Abstract
Cyclooxygenase-2 (COX-2), a key enzyme of prostanoid biosynthesis, plays an important role in both hereditary and spontaneous colon cancer. Individuals with ulcerative colitis are also at high risk for colorectal cancer. To investigate the role of Cox-2 in colitis-associated colon cancer, we subjected Cox-2 luciferase-knock-in mice and Cox-2-knockout mice to a well-known mouse model of colitis-associated cancer in which animals are treated with a single-azoxymethane (AOM) injection followed by dextran sulfate sodium (DSS) administration. Tumors induced by AOM and DSS expressed significantly higher Cox-2 levels when compared with surrounding areas of colon, as detected both by luciferase reporter gene expression driven from the endogenous Cox-2 promoter and by western blotting of COX-2 protein in Cox-2 luciferase heterozygous knock-in mice. Immunofluorescence revealed that tumor stromal fibroblasts, macrophages and endothelial cells express COX-2 protein. In contrast, little COX-2 expression was observed in myofibroblasts or epithelial cells. Despite a significant elevation of COX-2 expression in AOM/DSS-induced colon tumors in wild-type mice, similar tumors developed in AOM/DSS-treated Cox-2−/−- and Cox-1−/−-knockout mice. These results indicate that cyclooxygenase-derived prostanoids are not major players in colitis-associated cancer. In contrast, tumor formation induced by multiple injections of AOM (with no DSS-induced colitis) did not occur in Cox-2−/−-knockout mice. Our data suggest that the mechanism of colorectal tumor promotion in colitis-associated cancer differs from the mechanism of tumor promotion for hereditary and sporadic colorectal cancer.
Introduction
Epidemiological studies indicate that regular administration of non-steroidal anti-inflammatory drugs (NSAIDs) reduces the risk of colon cancer (1,2). The target molecules of the NSAIDs are cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). These enzymes catalyze the conversion of arachidonic acid to prostaglandin H2, the common intermediate for the synthesis of the various prostanoids (3). COX-1 is constitutively expressed in most tissues, whereas COX-2 expression is induced in response to a wide range of physiological inducers (4). Although a role for COX-1 in inflammation, cancer and various neurodegenerative diseases is also probably to exist, exaggerated COX-2 expression plays a major modulatory role in many pathological conditions.
Human colorectal cancer is one of the most common fatal malignancies. Colon cancer can be classified by etiology in three groups: sporadic, inherited, including familial adenomatous polyposis, and inflammatory. Inflammation is recognized as an important causal factor for many types of cancer (5,6). Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis, significantly increases the risk of colon cancer. Colitis-associated colorectal cancer accounts for up to 5% of all colorectal cancers. The cumulative incidence of colitis-associated cancer in ulcerative colitis patients 25–30 years after diagnosis ranges from 8–43% (7). Crohn's disease patients also have a 40-fold excess risk for small bowel cancer (8).
COX-2 is significantly overexpressed in variety of tumors, including colon cancer. Pharmacologic and genetic elimination of COX-2 activity reduces the progression of spontaneous and hereditary non-inflammatory intestinal cancer, both in animal models and in patients (9). Deletion of the Cox-2 gene in Apc (adenomatous polyposis coli) gene-mutant mice, a model for human familial adenomatous polyposis, results in significant reduction in both the number and the size of polyps (10). Numerous studies have also demonstrated that NSAIDs and COX-2-specific inhibitors suppress intestinal polyp formation in Apc-knockout mice, in a chemical carcinogen-induced tumor model and in xenograft colon cancer cell growth (11). The results of animal experiments demonstrating suppression of tumor formation in Apc-mutant mice (10) were confirmed in a clinical trial of familial adenomatous polyposis patients treated with COX-2 inhibitors (12).
Oral administration of a dextran sulfate sodium (DSS) solution to rodents is a widely used IBD model (13). DSS dissolved in drinking water is toxic to the epithelial lining of the mouse colon, resulting in severe colitis characterized by body weight loss and bloody diarrhea. Colitis-associated cancer development in the DSS colitis model typically requires long exposure periods or many cycles of DSS administration, and the incidence is relatively low. Inflammation-associated reactive oxygen and nitrogen species have been proposed to damage DNA and promote tumorigenesis (14). These steps can be accelerated in an animal model that combines a single exposure to the mutagen azoxymethane (AOM) with subsequent DSS administration, resulting in an efficient animal model for colitis-associated carcinogenesis (15,16).
