Abstract
Introduction:
The biobehavioral mechanism(s) mediating bupropion’s efficacy are not well understood. Behavioral economic measures such as demand curves have proven useful in investigations of the reinforcing effects of drugs of abuse. Behavioral economic measures may also be used to measure the effect of pharmacotherapies on the reinforcing effects of drugs of abuse.
Methods:
The effects of bupropion on simulated demand for cigarettes were investigated in a placebo-controlled double-blind clinical trial. Participants reported the number of cigarettes they would purchase and consume in a single day at a range of prices. The effects of medication on the subjective effects of smoking were also explored.
Results:
Demand for cigarettes was well described by an exponential demand equation. Bupropion did not significantly decrease the maximum number of cigarettes that participants said they would smoke in a single day nor did it significantly alter the relation between price per cigarette and demand. Baseline demand elasticity did not predict smoking cessation, but changes in elasticity following 1 week of treatment did. Medication group had no effect on any subjective effects of smoking.
Discussion:
Bupropion had no significant effects on demand for cigarettes. The exponential demand equation, recently introduced in behavioral economics, proved amenable to human simulated demand and might be usefully employed in other pharmacotherapy studies as it provides a potentially useful measure of changes in the essential value of the drug as a reinforcer. Such changes may be useful in predicting the efficacy of medications designed to reduce drug consumption.
Clinical trials have demonstrated the efficacy of bupropion for smoking cessation (e.g., Hurt et al., 1997; Jorenby et al., 1999). In a meta-analysis, bupropion doubled the rate of long-term cessation over placebo (Fiore et al., 2008). While the biobehavioral mechanism(s) mediating bupropion’s efficacy are not well understood (Warner & Shoaib, 2005), there is some evidence from animal studies that chronic administration of bupropion attenuates the reinforcing efficacy of nicotine (Rauhut, Dwoskin, & Bardo, 2005), although findings have been inconsistent (Paterson, Balfour, & Markou, 2008; Shoaib, Sidhpura, & Shafait, 2003). At the same time, animal studies have found that bupropion attenuates nicotine-induced enhancement of brain reward systems (i.e., intracranial self-stimulation thresholds) and reverses the anhedonic and somatic symptoms of nicotine withdrawal (e.g., Paterson et al., 2008), although, again, results have been mixed (Paterson, Balfour, & Markou, 2007).
Human studies of the biobehavioral mechanism(s) mediating bupropion’s efficacy have been limited. In a laboratory study with non–treatment-seeking smokers, acute administration of 300 mg bupropion affected some subjective effects (i.e., reduced “intensity of cigarette” and “satisfaction” relative to placebo) during ad libitum smoking of participants’ preferred-brand cigarette (Cousins, Stamat, & de Wit, 2001). A 300 mg dose had no effect in a second study, although participants smoked experimental cigarettes rather than their usual brand (Rukstalis et al., 2005). Results of placebo-controlled clinical trials with bupropion have generally supported a role for bupropion in promoting abstinence by reducing craving in the postquit period (McCarthy et al., 2008; Piper et al., 2008). However, results have been somewhat inconsistent across trials regarding the effect of bupropion on withdrawal distress and whether these effects mediate outcome (e.g., McCarthy et al.; Piper et al.).
Bupropion acts as an inhibitor of dopamine and norepinephrine transporters (Dwoskin, Rauhut, King-Pospisil, & Bardo, 2007). In addition, Miller, Sumithran, and Dwoskin (2002) and others (Fryer & Lukas, 1999; Slemmer, Martin, & Damaj, 2000) have demonstrated that bupropion acts as an antagonist on several nicotinic acetylcholine receptors. This latter effect suggests that bupropion may attenuate the reinforcing properties of nicotine (Paterson, 2009). Consistent with this hypothesis, bupropion reverses the nicotine-induced decrease in brain stimulation reward threshold in animal models (Epping-Jordan, Watkins, Koop, & Markou 1998). However, to our knowledge, no clinical trial has investigated the effect of bupropion on the reinforcing efficacy of nicotine or smoking.
