Abstract
Introduction:
A vaccine against nicotine may soon be available to smokers who want to quit. The vaccine stimulates the production of antibodies that bind to nicotine, thereby impeding nicotine from crossing the blood-brain barrier and exerting psychoactive effects. The primary purpose of this study was to evaluate intentions to try a nicotine vaccine if one were to become available among a nationally representative sample of smokers. The secondary purpose was to assess whether information about genetic susceptibility to nicotine addiction had an effect on smokers’ interest in receiving the vaccine.
Methods:
Four hundred and twenty-seven adults were randomized to read one of two versions of a short description about the vaccine. One version framed addiction as genetically influenced, while the other framed it as environmentally influenced. Smokers were then asked about their intentions to use a nicotine vaccine if one were to become available in the future.
Results:
Across both groups, 53% indicated that they would be likely or very likely to try the vaccine. Using multivariate linear regression, the strongest predictors of vaccination intention were having a favorable attitude toward a nicotine vaccine (β = .41) and having a favorable attitude toward vaccination in general (β = .22). There were no significant effects of the framing conditions on intention to receive the vaccine.
Discussion:
Intentions to try a nicotine vaccine as a cessation method are relatively high among smokers. If the vaccine becomes available, specific groups of smokers may be more interested than others; education and recruitment efforts could be targeted appropriately.
Introduction
Data from the 2007 National Health Interview Survey show that an estimated 20% of Americans, or slightly more than 43 million adults, are current cigarette smokers (Centers for Disease Control and Prevention, 2008). Of these, 80% report smoking every day and 20% smoke some but not all days. The findings also indicated that 39.8% of current smokers, or more than 13 million Americans, had stopped smoking for at least 1 day in the preceding 12 months because they were trying to quit. However, even with currently available medications and counseling, only about one in four smokers who use these therapeutics is able to quit and maintain long-term abstinence (Schnoll & Lerman, 2006). Thus, there is a critical need to develop new approaches to the treatment of nicotine dependence (Lerman et al., 2007).
Current research
Therapeutic vaccines for drug addiction, mainly for cocaine addiction, have been in development for years. Researchers have been exploring immunotherapy for smoking addiction, primarily in the form of a vaccine against nicotine. Multiple nicotine vaccines are currently under development (Frishman, 2009). The vaccine stimulates the immune system to produce antibodies against nicotine, and the nicotine–antibody molecules are too large to pass from the blood into the brain. Preclinical studies of short- and long-term administration of nicotine found that one of the nicotine vaccines reduced the distribution of nicotine into the brain in rats by up to 65% (Pentel & Malin, 2002). Studies of rats taught to self-administer nicotine found that vaccinated rats self-administered nicotine at statistically lower levels than unvaccinated rats (Le Sage et al., 2006).
Two studies have shown that the vaccine is well tolerated and highly immunogenic in human smokers (Cornuz et al., 2008; Hatsukami et al., 2005). Cornuz et al. showed that point prevalence of abstinence 2 months after vaccination was different, although not statistically, between smokers who received the vaccine versus those who received the placebo. Not all smokers achieved high antibody levels. When cessation rates were analyzed based on antibody levels, smokers with the highest antibody levels showed significantly higher continuous abstinence from Month 2 to Month 6 than those with medium or low antibody levels. Yet, despite the success of the vaccine in early trials, whether smokers would intend to try this new form of cessation therapy has yet to be explored.
Communication effects: Framing
The ways in which information is presented, or “framed,” can influence individuals’ perceptions or understanding of an issue (Gamson & Wolfsfeld, 1993). For example, information framed in gain or loss terms, which is otherwise logically equivalent, can have consequences on compliance and acceptance (Rothman, Bartels, Wlaschin, & Salovey, 2006). Frames can also offer a particular way to understand an issue and can create and shape individuals’ thoughts and opinions on particular issues (Entman, 1993; Kinder, 1998). Framing has been shown to influence a myriad of health decisions, such as HIV testing, cancer screening, flu shots, sunscreen use, and safe driving (Apanovitch, McCarthy, & Salovey, 2003; Detweiler, Bedell, Salovey, Pronin, & Rothman, 1999; Finney & Iannoti, 2002; McCall, Johnson, & Rothman, 2002; Meyerowitz & Chaiken, 1987; Millar & Millar, 2000; O’Keefe & Jensen, 2006). In our study, framing the cause of nicotine addiction as either genetically or environmentally influenced could affect smokers’ intentions to try a nicotine vaccine if one becomes available.