NSAIDs and COX-2-specific inhibitors exacerbate DSS-induced colon injury in rodent models (17). Cox-2-knockout mice exposed to DSS have exacerbated weight loss and clinical symptoms (18). Although there are contradictory reports, clinical data also suggest that treatment with COX-2 inhibitors is associated with a high incidence of exacerbation of the underlying IBD (19). These data suggest that endogenous COX-2 activity plays a beneficial, rather than a proinflammatory, role in colitis. COX-2 is also expressed in neoplastic lesions from ulcerative colitis patients (20) and AOM/DSS-induced colon tumors (16). Although NSAIDs and COX-2 inhibitors exacerbate DSS-induced colon inflammation, they have been reported to reduce colon cancer if administered subsequent to inflammatory promotion of initiated colorectal cancer precursor cells; despite exacerbation of DSS-induced inflammation, nimesulide (a preferential COX-2 inhibitor) is reported to suppress both the incidence and multiplicity of subsequent colon tumors (21,22). These data are also supported by clinical observations; NSAID therapy is reported to reduce colitis-associated colorectal cancer risk of by 75–81% (7). In this study, we used Cox-2−/− and Cox-1−/−-knockout mice to clarify the role of cyclooxygenases in colitis-associated tumors. Colitis-associated tumors induced by AOM/DSS can fully develop without either COX-1 or COX-2 expression.
Materials and methods
Mice
Mice carrying a knock-in allele of the firefly luciferase-coding region in the Cox-2 gene (Cox-2luc) and mice in which we knocked out the Cox-2 gene were created in our laboratory (23). Commercially available Cox-1- and Cox-2-knockout mice were obtained from Taconic (Hudson, NY). Animal experiments were carried out with Animal Research Committee approval at University of California, Los Angeles.
Mouse models of colon cancer
For the colitis-associated colon cancer model, mice were injected intraperitoneally with 10 mg/kg of AOM (Sigma, St Louis, MO) in phosphate-buffered saline. One week later, 2% DSS (MP biomedicals, Solon, OH) was given in the drinking water for 7 days. The mice were killed at 12 weeks after injection of AOM.
For the non-inflammatory ‘spontaneous’ colon cancer model, mice were injected intraperitoneally with 10 mg/kg of AOM in phosphate-buffered saline once a week for 6 weeks (15,16). Mice were killed 20 weeks after the first AOM injection.
For macroscopic and histological examination of tumors, the colons were isolated, rinsed with phosphate-buffered saline, filled with 4% paraformaldehyde and then opened longitudinally. Tumors were counted and size of each tumor was determined using a dissection microscope.
Detection of luciferase activity
For ex vivo colon imaging, mice were anesthetized by intraperitoneal administration of a ketamine (80 mg/kg, Phoenix Pharmaceutical, St Joseph, MO) and xylazine (4 mg/kg, Phoenix Pharmaceutical) mixture. Anesthetized mice were injected intraperitoneally with D-luciferin (125 mg/kg, Caliper Life Sciences, Hopkinton, MA) and placed in the light-tight box of the IVIS imaging system (Caliper Life Sciences). Whole body 1 minute images were acquired repeatedly. After the photon number during the 1 minute scans reached a maximum, the mice were killed and the colons were rapidly excised. Isolated colons were placed on culture dishes and imaged with the IVIS system. Collected photon number and images were analyzed using LIVING IMAGE software (Caliper Life Sciences). Tumors and normal colon tissue samples were then homogenized in passive lysis buffer (Promega, Madison, WI) and assayed using the Luciferase Assay System (Promega). Relative light units for each sample were normalized by lysate protein concentration (Bio-Rad, Hercules, CA).
