Abstract
The present study investigated the effects of a preparation of a γ-tocopherol-rich mixture of tocopherols (γ-TmT) on chemically induced lung tumorigenesis in female A/J mice and the growth of H1299 human lung cancer cell xenograft tumors. In the A/J mouse model, the lung tumors were induced by either 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; intraperitoneal injections with 100 and 75 mg/kg on Week 1 and 2, respectively) or NNK plus benzo[a]pyrene (B[a]P) (8 weekly gavages of 2 μmole each from Week 1 to 8). The NNK plus B[a]P treatment induced 21 tumors per lung on Week 19; dietary 0.3% γ-TmT treatment during the entire experimental period significantly lowered tumor multiplicity, tumor volume and tumor burden (by 30, 50 and 55%, respectively; P < 0.05). For three groups of mice treated with NNK alone, the γ-TmT diet was given during the initiation stage (Week 0 to 3), post-initiation stage (Week 3 to 19) or the entire experimental period, and the tumor multiplicity was reduced by 17.8, 19.7 or 29.3%, respectively (P < 0.05). γ-TmT treatment during the tumor initiation stage or throughout the entire period of the experiment also significantly reduced tumor burden (by 36 or 43%, respectively). In the xenograft tumor model of human lung cancer H1299 cells in NCr-nu/nu mice, 0.3% dietary γ-TmT treatment significantly reduced tumor volume and tumor weight by 56 and 47%, respectively (P < 0.05). In both the carcinogenesis and tumor growth models, the inhibitory action of γ-TmT was associated with enhanced apoptosis and lowered levels of 8-hydroxydeoxyguanine, γ-H2AX and nitrotyrosine in the tumors of the γ-TmT-treated mice. In cell culture, the growth of H1299 cells was inhibited by tocopherols with their effectiveness following the order of δ-T > γ-TmT > γ-T, whereas α-T was not effective. These results demonstrate the inhibitory effect of γ-TmT against lung tumorigenesis and the growth of xenograft tumors of human lung cancer cells. The inhibitory activity may be due mainly to the actions of δ-T and γ-T.
Introduction
Lung cancer is a major disease worldwide and is the leading cause of cancer death in the Western world (1). Prevention of lung cancer by dietary constituents and nutrients could be a safe, feasible and economical approach. It has been suggested that tocopherols (vitamin E) have cancer preventive activities (2–4). Tocopherols are a family of phenolic compounds, each containing a chromanol ring system and a 16-carbon tail (5). They function as chain-breaking antioxidants that prevent the propagation of free radical reactions (6). The major dietary sources of tocopherols are vegetable oils, such as oils from soybean, corn, cottonseeds and nuts (7). There are four forms of tocopherols. γ-Tocopherol (γ-T) is the major form of tocopherol in our diet. α-Tocopherol (α-T) is the major tocopherol found in human blood and tissues, and it has superior activity over other tocopherols in the classic fertility-restoration assay (6). Therefore, α-T has previously received the most attention, and its possible cancer-preventive activities have been studied extensively (8). Recent results from large-scale intervention studies with high doses of α-T, however, have been disappointing (9,10).
As pointed out in several recent reviews, γ-T has stronger anti-inflammatory activities than α-T, and it may be more effective in the prevention of cancer, as well as cardiovascular and neurodegenerative diseases (3,4,11,12). Recent results from our collaborative group at Rutgers University have demonstrated the inhibition of colon, prostate and mammary carcinogenesis in animal models by a γ-T-rich mixture of tocopherols (γ-TmT) (2,13–16). For example, dietary γ-TmT treatment (0.3 and 0.17% in AIN93M diet) significantly inhibited inflammation and colon carcinogenesis in mice that had been previously treated with azoxymethane and dextran sulfate sodium (2).
The effects of tocopherols on lung cancer chemoprevention have not been sufficiently studied. There have been four case–control studies (17–20) and three cohort studies (21–23) on the relationship between dietary or blood levels of tocopherols and risk of lung cancer. Three case–control studies (18–20) and two cohort studies (22,23) reported a significant inverse association between dietary intake of vitamin E and the risk of lung cancer. In the two cohort studies with the positive findings, the protective effects were found in current smokers, suggesting a preventive effect of dietary vitamin E against insult from cigarette smoking (22,23). In the α-Tocopherol, β-Carotene Cancer Prevention Study, daily supplement of 50 mg of all-racemic-α-tocopheryl acetate did not produce a significant effect on the incidence of lung cancer (24). One animal study reported that supplementation with α-T did not inhibit lung metastasis of intravenously injected murine colon adenocarcinoma cells in BALB/C mice (25). However, in our recent study, γ-TmT inhibited the growth of CL-13 murine lung cancer cells injected subcutaneously in A/J mice (26).
In the present study, we investigated the effects of γ-TmT in chemically induced lung tumorigenesis in A/J mice and in H1299 human lung cancer cell xenograft tumors. The effects of γ-TmT treatment on oxidative stress, nitrosative stress and apoptosis in the tumors were studied. We also characterized the inhibitory effects of different forms of pure tocopherols against human lung cancer H1299 cells.