Genetic risk and smoking cessation
Studies that have investigated the effects of genetic susceptibility feedback on smokers’ motivation and ability to quit smoking, although not in the context of a nicotine vaccine, have produced mixed results. Lerman et al. (1997) found that those given biomarker feedback about lung cancer susceptibility had higher levels of perceived risk, perceived quitting benefits, and fear arousal than those in other conditions but were no more likely to have quit smoking than those who did not receive biomarker information. A study of smokers participating in a nicotine replacement therapy trial found that those who attributed their smoking to genetic causes had lower perceived behavioral control but similar quit rates to other smokers, indicating that genetic information may have psychological implications but not deter quit attempts (Wright et al., 2007). Sanderson et al. (2009) found that smokers who were told that they were at higher risk of lung cancer had significantly lower rates of response efficacy than those who were told that they were at low risk but that uptake of cessation services did not significantly differ between the two groups. Based on these findings, we hypothesized that smokers who read about the vaccine in the context of a genetic addiction to nicotine would show similar or increased intentions to try a vaccine than those who read about the vaccine in the context of an environmental addiction but that they would exhibit lower rates of self-efficacy than the other group.
Methods
Four hundred and twenty-seven adult smokers completed an online survey in August 2006 about their intentions to try a nicotine vaccine if one were to be available in the future. The survey was part of the second wave of a two-wave study of topics related to tobacco advertising and public service announcements about quitting. Survey participants were part of a previously established Web-enabled research panel assembled by an independent research firm; the panel was designed to be representative of the U.S. population. The panel recruitment rate was 48%, and the survey completion rate for the study was 83%. Further description of the sampling methodology is described elsewhere (Pineua & Dennis, 2007). The survey and methodology were approved by the Institutional Review Board at the University of Pennsylvania. Previous work verified that there were no significant differences between panel responders and nonresponders (Weibe, Eyerman, & Loft, 2001).
To ensure that participants were current and regular smokers, inclusion criteria were the following: (a) currently smoking cigarettes, (b) had smoked an average of 5 or more cigarettes/day in the past week, and (c) had smoked at least 100 cigarettes in their lifetime.
Participants responded to questions related to their own smoking habits and their family history of smoking, as well as their general attitude toward vaccines. Next, participants were randomly assigned to read one of two paragraphs describing the nicotine vaccine. Supplementary material contains full-text wordings of each paragraph. Both versions explained the vaccine, mentioned safety and efficacy, and the possibility of periodic booster shots for long-term efficacy. One version of the paragraph framed nicotine addiction in terms of genetic susceptibility, while the other version framed nicotine addiction in terms of environmental influences. The “genetic” version was 166 words, and the “environmental” version was 164 words. Participants were then asked about their intentions to get a nicotine vaccine if one were to become available in the future. Main effects of the experimental manipulation were assessed using analysis of variance, and predictors of intention to vaccinate were determined using multivariate linear regression.
Measures
Measures asked prior reading the framing paragraph
Number of previous quit attempts.
Participants were asked, “How many times have you previously quit smoking on purpose for more than one complete day?” Based on distribution of responses, participants were placed into one of four categories: 0, 1–2, 3–4, or 5 or more previous quit attempts.
Previous quit methods.
Participants were asked, “Have you ever tried any of the following methods to quit smoking.” Response options were: (a) counseling or calling a quitline; (b) going “cold turkey” without using any products; (c) nicotine patch; (d) nicotine gum; (e) nicotine lozenge; (f) nicotine nasal spray; (g) nicotine inhaler; and (h) medications, such as Zyban or Wellbutrin. Participants could choose multiple quit methods if necessary.
Nicotine dependence.
Nicotine dependence was measured using the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991). Based on distribution, participants’ scores were separated into three categories of nicotine dependence: low (0–3), medium (4–5), and high (6–10). Internal consistency of the FTND has been previously reported as 0.61 (Heatherton et al.).
Personal vulnerability to smoking-related illness.
Participants were asked, “To what extent do you feel your overall health has been affected by smoking.” Response options were 1 (not at all), 2 (a little), 3 (somewhat), 4 (quite a bit), or 5 (very much). Previous studies have used this measure to assess how people process information about smoking cessation products and relate it to their own personal need for cessation (Shadel, Niaura, & Abrams, 2001; Shadel et al., 2006).
Readiness to quit smoking.
Participants’ level of readiness to quit was measured using a modified version of the ladder of contemplation (Biener & Abrams, 1991). The 11-point ladder is anchored at 0 (I have no thoughts about quitting smoking) to 10 (I am taking action to quit smoking). The different statements on the contemplation ladder reflect the different stages of change described by Prochaska and DiClemente (1983). For analyses, participants were separated into three categories of interest in quitting: low (0–3), medium (4–5), and high (6–10).
