Abstract
Introduction:
Stress is thought to influence use of drugs, including cigarette smoking, but the mechanisms by which it does so are not clear. In this study, we investigated the effects of acute psychosocial stress on cigarette craving, the subjective effects of smoking, and smoking behavior in daily smokers.
Methods:
Healthy male and female smokers participated in two experimental sessions in which they were exposed to the Trier Social Stress Test or a nonstressful control task. For 2 hr after each task, they had repeated opportunities to either smoke or earn money. Physiological (heart rate, cortisol, and alpha-amylase) and subjective (anxiety and desire to smoke) measures were obtained before and after the tasks and after each smoking opportunity.
Results:
Stress significantly increased cigarette craving but it did not increase smoking. When individual differences in nicotine dependence were taken into account, stress influenced CO boost and pleasure from smoking the first cigarette.
Discussion:
Our results support previous evidence that acute psychosocial stress increases smoking desire.
Introduction
Psychological stress influences the development of substance dependence, including tobacco dependence (Sinha, 2008). A common belief is that smoking reduces aversive effects of stress, particularly negative affect (Kassel, Stroud, & Paronis, 2003), but there is relatively little behavioral evidence of this (Jarvik, Caskey, Rose, Herskovic, & Sadeghpour, 1989; Perkins, Grobe, Fonte, & Breus, 1992; Pomerleau & Pomerleau, 1987; Pomerleau, Turk, & Fertig, 1984). Actually, acute nicotine activates stress systems and prolongs physiological stress responses (Fuxe, Andersson, Eneroth, Harfstrand, & Agnati, 1989; Pomerleau & Pomerleau, 1990). Others propose that smoking increases stress, which drives smoking. The deprivation reversal (Schachter, 1978; Silverstein, 1982) and stress induction (Parrott, 1999) models posit that smokers experience nicotine deprivation, including negative affect, between smoking episodes, which motivates smoking. Thus, by mimicking nicotine deprivation, acute stress may stimulate smoking. Together, these studies illustrate that stress–smoking relationships are complex.
Acute physical or psychological stress and behavioral challenges increase subjective desire to smoke, smoking rate, and nicotine intake (al’Absi, Amunrud, & Wittmers, 2002; al’Absi, Wittmers, Erickson, Hatsukami, & Crouse, 2003; Buchmann et al., 2008; Cherek, 1985; Pomerleau & Pomerleau, 1987; Rose, Ananda, & Jarvik, 1983; Schachter, Silverstein, & Perlick, 1977), which is thought to be driven by the negative subjective effects of stress (Conklin & Perkins, 2005; Niaura, Shadel, Britt, & Abrams, 2002; Payne, Schare, Levis, & Colletti, 1991; Willner & Jones, 1996). However, stress may influence smoking by altering the effects of nicotine. Acute stress (Flemmer & Dilsaver, 1989; Peck, Dilsaver, & McGee, 1991) and corticosteroid administration (Caggiula et al., 1993, 1998; Grun, Pauly, Bullock, & Collins, 1995; Pauly, Ullman, & Collins, 1988; Shoaib & Shippenberg, 1996) regulate sensitivity to nicotine’s effects in laboratory animals. In humans, withdrawal- or stress-induced cortisol release modulates smoking desire and satisfaction (Buchmann et al.; Reuter et al., 2002). Thus, stress could increase smoking by dampening responses to nicotine, that is, smokers may smoke more after stress to compensate for attenuated effects.
Here, we investigated the effects of acute psychosocial stress on cigarette craving, subjective effects of smoking, and smoking behavior. Participants could choose to either smoke or earn money after participating in stressful or nonstressful tasks. We hypothesized that stress would increase cigarette craving, dampen the subjective effects of smoking, and increase smoking.
Methods
Procedure
Daily smokers (male = 13, female = 4) were recruited without regard to race or ethnicity excluding a current or prior (within 2 years) diagnosis of Major Axis I DSM-IV (American Psychological Association, 1994) disorder (except nicotine dependence, assessed using The Fagerström Test for Nicotine Dependence [FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991] and the Nicotine Dependence Syndrome Scale [NDSS; Shiffman, Waters, & Hickcox, 2004]), regular medications, history of cardiovascular problems, night-shift work, pregnancy, lactation, or hormonal contraceptive use (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999).