Materials and methods
Animal treatment
Female A/J mice (5 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). The animals were fed an AIN-93M diet and maintained at 20 ± 2°C with a relative humidity of 50 ± 10% and with an alternating 12 h light/dark cycle. They were acclimated in our animal facility for 1 week, and carcinogenesis was initiated by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) plus benzo[a]pyrene (B[a]P) (2 μmole each in saline or glycerol trioctanoate, respectively, for 8 weekly gavages from Week 1 to 8) or NNK (intraperitoneal injections with 100 and 75 mg/kg in saline on Week 1 and 2, respectively). The experiment was carried out under protocol 91-024 and designed as shown in Figure 1A. The mice treated with NNK plus B[a]P were maintained on either an AIN93M diet or AIN93M diet containing 0.3% γ-TmT starting 1 week before the first carcinogen treatment (Week 0) to the end of the experiment (Week 19). The mice treated with NNK were given 0.3% γ-TmT in the AIN93M diet during the initiation stage (Week 0 to Week 3), post-initiation stage (Week 3 to Week 19) or during the entire experimental period (Week 0 to Week 19). The γ-TmT used was a mixture containing 57% γ-T, 24% δ-T, 13% α-T and 1.5% β-T (Cognis Corporation, Cincinnati, OH). Body weights, food and fluid consumption and general health status were monitored weekly. The animals were killed by CO2 asphyxiation at Week 19. The lungs of each animal were removed, inflated and fixed in 10% buffered formalin. Blood was taken by cardiac puncture and centrifuged for 15 min at 3000g for the preparation of plasma. The livers and the omentum fat pad were also removed and weighed. Visible tumors (>0.1 mm in diameter) on the surface of the lungs were counted, and their sizes were measured. For histopathological analysis, the formalin-fixed lungs were embedded in paraffin, dorsal sides facing down, so that most of the tumors were sectioned in 60 serial 5 μm sections. Two sections (each covering all five lobes of the lung) were mounted on every glass slide. Three slides (taken from serial sections; numbers 1, 15 and 30) from each sample were stained with hematoxylin and eosin for histopathological analysis. NNK-induced lung tumors in A/J mice are alveolar type II cell-derived adenomas (27). The tumor diagnosis will be based on our previous criteria (28).
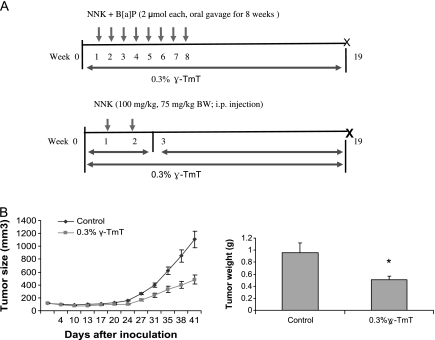
Fig. 1.
(A) Study design for evaluating the chemopreventive efficacy of γ-TmT against NNK plus B[a]P- or NNK-induced lung tumorigenesis in the A/J mouse. In Experiment 1, the mice were treated with NNK plus B[a]P, and the γ-TMT diet was started 1 week before the first carcinogen treatment (Week 0) to the end of the experiment (Week 19). In Experiment 2, the mice treated with NNK alone were given 0.3% γ-TmT diet in the initiation stage (Week 0 to Week 3), post-initiation stage (Week 3 to Week 19) or during the entire experimental period (Week 0 to Week 19). (B) Effects of γ-TmT on the tumor weight and tumor volume in H1299 cell xenograft tumors. γ-TmT diet significantly reduced the tumor volume starting from Week 3. The tumor weight was measured at the end of the experiment after the tumors were dissected. *P < 0.05 by Student’s t-test.
Xenograft experiment
Male NCr-nu/nu mice (5 weeks old) were purchased from Taconic Farm (Germantown, NY). Human lung cancer H1299 cells were obtained from American Type Cell Collection (Manassas, VA). All animals were housed five to a plastic cage with a filter top. Conditions of the animal room were the same as the experiments with A/J mice described above. After 1 week of acclimation, animals were randomly distributed into control and experimental groups (10 per group) and H1299 cells (1 × 106 cells per site in 100 μl mixture of matrigel and culture medium at a 1:1 ratio) were injected subcutaneously to both flanks of the mice. One day after the injection, animals were fed the AIN93M diet or the same diet containing 0.3% γ-TmT. Tumor size (length and width) was measured by a caliper and calculated based on tumor volume = 0.5 × width2 × length. Body weight and food consumption were monitored once a week, until the termination of the study. All animals were killed by CO2 asphyxiation 7 weeks after tumor injection, and tumors were harvested and weighed.
Immunohistochemistry
Immunohistochemistry was performed on lung tissue sections and xenograft tumor sections using specific antibodies to detect the localization and to quantify the levels of the positive stainings. In brief, antigens were unmasked in antigen unmasking solution (DAKO, Glostrup, Denmark). Endogenous peroxidase was quenched using 3% H2O2 in distilled water. Sections were then blocked for 1 h at room temperature in phosphate-buffered saline containing 3% serum and incubated with primary antibody overnight at 4°C. Biotin-conjugated secondary antibody (1:200) and avidin–biotin peroxidase complex were then applied to the sections. Diaminobenzidine (Sigma-Aldrich, St Louis, MO) was used as a chromogen. Negative controls were processed in the absence of the primary antibody.
Analysis of tocopherols by high-performance liquid chromatography
Our previous procedure was used for the determination of tocopherol levels (16). In brief, the fat-soluble materials in plasma or tumor samples were extracted with hexane, redissolved in ethanol and then analyzed by high-performance liquid chromatography. The tocopherol concentrations in mouse plasma and tissues were determined by comparison with the peak heights of the ‘standard plasma’ established in our laboratory (16).
