the consequences of inhibiting CARTp progression along the secretory pathway using BFA. In the presence of BFA there was localization of CARTp-immunoreactivity to a perinuclear region consistent with failure to progress from the endoplasmic reticulum, and TGN38 exhibited a dispersed cytosolic distribution consistent with disruption of the trans-Golgi network (supplementary Figure 2). In BFA-treated cells, there was inhibition of the action of CCK8s in stimulating CART-Luc (Figure 5D) and Y2R-Luc (Figure 5E).
Figure 5.
CARTp mediates excitatory effects of CCK on cultured vagal afferent neurons. (A) CCK8s (10 nM, 2 h) stimulated expression of 3.45 kb CART-Luc; co-transfection with CART siRNA (50 nM), but not a scrambled sequence, reduced the number of CART positive cells by 36 ± 3.9 % (p<0.05) and reduced fluorescence intensity in most of the remainder, and significantly inhibited the response. (B) Similarly, CCK8s (10 nM, 2 h) stimulated expression of 3.034 kb Y2R-Luc and co-transfection with CART siRNA inhibited this. But, (C) CCK8s (10 nM, 2 h) inhibited expression of 1.6 kb MCH-Luc and CART siRNA had no effect on the response. (D) Pretreatment of cells with brefeldin A (BFA, 10 µg/ml) inhibited the CART-Luc and, (E) the Y2R-Luc responses to CCK8s, respectively. ** p<0.01, ***p<0.001.
CARTp acts via pCREB and EGR1 to stimulate the CART and Y2R promoters
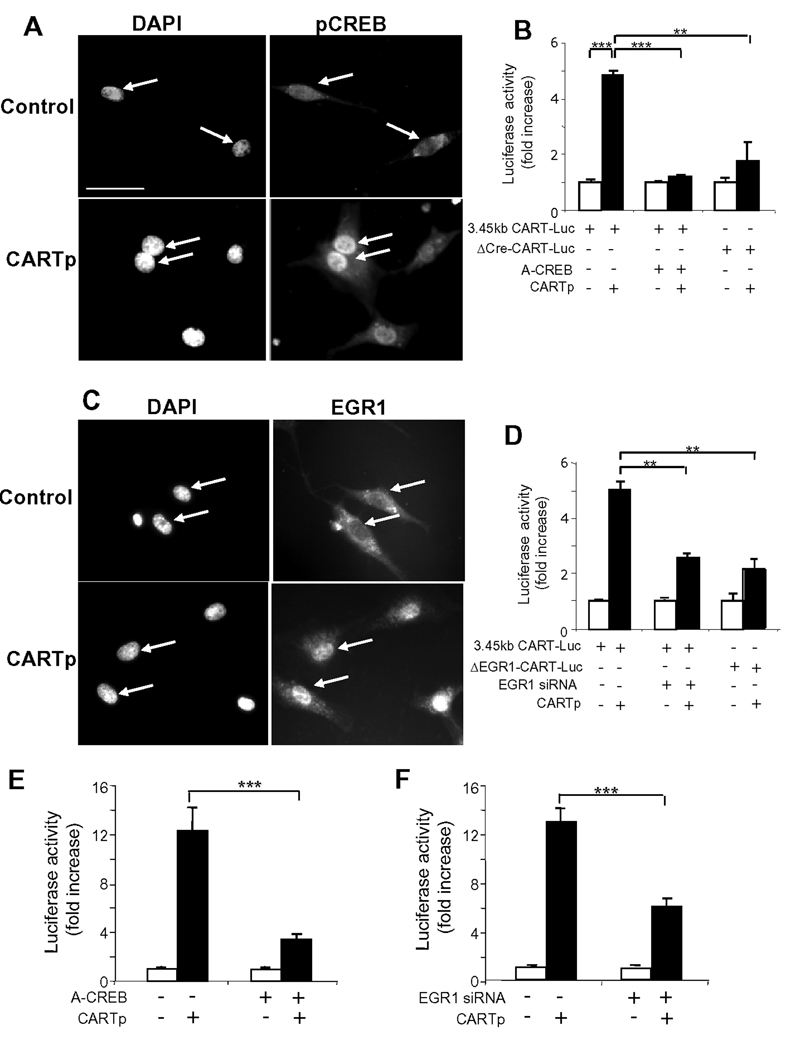
To identify putative transcriptional mechanisms we examined the possibility that CARTp acted by phosphorylation of CREB and activation of the immediate early gene EGR1 which we had identified in these neurons in preliminary studies (unpublished observations). In response to CARTp there was nuclear accumulation of both pCREB and EGR1 (Figure 6A,C). Mutation of either the putative Cre (Figure 6B) or EGR1 (Figure 6D) response elements in CART-Luc substantially reduced the response to CARTp. Moreover, inhibition of CREB by co-transfection of cells with A-CREB substantially inhibited responses to CARTp (Figure 6B). In the case of EGR1, treatment of cells with EGR1 siRNA substantially reduced the number of EGR1-expressing cells detected by immunocytochemistry and significantly inhibited the response of CART-Luc to CARTp (Figure 6D), so that both phosphoCREB and EGR1 are implicated in CARTp control of CART expression. Similarly ACREB (Figure 6E) and EGR1 siRNA (Figure 6F) inhibited CARTp stimulation of Y2R-Luc expression.
