Abstract
Introduction:
Potential-reduced exposure products (PREPs) are marketed as a way for smokers to continue using tobacco while possibly lessening their tobacco toxicant intake. Some tobacco-based PREPs are combustible and intended to be smoked, while others are noncombustible and intended to be administered orally (e.g., Camel Snus [CS] tobacco sachets and Ariva tobacco tablets). The ability of these noncombustible PREPs to reduce smokers’ exposure to cigarette-delivered toxicants and suppress tobacco abstinence symptoms effectively is unclear. Clinical laboratory methods have been used to measure combustible PREP-associated toxicant exposure and abstinence symptom suppression and could be applied to evaluating the effects of orally administered noncombustible PREPs.
Methods:
In this study, 21 smokers (6 women) participated in four 5-day conditions that differed by product used: CS, Ariva, own brand cigarettes, or no tobacco. Measures included expired-air carbon monoxide (CO), the urinary metabolite of nicotine (cotinine), the urinary metabolite of the carcinogen NNK (NNAL-T), and subjective effect ratings.
Results:
Relative to own brand, all other conditions were associated with CO and cotinine levels that were lower and abstinence symptom ratings that were greater. Only no-tobacco use was associated with significantly lower NNAL levels. Acceptability ratings were also lower in all conditions relative to own brand.
Discussion:
Although these oral products reduce exposure to CO, their ineffective abstinence symptom suppression and low acceptability may limit their viability as PREPs. As with combustible PREPs, clinical laboratory study of orally administered noncombustible PREPs will be a valuable part of any comprehensive PREP evaluation strategy.
Introduction
Tobacco use costs more than 400,000 American lives annually (United States Department of Health and Human Services, 2004), primarily due to exposure to smoke’s lethal chemical compounds. Chronic carbon monoxide (CO) exposure has been implicated in cardiovascular disease (Lakier, 1992), while tobacco-specific nitrosamines (TSNAs) cause cancer (Wogan, Hecht, Felton, Conney, & Loeb, 2004). Toxicant exposure and health risks are reduced when smokers quit, but most quit attempts end in relapse (Bolliger et al., 2002). High relapse rates have led to an increasing interest in harm reduction: continued tobacco use with less disease (Stratton, Shetty, Wallace, & Bondurant, 2001).
Harm reduction may have been the impetus behind the release of “low-yield” cigarettes decades ago. However, despite widespread acceptance by consumers, long-term epidemiological data demonstrate that these products did little to decrease cigarette-caused morbidity and mortality (e.g., Thun & Heath, 1997). The failure of low-yield cigarettes as a harm-reduction strategy might have been predicted: Later clinical research demonstrated that these products did not reduce smokers’ toxicant exposure (National Cancer Institute, 2001; Stratton et al., 2001). This experience highlights the value of studying the effects of new harm-reduction strategies for smokers, including the many potential-reduced exposure products (PREPs) now marketed as a way for smokers to lessen their intake of cigarette-delivered toxicants (Warner, 2002). Specifically, clinical research may help predict the success or failure of these novel harm-reduction strategies.
While some PREPs for smokers involve tobacco combustion (e.g., Breland, Kleykamp, & Eissenberg, 2006), others involve orally administered noncombustible tobacco products. For example, Star Scientific markets Ariva, a compressed tobacco tablet intended for “adult smokers in situations where they cannot or choose not to smoke.” According to the manufacturer, the tobacco used in Ariva is cured such that the formation of TSNAs is limited; laboratory study reveals that this product delivers nicotine, although at a dose lower than a typical cigarette (Blank, Sams, Weaver, & Eissenberg, 2008; Cobb, Weaver, & Eissenberg, 2009; Kotlyar et al., 2007). Another noncombustible PREP is R. J. Reynolds’ Camel Snus (CS), a sachet of tobacco that is marketed for “pleasure for wherever.” CS is made in Sweden where use of this type of smokeless tobacco may account for decreased rates of smoking-related disease (Lewin et al., 1998; Schildt, Eriksson, Hardell, & Magnuson, 1998). Like Ariva, laboratory study reveals that CS delivers less nicotine than a cigarette (Cobb et al.). Importantly, as with low-yield cigarettes, these and other noncombustible PREPs have been marketed to U.S. smokers despite the dearth of objective data regarding their toxicant exposure and effects.
Measuring PREP effects includes assessing the levels of toxicants to which users are exposed as well as the PREP’s ability to suppress cigarette abstinence symptoms. Those PREPs that fail to suppress cigarette abstinence are unlikely to replace smokers’ cigarette intake fully (e.g., Hughes & Keely, 2004). For combustible PREPs, methods exist for measuring user toxicant exposure and cigarette abstinence symptom suppression over several days (Breland et al., 2006; Hatsukami et al., 2004). These methods may also be valuable for evaluating noncombustible PREPs. Thus, the purpose of this study was to adapt these methods in order to measure the toxicant exposure and abstinence symptom suppression associated with the use of orally administered noncombustible PREPs for smokers. Participants completed four 5-day conditions that differed by product used: Ariva, CS, own brand cigarettes, or no tobacco. It was hypothesized that, relative to own brand, the noncombustible PREPs would be associated with lower toxicant exposure but higher magnitude of tobacco abstinence symptom ratings.