Family history of smoking.
Participants were asked if their mother, father, or siblings had ever smoked on a daily basis for 1 month or more. Response options were yes, no, or Not Applicable. A family history mean score was created by dividing the number of family members who smoke by the total number of family members.
Attitude toward vaccines.
Participants were asked the following items to assess their general attitude toward vaccines: (a) Any type of vaccine against a health threat is a good thing and (b) vaccines are worth the risk of side effects. Response options were on a 5-point scale ranging from 1 (strongly disagree) to 5 (strongly agree). Responses from each item were averaged to create a mean score for each participant. The correlation between the two items was .65 (p < .001).
Measures asked after reading the framing paragraph
Attitudes toward nicotine vaccine.
Participants were asked about their attitudes toward getting a nicotine vaccine by answering the following item: My getting a nicotine vaccine would be (a) bad versus good, (b) unpleasant versus pleasant, and (c) difficult versus easy. The response pairs were at opposite ends of a 5-point scale so that the response options were, for example, 1 (very bad), 2 (somewhat bad), 3 (neither bad nor good), 4 (somewhat good), or 5 (very good). Responses from (a) and (b) were averaged together to get a combined attitude score for each participant and were used in subsequent analyses. The correlation between (a) bad–good and (b) pleasant–unpleasant was .65 (p < .001).
Genetic and environmental susceptibility to nicotine addiction.
Participants were asked to respond to the following two items: “How likely is it that you have inherited genes that cause smoking addiction?” and “How likely is it that factors, like the things around you, contribute to smoking addiction?” Response options ranged from 1 (very unlikely) to 5 (very likely).
Intention to vaccinate.
Participants were asked, “If a vaccine becomes available in the future, how likely is it that you would get a nicotine vaccine to help you quit smoking?” Response options ranged from 1 (very unlikely) to 5 (very likely).
Intention to quit smoking.
Intention to quit smoking was based on the integrative model of behavioral prediction (Fishbein, 2000), an extended version of the theory of planned behavior (Ajzen, 1991). Participants were asked, “How likely is it that you will try to quit smoking in the next three months?” and “How likely is it that you will quit smoking in the next three months?” The items were scored on a 5-point scale ranging from 1 (very unlikely) to 5 (very likely).
Self-efficacy to quit smoking.
Each participant was asked how sure they were that in the next 3 months they could: (a) quit smoking completely and permanently, (b) avoid smoking when they were craving a cigarette, and (c) avoid smoking when they were with friends who smoke. Responses were on a 4-point scale ranging from 1 (not at all sure) to 4 (completely sure). These items were taken from a previously validated scale (Velicer, DiClemente, Rossi, & Prochaska, 1990).
Results
The survey was completed by 427 adults (54.1% male). Participants ranged in age from 18 to 87 years (mean age = 56.1 years, SD = 11.8). Most were non-Hispanic White (85.5%), while 6.3% identified themselves as non-Hispanic Black and 4.7% identified themselves as Hispanic. More than half (57.6%) reported having some form of education beyond high school; 31.8% reported having an annual household income greater than $50,000.
The mean number of cigarettes that participants smoked each day for the past 7 days was 25 (M = 25.21, SD = 29.18), the mean age of smoking onset was 16 years (M = 16.24, SD = 4.39), and the mean score on the FTND was 4.10 (M = 4.10, SD = 2.14). The majority of participants (82.4%) have tried to quit smoking at least once (M = 7.6 quit attempts, SD = 29.34). Almost three quarters of smokers (72.4%) tried to quit “cold turkey,” 31.4% tried using a nicotine patch, 24.8% tried nicotine gum, 18.3% took medications (Zyban and Wellbutrin), and 9.6% sought counseling or called a quitline in the past.
Univariate analyses
If a nicotine vaccine were to become available in the future, 54.8% of smokers responded that they would be likely or very likely to try the vaccine in an attempt to quit smoking. Intentions to vaccinate are reported in Table 1. About 20% of smokers (21.5%) were unlikely or very unlikely to try the vaccine, and 23.7% had neutral opinions about the vaccine. Differences in intentions to vaccinate did not vary by demographic characteristics, such as age, gender, race, and educational attainment.
Table 1.