Figure 6.
CARTp activates CREB and EGR1 to stimulate CART and Y2R expression. (A) Strong nuclear staining of phosphoCREB in vagal afferent neurons (arrows) transferred to serum-free medium (2 h, control) and stimulated with CARTp (2 nM). (B) Co-transfection with A-CREB (1 µg DNA per well) inhibited CARTp (2 nM, 16 h) stimulation of 3.45 kb CART-Luc, and CARTp only weakly stimulated expression of a Cre-deletion mutant of CART-Luc (ΔCre-CART-Luc). (C) CARTp (2 nM, 2 h) stimulated nuclear translocation of EGR1. (D) Mutation of a putative EGR1 binding site in CART-Luc (ΔEGR1-CART-Luc) reduced responses to CARTp and so too did pretreatment with EGR1 siRNA. (E) CARTp (2 nM, 16 h) stimulated expression of 3.034 bp Y2R-Luc and co-transfection with A-CREB inhibited this. (F) Pretreatment with EGR1 siRNA reduced CARTp stimulation of Y2R-Luc. Mean ± SE, n=6 independent experiments; ** p<0.01, *** p<0.001.
CARTp potentiates CCK-inhibition of food intake
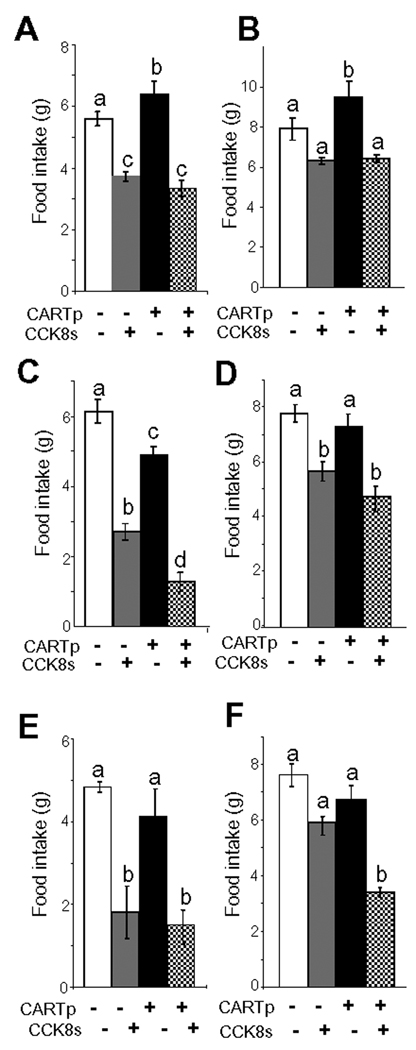
On the basis of the data presented above, we hypothesised that CARTp has peripheral effects on food intake acting co-operatively with CCK over time courses compatible with modulation of gene expression. In testing this we took into account the fact that the neurochemical phenotype of vagal afferent neurons depends on the duration of prior food withdrawal.16, 20 Thus we examined food intake in rats after either a long duration fast (15 h) or after a short duration fast (2.5 h). In both cases, CCK8s inhibited food intake (Figure 7A–F; supplementary Figure 3A–C). When CARTp was administered alone 2 h before the feeding trial, there was a small effect in decreasing food intake after a short fast (Figure 7C) but after the prolonged fast it slightly increased food intake (Figure 7A); it had no significant effect on CCK-inhibition of food intake after a long term fast (Figure 7A,B). However, CARTp significantly enhanced the effect of CCK8s in inhibiting food intake following a short duration fast: depending on the timing of administration of CARTp relative to CCK8s, the action of CCK8s was either increased or prolonged. Thus when administered 2 h before CCK8s (following a short duration fast) the inhibitory action of CCK8s was increased (Figure 7C,D); and when CARTp and CCK8s were administered together (after a short duration fast) the action of CCK8s was prolonged (Figure 7E,F).
Figure 7.