In recent years, behavioral economists have quantified consumer demand for addictive substances by studying how price affects labor supplied (i.e., spending) in the pursuit of drugs (e.g., Hursh & Silberberg, 2008). These studies generally find that drug consumption is sensitive to price changes: a finding characterized by a demand curve across which drug consumption decreases as price increases (e.g., Bickel & Madden, 1999a). Quantifying properties of the demand curve (e.g., steepness of the curve) have proven useful in determining the reinforcing efficacy of drugs of abuse (e.g., Hursh & Winger, 1995). Quantitative properties of drug demand curves may also be used to measure the effect of pharmacotherapies on drug consumption.
Responding to practical difficulties of assessing an entire drug demand curve in human drug users (Bickel, Marsch, & Carroll, 2000), a number of researchers have usefully employed a simulated demand measure, sometimes referred to as a purchase task, in which participants indicate daily drug consumption at a range of drug prices (e.g., Jacobs & Bickel, 1999; MacKillop et al., 2009; Murphy & MacKillop, 2006). The outcomes of these purchase task studies have been in general agreement with studies in which participants expend real effort to consume real drugs (e.g., Jacobs & Bickel vis. Bickel & Madden, 1999b), and some evidence suggests that purchase task outcomes have predictive validity (MacKillop & Murphy, 2007; Tucker, Vuchinich, & Rippens, 2002).
The purpose of this study was to determine the effect of bupropion on the reinforcing efficacy of cigarettes in treatment-seeking smokers. A purchase task procedure was used. We sought to determine if bupropion would render demand for cigarettes more sensitive to price increases. A secondary aim was to determine the effect of bupropion on the subjective effects of smoking. To our knowledge, this is the first smoking cessation study to use a laboratory procedure to investigate biobehavioral mechanisms that may mediate bupropion’s efficacy as a smoking cessation medication.
Methods
Participants
Participants were recruited to participate in a double-blind placebo-controlled smoking cessation clinical trial of smokers with a history of alcohol dependence. Prior to enrollment, all participants were medically screened by the study physician who also monitored adverse events throughout a participant’s involvement in the study. Participants were randomly assigned to bupropion (N = 33) or placebo (N = 27) for 8 weeks.
To be eligible for the trial, participants must have smoked at least 10 cigarettes/day, have a history of alcohol dependence, and between 2 and 12 months of abstinence from alcohol prior to enrollment. Dependence was determined by administering the Alcohol Disorders section of the Structured Clinical Interview for Diagnosis for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995). Exclusion criteria were (a) older than age 70, (b) diagnosis of schizophrenia, (c) current psychotic episode, (d) cardiac problems in the past 3 months, (e) uncontrolled hypertension, (f) history of seizure, (g) history of head injury, and (h) use of medications that lower the seizure threshold. Only use of antidepressant medication that lowered the seizure threshold was excluded. The study was approved by the Institutional Review Boards of Boston University, University of Massachusetts, the Edith Nourse Rogers Memorial Veterans Administration Medical Center (ENRM VAMC), and the University of Kansas. For the larger clinical trial, participants were recruited from Boston University Medical Center (BUMC) and the ENRM VAMC. However, the present study was only conducted at the ENRM VAMC due to a smoking restriction policy at BUMC, which prevented the administration of the procedures described here.
Measures
Purchase task
Participants reported the number of cigarettes they would purchase and smoke each day if a single cigarette ranged in price from $0 to $1,120. Twenty-six different prices were investigated: $0, $0.01, $0.05, $0.13, $0.15, $0.25, $0.35, $0.5, $1, $1.50, $2, $2.50, $3, $4, $5, $6, $7, $8, $9, $11, $35, $70, $140, $280, $560, and $1,120. Participants were instructed to report only those cigarettes that they themselves would smoke and were told to assume that the price indicated was the price of all cigarettes that they could purchase from any source. These instructions that did not change between assessments and double-blind procedures ensured that the experimenter did not know if participants had taken bupropion or placebo at the second assessment.
Subjective effects questionnaire
Two items from the Cigarette Evaluation Scale (i.e., “was the cigarette satisfying,” “did the cigarette taste good”) were used to assess smoking satisfaction (Westman, Levin, & Rose, 1992). In addition, participants rated the effects of smoking on the following mood states: jittery, relaxed, stimulated, dizzy, buzzed, and high. One item queried each mood, and all items were rated on a 5-point scale (not at all to extremely). These mood states are commonly assessed in studies of the subjective effects of smoking (Kalman, 2002).
