Abstract
Introduction:
Postpartum relapse rates are high among women who spontaneously quit smoking during pregnancy. This randomized clinical trial tested a Motivation and Problem-Solving (MAPS) treatment for reducing postpartum relapse among diverse low-income women who quit smoking during pregnancy (N = 251; 32% Black, 30% Latino, and 36% White; 55% <$30,000/year household income).
Methods:
Pregnant women were randomly assigned to MAPS/MAPS+ or Usual Care (UC). Continuation ratio logit models were used to examine differences in biochemically confirmed continuous abstinence at Weeks 8 and 26 postpartum by treatment group and moderators of the treatment effect. Analyses controlled for age, race/ethnicity, partner status, education, smoking rate, and the number of smokers in the participant’s environment.
Results:
MAPS/MAPS+ was more efficacious than UC in the prevention of postpartum relapse (p = .05). An interaction between treatment and the number of cigarettes smoked per day before quitting approached significance (p = .09), suggesting that the MAPS/MAPS+ treatment effect was stronger among women who smoked more cigarettes per day.
Discussion:
MAPS, a holistic and dynamic approach to changing behavior using a combined motivational enhancement and social cognitive approach, is a promising intervention for postpartum smoking relapse prevention among low-income women, which may have particular relevance for women with higher prequit smoking rates.
Introduction
Although public health efforts have produced dramatic declines in the prevalence of cigarette smoking over the last several decades, smoking remains the leading cause of preventable disease and death in the United States (Ries et al., 2004). Smoking is associated with a number of negative health outcomes related to pregnancy, including premature birth, restricted fetal growth, pregnancy complications, and sudden infant death syndrome (Fiore, Jaen, & Baker, 2008; Tong, Jones, Dietz, D’Angelo, & Bombard, 2009). Approximately 20% of women smoke in the months immediately prior to pregnancy, with one third to one half spontaneously suspending or quitting smoking during pregnancy (Tong et al.). Unfortunately, more than 50% of these women relapse by 6 months, and up to 80% relapse within 12 months of childbirth (Centers for Disease Control and Prevention [CDC], 2007; Fiore et al., 2000; Mullen, 2004; Mullen, Quinn, & Ershoff, 1990; Ockene, 1993; Stotts, DiClemente, Carbonari, & Mullen, 2000). Thus, pregnancy represents a unique opportunity to capitalize on high rates of spontaneous smoking cessation by facilitating the postpartum continuation of abstinence.
Unfortunately, meta-analytic studies evaluating relapse prevention interventions among pregnant and postpartum women have failed to support their efficacy, regardless of the timing of the intervention along the pregnancy–postpartum continuum (Hajek, Stead, West, Jarvis, & Lancaster, 2009; Lancaster, Hajek, Stead, West, & Jarvis, 2006). As a result, how to best intervene to prevent postpartum relapse among spontaneous quitters is unclear (Melvin & Gaffney, 2004). Given the adverse health consequences of smoking to the fetus, child, and mother (e.g., Cnattingius, 2004; Floyd, Rimer, Giovino, Mullen, & Sullivan, 1993; Roza et al., 2007; Shah, Sullivan, & Carter, 2006), there is a strong need to develop innovative treatments to reduce smoking relapse within this population (Fiore et al., 2008). Moreover, because low-income women are more likely than their higher income counterparts to both smoke during pregnancy and relapse after childbirth (Tong et al., 2009), they represent an important target for postpartum relapse prevention interventions. The purpose of this study was to test the efficacy of a Motivation and Problem-Solving (MAPS) treatment to facilitate relapse prevention among pregnant/postpartum women, in a sample of racial/ethnically diverse, predominantly low-income women.
Based on social cognitive theory (Bandura, 1977, 1986), the relapse prevention model (Marlatt & Gordon, 1985; Witkiewitz & Marlatt, 2004) is perhaps the most prominent theory of smoking cessation and relapse. In this model, individual and contextual factors are hypothesized to increase smoking motivation and produce high-risk situations, thereby increasing the likelihood of smoking. Self-efficacy is viewed as the principal causal determinant of successful coping with high-risk situations and one of the best predictors of smoking cessation (DiClemente, Fairhurst, & Piotrowski, 1995; Fiore et al., 2008; Shiffman, 1984). This model has generated a tremendous amount of intervention research demonstrating that social cognitive/relapse prevention–based treatments for smoking cessation are effective (Carroll, 1996; Curry & McBride, 1994; Fiore et al., 2008). Nevertheless, these interventions have not yielded consistently superior results relative to other treatment approaches (Carroll; Lichtenstein & Glasgow, 1992).
One attribution for the lack of superiority of social cognitive approaches is that the translation of theory into specific treatment components has been incomplete. The relapse prevention model posits that the avoidance of specific high-risk situations or the performance of coping behaviors during such situations requires that the individual is sufficiently motivated to avoid smoking (Marlatt & Gordon, 1985). Thus, the effective treatment of smoking requires both enhancing the motivation to achieve and maintain change and developing the self-efficacy and skills necessary to do so. However, current interventions for smoking cessation often focus largely on either motivation or problem-solving/skills training despite the strong theoretical and empirical bases for focusing on both (Miller, Zweben, DiClemente, & Rychtarik, 1995). Those treatment models that address both motivation and problem-solving/skills training, such as the transtheoretical model, utilize motivational enhancement techniques largely to motivate individuals to make a quit attempt and problem-solving/skills training largely during the preparation, action, and maintenance stages (Prochaska, DiClemente, & Norcross, 1992). Likewise, the Treating Tobacco Use and Dependence Clinical Practice Guideline (the Guideline) has no specific recommendations for assessing or addressing motivation during a quit attempt or for preventing relapse (Fiore et al., 2008).