Enzyme immunoassay
Enzyme immunoassays (EIA) for prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) were conducted following the manufacturer’s instructions. In brief, the plasma samples were mixed with ethyl acetate, vortexed for 30 s and then centrifuged at 10 000g for 20 min. The organic layer was collected and dried using a Speed Vacuum Evaporator (VWR International, West Chester, PA). The dried samples were then reconstituted in EIA buffer (Cayman Chemical, Ann Arbor, MI), and levels of PGE2 and LTB4 were determined using EIA kits (Cayman Chemical).
Cell culture experiments
Human large-cell lung carcinoma H1299 cells were maintained in RPMI 1640 media (Mediatech Inc, Herndon, VA) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech Inc), 100 U/ml of penicillin and 0.1 mg/ml of streptomycin (Sigma-Aldrich) at 37°C with 5% CO2 and 95% humidity. Cells were kept subconfluent and media were changed every other day. Dimethyl sulfoxide was used as the vehicle to deliver tocopherols, and the final concentration of dimethyl sulfoxide in all experiments was 0.2%. H1299 cells (1500 cells/well or 5000 cells/well) were seeded in 96 well plates. After 24 h, cells were treated with different concentrations of specific tocopherols (γ-T, purified in our laboratory from γ-TmT; δ-T, Sigma-Aldrich; α-T, MP Biomedicals, Solon, CA) in 200 μl of serum complete media. At 48 and 72 h after treatment, cells were subject to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Media were replaced by 100 μl fresh media containing 0.5 mg/ml of MTT (Sigma-Aldrich). After 1–2 h incubation at 37°C, MTT-containing media was removed and the reduced formazan dye was solubilized by adding 100 μl of dimethyl sulfoxide to each well. After gentle mixing, the absorbance was monitored at 550 nm using a plate reader (TECAN, Phenix Research Products, Candler, NC). The comparison between the treated and control groups is expressed as the percentage of viable cells.
Statistical analysis
SPSS software was used to perform statistical analyses. For simple comparisons between two groups, the two-tailed Student’s t-test was used. One-way analysis of variance was used for comparisons among multiple groups.
Results
Effects of dietary γ-TmT treatment on general health and lung tumor formation in A/J mice
The possible inhibitory actions of dietary γ-TmT treatment on lung tumorigenesis were examined in two animal models. γ-TmT-enriched diet had no effect on food consumption or the body weights of the mice. The liver and omentum fat pad over body weight ratios were not affected by γ-TmT treatment (data not shown). All the carcinogen-treated mice developed lung tumors at the end of the experiment. The tumors were diagnosed as lung adenomas based on our previous criteria (28). Lung adenomas were in a solid, papillary or mixed growth pattern and were generally composed of well-differentiated cells. Treatment with γ-TmT did not significantly change tumor morphology or tumor incidence in either model. The oral administration of the combination of NNK and B[a]P induced 21 tumors per lung at Week 19 (Table I). Treatment of the mice with 0.3% γ-TmT in the diet during the entire experimental period significantly lowered the tumor multiplicity to 14.8 (30% inhibition, P < 0.05). Tocopherol treatment also significantly reduced the average tumor volume from 0.08 to 0.04 mm3 (50% inhibition, P < 0.05) and tumor burden (the total tumor volume per mouse) from 1.71 to 0.77 mm3 (55% inhibition, P < 0.05) in this experiment.
Table I.
The inhibitory effects of γ-TmT on tumorigenesis in A/J mice
| Group | Mice (No.) | Tumor multiplicity (% inhibition) | Tumor volume (mm3) (% inhibition) | Tumor burden (mm3) (% inhibition) |
| NNK + B[a]P | 22 | 21.0 ± 3.0 | 0.08 ± 0.01 | 1.77 ± 0.46 |
| NNK + B[a]P + 0.3% TmT | 22 | 14.8 ± 1.4a (30%) | 0.04 ± 0.01a (51%) | 0.71 ± 0.17a (60%) |
| NNK | 24 | 20.8 ± 1.2 | 0.20 ± 0.02 | 4.12 ± 0.49 |
| NNK + 0.3% TmT (initiation) | 24 | 17.1 ± 1.0b (18%) | 0.16 ± 0.02 | 2.64 ± 0.30b (36%) |
| NNK + 0.3% TmT (post-initiation) | 24 | 16.7 ± 1.6b (20%) | 0.19 ± 0.02 | 4.01 ± 0.68 |
| NNK + 0.3% TmT (entire period) | 24 | 14.7 ± 1.7b (30%) | 0.16 ± 0.02 | 2.33 ± 0.35b (43%) |
| Glycerol trioctanoate control | 6 | 0 | 0 | 0 |
| Saline control | 6 | 0 | 0 | 0 |
Values are the mean ± SE per mouse. Gross tumor data were analyzed under a dissection microscope. Tumors >0.1 mm were counted. Tumor volumes (mm3) were measured using the formula V = 4/3 r3, where r is the radius of the tumor determined by the mean values of the longest and shortest diameters.
P < 0.05 by Student’s t-test compared with the NNK plus B[a]P group.
P < 0.05 by Student’s t-test compared with the NNK group.
In the NNK-induced carcinogenesis experiment, the 0.3% γ-TmT diet was given to three groups of mice: during the initiation stage, the post-initiation stage or the entire experimental period. γ-TmT treatment significantly reduced the tumor multiplicity for all three treatment time schedules (to 17.1, 16.7 and 14.7, respectively) as compared with the NNK control group (20.8, P < 0.05). Moreover, the tumor burden was reduced by the tocopherol treatment given during the tumor initiation stage or during the entire experimental period (36 or 43% inhibition, respectively; P < 0.05). No lung tumors were found in the saline- or glycerol trioctanoate-treated control animals that did not receive carcinogen treatment.