Participants also completed the six-item Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), provided demographic information (age and gender), and reported the number of cigarettes they smoked per day.
Procedure
Prior to the study, participants provided informed consent. At the onset of Week 1, participants began taking the study medication (bupropion or placebo) and attending a counseling sessions that occurred once per week. They were instructed to take one tablet (150 mg) per day for 3 days and then two tablets (300 mg) per day for the remainder of Week 1. Placebo tablets were identical in shape and color to active medication. At the start of Week 2, participants were instructed to quit smoking and were given a 7-week supply of the nicotine patch. Participants continued to attend weekly counseling sessions and take bupropion (300 mg) for the 7-week quit period. At Week 10, participants returned to the clinic to provide a carbon monoxide breath sample and to report on their smoking status.
Participants attended two laboratory sessions, each lasting ∼60 min. The first session was conducted during the baseline assessment (Week 0), prior to the administration of the study medication. The second was conducted on the last day of Week 1 (i.e., 7 days after participants began taking study medication and the day before their quit day). In each session, participants smoked one preferred-brand cigarette, sat quietly for 30 min, smoked another cigarette, and then completed the demand simulation measure. The first cigarette was smoked ad libitum. Following Schupp, Mucha, and Pauli (1996), a paced puffing procedure was used for the second cigarette. Participants took 10 puffs from the cigarette, each held for 3 s with 30 s between puffs. This procedure represents a middle ground between ad libitum smoking (which greatly reduces experimenter control over dosing) and smoking via a controlled smoking device (a procedure that attenuates nicotine’s effects; see Grobe & Perkins, 2000). In the second session, participants also completed the subjective effects measures. Participants who required dose adjustments to their medication during the course of the clinical trial (n = 7) were not eligible for inclusion in this study.
Statistical methods
Following Hursh and Silberberg (2008), we used the following exponential demand equation to fit the simulated demand data provided by individual participants:
| (1) |
Equation (1) contains a single free parameter, α, which quantifies the rate of change in consumption (i.e., number of cigarettes smoked per day) across the range of cigarette price increases. This provides a quantitative index of the degree to which an individual will expend resources to defend prior levels of smoking. As such, Hursh and Silberberg (2008) argued that α may be interpreted as the essential value of a commodity. The α parameter was quantified for individual participants using GraphPad Prism 5 curve-fitting software and a file provided to the first author by S. R. Hursh.
With respect to the other components of equation (1), Q is the number of cigarettes smoked per day, Q0 is peak level of smoking when cigarettes are freely available, and k is the obtained range of Q (from 0 to Q0) expressed in logarithmic units (note that for the purpose of fitting curves to individual participant data, 0.9 was substituted for 0 at the first price at which no cigarettes were purchased; all subsequent prices were ignored as the log of 0 is undefined). One bupropion participant who indicated that he/she would smoke 40 cigarettes/day even if the price of each cigarette was $1,120 was excluded as an outlier.
The price of a cigarette, Ps, is normalized to the cost of obtaining the peak level of smoking (Q0) at each nominal price (Ps = Q0 × P). Normalizing price ensures that α is independent of changes in Q0. Equation (1) accurately describes consumption of drug and nondrug commodities in human and animal subjects (R2 values typically exceeding .90; Hursh & Silberberg, 2008). A 2 (baseline vs. follow-up) × 2 (placebo vs. bupropion group) mixed factor analysis of variance (ANOVA) was used to evaluate changes in smoking measures (e.g., Q0, α). Distributions of these parameter values met the equality of variance assumptions of these ANOVAs.
Results
Purchase task
At intake, no significant differences in gender, age, number of cigarettes smoked per day, or FTND scores were observed across participants assigned to the bupropion and placebo groups (p > .05 in all cases; see Table 1). Because the difference in age approached significance [t(58) = 1.7, p = .1], it was included as a covariate in subsequent ANOVAs. At baseline, there were no significant differences between groups in either the number of cigarettes that would be smoked if they were free [F(1, 57) = 1.74, p > .05], the maximum amount of money that would be spent on cigarettes in a single day [F(1, 57) = 0.51, p > .05] or the α parameter of baseline demand curves [F(1, 57) = 0.84, p > .05].
Table 1.