Recent evidence, however, demonstrates that motivation can change rapidly (Hughes, Keely, Fagerstrom, & Callas, 2005). For example, 41% of smokers in the United States report that their motivation to quit smoking changes daily (Werner, Lovering, & Herzog, 2004), and half or more of quit attempts are unplanned (Larabie, 2005; West & Sohal, 2006). These findings are consistent with a recent model of smoking motivation positing that motivation is dynamic and characterized by frequent fluctuations (West & Sohal). Importantly, motivational deficits are important in determining the maintenance of abstinence: 24% of all relapse episodes are characterized by a distinct lack of motivation to maintain abstinence in that situation (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996), and a decline in motivation over time prospectively predicts relapse (McBride, Pirie, & Curry, 1992). Thus, an intervention that is responsive to motivational fluctuations and includes the skill-based components of the relapse prevention model may enhance treatment efficacy.
Treatment efficacy among low-income pregnant/postpartum women might be further enhanced by addressing the myriad of stressors common among such populations, including high levels of stress, negative affect and depression, and low levels of social support (Allen, Prince, & Dietz, 2009; Park et al., 2009; Reitzel et al., 2007). These, and other stressors (e.g., partner relational problems, financial difficulty), often derail women’s attempts to maintain smoking abstinence (Ripley-Moffitt et al., 2008) and clearly warrant attention within a broader based treatment program. Moreover, a lack of reliable transportation, high mobility, and lower rates of routine clinic-based care are prevalent among low-income pregnant/postpartum women (Beck et al., 2002; Gazmararian, Arrington, Bailey, Schwarz, & Koplan, 1999; Williams et al., 2003). Therefore, interventions directed toward low-income women that minimize clinic-based face-to-face contact, such as telephone counseling, may be useful in increasing the dosage of treatment and improving adherence (Parker et al., 2007).
MAPS is a holistic dynamic approach to facilitating and maintaining behavior change that utilizes a combined motivational enhancement and social cognitive approach based on Motivational Interviewing (MI; Miller & Rollnick, 2002; Rollnick & Miller, 1995), the Guideline (Fiore et al., 2008), and social cognitive/relapse prevention theory (Marlatt & Donovan, 2005; Witkiewitz & Marlatt, 2004). MAPS is a general intervention approach that has evolved from our previous work (e.g., McClure, Westbrook, Curry & Wetter, 2005; Wetter et al., 2007) and that can be adapted for different populations and target behaviors. In this study, MAPS was adapted for use with all pregnant/postpartum women regardless of motivation to change and was designed to specifically target social cognitive constructs and other key factors of particular relevance to low-income pregnant/postpartum women within an overarching motivational enhancement framework. The current research represents the first clinical trial in which the efficacy of MAPS was evaluated for the prevention of postpartum relapse among pregnant women who quit smoking as a result of their pregnancy.
MAPS conceptualizes motivation as fluctuating dynamically and rapidly, such that the counselor switches between practical problem-solving/coping skills training and MI techniques based on an individual’s motivation. That is, MAPS requires the counselor to carefully attend to subtle changes in motivation so that they can be addressed in the moment as they emerge. Although stage-based conceptualizations of behavior change also emphasize both the enhancement of motivation and the skills training (Prochaska et al., 1992), motivational shifts in MAPS are viewed as much more volatile and less stable (i.e., less “stage like”). Although MI is not stage-based intervention (Miller & Rollnick, 2009), it has two distinct phases of treatment—building motivation (Phase 1) and strengthening commitment (Phase 2; Miller & Rollnick, 2002). While the transition to Phase 2 is prompted by participant cues of readiness to change, the initiation of Phase 2 is a process entailing recapitulation, asking key questions, developing a change plan, and so forth (Miller & Rollnick, 2002). In contrast, MAPS counselors may move back and forth between MI and problem-solving/coping skills training from moment to moment in response to participant cues, relying less on preemptive recapitulation and other strategies.
Another unique component of MAPS is the creation of a “wellness plan” in collaboration with each woman, which entails the formation of treatment goals related to smoking abstinence, as well as other salient concerns such as anxiety, stress, depression, interpersonal issues, family problems, financial concerns, and so forth. Thus, in addition to directly targeting smoking abstinence, MAPS assists women with their various life stressors that may ultimately affect abstinence (Drobes, Meier, & Tiffany, 1994; Shiffman & Waters, 2004; Wetter et al., 1999). Prioritizing and addressing these prominent concerns may also increase participants’ overall wellness and help them to maintain their investment in the counseling process.
The current randomized clinical trial (RCT) tested the efficacy of two versions of MAPS (i.e., MAPS and MAPS+) versus Usual Care (UC) for the prevention of postpartum smoking relapse. Participants were racially/ethnically diverse, predominantly low-income women who spontaneously quit smoking prior to their 30th week of pregnancy.
Methods
Participants
Participants were proactively recruited from within the Houston metropolitan area through a local health care system and via newspaper, radio, bus, and clinic advertisements. Participants (N = 251) were English-speaking pregnant women aged 18 years or older who stopped smoking either during their pregnancy or within 2 months prior to becoming pregnant. Participants smoked an average of at least one cigarette daily for the year prior to pregnancy and were in their 30th to 33rd week of pregnancy at the time of study enrollment. Women reporting a high-risk pregnancy were excluded.