Effects of γ-TmT on H1299 cell xenograft tumors in nude mice
The possible inhibitory effect of γ-TmT on the growth of H1299 human lung cancer cells was studied in a xenograft tumor model. The 0.3% γ-TmT diet was given to the mice 1 day after cancer cell implantation until the end of the experiment. No significant changes were observed in the body weight of treated mice as compared with the mice fed with control diet (data not shown). An inhibitory effect of γ-TmT was first observed 3 weeks after implantation of the cancer cells. After 6 weeks, tumor size and tumor weight were significantly reduced (Figure 1B). As compared with the control group, the tumor size was decreased by 56%, and the tumor weight was reduced by 47%.
Effects of γ-TmT on cell apoptosis and proliferation in the A/J mouse model and xenograft tumor model
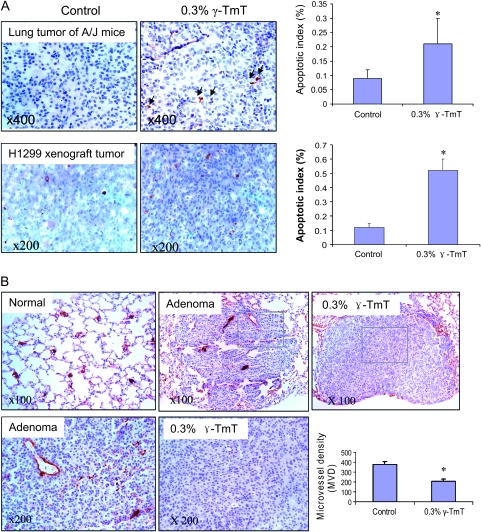
Cell apoptosis was detected by immunohistochemistry with an antibody against cleaved caspase 3 (Figure 2A) and then analyzed by (Image-Pro system, Bethesda, MD). In the NNK plus B[a]P-treated control group, 0.09% tumor cells were positively stained (apoptotic index), and the dietary γ-TmT treatment significantly increased the apoptotic index to 0.25% (155% increase, P < 0.05). Similarly, a 3.3-fold increase in apoptotic index was observed in the H1299 xenograft tumors of mice treated with γ-TmT (0.13% in the control diet group versus 0.55% in the γ-TmT treated group). Cell proliferation was measured by immunohistochemistry with anti-Ki-67 antibody in both the NNK plus B[a]P-induced tumors and the xenograft tumors. The percentage of Ki-67-positive cells was not significantly changed by γ-TmT treatment in both the carcinogenesis and xenograft models (data not shown).
Fig. 2.
Effects of γ-TmT on apoptosis and angiogenesis in NNK plus B[a]P-induced lung tumors and H1299 cell xenograft tumors. (A) Apoptosis was studied by immunohistochemistry with anti-caspase-3 antibody. Light photographs of caspase-3 staining in lung tumors of A/J mice and H1299 cell xenograft tumors (left panel). Bar graph showing that treatment with γ-TmT enhanced the apoptosis indexes in lung tumors and xenograft tumors (right panel). Data shown are mean ± SE from 50 lung tumors in each group of A/J mice or 10 xenograft tumors of each group; *P < 0.05 by Student’s t-test. (B) Immunohistochemical analysis of angiogenesis in lung adenomas with anti-CD31 antibody. CD-31-stained blood capillaries were visualized as brown color staining. Positively stained microvessels in lung adenomas are expressed as MVD, which is the number of the blood vessels per area of adenoma measured (blood vessels per square millimeter). Bar graph showing that treatment with γ-TmT reduced the MVD in lung tumors. Data shown are mean ± SE from 40 lung tumors in each group of A/J mice; *P < 0.05 by Student’s t-test.
Effects of γ-TmT on angiogenesis in the A/J mouse model
The formation of microvessels in lung adenomas was analyzed immunohistochemically using anti-endothelial cell CD31 antibodies. Morphologically, CD31-labeled capillary clusters and blood vessels were more frequently observed in the peripheral area of the adenoma than in the central area of the tumor (Figure 2B). Positively stained microvessels in lung adenomas are expressed as microvessel density (MVD), which is the number of blood vessels per area of adenoma measured (blood vessels per square millimeter). In the NNK plus B[a]P-treated control group, the MVD was 375. The dietary γ-TmT treatment significantly reduced the MVD to 208 (44.5% reduction, P < 0.05). In the xenograft tumors, we did not observe significant changes of MVD by treatment with γ-TmT, which might be due to the relatively large size and necrosis of the tumors caused by the relatively long time of tumor growth.