Demographic characteristics and severity of nicotine dependence at baseline
| Variable | Placebo | Bupropion |
| Age (years) | 51.2 (6.6) | 48.6 (7.2) |
| Gender (% male) | 92.6 | 81.8 |
| Nicotine dependence | 5.1 (1.7) | 5.7 (1.9) |
Note. Nicotine dependence was measured with the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991). Unless otherwise noted, all data are means and SDs.
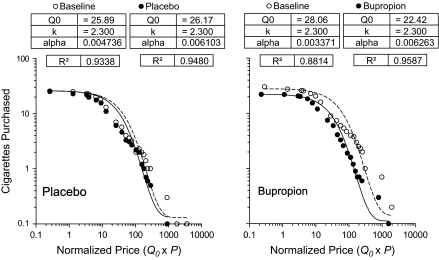
The left and right graphs in Figure 1 show the average number of simulated cigarettes purchased in baseline (open symbols) and treatment assessments for participants in the placebo and bupropion groups, respectively. Demand curves were fit to these group averages using equation (1), where Q0 was the number of free cigarettes that participants said they would smoke per day in each condition. Parameters of these fits are provided in each panel. In all cases, equation (1) provided a good fit of the data, with greater than 88% variance accounted for. Equation (1) also provided a good fit of individual participant’s simulated demand data (median R2 = .88, interquartile range = .70–.93). Baseline α parameter values derived from simulated demand curves (a measure of the essential value of cigarettes) were significantly correlated with baseline FTND scores (Spearman r = −.255, p < .05). The correlation between α parameter values and the number of cigarettes participants reported smoking each day at intake approached significance (Pearson r = −.232, p = .07).
Figure 1.
Simulated demand for cigarettes across the programmed range of normalized prices for participants in the placebo and bupropion groups. Curves are fit using the demand equation proposed by Hursh and Silberberg (2008), see text for details.
A mixed factor ANOVA applied to peak smoking (Q0) revealed no significant main effects of time (baseline vs. follow-up, p = .09) or group (bupropion vs. placebo, p = .76). The Time × Group interaction was not significant [F(1, 57) = 1.74, p = .19]. The same mixed factor ANOVA was applied to α values derived from individual participants’ demand curves. No significant main effects of time [F(1, 57) = 1.15, p = .29] or group [F(1, 57) = 2.75, p = .10] were detected. Likewise, the Time × Group interaction did not approach significance [F(1, 57) = 1.29, p = .26]. In sum, bupropion did not decrease peak smoking or the essential value of cigarettes.
Pmax and Omax values were calculated from the derived parameters of the demand curves and were subjected to the same mixed factor ANOVA. Pmax is the cigarette price (nonnormalized for the purpose of this analysis) at which the demand curve has a slope of −1.0. More importantly, it is the price at which spending on cigarettes is predicted to asymptote; at higher prices, spending should decline. Omax is the predicted maximum amount that would be spent on cigarettes in a single day. Pmax and Omax were calculated using the spreadsheet written by S. R. Hursh (available at http://www.ibrinc.org/centers/bec/BEC_demand.html).
No significant main effects of time were detected on either Pmax or Omax (p > .4 in both cases). Likewise, no main effect of group was detected (p > .4 in both cases), and the Time × Group interaction did not approach significance (p > .4 in both cases). The correlations between derived and obtained measures of peak spending and price at which peak spending occurred were strong (Omax: Pearson r = .64, p < .0001; Pmax: Pearson r = .79, p < .0001), and a mixed factor ANOVA applied to obtained measures did not reveal a Time × Group interaction (p > .2 in both cases). Thus, an effect of bupropion on cigarette purchases was not missed by using measures derived from the demand curves.
Subjective effects
Because the measures of subjective effects of smoking were assessed only in the second session (when participants had taken bupropion or placebo), one-way ANOVA was conducted to determine if groups differed on these measures. There were no significant differences in Cigarette Evaluation Scale measures of satisfaction (p = .46) or taste (p = .97). Likewise, mood states of jittery (p = .13), relaxed (p = .14), stimulated (p = .27), dizzy (p = .63), buzzed (p = .86), and high (p = .22) did not differ across groups.