Procedures
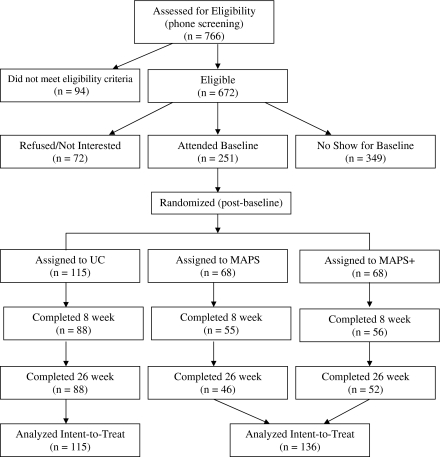
The University of Texas M.D. Anderson Cancer Center Institutional Review Board approved this study. Participants were enrolled from October 2004–April 2008 by research personnel. Written informed consent was obtained before data collection. Following baseline data collection, participants were randomized by computer into UC (n = 115), MAPS (n = 68), or MAPS+ (n = 68) using a form of adaptive randomization called minimization. Minimization provides for balanced treatment groups throughout the randomization process in trials with extended accrual periods. Randomization in the current study was based on age, education, race/ethnicity, current depression (measured at screening and assessed by the Center for Epidemiological Studies Depression Scale; Radloff, 1977), and prequit smoking rate. Neither participants nor research personnel was blind to treatment condition assignment following randomization. Participant flow through the study is detailed in Figure 1.
Figure 1.
CONSORT flowchart of recruitment and attendance at follow-up appointments.
Participants attended three on-site assessment visits (baseline [30- to 33-weeks pregnant] and Weeks 8 and 26 postpartum) were provided with $40 gift cards and small items of compensation (e.g., diapers) upon completion of each assessment visit. Babies and children could accompany mothers for assessment visits or could be left with on-site childcare providers free of charge. Participants were also given bus tokens or parking vouchers.
All participants were given self-help materials and 5–10 min of Guideline-based (Fiore et al., 2008) brief relapse prevention advice. The MAPS and MAPS+ groups also received six telephone-based counseling sessions (Weeks 34 and 36 prepartum and Weeks 2, 4, 7, and 16 postpartum), and the MAPS+ group received two additional in-person counseling sessions (baseline and Week 8 postpartum). The MAPS and MAPS+ groups covered identical treatment content, differing only with respect to the two in-person counseling sessions. MAPS/MAPS+ counseling sessions lasted an average of 22 min (SD = 13.55), with a modal length of 15 min.
Treatment delivery and integrity
Research personnel with Tobacco Treatment Specialist (TTS) training provided the brief advice that formed the basis of UC. Brief advice consisted of encouragement to remain quit, a review of the risks of smoking and the rewards of remaining abstinent, and a review of the Guideline-based self-help materials.
Master’s (n = 2) or doctoral-level (n = 4) counselors received MI, TTS, and MAPS/MAPS+ protocol training. MI training consisted of an introductory, intermediary, and advanced course in MI practice. TTS training consisted of more than 40 hr of intensive training designed to prepare counselors to address tobacco dependence and relapse prevention, as offered by nationally recognized training and certification programs. MAPS/MAPS+ protocol training was standardized and consisted of approximately 80 hr of manual review and role playing with a MI expert, hereafter referred to as supervisor, by which time proficiency was reached in all cases.
The study had at least two active female counselors at any given time, and counselor assignment varied between but not within participants. Counselors met twice monthly with a supervisor for case review and practice (e.g., role plays), as recommended (Velasquez et al., 2000). The supervisor was also available for consultation between scheduled appointments. Counselors sought feedback from participants throughout counseling via open-ended questions, and problems with participant comprehension of treatment strategies were addressed in vivo.
Counseling sessions were tape-recorded, and two randomly selected tapes per month per counselor were coded with an adapted version of the Motivational Interviewing Treatment Integrity (MITI) Code (2.0; Moyers, Martin, Manuel, & Miller, n.d.), which is a brief system for coding MI treatment fidelity. Coding used the global indices (empathy/understanding and MI spirit) and a study-specific index to assess adherence to the MAPS/MAPS+ treatment manual, capturing the “desirable shifting” between MI and relapse prevention as described above. Possible ratings on indices ranged from 1 (low) to 7 (high). Counselors maintained adequate adherence to MI (empathy/understanding M = 5.39 [SD = 0.62] and MI spirit M = 5.50 [SD = 0.57]), which compares favorably with other intervention studies in similar populations (Thyrian et al., 2007). Counselors maintained excellent adherence (Moyers et al., n.d.) to the MAPS/MAPS+ treatment manual (M = 6.77 [SD = 0.50]) throughout the study.
Overall, 47% of MAPS/MAPS+ participants completed all six counseling calls, 67% completed at least five, and 80% completed at least four of the calls. Of those in MAPS+, 100% completed the baseline in-person counseling session and 81% completed the 8-week session. The number of completed calls did not differ by demographics or prequit smoking rate.
Measures
Questionnaires were administered and completed via computer. Data collection was completed by January 2009. Variables of interest are below.
Demographics
Demographics collected at baseline included age, race/ethnicity, partner status, total annual household income, and educational level.
Smoking rate
The average number of cigarettes smoked per day prior to quitting was assessed by self-report at baseline.
Smokers in the environment
The number of smokers around the participants on typical weekdays and weekends, respectively, was assessed by self-report at baseline.
Smoking abstinence
Continuous abstinence from smoking, defined as no smoking since the delivery date, was assessed at Weeks 8 and 26 postpartum via self-report. Abstinence was biochemically verified through expired carbon monoxide levels (CO) <10 ppm (Hajek et al., 2001) and/or a cotinine value of <20 ng/ml (McBride et al., 1999). Cotinine, collected in a saliva sample by mail, was used if a participant had relocated and was unable to return to the clinic for a CO assessment (n = 8 at Week 8 and n = 11 at Week 26), a method with precedence in prior research (McBride et al., 1999).
Data analysis
This study tested the efficacy of MAPS and MAPS+ versus UC for postpartum relapse prevention. Sample size was based on the ability to detect increments of 15–20 percentage points between treatment groups at any single point in time. All analyses were performed using Statistical Analysis Software (version 9.1). Because continuous abstinence was the outcome, continuation ratio (CR) logit models (SAS PROC GENMOD; Agresti, 2002; Allison, 2001 Bender & Bender, 2000; McGowan, 2000) were used for analyses. CR logit models are appropriate when ordered categories (e.g., relapsed at Week 8, abstinent at Week 8 but relapsed at Week 26, and abstinent through Week 26) represent a progression through stages (Agresti; Bender & Bender; McGowan). The CR logit models operate by modeling the conditional probability of being abstinent at the current assessment point, given that a participant has been abstinent through the most recent assessment point. An intention-to-treat procedure was followed, whereby those lost to follow-up were considered relapsed. Both unadjusted analyses and analyses adjusted for the covariates of age, race/ethnicity, partner status, education, smoking rate, and smokers in the environment were conducted.