CARTp augments the inhibitory action of CCK on food intake after a short but not long fast. (A,B) Cumulative food intake over 0–40 and 0–80min, respectively, in rats fasted for 13 h and receiving CARTp (2 nmol, ip; ie “CART priming”) or saline followed 2 h later by CCK8s (10 nmol, ip) or saline and refeeding; CCK8s inhibited food intake over 0–40 min and CARTp had no effect on this but slightly increased food intake on its own. (C,D) Food intake over 0–40 and 0–80min, respectively, in rats fasted for 30 min and receiving a priming dose of CARTp or saline followed 2 h later by CCK8s (or saline) and refeeding; CARTp alone modestly inhibited food intake over 0–40 min compared with saline; CCK8s also inhibited food intake over the same period and CARTp enhanced this. (E,F) Food intake over 0–40 and 0–80 min, respectively, in rats fasted for 2.5 h and receiving CARTp or CCK8s (or saline) immediately before refeeding. CARTp alone did not significantly change food intake compared with saline, CCK8s inhibited food intake over 0–40 min and CARTp prolonged the action of CCK. N= 6 rats; a,b,c,d, Different letters denote significant differences between groups (p <0.05) ie a vs b; c vs a or b; d vs a, b or c.
Discussion
Vagal afferent neurons serving the gut mediate the effects of a variety of peripheral signals on food intake and gastric emptying. One of the best studied hormones stimulating these neurons is CCK and previous studies have shown that this is associated with inhibition of gastric emptying,5, 6 inhibition of food intake,8, 34 stimulation of pancreatic secretion35 and inhibition of gastrointestinal inflammatory responses.36 We have reported that CCK stimulates expression in vagal afferent neurons of the satiety peptide CARTp.16 We now show that CCK releases CARTp from these neurons, that CARTp on its own replicates the stimulatory effects of CCK on expression of Y2R and CART in them, and that blocking CART expression or its release inhibits the action of CCK. Moreover peripheral administration of CARTp potentiated CCK-inhibition of food intake following a short duration fast. Importantly, however, CART did not replicate the inhibitory effect of CCK on CB1R, MCH1R or MCH expression in vagal afferent neurons. We propose that CCK activates an autocrine positive feedback loop in vagal afferent neurons that is mediated by CARTp and that selectively augments the satiety action of CCK in partially satiated rats.