Methods
Participants
Seventy-five individuals provided informed consent and agreed to participate in this Institutional Review Board-approved study. Sixteen did not begin the study either because they failed to meet inclusion/exclusion criteria, voluntarily withdrew before the first laboratory visit, or were otherwise ineligible. Of the 59 participants who began the study, 30 did not complete it because they could not comply with study procedures when using their own brand of cigarettes (n = 3) or were unable to abstain from other tobacco in conditions where they could not use any tobacco (n = 13), use CS only (n = 7), or use Ariva only (n = 7). In addition, post-study analysis of CO levels, quantitative urine cotinine concentrations, and/or participant self-report revealed that seven participants who completed the study failed to comply with study procedures in conditions where they were required to smoke their own brand of cigarette (n = 1), not use any tobacco (n = 4), use CS only (n = 1), or use Ariva only (n = 2). Thus, all subsequent analyses and discussion are based on the 21 participants who completed the protocol and complied with all study procedures.
Of the 21 participants, 15 were men (9 non-White) and 6 were women (3 non-White). They were between 18 and 55 years (M = 33.3, SD = 13.0) and reported smoking ≥15 cigarettes /day (M = 20.4, SD = 5.3) for at least 1 year (M = 8.0, SD = 7.1). Smoking status was confirmed at screening with an expired-air CO level of ≥15 ppm (M = 24.4 ppm, SD = 8.4) and an average score of 5.8 (SD = 1.7) on the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Exclusion criteria included current attempts at reducing cigarette intake, current use of tobacco products other than cigarettes, past use of Ariva (i.e., more than one pack), history of chronic health or psychiatric conditions, active menopause, and pregnancy or breast feeding.
Product descriptions and administration instructions
Ariva (A; mint flavor; Star Scientific, Inc., Chesterfield, VA) is a pressed tobacco tablet and was obtained from local retail sources. Each tablet contains 0.6 mg/g free nicotine (Hatsukami, Ebbert, Feuer, Stepanov, & Hecht, 2007). Per package instructions, participants were asked to place the product in their mouth and allow it to dissolve (∼15-min duration) without chewing or swallowing.
CS (R. J. Reynolds Tobacco Company, Winston-Salem, NC) is a sachet of tobacco that is produced in Sweden. At the time of this study, CS was not available in local retail stores and so was obtained at no cost from R. J. Reynolds. During a sampling session at screening, participants chose one of three flavors: “original” (n = 9), “frost” (n = 10), and “spice” (n = 2). Each sachet contains 6.1 (original), 9.2 (spice), or 6.4 (frost) mg/g free nicotine (Stepanov, Jensen, Hatsukami, & Hecht, 2008). Participants were asked to place and hold each pouch between their lip and gum for 15 min.
For own brand cigarettes (OWN), participants reported smoking either “light” (n = 7) or “full-flavor” (n = 14) cigarettes yielding, on average, 1.04 mg nicotine (SD = 0.23), 14.25 mg tar (SD = 3.18), and 14.16 mg CO (SD = 2.97) by the Federal Trade Commission (2000) method.
Procedure
Participants completed four 5-day (Monday through Friday) conditions that were ordered by Latin square and that differed by product used: A, CS, OWN, or no tobacco/nicotine (No-T). Weekends, when participants use their own brand of cigarettes, were considered washout periods (as in Breland, Buchhalter, Evans, & Eissenberg, 2002, 2006). Participants visited the laboratory for ∼1 hr on each of Days 1–5. Immediately following arrival, participants provided breath and urine samples. Compliance in all nonsmoking conditions was verified by ensuring that CO levels decreased on each successive day (or did not increase when the previous days’ expired-air CO level was ≤5 ppm). Additionally, a portion of the urine sample was stored for later analysis and the remainder used for immediate semiquantitative assessment of cotinine level (Nicalert; Nymox, Maywood, NJ). The semiquantitative test yields a whole number value between 0 and 6, with each number corresponding to a range of cotinine levels (e.g., 0 indicates 0–10 ng/ml; 6 indicates greater than 1,000 ng/ml; see Acosta, Buchhalter, Breland, Hamilton, & Eissenberg, 2004). In the No-T condition, semiquantitative cotinine values were expected to decrease from Day 1 to Day 5 for confirmation of compliance, while no decrease was expected in OWN. After compliance was assessed, subjective questionnaires were administered. On Days 1–4, participants were given a 24-hr supply of product (i.e., same number of tablets or sachets as reported number of cigarettes per day). On Days 2–5, product use was assessed by counting returned unused tablets and sachets (A or CS conditions) or smoked cigarette “butts” (OWN condition). When deemed noncompliant, one opportunity to repeat the condition was given. Participants were paid for their time/compliance on Day 3 ($30) and Day 5 ($70) in each condition and after study completion ($100, total of $500).