Effects of γ-TmT treatment on 8-OH-dG, γ-H2AX and nitrotyrosine levels
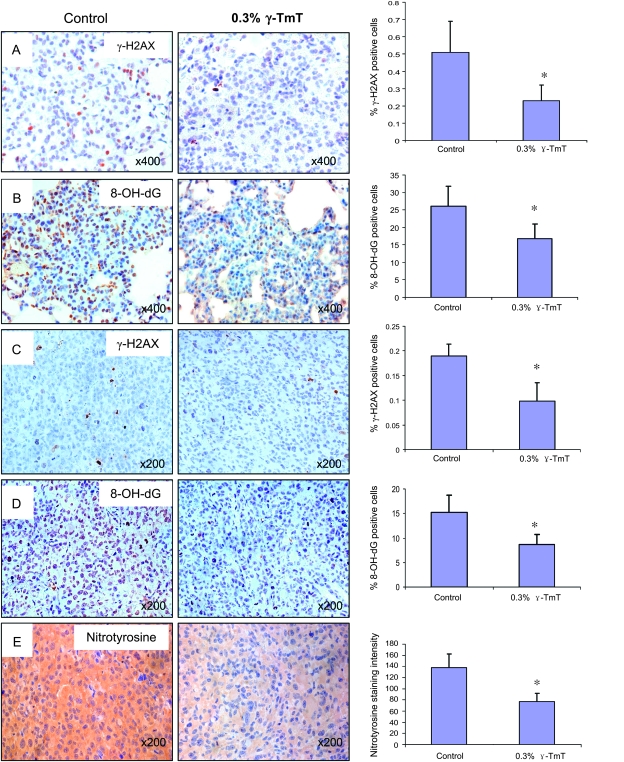
In order to study the effects of dietary γ-TmT treatment on reactive oxygen and nitrogen species, the levels of 8-OH-dG, γ-H2AX and nitrotyrosine were determined by immunohistochemistry and analyzed by Image-Pro system. As shown in Figure 3, the antibody against 8-OH-dG showed a strong nuclear staining in the NNK plus B[a]P-induced lung tumors. The percentage of 8-OH-dG-positive cells in these lung tumors was ∼26%, which was much higher than that of the normal lung tissues (9.5%). The treatment with γ-TmT significantly reduced the percentage of 8-OH-dG-positive cells in lung tumors to 16.8% (35% reduction, P < 0.05), but not in the normal lung tissues. The γ-H2AX staining, which reflects DNA double-strand break-induced DNA repair, also showed a nuclear staining pattern (Figure 3). Most of the γ-H2AX-positive cells were found in lung tumors, but very few normal lung tissue cells were stained. Similarly, treatment with γ-TmT significantly reduced the γ-H2AX-positive cells from 0.51% in the control group to 0.23% in the γ-TmT-treated group (55% reduction, P < 0.05). The nitrotyrosine-positive staining was found only in the cytoplasm of pulmonary macrophages and some type II cells, but not in lung tumor cells. There was no difference in nitrotyrosine staining found between the γ-TmT-treated and control groups.
Fig. 3.
Effects of γ-TmT treatment on 8-OH-dG (A and C), γ-H2AX (B and D) and nitrotyrosine (E) levels in lung tumors of A/J mice and xenofraft tumors. (left) Light photographs of immunohistochemistry with corresponding antibodies. (right) Bar graphs showing that treatment with γ-TmT reduced 8-OH-dG- and γ-H2AX-positive cell indexes and nitrotyrosine-positive intensity. Data shown are mean ± SE from 50 lung tumors in each group of A/J mice or 10 xenograft tumors from each group; *P < 0.05 by Student’s t-test.
The staining patterns of 8-OH-dG and γ-H2AX in the H1299 cell xenograft tumors were similar to those in the lung tumors of A/J mice as described above. Significant reductions in the levels of 8-OH-dG and γ-H2AX by the γ-TmT treatment were observed as compared with the tumors in mice on the control diet. Dietary γ-TmT treatment decreased the percentage of 8-OH-dG- and γ-H2AX-positive cells by 52 and 57%, respectively. Strong cytoplasm staining of nitrotyrosine was observed in xenograft tumor cells. The staining intensity was 44% lower in the tumors of mice treated with 0.3% γ-TmT compared with the control (Figure 3).
Effects of γ-TmT on plasma PGE2 and LTB4 levels
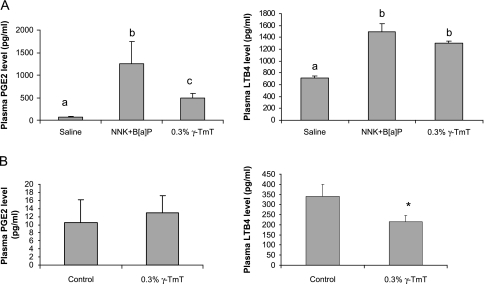
The plasma levels of PGE2 and LTB4 in the A/J mouse model and xenograft tumor model were assayed by EIA (Figure 4). The plasma PGE2 and LTB4 levels were significantly higher in tumor-bearing A/J mice compared with the mice without carcinogen treatment (21-fold and 51% higher, respectively; P < 0.05). In the tumor-bearing mice, the γ-TmT treatment resulted in lower plasma levels of PGE2 (by 61%, P < 0.05) and LTB4 (by 12.7%, P < 0.1). In the xenograft tumor model, γ-TmT significantly reduced the plasma LTB4 level to 63.5% of the control mice (P < 0.05). The plasma PGE2 levels of the thymus-deficient nude mice were very low, and no difference was found between control and γ-TmT treated mice.
Fig. 4.
Effects of γ-TmT on plasma PGE2 and LTB4 levels. (A) PGE2 and LTB4 levels in the plasma of NNK plus B[a]P-treated A/J mice. (B) PGE2 and LTB4 levels in the plasma of Ncr-nu/nu mice. The data are shown as mean ± SE. Different notations (a, b, c) indicate statistical significance by one-way analysis of variance (P < 0.05). *P < 0.05 by Student’s t-test.