Following the assessment of the main effect of treatment, age, race/ethnicity, partner status, education, smoking rate, and smokers in the environment were examined as potential moderators in unadjusted and adjusted CR logit models.
Results
Participant characteristics
Participants were predominantly of low income (55% reported <$30,000/year in annual household income) and racially/ethnically diverse (32% Black, 30% Latino, and 36% White; Table 1). The average prequit smoking rate was 10.2 (SD = 7.6) cigarettes/day. Some women (7.6%) reported quitting smoking within 4 (SD = 2.05) weeks of pregnancy, but most (92.4%) quit smoking about 8 (SD = 5.70) weeks after pregnancy.
Table 1.
Participant characteristics by treatment group
| Usual care |
MAPS/MAPS+ |
Total group |
||
| n = 115 | n = 136 | N = 251 | p value | |
| Demographics | ||||
| Age, mean years (M ± SD) | 24.6 (5.5) | 24.6 (5.2) | 24.6 (5.3) | .96 |
| Race (%) | .93 | |||
| White | 34.8 | 36.0 | 35.5 | |
| Black | 32.2 | 32.4 | 32.3 | |
| Latino | 30.4 | 30.1 | 30.3 | |
| Other | 2.6 | 1.5 | 2.0 | |
| Partner status (%) | .49 | |||
| Partner | 65.2 | 61.0 | 62.9 | |
| No Partner | 34.8 | 39.0 | 37.1 | |
| Household income (%) | .53 | |||
| <$30,000/year | 54.7 | 55.2 | 55.0 | |
| ≥$30,000/year | 45.3 | 44.8 | 45.0 | |
| Education (%) | .14 | |||
| <High school/GED | 14.8 | 22.1 | 18.7 | |
| ≥High school/GED | 85.2 | 77.9 | 81.3 | |
| Smoking rate | ||||
| Cigarettes per day (M ± SD) | 10.7 (8.2) | 9.7 (7.1) | 10.2 (7.6) | .28 |
| Smokers in the environment | ||||
| On weekdays (M ± SD) | 2.6 (4.7) | 1.9 (3.0) | 2.2 (3.9) | .19 |
| On weekends (M ± SD) | 2.5 (3.1) | 2.2 (2.8) | 2.4 (2.9) | .38 |
| Depression | ||||
| Screening CES-D (M ± SD) | 18.6 (18.0) | 18.0 (11.9) | 18.2 (11.3) | .67 |
Note. p values for continuous variables are based on t tests, and p values for categorical variables are based on chi-square tests. CES-D = Centers for Epidemiological Studies Depression Scale; GED = general equivalency degree; MAPS = Motivation and Problem-Solving.
Participant attrition
Participant attrition was 20.7% (n = 52) at Week 8 and 25.9% (n = 65) at Week 26. At Weeks 8 and 26, completers were more educated, F(1, 249) = 10.21, p < .01; F(1, 249) = 6.63, p = .01, and reported smoking fewer cigarettes per day, F(1, 249) = 4.07, p = .05; F(1, 249) = 3.79, p = .05, than those lost to follow-up.
MAPS/MAPS+ group comparison
The MAPS and MAPS+ groups did not differ on baseline characteristics, number of completed calls, counseling session length, and percentage of participants who were abstinent at 8- and 26-weeks postpartum. As such, MAPS and MAPS+ were combined for analyses.
UC and MAPS/MAPS+ baseline differences
t tests and chi-square tests were used to evaluate differences between UC and MAPS/MAPS+ at baseline on demographics, smoking-related variables, and depression. No significant differences were found between the groups (Table 1).
Treatment effects
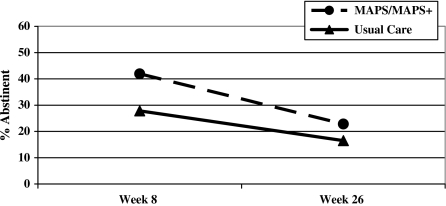
Abstinence rates were 27.8% in UC versus 41.9% in MAPS/MAPS+ at 8 weeks and 16.5% in UC versus 22.8% in MAPS/MAPS+ at 26 weeks (Figure 2). The main effect of treatment approached significance in the unadjusted analyses, χ2(1) = 3.10; p = .08, and was significant in analyses adjusted for age, race/ethnicity, partner status, education, smoking rate, and smokers in the environment, χ2(1) = 3.79; OR = 1.60 (95% CI = 1.00–2.58); p = .05. The treatment by time interaction was not significant, indicating no significant variation of the treatment effect over time.
Figure 2.
Abstinence rates by treatment group at 8 and 26-weeks postpartum.
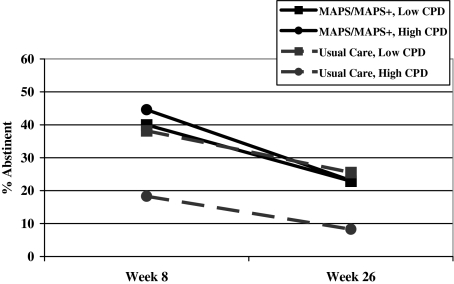
Moderator analyses were performed to test for significant interactions of treatment group with demographics, screening depression, smoking rate, and smokers in the environment in predicting abstinence. The smoking rate by treatment group interaction was significant in unadjusted analyses, χ2(1) = 3.86; OR = 1.07 (95% CI = 1.00–1.15); p = .05, and approached significance in adjusted analyses, χ2(1) = 2.82; p = .09. Follow-up analyses on raw (unadjusted) data using a median split for smoking rate (≤7.5 vs. >7.5 cigarettes/day) indicated MAPS/MAPS+ was more efficacious than UC among women who smoked more cigarettes per day, χ2(1) = 7.37; OR = 2.87 (95% CI = 1.34–6.13); p = .007, but not among women who smoked fewer cigarettes per day, χ2(1) < 0.01; p = .99 (Figure 3). No other potential moderator variables were significant or near significant in unadjusted or adjusted analyses.