Outcome measures
Carbon monoxide
Expired-air CO levels were measured on Days 1 through 5 via a BreathCo monitor (Vitalograph, Lenexa, KS).
Urine cotinine and NNAL
Urine samples from Days 1 through 5 were stored at −70 °C and analyzed for the nicotine metabolite cotinine using an adaptation of a method reported elsewhere (Naidong, Shou, Chen, & Jiang, 2001; limit of quantitation = 1.0 ng/ml). Samples from Days 1, 3, and 5 only were analyzed for metabolites of the TSNAs NNK (NNAL and NNAL-glucuronide reported here as total NNAL or NNAL-T) using liquid chromatography–mass spectrometry/mass spectrometry (MDS Pharma Services, Lincoln, NE; method described in Roethig et al., 2007; limit of quantitation = 20 pg/ml).
Product use
For A and CS, unused products were returned, counted, and the total subtracted from the amount provided to the participant on the previous day for a measure of PREP use. For OWN, participants returned smoked cigarette butts and these were counted as a measure of cigarettes smoked each day.
Hughes–Hatsukami questionnaire
The Hughes and Hatsukami (1986) questionnaire, administered to participants on Days 1 through 5, consists of 11 Visual Analog Scale (VAS) items (see Table 1). Items are presented as a word or phrase centered above a horizontal line that ranges from 0 (not at all) to 100 (extremely). Participants used a computer mouse to place a vertical mark anywhere along the horizontal line; the score is the distance of the vertical mark from the left anchor, expressed as a percentage of total line length.
Table 1.
Statistical analysis results for all outcome measures
| Conditiona |
Dayb |
Condition × Day |
||||
| F | p | F | p | F | p | |
| Physiological measures | ||||||
| Expired-air COc | 26.2 | <.001 | 56.1 | <.001 | 24.5 | <.001 |
| Cotininec | 18.4 | <.001 | 5.1 | <.01 | 9.2 | <.001 |
| NNAL-Td | 5.8 | <.01 | 5.1 | <.05 | 3.0 | <.05 |
| Amount product usede | 23.1 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Hughes and Hatsukamic | ||||||
| Anxious | 2.8 | <.05 | <1.0 | n.s. | 1.3 | n.s. |
| Craving | 10.5 | <.001 | 3.6 | <.05 | 2.4 | <.05 |
| Depression/feeling blue | 2.6 | n.s. | 3.0 | <.05 | 1.3 | n.s. |
| Desire for sweets | 6.1 | <.001 | 4.5 | <.001 | 2.7 | <.001 |
| Difficulty concentrating | 2.6 | n.s. | 3.4 | <.05 | 1.5 | n.s. |
| Drowsy | 2.3 | n.s. | 5.2 | <.01 | 2.1 | <.05 |
| Hunger | 5.2 | <.01 | 3.4 | <.05 | <1.0 | n.s. |
| Impatient | 2.6 | n.s. | 1.6 | n.s. | 3.3 | <.001 |
| Increased eating | 8.5 | <.001 | 9.8 | <.001 | 2.3 | <.05 |
| Insomnia | 2.5 | n.s. | 5.9 | <.01 | 3.4 | <.001 |
| Irritability/frustration/anger | 5.7 | <.01 | 6.3 | <.001 | 2.2 | n.s. |
| Restless | 3.5 | <.05 | 3.1 | <.05 | 2.2 | n.s. |
| Urges to smoke | 7.0 | <.01 | 1.5 | n.s. | 2.2 | <.05 |
| Direct effects of nicotinee | ||||||
| Are the tobacco products you are using this week satisfying? | 43.5 | <.001 | <1.0 | n.s. | 1.4 | n.s. |
| Are the tobacco products you are using this week pleasant? | 50.3 | <.001 | <1.0 | n.s. | 1.9 | n.s. |
| Do the tobacco products you are using this week taste good? | 40.3 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week make you dizzy? | <1.0 | n.s. | 2.9 | <.05 | <1.0 | n.s. |
| Do the tobacco products you are using this week calm you down? | 38.2 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week help you concentrate? | 35.0 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week make you feel more awake? | 29.9 | <.001 | <1.0 | n.s. | 1.7 | n.s. |
| Do the tobacco products you are using this week reduce your hunger for food? | 9.0 | <.01 | <1.0 | n.s. | 1.1 | n.s. |
| Do the tobacco products you are using this week make you sick? | 6.9 | <.01 | 1.4 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week taste like your own brand of cigarette? | 284.4 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week feel like your own brand of cigarette? | 278.6 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do the tobacco products you are using this week feel as harsh as your own brand of cigarette? | 12.8 | <.001 | <1.0 | n.s. | 1.2 | n.s. |
| Do the tobacco products you are using this week feel as mild as your own brand of cigarette? | 30.6 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Do you like the tobacco products you are using this week? | 56.3 | <.001 | 1.3 | n.s. | 1.1 | n.s. |
| Do you dislike the tobacco products you are using this week? | 27.3 | <.001 | <1.0 | n.s. | <1.0 | n.s. |
| Tiffany–Drobes Questionnaire of Smoking Urgesc | ||||||
| Factor 1 | 2.2 | n.s. | 3.5 | <.05 | 1.9 | n.s. |
| Factor 2 | 7.2 | <.001 | 6.2 | <.01 | 1.9 | n.s. |
Note. A = Ariva; CO = carbon monoxide; CS = Camel Snus; n.s. = nonsignificant.