Demand as a predictor of treatment outcome
To determine if α values could be used to predict the outcome of a smoking cessation trial, we compared intake α values of those who were and were not smoking at follow-up. A one-tailed t test revealed that the quitters’ baseline demand for cigarettes was no more elastic than those who failed to quit [t(58) = 0.4, p > .05]. However, demand for cigarettes at Week 2 (after one counseling session and 1 week of bupropion or placebo) became more elastic in those who quit than those who did not [i.e., prepost α difference scores; one-tailed t(58) = 1.63, p = .05]. The change in Q0 from Weeks 1 to 2 did not achieve traditional levels of significance across those who quit and failed to quit [one-tailed t(58) = 1.3, p = .11].
Discussion
The current investigation revealed that bupropion neither reduced peak smoking (Q0) nor decreased the essential value (α) of cigarettes as measured by a purchase task. Bupropion also did not decrease peak spending on cigarettes (Omax) or the cigarette price at which spending would begin to decline (Pmax). There were no differences between the bupropion and placebo groups on any subjective effect measure of smoking. However, demand for cigarettes following 1 week of treatment (bupropion or placebo) became more elastic in those who quit than those who did not.
Animal studies have reported mixed results regarding the effects of chronic bupropion administration on the reinforcing effects of nicotine (Rauhut et al., 2005; Shoaib et al., 2003). In a small clinical study, Hawk et al. (2008) reported a decline in cigarette consumption as a function of length of bupropion treatment prior to quitting. Significantly greater smoking reduction was observed in participants who were randomly assigned to receive 4 weeks versus 1 week of bupropion treatment prior to quitting. Extended pretreatment also enhanced abstinent outcomes (mediational analyses were not conducted, however). If bupropion attenuates the reinforcing effects of nicotine, as suggested by its action as a nicotinic antagonist, extended pretreatment may be needed for these effects to emerge. It may, therefore, be worthwhile to determine if peak smoking (Q0) would decline and elasticity (α) would increase with longer precessation exposure to bupropion. Because the purchase task was not completed weekly in the present study, this analysis will have to await further research.
A few clinical trials have investigated the effect of bupropion on the subjective effects of smoking with mixed results. In two studies, nonabstinent smokers receiving bupropion reported decreased “psychological reward” and “smoking satisfaction” compared with their counterparts in the placebo condition (Jorenby et al., 2006; West, Baker, Cappelleri, & Bushmakin, 2008). A third study found an effect but only for “psychological reward” (Gonzales et al., 2006), and a fourth study found no effect on any of their measures of subjective effects (McCarthy et al., 2008). In the latter study, there was also no evidence that bupropion slowed or prevented progression from a lapse to a relapse, an effect that would be expected if bupropion attenuated the reinforcing efficacy and/or subjective effects of smoking (McCarthy et al.).
Finally, the present findings offer some support for the utility of the purchase task. First, simulated demand for cigarettes in the purchase task conformed to the positively decelerating demand curves that have been observed with humans smoking real cigarettes (Bickel & Madden, 1999a) and nonhumans consuming a variety of drug and nondrug reinforcers (Hursh, 2000). Second, in accordance with previous purchase task data (e.g., Murphy & MacKillop, 2006), simulated demand for cigarettes was well described by a quantitative demand curve, in this instance the exponential demand equation of Hursh and Silberberg (2008). Third, measures derived from the demand curves (Pmax and Omax) were well correlated with obtained measures. Fourth, baseline α values were modestly correlated with intake FTND scores, but they were not predictive of which participants would successfully quit smoking regardless of the medication received. Interestingly, the change in α values after the first week of the study (a week in which participants completed one counseling session and took bupropion or placebo) was predictive of cigarette abstinence at follow-up. Those participants who successfully quit smoking showed larger increases in α values after the first week. Their purchase task responses indicated that at Week 2, their demand for cigarettes was more likely to decline in the face of price increases when compared with those who failed to quit.