Figure 3.
Illustration of the interaction effect of treatment group with prequit cigarettes per day (CPD) in unadjusted analyses.
Discussion
This RCT evaluated the efficacy of MAPS/MAPS+ versus UC for postpartum smoking relapse prevention among a diverse sample of low-income women who quit smoking as a result of their pregnancy. Results of analyses adjusted for demographics, prequit smoking rate, and the number of smokers in the women’s environment indicated that MAPS/MAPS+ significantly increased the maintenance of postpartum smoking abstinence over UC through 26-weeks postpartum. MAPS/MAPS+ integrates MI (Miller & Rollnick, 2002) and relapse prevention (Marlatt & Donovan, 2005) techniques in a way that adjusts for participants’ in-the-moment motivational shifts. As such, MAPS/MAPS+ is responsive to research indicating that motivation for quitting and maintaining abstinence varies over short periods of time (Hughes et al., 2005; Werner et al., 2004) and differs from other interventions that conceptualize motivation as stage based and less volatile (e.g., Hajek et al., 2001; Morasco, Dornelas, Fischer, Oncken, & Lando, 2006; Valanis et al., 2001). Similar to other emerging interventions (Bullock et al., 2009; Chalmers et al., 2004; Gaffney, 2006), MAPS/MAPS+ is a holistic approach in which numerous issues of relevance to pregnant and postpartum women are addressed. Results suggest that an intervention that treats women’s smoking within a larger framework of wellness, and that recognizes and adapts to moment-to-moment changes in motivation, is more efficacious than UC for relapse prevention among women who spontaneously quit smoking during pregnancy. These findings are of clinical importance because the majority of postpartum relapse prevention studies have failed to demonstrate a significant treatment effect (Hajek et al., 2009; Lancaster et al., 2006). However, similar to other studies in this area, our results indicated increasing rates of relapse over time among all participants (CDC, 2007; Fiore et al., 2000; Mullen, 2004; Mullen et al., 1990; Ockene, 1993; Stotts et al., 2000) and suggest the diminishing effect of treatment over time. Future research should focus on evaluating the efficacy of MAPS/MAPS+ for relapse prevention beyond 26-weeks postpartum.
Results of unadjusted analyses indicated that MAPS/MAPS+ was particularly efficacious among women with higher prequit smoking rates, although this effect only approached significance in adjusted analyses. Treatments that effectively address postpartum smoking relapse prevention among women with higher prequit smoking rates, potentially indicative of greater dependence on tobacco, are essential as these women are at increased risk of postpartum relapse compared with less dependent women (Fang et al., 2004; Ripley-Moffitt et al., 2008; Severson, Andrews, Lichtenstein, Wall, & Zoref, 1995). Telephone-based smoking cessation interventions have previously shown more promise among less dependent pregnant women (Ershoff et al., 1999; Rigotti et al., 2006). The current study extends the efficacy of a largely telephone-based counseling protocol to relapse prevention among all pregnancy-based spontaneous quitters, and suggests that MAPS/MAPS+ may be particularly efficacious for those with higher prequit smoking rates (and potentially greater tobacco dependence).
On average, about 40% of spontaneous quitters are abstinent at 6-months postpartum (Solomon & Quinn, 2004), with continuous abstinence rates in individual studies typically ranging between 33% (McBride et al., 1999) and 43% (Morasco et al., 2006). Thus, the continuous abstinence rate of 23% in the MAPS/MAPS+ group at 26-weeks postpartum appears relatively low. However, many previous studies have been limited by the failure to biochemically confirm self-reports of smoking abstinence (e.g., Valanis et al., 2001) and by smaller sample sizes than the current study (Morasco et al.), which could result in inflated and unstable abstinence rates (cf., Secker-Walker et al., 1995). The MAPS/MAPS+ abstinence rate, however, is identical to that found in a midwife-delivered cessation intervention that used carbon monoxide readings to verify abstinence (Hajek et al., 2001). Additionally, as with MAPS/MAPS+ abstinence rates, UC abstinence rates in this study were also low (17%). For example, previous studies have cited UC continuous abstinence rates at 6-months postpartum of 25% (Hajek et al., 2001) and 32% (Morasco et al.). The lower abstinence rates for both MAPS/MAPS+ and UC in the present study suggest that our participants may have been at higher risk of relapse in general as compared with women in previous studies, a potential consequence of the high proportion of low-income women, a group that is at elevated risk for postpartum relapse (Tong et al., 2009).
Another important finding from the present study concerns the acceptability of MAPS/MAPS+ among racially/ethnically diverse, predominately low-income women. In total, more than 80% of the sample completed at least four counseling calls (67% of the intervention dose in MAPS). Moreover, the completion of counseling calls was not related to age, race/ethnicity, partner status, income, or education, suggesting that women of various demographic backgrounds found the intervention equally acceptable. In addition to call completion, a high proportion of our sample completed study follow-up assessments: 79% at Week 8 and 74% at Week 26. This is an excellent long-term retention rate for a sample of largely low-income women, who can be difficult to track due to high mobility.
A major strength of MAPS/MAPS+ is its potential for dissemination. MAPS/MAPS+ entailed six counseling calls with a modal duration of 15 min each, which is very similar to smoking cessation services provided by quitlines throughout the country. Therefore, MAPS/MAPS+ has widespread dissemination potential as it can easily be incorporated into a population-based smoking treatment program or a state quitline.