Demographic characteristics of the sample population and likelihood of getting a nicotine vaccine if one were available
| Sample characteristics, N = 427 | Likelihood of vaccination (%), N = 427 |
||||||
| Very unlikely | Unlikely | Neither likely or unlikely | Likely | Very likely | χ2 (df) | ||
| Overall | 12.4 | 9.1 | 23.7 | 32.8 | 22.0 | ||
| Gender | |||||||
| Male | 54.1 | 13.4 | 9.5 | 22.5 | 33.8 | 20.8 | 1.27 (4) |
| Female | 45.9 | 11.2 | 8.7 | 25.0 | 31.6 | 23.5 | |
| Age (years) | |||||||
| 18–29 | 1.9 | 12.5 | 12.5 | 37.5 | 25.0 | 12.5 | 15.52 (12) |
| 30–44 | 14.4 | 18.3 | 5.0 | 35.0 | 26.7 | 15.0 | |
| 45–59 | 44.0 | 9.6 | 8.0 | 20.2 | 36.2 | 26.1 | |
| 60+ | 40.0 | 13.5 | 11.7 | 22.8 | 31.6 | 20.5 | |
| Race/ethnicity | |||||||
| Non-Hispanic White | 85.5 | 12.1 | 9.6 | 24.1 | 33.4 | 20.8 | 10.09 (12) |
| Non-Hispanic Black | 6.3 | 11.1 | 3.7 | 33.3 | 33.3 | 18.5 | |
| Hispanic | 4.7 | 15.0 | 10.0 | 10.0 | 25.0 | 40.0 | |
| Other, more than 1 race | 3.5 | 20.0 | 6.7 | 13.3 | 26.7 | 33.3 | |
| Education | |||||||
| HS degree or less | 42.4 | 16.0 | 11.0 | 23.8 | 30.4 | 18.8 | 6.60 (4) |
| More than HS degree | 57.6 | 9.8 | 7.7 | 23.6 | 34.6 | 24.4 | |
| Nicotine dependence | |||||||
| Low | 38.2 | 16.1 | 9.9 | 24.8 | 31.7 | 17.4 | 7.17 (8) |
| Medium | 34.2 | 9.0 | 9.0 | 22.9 | 34.0 | 25.0 | |
| High | 27.6 | 9.5 | 8.6 | 22.4 | 34.5 | 25.0 | |
| Previous quit attempts | |||||||
| 0 | 17.7 | 25.3 | 14.7 | 32.0 | 14.7 | 13.3 | 42.33 (12)* |
| 1–2 | 31.7 | 11.2 | 6.7 | 20.9 | 38.8 | 22.4 | |
| 3–4 | 21.5 | 6.6 | 11.0 | 28.6 | 37.4 | 16.5 | |
| 5 or more | 29.1 | 8.9 | 7.3 | 17.9 | 34.1 | 31.7 | |
| Previous quit methods | |||||||
| 0 | 53.7 | 16.8 | 12.7 | 30.5 | 30.9 | 9.1 | 64.19 (12)* |
| 1 | 21.7 | 7.9 | 5.6 | 15.7 | 39.3 | 31.5 | |
| 2 | 11.2 | 10.9 | 8.7 | 21.7 | 23.9 | 34.8 | |
| 3 or more | 13.4 | 1.8 | 0.0 | 18.2 | 38.2 | 41.8 | |
| Readiness to quit | |||||||
| Low | 32.5 | 20.3 | 13.8 | 27.5 | 24.6 | 13.8 | 33.08 (8)* |
| Medium | 32.7 | 9.4 | 7.2 | 22.3 | 40.3 | 20.9 | |
| High | 34.8 | 6.8 | 6.8 | 21.6 | 33.8 | 31.1 | |
| Vaccine attitudes | |||||||
| Unfavorable | 33.8 | 25.7 | 13.9 | 24.3 | 23.6 | 12.5 | 59.75 (8)* |
| Neutral | 20.4 | 9.2 | 6.9 | 29.9 | 34.5 | 19.5 | |
| Favorable | 45.8 | 3.6 | 6.7 | 20.5 | 39.0 | 30.3 | |
| Nicotine vaccine attitudes | |||||||
| Unfavorable | 14.3 | 44.1 | 20.3 | 16.9 | 11.9 | 6.8 | 164.25 (8)* |
| Neutral | 27.8 | 13.0 | 18.3 | 40.0 | 22.6 | 6.1 | |
| Favorable | 57.9 | 3.8 | 2.1 | 18.0 | 43.1 | 33.1 | |
Note. HS = high school.
*p < .001.