Condition factor: 3 (OWN, A, and CS for direct effects and amount product used); 4 (OWN, A, CS, and No-T for all other measures).
Day factor: 1–5 (CO, cotinine, and withdrawal-related items); 2–5 (direct effects and amount product used); 1, 3, and 5 (NNAL-T).
dfcondition = (3, 60); dfday = (4, 80); dfday = (12, 240).
dfcondition = (3, 60); dfday = (2, 40); dfday = (6, 120).
dfcondition = (2, 40); dfday = (3, 60); dfday = (6, 120).
Tiffany–Drobes Questionnaire of Smoking Urges
The Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991) was administered to participants on Days 1 through 5. This questionnaire consists of 32 items rated on a 7-point scale (0 = strongly disagree to 6 = strongly agree). Items were collapsed into two factors that have been defined previously by factor analysis: “intention to smoke” (Factor 1) and “anticipation of relief from withdrawal” (Factor 2).
Direct effects of nicotine scale
This 15-item VAS (see Table 1 for items) was developed to assess the incidence of nicotine-related side effects (see Evans, Blank, Sams, Weaver, & Eissenberg, 2006; Pullan et al., 1994). The scale was administered on Days 2–5 of A, CS, and OWN conditions.
Data analysis
Six datapoints (<1% of total) were missing from the final dataset. Most of these missing points were estimated by using the mean of the surrounding points. For missing Day 1 datapoints (i.e., before subjects began using the assigned product that week), the average of all other Day 1 datapoints for that participant was used. For missing Day 5 datapoints, the value from Day 3 for that participant in that condition was used. All data were analyzed using two -factor (condition and day) repeated measures analysis of variance where the number of levels depended upon measure. For the condition factor, there were four levels (A, CS, OWN, and No-T) for all measures except direct effects of product and amount of product used for which there were three levels (A, CS, and OWN). For the day factor, there were five levels (Days 1 through 5) for expired-air CO, urine cotinine, and abstinence effects; there were four levels (Days 2 through 5) for amount used and direct effects of product; and there were three levels (Days 1, 3, and 5) for NNAL-T. Corrections by Huynh and Feldt (1976) were used to adjust for violations of the sphericity assumption. Differences between means were examined using Tukey’s Honestly Significant Difference (HSD; Keppel, 1991). Comparisons for which p < .05 are reported as significant.
Results
Statistical analyses results for all measures are displayed in Table 1. The results of primary interest involve significant condition by day interactions, meaning that changes in outcome measures across days depended upon the tobacco product used in each condition.
Physiological measures
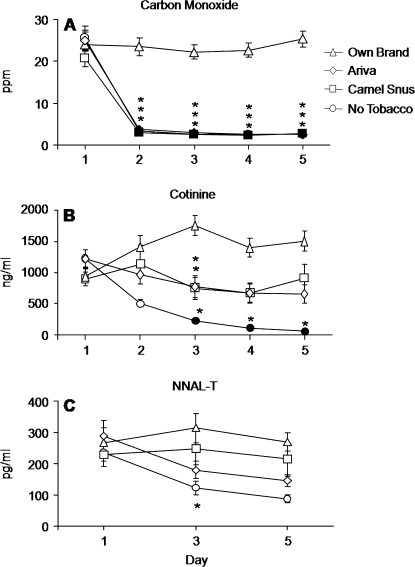
As displayed in Figure 1A, average CO levels were similar on Day 1 for all conditions (n.s., Tukey’s HSD; collapsed across conditions M = 23.7, SEM = 1.3). Relative to Day 1, CO did not differ across days for OWN but decreased significantly on Days 2 through 5 for A, CS, and No-T (p < .05, Tukey’s HSD). In each of these nonsmoking conditions, average CO decreased by at least 86% from Day 1 to Day 2 and remained low through Day 5. Across conditions, average CO for A, CS, and No-T was significantly lower on Days 2 through 5, relative to OWN (p < .05, Tukey’s HSD).
Figure 1.
Mean data (±1 SEM) for expired-air carbon monoxide (CO; A), cotinine (B), and NNAL-T (C). Data are presented as condition (own brand, Ariva, Camel Snus, and no tobacco) by day (Days 1, 3, and 5 for NNAL-T and Days 1–5 for CO and cotinine). Filled symbols indicate a significant difference from Day 1 within that condition, and asterisks indicate a significant difference from own brand on that day (all ps < .05, Tukey’s Honestly Significant Difference post-hoc test).