The study consisted of an orientation session and two 4.5-hr experimental sessions, beginning at noon and scheduled at least 72 hr apart. The Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993a) was administered on one session and a control task on the other, in randomized order. At the orientation session, volunteers gave written informed consent and completed a computer questionnaire to determine the monetary value of a single cigarette (Acheson, Mahler, Chi, & de Wit, 2006). Before each session, participants could smoke as normal but not eat for 1 hr. On arrival, participants completed drug and alcohol tests; no one tested positive. Sessions began with volunteers smoking a single cigarette of their usual brand to standardize the time since last smoking (t = 0). Fifteen minutes later (t = 15), baseline measures were recorded. Then, an assistant read instructions for the task to follow (t = 30). The TSST instructions stated that subjects would perform a mock job interview consisting of a speech and mental arithmetic before two examiners and a video camera (see Childs & de Wit, 2009, for a detailed description). The control task instructions stated that they would describe their favorite book, film, and so on to the assistant for 5 min, followed by a computer game (solitaire), without evaluation or videotaping. Ten minutes after the instructions, participants were escorted to an adjacent room to perform the tasks (t = 40). Afterward, they returned to the testing room where measures were recorded (t = 50). Ten minutes later (t = 60), the 2-hr smoking choice procedure began. Once every 15 min, individuals chose between smoking a half-cigarette of their usual brand or receiving an equivalent amount of money. Dependent measures were obtained before and after participants smoked their first half-cigarette. At the end of the session, subjects received the money earned during that session. At the end of the study, the study director explained the study aims and paid the participants.
Dependent measures
Physiological
Heart rate and blood pressure were measured using a monitor, and saliva samples were collected using cotton wads. Samples were assayed for cortisol (Salimetrics LLC, State College, PA; sensitivity = 0.003 ug/dL) and for alpha-amylase (Salimetrics LLC). Alpha-amylase, released from the salivary gland after autonomic nervous system activation, provides a noninvasive biomarker of acute stress responses (Granger, Kivlighan, el-Sheikh, Gordis, & Stroud, 2007; Nater & Rohleder, 2009). CO in expired air was measured using a Smokerlyzer (Bedfont Scientific Ltd, Rochester, United Kingdom).
Subjective
Visual Analogue Scales (VAS; Folstein & Luria, 1973) assessed mood (jittery, relaxed, able to concentrate, and irritable), cigarette cravings (cigarette craving), and subjective smoking effects (pleasure, head rush, taste, satisfaction, harshness, smell, and intensity). We (Childs & de Wit, 2009; Cousins, Stamat, & de Wit, 2001) and others (Mendelson et al., 2008; Perkins et al., 1994) have shown that single-item VAS are sensitive to stress and smoking and correlate well with standardized craving questionnaires (King & Meyer, 2000; Singleton, Anderson, & Heishman, 2003).
Data analysis
Stress task efficacy was confirmed by comparing pre- with posttask changes between sessions using Student’s paired t tests. Pretask baselines did not significantly differ between the sessions (Student’s paired t tests), and session order did not influence results (two-factor, Task × Order, repeated measures analysis of variance [ANOVA]).
The primary smoking outcome measures were the proportion of participants smoking at the first opportunity, the CO boost (pre- to postcigarette) from and subjective effects of the first cigarette smoked, and the total number of half-cigarettes smoked. These measures were compared between sessions using Student’s paired t test and binomial test. Habitual smoking and dependence levels were included in the analysis using repeated measures analysis of covariance (ANCOVA).
Analyses were performed using SPSS version 16 for Windows. Repeated measures ANOVAs were performed with Greenhouse–Geisser correction when violations of sphericity were detected. All findings remained significant after adjustment; thus, we report degrees of freedom from uncorrected analyses.
Results
Most participants were European Americans (59%) in their early twenties (mean age 24.2 ± 1.4 years). They smoked approximately 10 cigarettes a day (11.0 ± 2.0, range 4–40), were light social drinkers (7.2 ± 1.4 alcoholic drinks/week), and reported some prior recreational drug use; 88% marijuana, 35% stimulants, 24% hallucinogens, 21% opiates, 12% inhalants, and 6% sedatives. The average FTND score of participants was 3.4 ± 0.5 (range 0–7), and the mean monetary value of a cigarette was $1.78 ± $0.38 (range $0.20–$5.00). Demographic characteristics did not influence responses to stress.