A limitation of the current study was the sample size. Future studies of MAPS/MAPS+ should include larger sample sizes. Despite high acceptability rates once women were enrolled, only 42% of eligible participants attended the enrollment visit and were randomized to treatment in this study. These rates, however, are comparable with those found among studies with similar populations (Morasco et al., 2006) and may reflect the many barriers faced by low-income women in accessing care (e.g., problems related to finding transportation). Post-hoc analyses (not shown) of enrolled women versus no-shows did not indicate differences between groups on demographics, prequit smoking rates, current depression, or “motivation” as assessed by postpartum abstinence goals.
MAPS/MAPS+ efficacy or effectiveness studies might benefit from the assessment of the specific treatment strategies reviewed with each patient, as well as the participant enactment of treatment skills, as these variables inform treatment fidelity and affect reliability and external validity (Bellg et al., 2004; Borrelli et al., 2005). The collection and coding of data on the achievement of nonsmoking-related goals and their association with smoking outcomes might also be of interest. Future studies might also explore the effect of altering the timing or number of counseling calls (e.g., increasing the number of calls over a longer duration of time, allowing women to negotiate the calling schedule based on their perceived needs). Finally, we lacked the sample size and power to adequately test the relative efficacy of counseling calls alone versus a combination of both calls and in-person sessions in the prevention of postpartum relapse. Future studies may wish to address this issue.
Results of this RCT supported the efficacy of MAPS/MAPS+, a motivational enhancement and relapse prevention–based treatment, in the prevention of postpartum smoking relapse among racially/ethnically diverse, predominantly low-income women through 26-weeks postpartum. Given the high postpartum relapse rate (Fingerhut, Kleinman, & Kendrick, 1990; McBride & Pirie, 1990; Melvin & Gaffney, 2004; Mullen et al., 1990; Ockene, 1993; Solomon & Quinn, 2004; Stotts et al., 2000) and the limitations of previous interventions (Hajek et al., 2009; Lancaster et al., 2006), the need for innovative and effective interventions to prevent postpartum relapse is paramount (Fiore et al., 2008). MAPS/MAPS+ shows promise as a relapse prevention intervention for women who spontaneously quit smoking during pregnancy and may be particularly efficacious among women with higher prequit smoking rates.
Funding
This manuscript was supported by grants from the National Cancer Institute (R01CA89350 to DWW; R25TCA57730 to MSB and DEK) and the CDC (K01DP001120 to LRR; K01DP000086 to JIV).
Declaration of Interests
None declared.
Acknowledgments
Authors are appreciative for the contributions of Patricia Figueroa, M.Ed., and Vantrese Camiso, M.Ed., who were counselors on the clinical trial. Ms. Figueroa also assisted with the coauthorship of the treatment manual. Authors also appreciate the contributions of Nanette Stephens, Ph.D., who assisted with the coauthorship of the treatment manual, supervised the counselors in this protocol, and coded therapy tapes to monitor integrity. Finally, authors appreciate the contributions of the research staff who assisted with the completion of the clinical trial.
References
- Agresti A. Categorical data analysis. 2nd ed. Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- Allen AM, Prince CB, Dietz PM. Postpartum depressive symptoms and smoking relapse. American Journal of Preventive Medicine. 2009;36:9–12. doi: 10.1016/j.amepre.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Allison PD. Logistic regression using the SAS system: theory and application. Cary, NC: SAS Institute and Wiley; 2001. [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychology Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Beck LF, Morrow B, Lipscomb LE, Johnson CH, Gaffield ME, Rogers M, et al. Prevalence of selected maternal behaviors and experiences, Pregnancy Risk Assessment Monitoring System (PRAMS), 1999. Morbidity and Mortality Weekly Report Surveillance Summary. 2002;51:1–27. [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH behavior change consortium. Health Psychology. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- Bender R, Bender A. Calculating ordinal regression models in SAS and S-Plus. Biometrical Journal. 2000;42:677–699. [Google Scholar]
- Borrelli B, Sepinwall D, Ernst D, Bellg AJ, Czajkowski S, Breger R, et al. A new tool to assess treatment fidelity and evaluation of treatment fidelity across 10 years of health behavior research. Journal of Consulting and Clinical Psychology. 2005;73:852–860. doi: 10.1037/0022-006X.73.5.852. [DOI] [PubMed] [Google Scholar]
- Bullock L, Everett KD, Mullen PD, Geden E, Longo DR, Madsen R. Baby BEEP: A randomized controlled trial of nurses’ individualized social support for poor rural pregnant smokers. Maternal and Child Health Journal. 2009;13:395–406. doi: 10.1007/s10995-008-0363-z. [DOI] [PubMed] [Google Scholar]
- Carroll K. Relapse prevention as a psychosocial treatment: A review of controlled clinical trials. Experimental and Clinical Psychopharmacology. 1996;4:46–54. [Google Scholar]
- Centers for Disease Control and Prevention. Preventing smoking and exposure to secondhand smoke before, during, and after pregnancy. Atlanta, GA: Centers for Disease Control and Prevention; 2007. Retrieved 4 February 2010, from http://www.cdc.gov/chronicdisease/resources/publications/fact_sheets/smoking.htm. [Google Scholar]