For cotinine, Day 1 levels did not differ significantly across conditions (n.s., Tukey’s HSD; see Figure 1B). In the No-T condition, relative to Day 1, cotinine decreased significantly by 81% on Day 3, 92% on Day 4, and 95% on Day 5 (p < .05, Tukey’s HSD). Relative to OWN, significantly lower cotinine levels were observed for A (56% decrease) and CS (58% decrease) on Day 3 only and for No-T on Days 3 through 5 (87%, 93%, and 96% decrease, respectively; ps < .05, Tukey’s HSD). No other between-condition effects were observed on this outcome measure.
Figure 1C also shows data for NNAL-T, and these data indicate that NNAL-T levels were similar on Day 1 for all conditions (n.s., Tukey’s HSD). The figure shows a trend toward decreasing urine NNAL-T levels from Day 1 to Day 5 for A (49.1% decrease) and No-T (63.1% decrease), although these decreases did not achieve conventional levels of statistical significance (i.e., p > .05, Tukey’s HSD). Across conditions, on Day 3, NNAL-T levels for No-T (M = 121.13, SEM = 21.77) were significantly lower relative to OWN on Day 3 (M = 313.2, SEM = 47.5; p < .05, Tukey’s HSD). No other between-condition effects were observed on this outcome measure.
Amount product used
The amount of product used differed by condition (p < .001), but the main effect of day and the condition by day interaction were not significant (Fs < 1.0; see Table 1). For OWN, the mean number of cigarettes smoked collapsed across the day factor was 21.9 (SEM = 0.77). This amount was significantly higher than that for A (M = 12.3, SEM = 0.88) and CS (M = 11.7, SEM = 0.79; p < .05, Tukey’s HSD). Specifically, on Days 1–2 of OWN, participants smoked 21.2 (SEM = 1.8) cigarettes; on Day 2–3, they smoked 21.4 (SEM = 1.3) cigarettes; on Days 3–4, they smoked 21.8 (SEM = 1.6) cigarettes; and on Days 4–5, they smoked 23.1 (SEM = 1.6) cigarettes. For Days 1–2 of A, participants used 12.7 (SEM = 1.6) tablets; on Days 2–3, they used 11.2 (SEM = 1.6) tablets; on Days 3–4, they used 13.1 (SEM = 1.8) tablets; and on Days 4–5, they used 12.0 (SEM = 2.1) tablets. For Days 1–2 of CS, participants used 12.0 (SEM = 1.5) sachets; on Days 2–3, they used 11.6 (SEM = 1.7) sachets; on Days 3–4, they used 11.3 (SEM = 1.7) sachets; and on Days 4–5, they used 11.7 (SEM = 1.7) sachets.
Nicotine/tobacco withdrawal effects
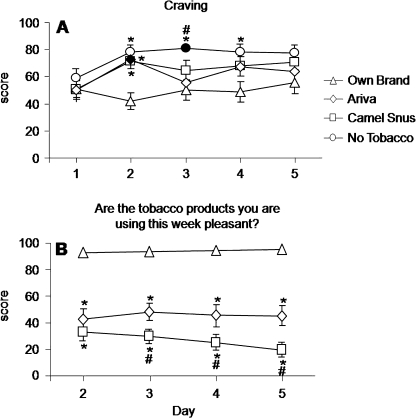
A significant condition by day interaction was observed for many withdrawal-related items (see Table 1). Mean results for the Hughes–Hatsukami item “craving” (item with the largest F value for condition by day) are shown in Figure 1A; mean scores did not differ across conditions on Day 1. However, mean scores increased relative to Day 1 on subsequent days in all conditions except for OWN. Relative to mean scores for OWN on Day 2 (M = 41.7, SEM = 6.3), significantly higher mean scores were observed for A (M = 72.4, SEM = 5.9), CS (M = 71.3, SEM = 5.6), and No-T (M = 78.1, SEM = 4.9; all ps < .05, Tukey’s HSD). For No-T (but not A or CS), mean scores remained significantly elevated relative to OWN on Days 3 and 4. A similar pattern of results (no differences across conditions on Day 1; increases relative to Day 1 for A, CS, and No-T; and higher scores relative to OWN on most days) was observed for the other withdrawal-related items on which a significant condition by day interaction was observed (see Table 1).
Significant main effects of day and condition were observed for three items of the Hughes–Hatsukami questionnaire (hunger, irritability/frustration/anger, and restless), and inspection of the data revealed a similar pattern of results across these items. For example, for irritability/frustration/anger, collapsed across day, mean scores were lower for OWN (18.2, SEM = 2.0), relative to A (28.2, SEM = 2.9), CS (28.4, SEM = 2.7), and No-T (35.5, SEM = 2.9; n.s., Tukey’s HSD). Collapsed across condition, scores were elevated from Day 1 (M = 17.5, SEM = 2.1) to Days 2 (M = 32.2, SEM = 3.3) through 5 (M = 27.6, SEM = 3.1; n.s., Tukey’s HSD). Results also revealed a significant main effect of day for items assessing “difficulty concentrating” and “depression/feeling blue” and a significant main effect of condition for “anxious,” although Tukey’s HSD revealed no significant differences among means.