There were, however, differences in intention to vaccinate by smoking characteristics. Those who have never tried to quit smoking were least likely to be interested in a nicotine vaccine (28.0%), while those who had tried most often to quit (5 or more quit attempts) were most likely to be interested (65.8%; χ2 = 42.33, df = 12, p ≤ .001). Those who had tried multiple different methods to quit smoking also had higher intentions to vaccinate. Smokers who had tried three or more different quit methods in the past were most likely to be interested in trying a nicotine vaccine (χ2 = 64.19, df = 12, p ≤ .001). Intentions to vaccinate did not vary significantly by nicotine dependence scores, measured by participants’ Fagerström scores (χ2 = 7.17, df = 8, p = .519). More than half of those (64.9%) who scored highest on the readiness to quit smoking scale reported that they were “likely” or “very likely” to try a nicotine vaccine compared with 38.4% of those who scored lowest on the quit scale (χ2 = 33.08, df = 8, p ≤ .001). Intentions were highest among those with favorable attitudes toward vaccines in general and the nicotine vaccine specifically. About 69% (69.3%) of those who had the most favorable attitude toward vaccines in general and 76.2% of those who had the most favorable attitude toward the nicotine vaccine reported that they were either “very likely” or “likely” to try the nicotine vaccine compared with 36.1% and 18.7% of those with unfavorable attitudes toward vaccines and the nicotine vaccine, respectively (χ2 = 59.75, df = 8, p ≤ .001; χ2 = 164.25, df = 8, p ≤ .001).
Mulitvariate analyses
A series of multiple regression models were carried out to predict intention to get a nicotine vaccine in the future. Predictors were entered in three steps: demographics, attitudes, and readiness to quit smoking. All three regression models were significant. Model 1 explained 14% of the variance in intention to vaccinate, F(10, 377) = 5.96, p ≤ .001, while Models 2, F(12, 375) = 19.89, p ≤ .001, and 3, F(13, 374) = 19.06, p ≤ .001, each explained 39% and 40% of the variance, respectively. The following factors were significant and positively associated with intentions to vaccinate in the final model (Model 3): education level, attitude toward the nicotine vaccine (using the combined attitude item), attitude toward vaccines in general, readiness to quit smoking, and variety of quit methods tried. The strongest predictors, seen when all variables were included in Model 3, were attitudes toward the nicotine vaccine and general attitudes toward vaccination. Results from the linear regression models are presented in Table 2.
Table 2.
Linear regression models of intention to use a nicotine vaccine
| Predictors | Model 1 β (standardized), n = 427 | Model 2 β (standardized), n = 427 | Model 3 β (standardized), n = 427 |
| Male | −0.04 | −0.06 | −0.07 |
| Black | 0.05 | 0.03 | 0.02 |
| Hispanic | 0.05 | 0.02 | 0.02 |
| Other race | 0.02 | 0.01 | 0.01 |
| Age | −0.03 | −0.05 | −0.05 |
| Education | 0.08 | 0.09* | 0.09* |
| Number of quit methods | 0.24** | 0.13** | 0.10* |
| Number of previous quit attempts | 0.15** | 0.10* | 0.08 |
| Fagerström score | 0.07 | 0.04 | 0.05 |
| Family history mean score | 0.08 | 0.05 | 0.06 |
| Attitude toward vaccines | — | 0.22** | 0.22** |
| Attitude toward nicotine vaccine | — | 0.42** | 0.41** |
| Readiness to quit | — | — | 0.11* |
| Adjusted R2 (model) | 0.14 | 0.39 | 0.40 |
| F (model) | 5.96** | 19.86** | 19.06** |
*p < .05; **p < .01.
Intention to get a nicotine vaccine was positively and significantly correlated with intentions to quit smoking in the next 3 months (r = .24, p < .01) but was negatively and nonsignificantly correlated with self-efficacy to quit (r = −.05, p = .28). Intention to quit smoking and self-efficacy to quit were more strongly correlated (r = .48, p < .01).
Framing manipulation—Main effects
Smokers who read the genetic version of the nicotine vaccine were no more likely to have intentions to vaccinate than those who read the environmental (nongenetic) version, F(1, 425) = 1.05, p = .307. Fifty-four percent of smokers who read the genetic version were likely or very likely to get a nicotine vaccine compared with 55.5% of those who read the environmental version. Smokers who read the environmental version had a significantly more favorable attitude toward vaccination than those who read the genetic version, F(1, 411) = 5.292, p = .02. However, attitudes about the ease of getting a nicotine vaccine were similar between the two groups, F(1, 385) = 1.15, p = .28. Intentions and efficacy to quit smoking varied little between those who read the genetic version and those who read the environmental version. Mean scores for intention to quit were 2.99 (SD = 1.33) for the genetic group and 2.95 (SD = 1.29) for the environmental group, F(1, 424) = 0.63, p = .801, while efficacy to quit smoking was identical in both groups, M = 1.34, SD = 0.69, F(1, 425) = 1.98, p = .16.