- Chalmers K, Gupton A, Katz A, Hack T, Hildes-Ripstein E, Brown J, et al. The description and evaluation of a longitudinal pilot study of a smoking relapse/reduction intervention for perinatal women. Journal of Advanced Nursing. 2004;45:162–171. doi: 10.1046/j.1365-2648.2003.02862.x. [DOI] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Curry SJ, McBride CM. Relapse prevention for smoking cessation: Review and evaluation of concepts and interventions. Annual Review of Public Health. 1994;15:345–366. doi: 10.1146/annurev.pu.15.050194.002021. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Fairhurst SK, Piotrowski NA. Self-efficacy and addictive behaviors. In: Maddux JE, editor. Self-efficacy, adaptation, and adjustment: Theory, research, and application. New York: Plenum Press; 1995. pp. 109–141. [Google Scholar]
- Drobes DJ, Meier EA, Tiffany ST. Assessment of the effects of urges and negative affect on smokers’ coping skills. Behavioral Research Therapy. 1994;32:165–174. doi: 10.1016/0005-7967(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Ershoff DH, Quinn VP, Boyd NR, Stern J, Gregory M, Wirtschafter D. The Kaiser Permanente prenatal smoking-cessation trial: When more isn’t better, what is enough? American Journal of Preventive Medicine. 1999;17:161–168. doi: 10.1016/s0749-3797(99)00071-9. [DOI] [PubMed] [Google Scholar]
- Fang WL, Goldstein AO, Butzen AY, Hartsock SA, Hartmann KE, Helton M, et al. Smoking cessation in pregnancy: A review of postpartum relapse prevention strategies. Journal of American Board of Family Practitioners. 2004;17:264–275. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. American Journal of Public Health. 1990;80:541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- Fiore M, Jaen C, Baker T. Treating tobacco use and dependence: 2008 Update. Clinical practice guidelines. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Floyd RL, Rimer BK, Giovino GA, Mullen PD, Sullivan SE. A review of smoking in pregnancy: Effects on pregnancy outcomes and cessation efforts. Annual Review of Public Health. 1993;14:379–411. doi: 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- Gaffney KF. Postpartum smoking relapse and becoming a mother. Journal of Nursing Scholarship. 2006;38:26–30. doi: 10.1111/j.1547-5069.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- Gazmararian JA, Arrington TL, Bailey CM, Schwarz KS, Koplan JP. Prenatal care for low-income women enrolled in a managed-care organization. Obstetrics & Gynecology. 1999;94:177–184. doi: 10.1016/s0029-7844(99)00237-9. [DOI] [PubMed] [Google Scholar]
- Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database of Systematic Reviews. 2009 doi: 10.1002/14651858.CD003999.pub3. CD003999. [DOI] [PubMed] [Google Scholar]
- Hajek P, West R, Lee A, Foulds J, Owen L, Eiser JR, et al. Randomized controlled trial of a midwife-delivered brief smoking cessation intervention in pregnancy. Addiction. 2001;96:485–494. doi: 10.1046/j.1360-0443.2001.96348511.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Fagerstrom KO, Callas PW. Intentions to quit smoking change over short periods of time. Addictive Behavior. 2005;30:653–662. doi: 10.1016/j.addbeh.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Lancaster T, Hajek P, Stead LF, West R, Jarvis MJ. Prevention of relapse after quitting smoking: A systematic review of trials. Archives of Internal Medicine. 2006;166:828–835. doi: 10.1001/archinte.166.8.828. [DOI] [PubMed] [Google Scholar]
- Larabie LC. To what extent do smokers plan quit attempts? Tobacco Control. 2005;14:425–428. doi: 10.1136/tc.2005.013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein E, Glasgow RE. Smoking cessation: What have we learned over the past decade? Journal of Consulting and Clinical Psychology. 1992;60:518–527. doi: 10.1037//0022-006x.60.4.518. [DOI] [PubMed] [Google Scholar]
- Marlatt G, Donovan D, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. 2nd ed. New York: Guilford Press; 2005. [Google Scholar]
- Marlatt GA, Gordon JR. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford Press; 1985. [Google Scholar]
- McBride CM, Curry SJ, Lando HA, Pirie PL, Grothaus LC, Nelson JC. Prevention of relapse in women who quit smoking during pregnancy. American Journal of Public Health. 1999;89:706–711. doi: 10.2105/ajph.89.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride CM, Pirie PL. Postpartum smoking relapse. Addictive Behavior. 1990;15:165–168. doi: 10.1016/0306-4603(90)90020-x. [DOI] [PubMed] [Google Scholar]
- McBride CM, Pirie PL, Curry SJ. Postpartum relapse to smoking: A prospective study. Health Education Research. 1992;7:381–390. doi: 10.1093/her/7.3.381. [DOI] [PubMed] [Google Scholar]
- McClure JB, Westbrook E, Curry SJ, Wetter DW. Proactive, motivationally enhanced smoking cessation counseling among women with elevated cervical cancer risk. Nicotine & Tobacco Research. 2005;7:881–889. doi: 10.1080/14622200500266080. [DOI] [PubMed] [Google Scholar]
- McGowan MJ. Ordinal outcomes with the continuation ratio model. 2000 , September. Paper presented at the Proceedings of the Northeast SAS Users Group Conference, Philadelphia, PA. [Google Scholar]
- Melvin CL, Gaffney CA. Treating nicotine use and dependence of pregnant and parenting smokers: An update. Nicotine & Tobacco Research. 2004;6:S107–S124. doi: 10.1080/14622200410001669231. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behavioural and Cognitive Psychotherapy. 2009;37:129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: USDHHS; 1995. [Google Scholar]
- Morasco BJ, Dornelas EA, Fischer EH, Oncken C, Lando HA. Spontaneous smoking cessation during pregnancy among ethnic minority women: A preliminary investigation. Addictive Behavior. 2006;31:203–210. doi: 10.1016/j.addbeh.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Moyers TB, Martin T, Manuel JK, Miller WR. The motivational interviewing treatment integrity (MITI) code: Version 2.0. Unpublished. Albuquerque, NM: University of New Mexico, Center on Alcoholism, Substance Abuse and Addictions.; n.d.. Retrieved 4 February 2010, from http://casaa.unm.edu/download/miti.pdf. [Google Scholar]