Plasma and tumor levels of tocopherol
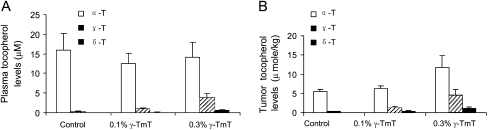
In a preliminary set of experiments, Ncr-nu/nu male mice were fed the AIN-93M diet or diet supplemented with 0.1 or 0.3% γ-TmT 1 week before the mice were injected with H1299 cells. γ-TmT, given at 0.3% in the diet for 7 weeks, inhibited the xenograft tumor growth (tumor size and tumor weight were 51 and 37% lower than the control groups), but 0.1% γ-TmT treatment did not (data not shown). The plasma and tumor levels of tocopherols were analyzed (Figure 5). In plasma, the γ-T levels were dose dependently increased by 0.1 and 0.3% γ-TmT diet (1.3- and 6.3-fold increase, respectively). A large increase was found in δ-T plasma levels after the treatment (14- and 107-fold increase, respectively), but α-T levels were not significantly altered by the dietary treatment. We also found that dietary treatment with γ-TmT dose dependently increased γ-T and δ-T levels in tumors. The α-T levels in tumors were also significantly increased by the 0.3% γ-TmT treatment (by 109%, P < 0.05). Assuming a volume of 1 ml for 1 g tumor tissue, the levels of α-, γ- and δ-T in the control group were 5.56, 0.36 and 0.08 μM and in the 0.3% γ-TmT group were 11.69, 4.59 and 1.07 μM, respectively.
Fig. 5.
Effect of γ-TmT on plasma and tumor tissue levels of tocopherols in Ncr-nu/nu mice. (A) Plasma tocopherol levels. (B) Tumor tissue tocopherol levels. The dietary γ-TmT treatment lasted for 7 weeks. Tocopherol levels were analyzed as described in Materials and Methods.
Inhibition of H1299 human lung cancer cell growth in culture by γ- and δ-tocopherol
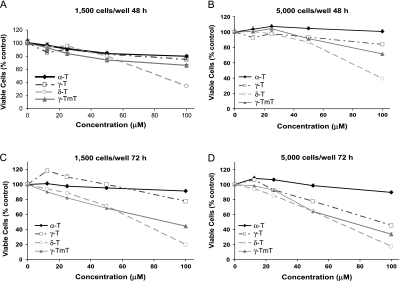
We studied the effect of three major tocopherol isoforms (α-, γ- and δ-T) and γ-TmT on the growth of H1299 human lung carcinoma cells. After 48 and 72 h treatment (5000 cells per well), α-T did not inhibit cell growth at all concentrations tested, but δ-T and γ-T dose dependently inhibited cell growth with δ-T having a stronger inhibitory effect than γ-T (Figure 6). γ-TmT also showed dose-dependent inhibition of cell growth, and the potency was lower than δ-T and higher than γ-T, except that at low concentrations (e.g. at 25 μM) with seeding of 1500 cells per well, γ-TmT appeared to be slightly more effective than δ-T and γ-T.
Fig. 6.
Effects of tocopherols on the growth of H1299 cells. (A–D) H1299 cells were seeded in 96 well plates, and after 24 h, cells were treated with different concentrations of tocopherols for 48 or 72 h. Viable cells were assayed by MTT as described in Materials and Methods. Data represent mean ± SE (n = 6). (A) 48 h treatment, 1500 cells per well (B) 48 h treatment, 5000 cells per well (C) 72 h treatment, 1500 cells per well (D) 72 h treatment, 5000 cells per well.
Discussion
The present study demonstrates the inhibitory effects of γ-TmT on chemically induced lung tumorigenesis in female A/J mice and the growth of human large-cell lung cancer H1299 cell xenograft tumors in NCr-nu/nu mice. To our knowledge, this is the first report on the inhibitory activities of a mixed tocopherol preparation against lung tumorigenesis in animals and tumor growth of human lung cancer cells in a xenograft model.
In this study, we found that γ-TmT given at the initiation stage reduced the tumor multiplicity and tumor burden. This result suggests that tocopherols can reduce the deleterious effects caused by carcinogen metabolism and can prevent the molecular or tissue damage by the carcinogens and their metabolites. For example, reactive oxygen species are generated in the lung by treatment with carcinogens, and tocopherols may act as antioxidants and scavenge these species (29). In a preliminary experiment, we have observed the protective effect of dietary γ-TmT (0.3%) against NNK or B[a]P-induced liver damage, which was reflected in elevated plasma alanine aminotransferase levels (data not shown). γ-TmT showed stronger effects in reducing tumor volume in the NNK plus B[a]P-induced lung tumorigenesis model as compared with the NNK-induced model. It appears that tissue tocopherols may be more effective at inhibiting the action of the eight smaller doses of carcinogen absorbed through the gastrointestinal tract than the two larger doses of NNK administered by intraperitoneal injection.
The tumor growth inhibitory effect of γ-TmT was also observed in the H1299 human lung cancer cell xenograft tumors. This result was consistent with a preliminary experiment we conducted under similar conditions, where 0.3% γ-TmT significantly decreased the tumor size and tumor weight by an average of 51 and 37%, respectively. Our group also found similar tumor inhibitory effects of γ-TmT in a syngeneic tumor model with CL-13 mouse lung adenocarcinoma cells implanted in the A/J mice, where dietary treatment with 0.1 and 0.3% γ-TmT for 7 weeks inhibited the tumor volume of CL-13 syngeneic tumors by 53.9 and 80.5%, respectively (26).