For Factor 2 of the QSU, a significant main effect of condition was observed, and this effect reflected generally lower scores for OWN (M = 28.1, SEM = 1.7), relative to A (M = 32.9, SEM = 1.9), CS (M = 33.4, SEM = 1.9), and No-T (M = 39.7, SEM = 1.7; all differences n.s., Tukey’s HSD). Additionally, a significant main effect of day was observed on both QSU factors, although Tukey’s HSD revealed no significant differences among means.
Direct effects of nicotine
The items in this scale describe effects of nicotine (e.g., “Do the tobacco products you are using this week make you dizzy?”) or sensory characteristics of a product (e.g., “Do the tobacco products you are using this week taste like your own brand of cigarette?”). A main effect of condition was observed for all items describing the effects of nicotine except “dizzy,” for which a main effect of day was observed. Generally, for these items, scores did not change across days within each condition, but, across conditions, mean scores for OWN were significantly different from A and CS. For example, for the item “Are the tobacco products you are using this week pleasant?” (nicotine-related item with the largest F value for condition main effect; Figure 2B), for OWN, Day 2 score was 92.7 (SEM = 3.0), Day 3 score was 93.6 (SEM = 2.3), Day 4 score was 94.2 (SEM = 2.2), and Day 5 score was 95.2 (SEM = 1.7). In contrast, for A, Day 2 score was 42.6 (SEM = 7.5), Day 3 score was 48.0 (SEM = 6.5), Day 4 score was 45.2 (SEM = 8.0), and Day 5 score was 45.1 (SEM = 7.4; relative to OWN, all ps < .05, Tukey’s HSD). Likewise, for CS, Day 2 score was 33.1 (SEM = 6.6), Day 3 score was 29.6 (SEM = 5.3), Day 4 score was 25.1 (SEM = 6.0), and Day 5 score was 19.5 (SEM = 5.5; relative to OWN, all ps < 0.05, Tukey’s HSD).
Figure 2.
Mean data (±1 SEM) for H–H item craving (A) and “Are the tobacco products you are using this week pleasant?” (B). Data are presented as condition (own brand, Ariva, Camel Snus, and no tobacco) by day (Days 1–5 for craving and Days 2–5 for “Pleasant”). Filled symbols indicate a significant difference from Day 1 within that condition; asterisks indicate a significant difference from own brand on that day; pound signs indicate a significant difference from Ariva on that day (all ps < .05, Tukey’s Honestly Significant Difference post-hoc test).
Similarly, scores for “Do the tobacco products you are using this week taste good?” were not significantly different across days in any condition but differed between OWN and each oral product on Days 2 through 5. For OWN, scores were 92.1 (SEM = 2.6) on Day 2, 93.6 (SEM = 2.4) on Day 3, 93.4 (SEM = 2.4) on Day 4, and 95.0 (SEM = 1.8) on Day 5. For A, scores were 52.4 (SEM = 7.1) on Day 2, 53.8 (SEM = 7.4) on Day 3, 49.9 (SEM = 7.3) on Day 4, and 50.2 (SEM = 7.7) on Day 5. For CS, scores were 37.9 (SEM = 6.8), 33.6 (SEM = 6.6), 35.0 (SEM = 6.8), and 28.7 (SEM = 5.5) for Days 2 through 5, respectively. Additionally, scores for CS on Days 3 and 5 were significantly lower than those for A (p < .05, Tukey’s HSD). For the item “Do the tobacco products you are using this week make you dizzy?”, scores on Days 3 through 5 were generally lower than scores on Day 2, although Tukey’s HSD revealed no significant differences among means.
Main effects of condition were observed on every item assessing product-related sensory characteristics. Generally, item scores for A and CS did not differ from each other, while scores for both products differed substantially from OWN. For example, for “Do the tobacco products you are using this week taste like your own brand of cigarette?” (item with the largest F value for condition main effect), scores for A (M = 7.7, SEM = 1.3) did not differ from those for CS (M = 11.2, SEM = 2.4). However, scores for both oral products were significantly lower than scores observed for OWN (M = 95.6, SEM = 1.7; p < .05, Tukey’s HSD). Average scores for “Do you dislike the tobacco products you are using this week?” were higher for A and CS on each day than for OWN. For OWN, “dislike product” scores were 8.1 (SEM = 4.3) on Day 2, 7.0 (SEM = 3.7) on Day 3, 6.7 (SEM = 4.2) on Day 4, and 8.2 (SEM = 4.7) on Day 5. For A, dislike product scores were 50.5 (SEM = 8.2) on Day 2, 46.5 (SEM = 8.5) on Day 3, 41.8 (SEM = 9.0) on Day 4, and 42.1 (SEM = 8.0) on Day 5. For the CS condition, scores were 64.7 (SEM = 6.9), 71.2 (SEM = 6.7), 69.1 (SEM = 7.2), and 68.1 (SEM = 7.1) for Days 2 through 5, respectively. Additionally, scores for CS on Days 2 through 5 were significantly higher than those for A (p < .05, Tukey’s HSD).