Five limitations of the present study should be noted. First, the purchase task involves hypothetical cigarettes, prices, and smoking, so one may reasonably question if real cigarette purchases would conform to the patterns of data reported here. While some evidence suggests that simulated demand outcomes are in general agreement with studies involving the expenditure of real effort to consume real drugs (e.g., Jacobs & Bickel, 1999 vis. Bickel & Madden, 1999b), addressing this issue will require studies designed to answer this specific question. Second, our participants were smokers with histories of alcohol dependence, and therefore, the results of the present study may differ in important ways from smokers without this history. Chronic heavy alcohol consumption is associated with changes in brain structure and function that, in turn, directly affect the brain reward circuitry that entrains drug-seeking behavior and possibly the reinforcing efficacy of smoking (Littleton, Barron, Prendergast, & Nixon, 2007; Makris et al., 2008; see also Hughes, Rose, & Callas, 2000). Therefore, caution should be exercised in generalizing the findings of this study to smokers without histories of alcohol dependence. Third, we did not examine the relationship between plasma bupropion levels and the reinforcing efficacy and subjective effects of smoking. Individual differences in these levels may have affected responses on our dependent measures. In addition, the reinforcing efficacy and subjective effects of the study medication on smoking were determined only 2 days after participants had begun taking the 300 mg dose of bupropion. Findings by Hawk et al. (2008), discussed above, suggest that longer medication treatment may produce more pronounced effects. Fourth, participants in our study were not smoking deprived during study assessments. It is possible that the effects of bupropion on study measures would be more pronounced under conditions of nicotine deprivation and withdrawal. Finally, cigarette deprivation was controlled by having participants smoke before completing the purchase task. Future research might examine the effects of bupropion under conditions of progressive cigarette deprivation (e.g., Hitsman et al., 2008). Longer duration of deprivation might be expected to render demand for cigarettes less elastic (c.f., Field, Santarcangelo, Sumnall, Goudie, & Cole, 2006; Giordano et al., 2002), and from this baseline, any effects of bupropion on elasticity (i.e., α values) may be more easily detected.
In conclusion, behavioral economic methods have been used extensively in animals and humans to study the reinforcing efficacy of drugs of abuse, including nicotine, and can be readily applied in human studies to investigate the effects of pharmacotherapies on nicotine reinforcement (Hursh & Silberberg, 2008). The present study suggests that bupropion has no detectable effect on demand for cigarettes. However, changes in elasticity from the first to the second week of participation were predictive of subsequent success in smoking cessation. Future laboratory studies might profitably use behavioral economic measures to investigate the effects of other medications on nicotine reinforcement, including studies in which the effects of two or more medications are compared. Behavioral economic approaches can also be used in clinical trials to investigate the relation between the effect of a medication on nicotine reinforcement and smoking cessation outcome. Future studies might also consider investigating the effects of medication on nicotine reinforcement during stress induction.
Funding
This research was supported by research grants (RO1 DA 017370 and R21 DA 023564) from the National Institute on Drug Abuse.
Declaration of Interests
None declared.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Administration Medical Center in Bedford, Massachusetts, where this study was conducted.
References
- Bickel WK, Madden GJ. The behavioral economics of smoking. In: Chaloupka FJ, Grossman M, Bickel WK, Saffer H, editors. The economic analysis of substance use and abuse: An integration of econometric and behavioral economic research. Chicago: University of Chicago Press; 1999a. pp. 31–61. [Google Scholar]
- Bickel WK, Madden GJ. A comparison of measures of relative reinforcing efficacy and behavioral economics: Cigarettes and money in smokers. Behavioural Pharmacology. 1999b;10:627–737. doi: 10.1097/00008877-199911000-00009. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA, Carroll ME. Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: A theoretical proposal. Psychopharmacology. 2000;153:44–56. doi: 10.1007/s002130000589. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Review. 2007;12:178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koop GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Field M, Santarcangelo M, Sumnall H, Goudie A, Cole J. Delay discounting and the behavioural economics of cigarette purchases in smokers: The effects of nicotine deprivation. Psychopharmacology. 2006;186:255–263. doi: 10.1007/s00213-006-0385-4. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services, Public Health Services, Public Health Service Report; 2008. [Google Scholar]
- First MD, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- Fryer JD, Lukas RJ. Antidepressants noncompetitively inhibit nicotine acetylcholine receptor function, acetylcholine receptor function. Journal of Neurochemistry. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Giordano LA, Bickel WK, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology. 2002;163:174–182. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. sustained-released bupropion and placebo for smoking cessation. Journal of American Medical Association. 2006;296:46–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grobe JE, Perkins KA. Behavioral factors influencing the effects of nicotine. In: Piasecki M, Newhouse PA, editors. Nicotine in psychiatry: Psychopathology and emerging therapeutics. Washington, DC: American Psychiatric Press; 2000. pp. 59–81. [Google Scholar]