Like other neuropeptides, CARTp is localised to secretory vesicles that progress from cell soma to terminals. The present data provide direct evidence that CCK stimulates CARTp release. Moreover, since CARTp also acts on vagal afferent neurons the idea arises that it is responsible for an autocrine positive feedback mechanism that is initially triggered by CCK. The evidence from studies in which CCK actions were determined after knockdown of CART expression by siRNA, or from inhibition of CARTp release by BFA treatment, supports this concept. Together the data suggest that in rats fed ad libitum, CCK releases CARTp from pre-existing stores in vagal afferent neurons and that CARTp in turn sustains and augments the excitatory effects of CCK on vagal afferent neurons leading to inhibition of food intake and increased expression of Y2R and of CART itself.
The action of CCK on vagal afferent neurons is potentiated by gastric distension,7 leptin33, 37 and urocortin38 and inhibited by ghrelin17 and orexin A39. In keeping with this pattern of response, the stimulation of CARTp release by CCK was increased in the presence of leptin and decreased in the presence of ghrelin. In addition, the actions of CARTp in stimulating its own expression and that of Y2R were also enhanced by leptin and inhibited by ghrelin. There is therefore modulation of CARTp actions by factors that are established vagal afferent modulators at the levels of peptide or receptor synthesis and control of neuropeptide release.
When plasma CCK is low (after fasting rats for 6–12 h or longer) there is decreased expression of CART and Y2R in vagal afferent neurons and increased CB1R, MCH1R and MCH expression;16, 20, 21 these changes are reversed by endogenous CCK released on refeeding. Since CB1R, MCH and MCH1R are associated with orexigenic effects there appears to be an enhanced capacity for orexigenic signalling by vagal afferent neurons in fasted rats. Until now, it has not been possible to dissociate the reciprocal actions of CCK in stimulating the expression of some genes associated with anorexic signalling in vagal afferent neurons and in inhibiting the expression of others associated with orexigenic signalling. The present data suggest that while CARTp exerts similar effects to CCK on Y2R expression and on its own expression, it does not influence expression of CB1, MCH1R and MCH. These data therefore provide clear evidence that the reciprocal expression of genes associated with orexigenic and anorexigenic signalling in response to fasting or CCK is dissociable. Moreover, we have identified phosphorylation of CREB and nuclear accumulation of the immediate early gene EGR1 as involved in CARTp stimulation of its own expression, and of Y2R.
Previous studies have established that CARTp acts in the brain stem to inhibit food intake29, 40 and to potentiate the effect of peripheral CCK.41 The different actions of CCK and CARTp in controlling expression of anorexic and orexigenic signalling molecules suggested to us that while CARTp may not be particularly effective in influencing food intake after a prolonged fast (>12 h when the capacity for orexigenic signalling is increased) it might nevertheless act peripherally to inhibit food intake in partially satiated rats. We reasoned therefore, that studies of the functional consequences of CARTp for food intake should take into account (a) the possibility of actions on food intake only after short duration fasting, (b) actions on food intake that might be somewhat delayed as they are secondary to changes in gene expression, and (c) should consider interactions with CCK. In keeping with this reasoning we found that after a long duration fast (defined on the basis of increased CB1R/MCH1R/MCH expression as >12 h) intraperitoneal CARTp alone did not inhibit food intake and may even have very modestly increased it when given 2 h before refeeding. However, after short duration fasting when given 2 h before refeeding it had a small inhibitory effect on food intake. As expected, CCK8s inhibited food intake whether given after either a long or short duration fast. Moreover, consistent with the reasoning above, after a short duration fast CARTp enhanced the anorexic effects of CCK8s. This action of CARTp was relatively long-lived since intraperitoneal administration of CARTp enhanced the action of CCK8s given 2 h later, and prolonged the action of CCK8s when administered together with it.
Bariatric surgery is well recognised as an effective treatment for weight loss in obese patients.42, 43 Modulation of gut-brain signalling pathways is likely to be involved and so it becomes attractive to consider the feasibility of developing non-surgical approaches to obesity that target the same signalling pathway. However, one potential difficulty is exemplified by the action of CCK which (on its own) is ineffective in long term inhibition of food intake because its effects are short-lived and are offset by increased meal frequency.44 The present data indicate that the actions of CCK on both the neurochemical phenotype of vagal afferent neurons, and on food intake, are enhanced by CART. They provide an illustration of a type of mechanism that has not previously received detailed consideration and they raise the possibility of developing novel therapies based on augmenting and prolonging the action of established gut-brain signalling molecules such as CCK.