Discussion
Methods used previously to evaluate the toxicant exposure and subjective effects of combustible PREPs (Breland et al., 2002, 2006) were adapted in this study for evaluating noncombustible PREPs for smokers. Results demonstrate that clinical laboratory methods can provide valuable information about the toxicant exposure and likely acceptability of these PREPs. For example, the inclusion of positive (OWN) and negative (No-T) control conditions provides necessary comparators for assessing the relative effects of noncombustible PREPs. In this study, participants’ usual level of CO exposure (i.e., OWN condition) was 23.7 ppm (collapsed across days, SEM = 1.0); these levels were significantly lowered by A (collapsed across days, M = 7.2, SEM = 1.0) and CS (collapsed across days, M = 6.1, SEM = 0.8) on all days. Both oral tobacco conditions were also associated with significantly lower levels of urine cotinine on Day 3 compared with participants’ usual levels as observed in the OWN condition (see also Cobb et al., 2009). Of course, even lower levels of urinary cotinine and NNAL-T were observed when participants abstained from all tobacco products (i.e., No-T condition).
Similarly, OWN and No-T control conditions provide a reference for which to compare the withdrawal suppression and user acceptability of noncombustible PREPs. Neither A nor CS was able to suppress symptoms of tobacco abstinence (e.g., irritability/frustration/anger, urge to smoke) as effectively as OWN (see Figure 1C). Participants also rated A and CS as significantly less pleasant than OWN (significantly lower ratings for direct product effects such as “Are the tobacco products you are using this week satisfying?” and “Do the tobacco products you are using this week taste good?”). These results are consistent with previous work in which these products were administered acutely in a laboratory setting (Cobb et al., 2009), demonstrating reliability across studies and methods.
Overall, results show that, relative to OWN, noncombustible PREPs for smokers reduce usual exposure to CO and may be able to reduce exposure to other toxicants (e.g., cotinine). Nonetheless, these PREPs were unable to suppress fully tobacco abstinence symptoms and were considered significantly less enjoyable than participants’ own brand of cigarette. These findings are supported by the observation that more participants were withdrawn from the study due to noncompliance during conditions when they were required to use oral tobacco products (n = 14) than when using their own brand of cigarettes (n = 3; see Methods section). Noncombustible PREPs that do not alleviate symptoms of withdrawal effectively and/or which produce less pleasant effects than normally marketed cigarettes are unlikely to substitute for cigarettes for the smokers represented by this study’s sample.
This study has several limitations, including compliance assessment and uncontrolled product use. Participants in this study used their products outside of the laboratory, not under careful observation. Although an inpatient study design might ensure compliance with study tobacco use restrictions, the importance of understanding PREP use in the smokers’ natural environment has been highlighted elsewhere (Hatsukami et al., 2005). Additionally, the biochemical measures used here cannot discriminate clearly between assigned versus other noncombustible PREPs used in a single condition. Evidence of product use, therefore, was assessed via a combination of measures (CO and cotinine levels, returned products, and self-report), a method used successfully in previous studies of PREP evaluation (Breland et al., 2006; Gray, Breland, Weaver, & Eissenberg, 2008). Products in each condition were also used ad libitum, as evidenced by significant differences in the amount of each product used: 44% less A tablets and 47% less CS pouches compared with number of own brand cigarettes. However, there currently exists no standardized method for measuring the topography of oral tobacco product use, and it would be challenging to compare topography for oral versus smoked products. Moreover, PREP research will benefit from the knowledge gained from studies examining toxicant uptake as a function of smokers’ natural product use. Finally, study results may also be influenced by the relatively high rates of attrition due to noncompliance observed in the No-T, A, and CS conditions. That is, if noncompliance with tobacco use restrictions in these three conditions reflects sensitivity to the aversive effects of tobacco/nicotine withdrawal, then completion of this study may reflect a relative insensitivity to these aversive effects. However, the fact that attrition in the two oral tobacco conditions was over triple that observed in the own brand condition is entirely consistent with the subjective effect data that, in completers and noncompleters, these oral tobacco products did not suppress withdrawal effectively.
In conclusion, this study demonstrates the utility of clinical laboratory research methods for noncombustible PREP evaluation. Findings support those of previous work (Cobb et al., 2009), and together, they suggest that currently available noncombustible PREPs are unlikely to substitute for smokers’ own brand of cigarette. To be a successful PREP, a noncombustible tobacco product for smokers will need to reduce toxicant exposure demonstrably and also will likely need to approximate a cigarette’s ability to suppress the aversive symptoms associated with cigarette abstinence. Fortunately, toxicant exposure and tobacco abstinence symptom suppression (as well as general product acceptability) can be assessed via established clinical laboratory research methods that will form an important part of a comprehensive PREP evaluation strategy.
Funding
This work was funded by the National Institute on Drug Abuse (PHS CA103827).
Declaration of Interests
As noted in the text, R. J. Reynolds Tobacco Company provided a supply of Camel Snus for testing at no cost.