Framing manipulation—Moderating effects
Readiness to quit and perceived vulnerability to the effects of smoking interacted with experimental condition on selected outcomes, two of which are briefly discussed. These findings suggest that genetic risk information can undermine efficacy in some subgroups while intensifying a commitment to engage in healthy behavior (quitting smoking) consistent with findings from past studies.
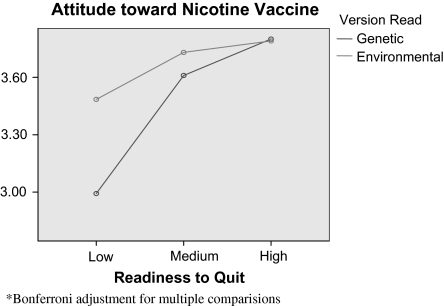
Participants’ scores on the readiness to quit scale interacted with experimental condition to produce differing effects on participants’ attitudes toward vaccination, F(3, 407) = 3.40, p = .06 (Figure 1). The association between readiness to quit and attitude toward vaccination is stronger in the genetic condition than in the environmental condition with those of lowest readiness receiving genetic risk information about addiction having the least favorable attitudes toward getting the vaccine (M [environmental, low] = 3.48, SD = 1.09 vs. M [genetic, low] = 2.99, SD = 1.08). Given the strong relationship between attitude toward the nicotine vaccine and the intention to try it, those with low readiness to quit and receiving genetic information about addiction are least likely to intend to vaccinate.
Figure 1.
Attitude toward nicotine vaccine.
Discussion
Nicotine vaccines are under development for tobacco dependence treatment and have shown some promising effects (Hatsukami et al., 2005). Our research with the proposed nicotine vaccine did not replicate the racial, ethnic, or educational differences in intention to vaccinate that have been seen in other studies (Riddiough, Willems, Sanders, & Kemp, 1981; Singleton, Greby, Wooten, Walker, & Strikas, 2000), possibly because the nicotine vaccine has a very different purpose than a vaccine against an infectious agent and is geared toward a segment of the population with a very powerful addiction. We did, however, find several differences within the population of smokers. These differences could be used by the medical and public health community when targeting which smokers would likely try the vaccine if and when one becomes available. Most notably, those who had tried to quit many times before, those who had tried several different cessation methods, and those who indicated that they were ready to try to quit had highest intentions to try a nicotine vaccine. Smokers with positive attitudes toward vaccines in general, and this vaccine more specifically, were also more receptive to the vaccine.
In this study, participants who read that genetics could play a role in nicotine addiction were no more likely to intend to vaccinate or quit smoking than those who read that environmental factors could play a role in addiction. The finding that genetic susceptibility had little or no direct impact on long-term intentions to quit smoking has been reported in other studies (McBride et al., 2002; Wright et al., 2007). In our study, we were only able to measure perceived susceptibility rather than actual susceptibility, and participants may not have completely inferred a genetic susceptibility to nicotine addiction. While those who read the genetic version thought that they were more likely to have inherited genes that contribute to smoking addiction than those who read the environmental version, they were also more likely to have thought that environmental factors played a role in their smoking addiction. The public’s understanding of the role of genetic susceptibilities is complex and not yet well understood (Parrott, Silk, & Condit, 2003).
The interaction effect observed between a predisposition and an important outcome measure pertinent to quitting smoking is consistent with the findings of other studies about the role of genetic risk information. Sometimes called the “teeter–totter model,” it holds that genetic risk information can increase intentions to engage in healthy behavior while undermining the sense of personal efficacy to accomplish those healthy behaviors (McBride, 2009). In the present data, the relatively mild manipulation of genetic versus environmental risk information produced weak evidence consistent with this model. The intention to quit smoking showed a steep decline as the Fagerström score increased for those receiving information about environmental risk but a much flatter slope when genetic risk was presented. On the other side of the teeter–totter model, smokers’ sense of personal efficacy and attitudes toward using an intervention that could be effective in quitting were undermined by the genetic risk information for some subgroups (low readiness to quit and high vulnerability to health problems from smoking). These results suggest a complex role for genetic risk information.
This study has several strengths, such as using a large nationally representative sample of adult smokers. Nonetheless, several factors should be considered in interpreting the results of this study. Foremost among these is that the vaccine is not available to the public, and therefore, we are unable to measure actual vaccination behavior. And while theories of health behavior change are premised on the idea that intention is the most immediate and important predictor of behavior (Ajzen & Fishbein, 1980) and meta-analyses of intention–behavior relationships show significant and strong associations (Armitage & Connor, 2001; Sheppard, Hartwick, & Warshaw, 1988), the findings of this study would be enhanced if actual vaccination behavior could have been measured. Also, the description of the vaccine is based on what limited information is currently available and may not reflect actual vaccine properties in the future. If the vaccine changes substantially from its current form under investigation, the intentions to vaccinate reported here might not be accurate. Lastly, the mean age of the sample was 56.1 years, which is somewhat higher than other studies involving smokers. Our results may not be generalizable to a younger population of smokers.