Supplementary Material
Acknowledgements
We are grateful to Drs M Kuhar and D Ginty for the gift of plasmids.
Grant support: Supported by grants from the BBSRC and MRC (GJD), and NIH (HER, NIHDK41004).
Abbreviations
- BFA
brefeldin A
- CART(p)
cocaine and amphetamine regulated transcript (peptide)
- CB1R
cannabinoid receptor 1
- CCK
cholecystokinin
- Cre
cAMP response element
- CREB
cAMP response element binding protein
- EGR1
early growth response protein-1
- MCH
melanin concentrating hormone
- MCH1R
melanin concentrating hormone receptor 1
- PMA
phorbol 12-myristate-13-acetate
- PYY3-36
peptide-tyrosine-tyrosine 3–36 fragment
- Y2R
receptor type 2 for neuropeptide Y and peptide-tyrosine-tyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts of interest exist.
Contribution of the authors: GdeL: acquisition of data in all parts of the study, analysis and interpretation of data in all parts of the study, contribution to drafting of the manuscript. RD: design and preparation of plasmids, analysis and interpretation of data, contribution to drafting of the manuscript. AV: design of some experiments, analysis and interpretation of data, contribution to drafting of the manuscript. HR: design of some experiments, analysis and interpretation of data, contribution to drafting of the manuscript. CBdlS: acquisition of data for feeding studies, analysis of interpretation of feeding studies; GD: overall supervision and project management, obtained funding, intellectual contribution throughout and major contribution to drafting of the manuscript.
Contributor Information
Guillaume de Lartigue, Physiological Laboratory, School of Biomedical Sciences, University of Liverpool, Crown St, Liverpool, L69 3BX, UK.
Rod Dimaline, Physiological Laboratory, School of Biomedical Sciences, University of Liverpool, Crown St, Liverpool, L69 3BX, UK.
Andrea Varro, Physiological Laboratory, School of Biomedical Sciences, University of Liverpool, Crown St, Liverpool, L69 3BX, UK.
Helen Raybould, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, UC Davis, California, USA.
Claire Barbier de la Serre, Department of Anatomy, Physiology and Cell Biology, School of Veterinary Medicine, UC Davis, California, USA.
Graham J. Dockray, Physiological Laboratory, School of Biomedical Sciences, University of Liverpool, Crown St, Liverpool, L69 3BX, UK
References
- 1.Dockray GJ. The brain-gut axis. In: Yamada T, editor. Textbook of Gastroenterology. 5th ed. Chichester, WestSussex: Wiley-Blackwell; 2009. pp. 86–102. [Google Scholar]
- 2.Moran TH, Kinzig KP. Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol. 2004;286:G183–G188. doi: 10.1152/ajpgi.00434.2003. [DOI] [PubMed] [Google Scholar]
- 3.Capasso R, Izzo AA. Gastrointestinal regulation of food intake: general aspects and focus on anandamide and oleoylethanolamide. J Neuroendocrinol. 2008;20 Suppl 1:39–46. doi: 10.1111/j.1365-2826.2008.01686.x. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs J, Young RC, Smith GP. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245:323–325. doi: 10.1038/245323a0. [DOI] [PubMed] [Google Scholar]
- 5.Forster ER, Green T, Elliot M, Bremner A, Dockray GJ. Gastric emptying in rats: role of afferent neurons and cholecystokinin. Am J Physiol. 1990;258:G552–G556. doi: 10.1152/ajpgi.1990.258.4.G552. [DOI] [PubMed] [Google Scholar]
- 6.Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GJ, McHugh PR, Moran TH. Gastric loads and cholecystokinin synergistically stimulate rat gastric vagal afferents. Am J Physiol. 1993;265:R872–R876. doi: 10.1152/ajpregu.1993.265.4.R872. [DOI] [PubMed] [Google Scholar]
- 8.Ritter RC, Ladenheim EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol. 1985;248:R501–R504. doi: 10.1152/ajpregu.1985.248.4.R501. [DOI] [PubMed] [Google Scholar]
- 9.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 10.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 11.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 12.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz GJ, McHugh PR, Moran TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol. 1991;261:R64–R69. doi: 10.1152/ajpregu.1991.261.1.R64. [DOI] [PubMed] [Google Scholar]