Acknowledgments
Portions of this work were presented at the 14th Annual Meeting of the Society for Research on Nicotine and Tobacco, February 15–18, 2006. All work was performed at Virginia Commonwealth University
References
- Acosta MC, Buchhalter AR, Breland AB, Hamilton DCP, Eissenberg T. Urine cotinine as an index of smoking status in abstinent smokers: Comparison of GC/MS with immunoassay test strip. Nicotine & Tobacco Research. 2004;6:615–620. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- Blank MD, Sams C, Weaver MF, Eissenberg T. Nicotine delivery, cardiovascular profile, and subjective effects of an oral tobacco product for smokers. Nicotine & Tobacco Research. 2008;10:417–421. doi: 10.1080/14622200801901880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger CT, Zellweger JP, Danielsson T, van Biljon X, Robidou A, Westin A, et al. Influence of long-term smoking reduction on health risk markers and quality of life. Nicotine & Tobacco Research. 2002;4:433–439. doi: 10.1080/1462220021000018380. [DOI] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: Clinical laboratory methodology. Nicotine & Tobacco Research. 2002;4:S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Cobb CO, Weaver MF, Eissenberg T. Evaluating the acute effects of oral, non-combustible potential reduced exposure products marketed to smokers. Tobacco Control. 2009 doi: 10.1136/tc.2008.028993. Published online 2 April; doi:10.1136/tc.2008.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptoms suppression: Nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. Tar, nicotine, and carbon monoxide of the smoke of 1294 varieties of domestic cigarettes for the year 1998. Washington, DC: 2000. [Google Scholar]
- Gray JN, Breland AB, Weaver MF, Eissenberg T. Potential reduced exposure products (PREPs) for smokeless tobacco users: Clinical evaluation methodology. Nicotine & Tobacco Research. 2008;10:1441–1448. doi: 10.1080/14622200802323258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Ebbert JO, Feuer RM, Stepanov I, Hecht SS. Changing smokeless tobacco products new tobacco-delivery systems. American Journal of Preventive Medicine. 2007;33(Suppl. 6):S368–S378. doi: 10.1016/j.amepre.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine & Tobacco Research. 2005;7:827–844. doi: 10.1080/14622200500266015. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, et al. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. Journal of the National Cancer Institute. 2004;96:844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP. The effect of a novel smoking system—Accord—on ongoing smoking and toxin exposure. Nicotine & Tobacco Research. 2004;6:1021–1027. doi: 10.1080/14622200412331296011. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Estimation of the box correction for degrees of freedom from sample data in randomized block and split-plot designs. Journal of Educational Statistics. 1976;1:69–82. [Google Scholar]
- Keppel G. Design and analysis, a researcher’s handbook. Englewood Cliffs, NJ: Prentice Hall; 1991. [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, et al. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tobacco Control. 2007;16:138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakier JB. Smoking and cardiovascular disease. American Journal of Medicine. 1992;93:8S–12S. doi: 10.1016/0002-9343(92)90620-q. [DOI] [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Bjorklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck. A population based case-referent study in Sweden. Cancer. 1998;82:1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen Y-L, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography B, Biomedical Sciences and Applications. 2001;754:387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. Risks associated with smoking cigarettes with low tar machine-measured yield of tar and nicotine: A report of the NCI expert committee. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 2001. Retrieved 11 August 2009, from http://cancercontrol.cancer.gov/tcrb/monographs/13/m13_complete.pdf. [Google Scholar]
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, et al. Transdermal nicotine for active ulcerative colitis. New England Journal of Medicine. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- Roethig HJ, Zedler BK, Kinser RD, Feng S, Nelson BL, Lian Q. Short-term clinical exposure evaluation of a second generation electrically heated cigarette smoking system. Journal of Clinical Pharmacology. 2007;47:518–530. doi: 10.1177/0091270006297686. [DOI] [PubMed] [Google Scholar]
- Schildt E-B, Eriksson M, Hardell L, Magnuson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. International Journal of Cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxcicant and carcinogen levels. Nicotine & Tobacco Research. 2008;10:1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the smoke: The science base for tobacco harm reduction, committee to assess the science base for tobacco harm reduction, board on health promotion and disease prevention. Washington, DC: National Academy Press; 2001. [Google Scholar]
- Thun MJ, Heath CW., Jr. Changes in from mortality in smoking from two American Cancer Society prospective studies since 1959. Preventative Medicine. 1997;26:422–426. doi: 10.1006/pmed.1997.0182. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. The health consequences of smoking: A report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- Warner KE. Tobacco harm reduction: Promise and perils. Nicotine & Tobacco Research. 2002;4:S61–S71. doi: 10.1080/1462220021000032825. [DOI] [PubMed] [Google Scholar]
- Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. Environmental and chemical carcinogenesis. Seminars in Cancer Biology. 2004;14:473–486. doi: 10.1016/j.semcancer.2004.06.010. [DOI] [PubMed] [Google Scholar]