This is the first study, to our knowledge, to assess smokers’ intentions toward a vaccine against nicotine addiction. If and when one becomes available in clinical practice, it will be important to identify which smokers would be most receptive to this form of cessation therapy. Smokers who have experimented with other quit methods in the past as well as smokers who have favorable attitudes toward vaccines may be the first group of smokers to target for this novel therapy. Whether or not a smoker believes in the underlying cause of nicotine addiction may not have an effect on their intention to use the vaccine or their self-efficacy to quit smoking. Based on current immunological studies and the work presented here, the vaccine holds promise for the millions of Americans who are addicted to smoking.
Funding
This work was supported by the National Institutes of Health (NCI P50-CA095856-05).
Declaration of Interests
None declared.
Supplementary Material
Supplementary material can be found at Nicotine and Tobacco Research online (http://www.ntr.oxfordjournals.org/).
Acknowledgments
The authors would like to thank Dorothy Hatsukami, Ph.D., for her expertise and guidance throughout the course of the study.
References
- Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–211. [Google Scholar]
- Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Upper Saddle River, NJ: Prentice Hall; 1980. [Google Scholar]
- Apanovitch AM, McCarthy D, Salovey P. Using message framing to motivate HIV testing among low-income, ethnic minority women. Health Psychology. 2003;22:60–67. doi: 10.1037//0278-6133.22.1.60. [DOI] [PubMed] [Google Scholar]
- Armitage CJ, Connor M. Efficacy of the theory of planned behaviour: A meta-analytic review. British Journal of Social Psychology. 2001;40:471–500. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults–United States, 2007. Morbidity and Mortality Weekly Report. 2008;57:1221–1226. [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klinger K, van Melle G, et al. A vaccine against nicotine for smoking cessation: A randomized clinical trial. PloS ONE. 2008;3:e2547.. doi: 10.1371/journal.pone.0002547. Retrieved 30 September 2009, From http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detweiler JB, Bedell BT, Salovey P, Pronin E, Rothman AJ. Message framing and sunscreen use: Gain-framed messages motivate beach-goers. Health Psychology. 1999;18:189–196. doi: 10.1037//0278-6133.18.2.189. [DOI] [PubMed] [Google Scholar]
- Entman RM. Framing: Toward clarification of a fractured paradigm. Journal of Communication. 1993;43:51–58. [Google Scholar]
- Finney L, Iannoti R. Message framing and mammography screening: A theory-driven intervention. Behavorial Medicine. 2002;28:5–14. doi: 10.1080/08964280209596393. [DOI] [PubMed] [Google Scholar]
- Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- Frishman WH. Smoking cessation pharmacotherapy. Therapeutic Advances in Cardiovascular Disease. 2009;3:287–308. doi: 10.1177/1753944709335754. [DOI] [PubMed] [Google Scholar]
- Gamson W, Wolfsfeld G. Movements and media as interacting systems. Annals of the American Academy of Political and Social Science. 1993;528:114–125. [Google Scholar]
- Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, et al. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clinical Pharmacology and Therapeutics. 2005;78:456–467. doi: 10.1016/j.clpt.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kinder DR. Communication and opinion. Annual Review of Political Science. 1998;1:167–197. [Google Scholar]
- Le Sage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, et al. The effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology. 2006;184:409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- Lerman C, Gold K, Audrain J, Lin TH, Boyd NR, Orleans CT, et al. Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: Effects on smoking-related cognitions, emotions, and behavior change. Health Psychology. 1997;16:87–99. doi: 10.1037//0278-6133.16.1.87. [DOI] [PubMed] [Google Scholar]
- Lerman C, Le Sage MG, Perkins KA, O’Malley SS, Siegel SJ, Benowitz NL, et al. Translational research in medication development for nicotine dependence. Nature Reviews: Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- McBride CM. Personal Genomics & Cancer Prevention. Philadelphia, PA: University of Pennsylvania; 2009, April. Presentation at the Annenberg School for Communication. [Google Scholar]