- Hawk LW, Mahoney MC, Ashare RL, Rhodes JD, Oliver JA, Cummings KM, et al. Preliminary evidence of extinction of smoking behavior with bupropion. 2008, February. Poster presented at the annual conference of the Society of Research on Nicotine and Tobacco, Portland, OR. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addictions. 1991;84:791–800. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hitsman B, MacKillop J, Lingford-Hughes A, Williams TM, Ahmad F, Adams S, et al. Effects of acute tyrosine/phenylalanine depletion on the selective processing of smoking-related cues and the relative value of cigarettes in smokers. Psychopharmacology. 2008;196:611–621. doi: 10.1007/s00213-007-0995-5. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Rose GL, Callas PW. Do former smokers respond to nicotine differently from never smokers? A pilot study. Nicotine & Tobacco Research. 2000;2:255–262. doi: 10.1080/14622200050147529. [DOI] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economic concepts and methods for studying health behavior. In: Bickel WK, Vuchinich RE, editors. Reframing health behavior change with behavioral economics. 2000. (pp. 27–60). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. Journal of the Experimental Analysis of Behavior. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JP, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jacobs EA, Bickel WK. Modeling drug consumption in the clinic using simulation procedures: Demand for heroin and cigarettes in opioid-dependent outpatients. Experimental and Clinical Pharmacology. 1999;7:412–426. doi: 10.1037//1064-1297.7.4.412. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs. placebo or sustained-release bupropion for smoking cessation. Journal of the American Medical Association. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Kalman D. The subjective effects of nicotine: Methodological issues, a review of experimental studies and directions for future research. Nicotine & Tobacco Research. 2002;4:25–70. doi: 10.1080/14622200110098437. [DOI] [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! shouldn’t we do something about it? Alcohol and Alcoholism. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug and Alcohol Dependence. 2007;89:227–233. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WK. Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology. 2009;203:33–40. doi: 10.1007/s00213-008-1367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Kim S, Hodge SM, Kennedy DN, Caviness VS, et al. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research. 2008;10:717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [3H] overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. Journal of Pharmacology & Experimental Therapeutics. 2002;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J. Relative reinforcing efficacy of alcohol among college student drinkers. Experimental and Clinical Psychopharmacology. 2006;14:219–227. doi: 10.1037/1064-1297.14.2.219. [DOI] [PubMed] [Google Scholar]
- Paterson NE. Behavioural and pharmacological mechanisms of bupropion’s anti-smoking effects: Recent preclinical and clinical insights. European Journal of Pharmacology. 2009;603:1–11. doi: 10.1016/j.ejphar.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion attenuated the anhedonic component of nicotine withdrawal in rats via inhibition of dopamine reuptake in the nucleus accumbens shell. European Journal of Neuroscience. 2007;25:3099–3108. doi: 10.1111/j.1460-9568.2007.05546.x. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine & Tobacco Research. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Piper ME, Federmen EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. Journal of Abnormal Psychology. 2008;117:94–105. doi: 10.1037/0021-843X.117.1.94. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self administration produced by repeated bupropion administration. Nicotine & Tobacco Research. 2005;7:901–907. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Strasser A, Lynch KG, Perkins K, Patterson F, et al. Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology. 2005;180:41–48. doi: 10.1007/s00213-004-2136-8. [DOI] [PubMed] [Google Scholar]
- Schupp PE, Mucha RF, Pauli P. Paced smoking in the laboratory and in the natural smoking setting: Differential situation-specific effects in light and heavy smokers. Psychopharmacology. 1996;127:238–288. [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine on rats. Psychopharmacology. 2003;165:405–412. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. Journal of Pharmacology & Experimental Therapeutics. 2000;295:321–327. [PubMed] [Google Scholar]
- Tucker JA, Vuchinich RE, Rippens PD. Predicting natural resolution of alcohol-related problems: A prospective behavioral economic analysis. Experimental and Clinical Psychopharmacology. 2002;10:248–257. doi: 10.1037//1064-1297.10.3.248. [DOI] [PubMed] [Google Scholar]
- Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addiction Biology. 2005;10:219–231. doi: 10.1080/13556210500222670. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Westman EE, Levin ED, Rose JE. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful? Clinical Research. 1992;40:871. [Google Scholar]