Stress significantly increased heart rate, t(15) = −4.9, p < .001, salivary cortisol, t(16) = 3.3, p < .01, and alpha-amylase, t(16) = −3.0, p < .01. It significantly decreased ratings of “relaxed,” t(16) = 4.0, p < .001, and “ability to concentrate,” t(16) = 4.8, p < .001, and increased “anxiety,” t(16) = −2.6, p < .05, “jitteriness,” t(16) = −3.9, p < .001, and “irritability,” t(16) = −3.2, p < .01.
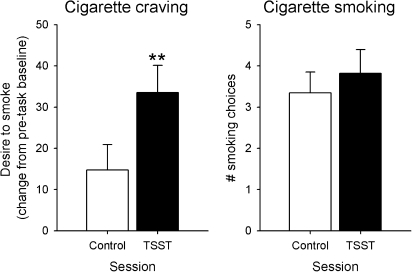
Stress significantly increased “desire to smoke,” t(16) = −3.5, p < .01, Figure 1, and nonsignificantly increased the proportion of participants smoking at the first opportunity (88.2% compared with 70.6% after the control task, p = .085). Stress did not change the total number of half-cigarettes smoked, t(16) = −1.6, p > .1, Figure 1.
Figure 1.
Changes in cigarette craving and the total number of smoking choices after control and stressful tasks. Data indicate mean ± SEM. Asterisks indicate a significant difference between the tasks (Student’s paired t test, p < .01). TSST = Trier Social Stress Test.
Participants’ level of smoking and FTND scores did not influence the findings. NDSS subscale scores were significantly associated with CO boost and “pleasure” from smoking. Stereotypy scores, reflecting a consistent pattern of smoking, influenced stress effects on pleasure from smoking, F(1, 14) = 6.2, p < .05; individuals with the highest Stereotypy scores exhibited the greatest pleasure from smoking after stress. Furthermore, Drive scores, reflecting smoking to relieve withdrawal and subjective compulsion, influenced stress effects on CO boost, F(1, 14) = 5.3, p < .05, and pleasure from smoking, F(1, 14) = 5.3, p < .05; individuals with the highest Drive scores exhibited the greatest stress-induced increases in CO boost and pleasure from smoking.
Discussion
Our findings support earlier studies showing that acute psychosocial stress increases smoking desire (Buchmann et al., 2008; Colamussi, Bovbjerg, & Erblich, 2007; Erblich, Boyarsky, Spring, Niaura, & Bovbjerg, 2003; Niaura et al., 2002; Perkins & Grobe 1992; Rose et al., 1983). Acute stress did not significantly influence smoking, yet individual differences in nicotine dependence influenced the subjective effects of smoking after acute stress. Participants with high NDSS Stereotypy scale scores (i.e., smoke in a highly consistent pattern) reported more pleasure from smoking after stress. This suggests that acute stress increased the rewarding effects of smoking in these individuals. Participants with high NDSS Drive scale scores (i.e., report subjective compulsion to smoke and smoking to relieve withdrawal and craving) exhibited greater stress-induced CO boost and pleasure from smoking. This suggests that stress increases nicotine intake (i.e., CO boost, McBride, Guyatt, Kirkham, & Cumming, 1984) and the subjective rewarding effects of smoking in these individuals. These findings may provide some support for the deprivation reversal model of stress-induced smoking, at least in these individuals. That is, the effects of acute stress may mimic withdrawal signs, which drive smoking (among these individuals) and enhance its rewarding value. We did not measure nicotine withdrawal signs throughout the sessions, which may have provided additional information about individuals’ motivations to smoke.
The present study had some limitations, which may have contributed to the lack of significant increases in smoking after stress. First, the smoking choice procedure may not have been sufficiently sensitive to detect changes in smoking. In our study, smoking choices began 10 min after tasks and again every 15 min for 2 hr. It is possible that the effects of the stress declined in the 10-min interval, although this is not consistent with previous studies utilizing the TSST, or it is possible that the subsequent smoking intervals were not timed to detect an effect. The 10-min interval between task performance and the first smoking opportunity was essential to assess physiological and subjective responses to the stress, and we and others (Buchman et al., 2009; Childs & de Wit, 2009; Kirschbaum, Strasburger, & Langkrar, 1993b) have shown that physiological and subjective responses to the TSST remain elevated well beyond this 10-min interval. Another potential methodological issue was the use of half-cigarettes instead of whole cigarettes. Half-cigarettes were used to minimize the effect of satiation, but their novelty may have had unknown other effects. Lastly, we did not control the topography of subjects’ smoking (e.g., puff volume), which may have influenced later choices to smoke. However, the CO boost from the first cigarette smoked and total cigarettes smoked provided empirical information about depth and frequency of smoking (Stepney, 1982).