- 14.Peters JH, Simasko SM, Ritter RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav. 2006;89:477–485. doi: 10.1016/j.physbeh.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Date Y, Toshinai K, Koda S, Miyazato M, Shimbara T, Tsuruta T, Niijima A, Kangawa K, Nakazato M. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology. 2005;146:3518–3525. doi: 10.1210/en.2004-1240. [DOI] [PubMed] [Google Scholar]
- 18.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28:11583–11592. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006;137:1405–1415. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- 22.Gomez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del AI, Cippitelli A, Nava F, Piomelli D, Rodriguez dF. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci. 2002;22:9612–9617. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–131. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Whited KL, Tso P, Raybould HE. Involvement of apolipoprotein A-IV and cholecystokinin1 receptors in exogenous peptide YY3 36-induced stimulation of intestinal feedback. Endocrinology. 2007;148:4695–4703. doi: 10.1210/en.2006-1665. [DOI] [PubMed] [Google Scholar]
- 25.Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: A putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci U S A. 1999;96:13506–13511. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 29.Zheng H, Patterson C, Berthoud HR. Fourth ventricular injection of CART peptide inhibits short-term sucrose intake in rats. Brain Res. 2001;896:153–156. doi: 10.1016/s0006-8993(00)03256-x. [DOI] [PubMed] [Google Scholar]
- 30.Dominguez G, Kuhar MJ. Transcriptional regulation of the CART promoter in CATH.a cells. Brain Res Mol Brain Res. 2004;126:22–29. doi: 10.1016/j.molbrainres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 31.Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine-and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80:885–893. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 32.Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol. 1998;18:967–977. doi: 10.1128/mcb.18.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters JH, Karpiel AB, Ritter RC, Simasko SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology. 2004;145:3652–3657. doi: 10.1210/en.2004-0221. [DOI] [PubMed] [Google Scholar]
- 34.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science. 1981;213:1036–1037. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology. 1994;107:525–531. doi: 10.1016/0016-5085(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 36.Luyer MD, Greve JW, hadfoune M, Jacobs JA, Dejong CH, Buurman WA. Nutritional stimulation of cholecystokinin receptors inhibits inflammation via the vagus nerve. J Exp Med. 2005;202:1023–1029. doi: 10.1084/jem.20042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–R837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- 38.Gourcerol G, Wang L, Wang YH, Million M, Tache Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology. 2007;148:6115–6123. doi: 10.1210/en.2007-0678. [DOI] [PubMed] [Google Scholar]
- 39.Burdyga G, Lal S, Spiller D, Jiang W, Thompson DG, Attwood S, Saeed S, Grundy D, Varro A, Dimaline R, Dockray GJ. Localization of orexin-1 receptors to vagal afferent neurons in the rat and humans. Gastroenterology. 2003;124:129–139. doi: 10.1053/gast.2003.50020. [DOI] [PubMed] [Google Scholar]
- 40.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–R1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 41.Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Pirnik Z, Kiss A, Zelezna B. Synergistic effect of CART (cocaine- and amphetamine-regulated transcript) peptide and cholecystokinin on food intake regulation in lean mice. BMC Neurosci. 2008;9:101–111. doi: 10.1186/1471-2202-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 43.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 44.Crawley JN, Beinfeld MC. Rapid development of tolerance to the behavioural actions of cholecystokinin. Nature. 1983;302:703–706. doi: 10.1038/302703a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.