The antioxidant and anti-inflammatory effects of tocopherols have been well documented. In the present experiment, we have also observed a strong anti-oxidative effect of γ-TmT in both A/J mice and the xenograft model, as lower 8-OH-dG, γ-H2AX and nitrotyrosine levels were observed after γ-TmT treatment. The oxidative stress levels in tumor cells are generally higher than normal tissues due to oncogene activation, aberrant metabolism, mitochondrial dysfunction, inflammation and other factors (30,31). The reactive oxygen species generated in tumor cells can function as secondary messenger molecules to transduce receptor-initiated signaling cascades, including vascular endothelial growth factor (32,33), platelet-derived growth factor (34) and cytokines, such as transforming growth factor (35) and hepatocyte growth factor (36). These signaling pathways control diverse cellular events that promote tumor growth (37–40). Thus, inhibition of reactive oxygen species by γ-TmT could retard tumor growth. Reactive oxygen species can also stimulate angiogenesis (40,41), and the antioxidant activity of γ-TmT may also contribute to its antiangiogenic effect observed in the lung tumors of A/J mice.
The plasma levels of proinflammatory eicosanoids, PGE2 and LTB4 were also found to be reduced, suggesting an anti-inflammatory effect of γ-TmT. We also observed enhanced apoptosis in γ-TmT treated animals in both models. These events may be related to the lung tumor inhibition by γ-TmT. However, significant changes were not observed in the levels of apoptosis-related proteins, such as the Bcl-2 family members Bcl-2, Bax, Bcl-xl or p-Bad by western blotting in NNK and B[a]P-induced lung tumors in A/J mice (data not shown). We did not observe the effects of γ-TmT on tumor cell proliferation in vivo, as the percentage of Ki-67-positive cell was not found significantly changed by immunohistochemistry (data not shown). The levels of key signals in cell signaling pathways, such as p-Erk1/2, p-C-Jun, p-JNK, p-Akt, NF-kB and p-Stat3, were studied by western blotting, but significant changes were not observed by treatment of γ-TmT.
We found that δ-T and γ-T inhibited tumor cell growth dose dependently in vitro, whereas α-T showed no significant effect. Of the three pure tocopherol isoforms, δ-T showed the strongest inhibitory effect. Previous studies have suggested that mixtures of tocopherols are superior to a single tocopherol at inhibiting inflammation (42,43). The tumor inhibitory effects of γ-TmT have been reported in other cancer cell lines and animal studies (2,13,14,16). In our study, however, γ-TmT was not found to be more effective than δ-T in most of the experiments. The possible synergistic actions among tocopherols need to be further studied. It has been reported that γ-T can inhibit cell growth (44–46), induce apoptosis (44,47), inhibit COX-2 activities and inflammation (43,48,49) and modulate the function of peroxisome proliferators-activated receptor (PPAR-γ) functions (44). The present study suggests that δ-T may have even more interesting activities. More detailed studies are needed to further elucidate the mechanism of action of δ-T, γ-T and γ-TmT.
In summary, we have demonstrated the inhibitory activity of a mixed tocopherol preparation (γ-TmT) against the formation and growth of lung cancer. The inhibition is proposed to be due to the antioxidant activity of all the tocopherols, the ability to quench reactive nitrogen species by δ-T and γ-T and the inhibition of COX-2 by δ-T, γ-T and their metabolites (43). Based on this analysis, we propose that tocopherol mixtures are better cancer prevention agents than pure α-T. This hypothesis warrants further testing, especially in light of recent large-scale human trials with high doses of α-T that failed to demonstrate any cancer preventive effect (9,10). Some epidemiological studies have demonstrated a protective effect of dietary tocopherols against cancer (8,23,50); the presently used γ-TmT preparation, with a similar tocopherol ratio as in our diet, may be a promising agent for human cancer prevention and warrants further studies.