Another noteworthy finding is that the TSST significantly increased salivary alpha-amylase among light smokers. This hormone is a relatively new biomarker of stress responses. In this study, levels of alpha-amylase were somewhat lower than those reported in previous studies (Nagler et al., 2000), possibly because of inactivation of alpha-amylase by acid aldehydes in tobacco smoke. However, it is important that we were still able to demonstrate a significant effect of stress on this measure suggesting that salivary alpha-amylase may be a useful noninvasive biomarker of stress-induced autonomic nervous system activation in light smokers.
Other limitations included that participants were young, and there was wide variation in habitual smoking and nicotine dependency severity. We chose to study young volunteers since smoking may be more susceptible to the influences of acute stress in these individuals, and the results may be different in an older or more dependent sample. Although there was large interindividual variation in habitual smoking and dependence severity, we were able to control for these and indeed demonstrated interesting relationships between dependence severity and influences of stress on smoking.
In conclusion, the findings from the present study support earlier findings that acute stress increases smoking desire. Acute stress did not significantly increase smoking; however, our findings suggest increased pleasure from smoking and deeper inhalations in more dependent smokers after stress. Our results extend previous findings and suggest relationships between the severity of nicotine dependence and influences of stress on smoking. Future studies should further investigate possible relationships between physiological and subjective responses to stress and stress-induced alterations in smoking.
Funding
This research was supported by National Institute on Drug Abuse (DA02812) and the University of Chicago Hospital’s General Clinical Research Center (US Public Health Service M01 RR00055).
Declaration of Interests
None declared.
Acknowledgments
We thank Nicholas Van Dam, Leslie Sidney, and Joshua Shulruff for their technical assistance.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology Biochemistry and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology Biochemistry and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. American Psychiatric Association diagnostic and statistical manual of psychiatry. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. Journal of Psychopharmacology. 2008 doi: 10.1177/0269881108095716. Epub ahead of print Nov 21. doi:10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, et al. The role of corticosteroids in nicotine’s physiological and behavioral effects. Psychoneuroendocrinology. 1998;23:143–159. doi: 10.1016/s0306-4530(97)00078-4. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Epstein LH, Antelman SM, Saylor S, Knopf S, Perkins KA, et al. Acute stress or corticosterone administration reduces responsiveness to nicotine: Implications for a mechanism of conditioned tolerance. Psychopharmacology. 1993;111:499–507. doi: 10.1007/BF02253543. [DOI] [PubMed] [Google Scholar]
- Cherek DR. Effects of acute exposure to increased levels of background industrial noise on cigarette smoking behavior. International Archives of Occupational and Environmental Health. 1985;56:23–30. doi: 10.1007/BF00380697. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J. Stress- and cue-induced cigarette craving: Effects of a family history of smoking. Drug and Alcohol Dependence. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA. Subjective and reinforcing effects of smoking during negative mood induction. Journal of Abnormal Psychology. 2005;114:153–164. doi: 10.1037/0021-843X.114.1.153. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98:657–664. doi: 10.1046/j.1360-0443.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- Flemmer DD, Dilsaver SC. Chronic injections of saline produce subsensitivity to nicotine. Pharmacology Biochemistry and Behavior. 1989;34:261–263. doi: 10.1016/0091-3057(89)90309-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychological Medicine. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Andersson K, Eneroth P, Harfstrand A, Agnati LF. Neuroendocrine actions of nicotine and of exposure to cigarette smoke: Medical implications. Psychoneuroendocrinology. 1989;14:19–41. doi: 10.1016/0306-4530(89)90054-1. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: Recent developments and applications. Annals of the New York Academy of Sciences. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Grun EU, Pauly JR, Bullock AE, Collins AC. Corticosterone reversibly alters brain alpha-bungarotoxin binding and nicotine sensitivity. Pharmacology Biochemistry and Behavior. 1995;52:629–635. doi: 10.1016/0091-3057(95)00157-r. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Caskey NH, Rose JE, Herskovic JE, Sadeghpour M. Anxiolytic effects of smoking associated with four stressors. Addictive Behaviors. 1989;14:379–386. doi: 10.1016/0306-4603(89)90025-7. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacology Biochemistry and Behavior. 2000;66:563–572. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacology Biochemistry and Behavior. 1993b;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- McBride MJ, Guyatt AR, Kirkham AJ, Cumming G. Assessment of smoking behaviour and ventilation with cigarettes of differing nicotine yields. Clinical Science (London, England: 1979) 1984;67:619–631. doi: 10.1042/cs0670619. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Goletiani N, Sholar MB, Siegel AJ, Mello NK. Effects of smoking successive low- and high- nicotine cigarettes on hypothalamic-pituitary-adrenal axis hormones and mood in men. Neuropsychopharmacology. 2008;33:749–760. doi: 10.1038/sj.npp.1301455. [DOI] [PubMed] [Google Scholar]
- Nagler R, Lischinsky S, Diamond E, Drigues N, Klein I, Reznick AZ. Effect of cigarette smoke on salivary proteins and enzyme activities. Archives of Biochemistry and Biophysics. 2000;379:229–236. doi: 10.1006/abbi.2000.1877. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addictive Behaviors. 2002;27:241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Does cigarette smoking cause stress? American Psychologist. 1999;54:817–820. doi: 10.1037//0003-066x.54.10.817. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Ullman EA, Collins AC. Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiology and Behavior. 1988;44:109–116. doi: 10.1016/0031-9384(88)90353-8. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: Effects on desire to smoke and topographical components of smoking behavior. Addictive Behaviors. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Peck JA, Dilsaver SC, McGee M. Chronic forced swim stress produces subsensitivity to nicotine. Pharmacology Biochemistry and Behavior. 1991;38:501–504. doi: 10.1016/0091-3057(91)90004-l. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. British Journal of Addiction. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Fonte C, Breus M. “Paradoxical” effects of smoking on subjective stress versus cardiovascular arousal in males and females. Pharmacology Biochemistry and Behavior. 1992;42:301–311. doi: 10.1016/0091-3057(92)90531-j. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Sexton JE, Reynolds WA, Grobe JE, Fonte C, Stiller RL. Comparison of acute subjective and heart rate effects of nicotine intake via tobacco smoking versus nasal spray. Pharmacology Biochemistry and Behavior. 1994;47:295–299. doi: 10.1016/0091-3057(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24:278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Cortisol response to a psychological stressor and/or nicotine. Pharmacology Biochemistry and Behavior. 1990;36:211–213. doi: 10.1016/0091-3057(90)90153-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Turk DC, Fertig JB. The effects of cigarette smoking on pain and anxiety. Addictive Behaviors. 1984;9:265–271. doi: 10.1016/0306-4603(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Reuter M, Netter P, Rogausch A, Sander P, Kaltschmidt M, Dorr A, et al. The role of cortisol suppression on craving for and satisfaction from nicotine in high and low impulsive subjects. Human Psychopharmacology. 2002;17:213–224. doi: 10.1002/hup.402. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addictive Behaviors. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Schachter S. Pharmacological and psychological determinants of smoking. Annals of Internal Medicine. 1978;88:104–114. doi: 10.7326/0003-4819-88-1-104. [DOI] [PubMed] [Google Scholar]
- Schachter S, Silverstein B, Perlick D. 5. Psychological and pharmacological explanations of smoking under stress. Journal of Experimental Psychology. General. 1977;106:31–40. doi: 10.1037//0096-3445.106.1.31. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters A, Hickcox M. The nicotine dependence syndrome scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Shippenberg TS. Adrenalectomy attenuates nicotine-induced dopamine release and locomotor activity in rats. Psychopharmacology. 1996;128:343–350. doi: 10.1007/s002130050143. [DOI] [PubMed] [Google Scholar]
- Silverstein B. Cigarette smoking, nicotine addiction, and relaxation. Journal of Personality and Social Psychology. 1982;42:946–950. doi: 10.1037//0022-3514.42.5.946. [DOI] [PubMed] [Google Scholar]
- Singleton EG, Anderson LM, Heishman SJ. Reliability and validity of the Tobacco Craving Questionnaire and validation of a craving-induction procedure using multiple measures of craving and mood. Addiction. 2003;98:1537–1546. doi: 10.1046/j.1360-0443.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepney R. Exposure to carbon monoxide in smokers of middle- and low-tar cigarettes. British Journal of Diseases of the Chest. 1982;76:390–396. doi: 10.1016/0007-0971(82)90075-4. [DOI] [PubMed] [Google Scholar]
- Willner P, Jones C. Effects of mood manipulation on subjective and behavioural measures of cigarette craving. Behavioural Pharmacology. 1996;7:355–363. doi: 10.1097/00008877-199608000-00007. [DOI] [PubMed] [Google Scholar]