- Mullen PD. How can more smoking suspension during pregnancy become lifelong abstinence? Lessons learned about predictors, interventions, and gaps in our accumulated knowledge. Nicotine & Tobacco Research. 2004;6:S217–S238. doi: 10.1080/14622200410001669150. [DOI] [PubMed] [Google Scholar]
- Mullen PD, Quinn VP, Ershoff DH. Maintenance of nonsmoking postpartum by women who stopped smoking during pregnancy. American Journal of Public Health. 1990;80:992–994. doi: 10.2105/ajph.80.8.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene JK. Smoking among women across the life span: Prevalence, interventions, and implications for cessation research. Annals of Behavioral Medicine. 1993;15:135–148. [Google Scholar]
- Park ER, Chang Y, Quinn V, Regan S, Cohen L, Viguera A, et al. The association of depressive, anxiety, and stress symptoms and postpartum relapse to smoking: A longitudinal study. Nicotine & Tobacco Research. 2009;11:707–714. doi: 10.1093/ntr/ntp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DR, Windsor RA, Roberts MB, Hecht J, Hardy NV, Strolla LO, et al. Feasibility, cost, and cost-effectiveness of a telephone-based motivational intervention for underserved pregnant smokers. Nicotine & Tobacco Research. 2007;9:1043–1051. doi: 10.1080/14622200701591617. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. American Psychologist. 1992;47:1102–1114. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Reitzel LR, Vidrine JI, Li Y, Mullen PD, Velasquez MM, Cinciripini PM, et al. The influence of subjective social status on vulnerability to postpartum smoking among young pregnant women. American Journal of Public Health. 2007;97:1476–1482. doi: 10.2105/AJPH.2006.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg LX, editors. SEER cancer statistics review, 1975-2001. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- Rigotti NA, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers: A randomized controlled trial. Obstetrics & Gynecology. 2006;108:83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- Ripley-Moffitt CE, Goldstein AO, Fang WL, Butzen AY, Walker S, Lohr JA. Safe babies: A qualitative analysis of the determinants of postpartum smoke-free and relapse states. Nicotine & Tobacco Research. 2008;10:1355–1364. doi: 10.1080/14622200802238936. [DOI] [PubMed] [Google Scholar]
- Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The generation R study. European Journal of Neuroscience. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Lepage SS, Goodwin GD, et al. Smoking relapse prevention counseling during prenatal and early postnatal care. American Journal of Preventive Medicine. 1995;11:86–93. [PubMed] [Google Scholar]
- Severson HH, Andrews JA, Lichtenstein E, Wall M, Zoref L. Predictors of smoking during and after pregnancy: A survey of mothers of newborns. Preventive Medicine. 1995;24:23–28. doi: 10.1006/pmed.1995.1004. [DOI] [PubMed] [Google Scholar]
- Shah T, Sullivan K, Carter J. Sudden infant death syndrome and reported maternal smoking during pregnancy. American Journal of Public Health. 2006;96:1757–1759. doi: 10.2105/AJPH.2005.073213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S. Cognitive antecedents and sequelae of smoking relapse crises. Journal of Applied Social Psychology. 1984;14:296–309. [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Solomon LJ, Quinn VP. Spontaneous quitting: Self-initiated smoking cessation in early pregnancy. Nicotine & Tobacco Research. 2004;6:S203–S216. doi: 10.1080/14622200410001669132. [DOI] [PubMed] [Google Scholar]
- Stotts AL, DiClemente CC, Carbonari JP, Mullen PD. Postpartum return to smoking: Staging a “suspended” behavior. Health Psychology. 2000;19:324–332. doi: 10.1037//0278-6133.19.4.324. [DOI] [PubMed] [Google Scholar]
- Thyrian JR, Freyer-Adam J, Hannover W, Roske K, Mentzel F, Kufeld C, et al. Adherence to the principles of motivational interviewing, clients’ characteristics and behavior outcome in a smoking cessation and relapse prevention trial in women postpartum. Addictive Behavior. 2007;32:2297–2303. doi: 10.1016/j.addbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy; pregnancy risk and monitoring system (PRAMS), United States, 31 sites, 2000–2005. Morbidity and Mortality Weekly Report. 2009;58:1–29. [PubMed] [Google Scholar]
- Valanis B, Lichtenstein E, Mullooly JP, Labuhn K, Brody K, Severson HH, et al. Maternal smoking cessation and relapse prevention during health care visits. American Journal of Preventive Medicine. 2001;20:1–8. doi: 10.1016/s0749-3797(00)00266-x. [DOI] [PubMed] [Google Scholar]
- Velasquez MM, Hecht J, Quinn VP, Emmons KM, DiClemente CC, Dolan-Mullen P. Application of motivational interviewing to prenatal smoking cessation: Training and implementation issues. Tobacco Control. 2000;9:36–40. doi: 10.1136/tc.9.suppl_3.iii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner J, Lovering A, Herzog TA. Measuring time frames to quit smoking. 2004. Annual Meeting of the Society for Research on Nicotine and Tobacco. Phoenix, AZ. [Google Scholar]
- West R, Sohal T. “Catastrophic” pathways to smoking cessation: Findings from national survey. British Medical Journal. 2006;332:458–460. doi: 10.1136/bmj.38723.573866.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Mazas C, Daza P, Nguyen L, Fouladi RT, Cofta-Woerpel L. Reaching and treating Spanish-speaking smokers though the national cancer institute’s cancer information service. Cancer. 2007;109:406–413. doi: 10.1002/cncr.22360. [DOI] [PubMed] [Google Scholar]
- Williams LM, Morrow B, Lansky A, Beck LF, Barfield W, Helms K, et al. Surveillance for selected maternal behaviors and experiences before, during, and after pregnancy. Pregnancy Risk Assessment Monitoring System (PRAMS), 2000. Morbidity and Mortality Weekly Report Surveillance Summary. 2003;52:1–14. [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: That was Zen, this is Tao. American Psychologist. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]