- McBride CM, Bepler G, Lipkus IM, Lyna P, Samsa G, Albright J, et al. Incorporating genetic susceptibility feedback into a smoking cessation program for African-American smokers with low income. Cancer, Epidemiology, Biomarkers, and Prevention. 2002;11:521–528. [PubMed] [Google Scholar]
- McCall KD, Johnson RJ, Rothman AJ. The effects of framing and action instructions on whether older adults obtain flu shots. Health Psychology. 2002;21:624–628. doi: 10.1037//0278-6133.21.6.624. [DOI] [PubMed] [Google Scholar]
- Meyerowitz BE, Chaiken S. The effect of message framing on breast self-examination attitudes, intentions, and behavior. Journal of Personality and Social Psychology. 1987;52:500–510. doi: 10.1037//0022-3514.52.3.500. [DOI] [PubMed] [Google Scholar]
- Millar MG, Millar K. Promoting safe driving behaviors: The influence of message framing and issue involvement. Journal of Applied Social Psychology. 2000;30:853–856. [Google Scholar]
- O’Keefe DJ, Jensen JD. The advantages of compliance or the disadvantages of noncompliance? A meta-analytic review of the relative persuasive effectiveness of gain-framed and loss-framed messages. Communication Yearbook. 2006;30:1–43. [Google Scholar]
- Parrott RL, Silk K, Condit C. Diversity in lay perceptions of the sources of human traits: Genes, environments, and personal behaviors. Social Science & Medicine. 2003;56:1099–1109. doi: 10.1016/s0277-9536(02)00106-5. [DOI] [PubMed] [Google Scholar]
- Pentel PR, Malin D. A vaccine for nicotine dependence: Targeting the drug rather than the brain. Respiration. 2002;69:193–197. doi: 10.1159/000063617. [DOI] [PubMed] [Google Scholar]
- Pineau V, Dennis M. Methodology for probability-based recruitment for a web-enabled panel. 2007. Retrieved 15 July 2009, from http://www.knowledgenetworks.com/ganp/docs/Knowledge%20Networks%20Methodology.pdf. [Google Scholar]
- Prochaska JO, DiClemente CC. Stages and processes of self-change in smoking: Toward an integrative model of change. Journal of Consulting and Clinical Psychology. 1983;51:390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- Riddiough MA, Willems JS, Sanders CR, Kemp K. Factors affecting the use of vaccines: Considerations for immunization program planners. Public Health Reports. 1981;96:528–535. [PMC free article] [PubMed] [Google Scholar]
- Rothman AJ, Bartels RD, Wlaschin J, Salovey P. The strategic use of gain- and loss-framed messages to promote healthy behavior: How theory can inform practice. Journal of Communication. 2006;56:S202–S220. [Google Scholar]
- Sanderson SC, O’Neill SC, White DB, Bepler G, Bastian L, Lipkus I, et al. Responses to online GSTM1 genetic test results among smokers related to patients with lung cancer: A pilot study. Cancer Epidemiology Biomarkers and Prevention. 2009;18:1953–1961. doi: 10.1158/1055-9965.EPI-08-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Lerman C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opinion on Emerging Drugs. 2006;11:429–444. doi: 10.1517/14728214.11.3.429. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Lerman C, Cappella JN, Strasser AA, Pinto A, Hornik R. Evaluating smokers’ reactions to advertising for new lower nicotine quest cigarettes. Psychology of Addictive Behaviors. 2006;20:80–84. doi: 10.1037/0893-164X.20.1.80. [DOI] [PubMed] [Google Scholar]
- Shadel WG, Niaura R, Abrams DB. How do adolescents process smoking and anti-smoking advertising? A social-cognitive analysis with implications for smoking initiation. Review of General Psychology. 2001;5:429–444. [Google Scholar]
- Sheppard BH, Hartwick J, Warshaw PR. The theory of reasoned action: A meta-analysis of past research with recommendations for modifications and future research. Journal of Consumer Research. 1988;15:325–343. [Google Scholar]
- Singleton JA, Greby SM, Wooten KG, Walker FJ, Strikas R. Influenza, pneumococcal, and tetanus toxioid vaccination of adults—United States, 1993-7. mmwr Morbidity and Mortality Weekly Report. CDC Surveillance Summaries. 2000;49:39–62. [PubMed] [Google Scholar]
- Weibe EF, Eyerman J, Loft J. Evaluating non-response in a web-enabled survey on health and aging. 2001. Conference of the American Association for Public Opinion Research. Montreal, Canada, May 17, 2001. [Google Scholar]
- Wright AJ, Aveyard P, Guo B, Murphy M, Brown K, Marteau TM. Is attributing smoking to genetic causes associated with a reduced probability of quit attempt success? A cohort study. Addiction. 2007;102:1657–1664. doi: 10.1111/j.1360-0443.2007.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer VF, DiClemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]