Funding
National Institutes of Health (CA122474 and CA 133021); shared facilities supported by Cancer Center Support Grant (CCSG CA72720) and Center Grant (ES 05022).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- B[a]P
benzo[a]pyrene
- T
tocopherol
- γ-TmT
γ-tocopherol-rich mixture of tocopherols
- PGE2
prostaglandin E2
- LTB4
leukotriene B4
- 8-OH-dG
8-hydroxydeoxyguanine
- EIA
enzyme immunoassay
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MVD
microvessel density
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Ju J, et al. A gamma-tocopherol-rich mixture of tocopherols inhibits colon inflammation and carcinogenesis in azoxymethane and dextran sulfate sodium-treated mice. Cancer Prev. Res. (Phila Pa) 2009;2:143–152. doi: 10.1158/1940-6207.CAPR-08-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich M, et al. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J. Am. Coll. Nutr. 2006;25:292–299. doi: 10.1080/07315724.2006.10719538. [DOI] [PubMed] [Google Scholar]
- 4.Hensley K, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Traber MG. Vitamin E. Modern Nutrition in Health and Disease, 10th ed. Baltimore, MD: Lippincott Williams and Wilkins; 2006. pp. 396–411. [Google Scholar]
- 6.Traber MG, et al. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eitenmiller T, et al. Vitamin E: Food Chemistry, Composition, and Analysis. New York, NY: Marcel Dekker, Inc.; 2004. [Google Scholar]
- 8.Ju J, et al. Cancer preventive activities of tocopherols and tocotrienols. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp205. Advanced access published September 11, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lippman SM, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaziano JM, et al. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell S, et al. Development of gamma (gamma)-tocopherol as a colorectal cancer chemopreventive agent. Crit. Rev. Oncol. Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- 12.Reiter E, et al. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newmark HL, et al. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr. Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 14.Suh N, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr. Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 15.Lee HJ, et al. Mixed tocopherols prevent mammary tumorigenesis by inhibiting estrogen action and activating PPAR-g. Clin. Cancer Res. 2009;15:4242–4249. doi: 10.1158/1078-0432.CCR-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barve A, et al. Gamma-tocopherol-enriched mixed tocopherol diet inhibits prostate carcinogenesis in TRAMP mice. Int. J. Cancer. 2009;124:1693–1699. doi: 10.1002/ijc.24106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comstock GW, et al. The risk of developing lung cancer associated with antioxidants in the blood: ascorbic acid, carotenoids, alpha-tocopherol, selenium, and total peroxyl radical absorbing capacity. Cancer Epidemiol. Biomarkers Prev. 1997;6:907–916. [PubMed] [Google Scholar]
- 18.Goodman GE, et al. The association between lung and prostate cancer risk, and serum micronutrients: results and lessons learned from beta-carotene and retinol efficacy trial. Cancer Epidemiol. Biomarkers Prev. 2003;12:518–526. [PubMed] [Google Scholar]
- 19.Menkes MS, et al. Serum beta-carotene, vitamins A and E, selenium, and the risk of lung cancer. N. Engl. J. Med. 1986;315:1250–1254. doi: 10.1056/NEJM198611133152003. [DOI] [PubMed] [Google Scholar]
- 20.Ratnasinghe D, et al. Serum tocopherols, selenium and lung cancer risk among tin miners in China. Cancer Causes Control. 2000;11:129–135. doi: 10.1023/a:1008977320811. [DOI] [PubMed] [Google Scholar]
- 21.Shibata A, et al. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br. J. Cancer. 1992;66:673–679. doi: 10.1038/bjc.1992.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woodson K, et al. Association between alcohol and lung cancer in the alpha-tocopherol, beta-carotene cancer prevention study in Finland. Cancer Causes Control. 1999;10:219–226. doi: 10.1023/a:1008911624785. [DOI] [PubMed] [Google Scholar]
- 23.Yong LC, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1997;146:231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 24.Albanes D, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J. Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 25.Ogasawara M, et al. Differential effects of antioxidants on the in vitro invasion, growth and lung metastasis of murine colon cancer cells. Biol. Pharm. Bull. 2007;30:200–204. doi: 10.1248/bpb.30.200. [DOI] [PubMed] [Google Scholar]
- 26.Lambert JD, et al. Inhibition of lung cancer growth in mice by dietary mixed tocopherols. Mol. Nutr. Food Res. 2009;53:1030–1035. doi: 10.1002/mnfr.200800438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belinsky SA, et al. Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in the A/J mouse. Cancer Res. 1992;52:3164–3173. [PubMed] [Google Scholar]
- 28.Yang G, et al. Characterization of early pulmonary hyperproliferation and tumor progression and their inhibition by black tea in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model with A/J mice. Cancer Res. 1997;57:1889–1894. [PubMed] [Google Scholar]
- 29.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 30.Szatrowski TP, et al. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 31.Kawanishi S, et al. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol. Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 32.Pani G, et al. Determination of intracellular reactive oxygen species as function of cell density. Methods Enzymol. 2002;352:91–100. doi: 10.1016/s0076-6879(02)52010-3. [DOI] [PubMed] [Google Scholar]
- 33.Colavitti R, et al. Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J. Biol. Chem. 2002;277:3101–3108. doi: 10.1074/jbc.M107711200. [DOI] [PubMed] [Google Scholar]
- 34.Choi MH, et al. Regulation of PDGF signalling and vascular remodelling by peroxiredoxin II. Nature. 2005;435:347–353. doi: 10.1038/nature03587. [DOI] [PubMed] [Google Scholar]
- 35.Rhyu DY, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 36.Arakaki N, et al. Involvement of oxidative stress in tumor cytotoxic activity of hepatocyte growth factor/scatter factor. J. Biol. Chem. 1999;274:13541–13546. doi: 10.1074/jbc.274.19.13541. [DOI] [PubMed] [Google Scholar]
- 37.Boonstra J, et al. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 38.Behrend L, et al. Reactive oxygen species in oncogenic transformation. Biochem. Soc. Trans. 2003;31((pt. 6),):1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- 39.Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- 40.Ushio-Fukai M, et al. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol. Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 41.Ushio-Fukai M, et al. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devaraj S, et al. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Q, et al. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl Acad. Sci. USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell SE, et al. Gamma (gamma) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (gamma) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gysin R, et al. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 46.Samandari E, et al. The effect of gamma-tocopherol on proliferation, integrin expression, adhesion, and migration of human glioma cells. Biochem. Biophys. Res. Commun. 2006;342:1329–1333. doi: 10.1016/j.bbrc.2006.02.110. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Q, et al. Gamma-tocopherol induces apoptosis in androgen-responsive LNCaP prostate cancer cells via caspase-dependent and independent mechanisms. Ann. N. Y. Acad. Sci. 2004;1031:399–400. doi: 10.1196/annals.1331.056. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q, et al. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 49.O’Leary KA, et al. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Mahabir S, et al. Dietary alpha-, beta-, gamma- and delta-tocopherols in lung cancer risk. Int. J. Cancer. 2008;123:1173–1180. doi: 10.1002/ijc.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]