Western immunoblotting
Portions of the lysates used for luciferase assays were separated in 10% sodium dodecyl sulfate–polyacrylamide gels and transferred to nitrocellulose membranes (100 μg total protein per lane). COX-2 was detected with polyclonal anti-murine COX-2 antibody (Cayman Chemical, Ann Arbor, MI); GAPDH was detected with polyclonal anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA). The enhanced chemiluminescence detection system (Thermo Scientific, Rockford, IL) was used to visualize the signals on X-ray film.
Histology and immunohistochemistry
Mouse colon tissues were fixed in 4% paraformaldehyde, paraffin-embedded and sectioned at 4 μm thickness. These sections were stained with hematoxylin and eosin or processed for immunostaining. COX-2 was detected with polyclonal anti-COX-2 antibody (Thermo Scientific). To detect macrophages, rat monoclonal antibody for F4/80 (Serotec, Oxford, UK) was used. To detect fibroblasts, goat anti-vimentin antibody (Sigma) was used. To detect endothelial cells, rat anti-mouse CD34 (BD Pharmingen, Franklin Lakes, NJ) was used. Staining signals were visualized by using Alexa Fluor 594- or Alexa Fluor 488-conjugated secondary antibodies (Molecular Probes, Eugene, OR). To detect myofibroblasts, mouse monoclonal anti-α-smooth muscle actin Cy-3 conjugated (Sigma) was used. To detect epithelial cells, monoclonal antibody for pan-keratin conjugated with Alexa Fluor 488 (Cell Signaling Technology, Danvers, MA) was used.
Results
COX-2 is expressed in AOM/DSS-induced colon tumors
Although COX-2 expression is normally expressed at low levels in colon tissue, significant elevation of COX-2 protein expression was detected by immunohistochemistry in AOM/DSS-induced (colitis-associated) tumors (16,21). We previously generated a Cox-2 luciferase-knock-in allele, Cox-2luc, in which the firefly luciferase reporter enzyme is expressed at the start site of translation of the endogenous Cox-2 gene, to analyze Cox-2 transcriptional activity in mouse (23). A single intraperitoneal AOM administration (10 mg/kg) and 1 week of DSS treatment (2% in the drinking water) induced development of multiple tumors in the middle to distal colon of Cox-2luc/+ heterozygous knock-in mice (Figure 1A). Tumor burden is clearly visualized by ex vivo imaging of luciferase activity (Figure 1B). Quantification of luciferase activity from the images indicates significantly higher luciferase signals from tumors when compared with normal areas of colon in the same mouse (Figure 1C). Luciferase enzymatic activity was then measured by conventional luciferase assay in lysates from each tumor or normal colon (Figure 1D). The same lysates were also analyzed for COX-2 protein expression by western blotting (Figure 1E). Substantial COX-2 protein expression is present in all tumor samples; in contrast, COX-2 expression in normal colon samples is absent or barely detectable.
Fig. 1.
COX-2 and luciferase expression in AOM/DSS-induced Cox-2luc/+ colon tumors. (A and B) Ex vivo imaging of luciferase expression in the colon of a Cox-2luc/+ mouse 12 weeks after AOM injection. Photo (A) shows a fixed opened colon with tumors. The color overlay on the image (B) illustrates the photons per second emitted from tissue, as shown in pseudocolor scales next to the image. (C) Quantification of luciferase activity determined by bioluminescent imaging. A region of interest (ROI) was manually selected on each tumor and each normal area. The area of the ROI was kept constant. Average photon number was measured. (D) Luciferase activity, measured by in vitro luciferase assay of tissue lysates, in tumors and normal colon regions. The same tissues analyzed in (C) were homogenized and lysates were assayed. (E) Western blot analysis of COX-2 protein expression in tumors and normal areas of the colon. Lysates used for luciferase assay in (D) were also used to detect COX-2 protein. GAPDH is a loading control.
Strong COX-2 immunohistochemical staining has been shown in stromal cells of AOM/DSS-induced mouse tumors (16,21); macrophages, fibroblasts and neutrophils are reported to express COX-2 in AOM/DSS-induced tumors (24). To determine the cell types that express COX-2 in our experiments, double staining of COX-2 with cell type-specific markers was performed on AOM/DSS-induced tumors from wild-type mice (Figure 2). Tumor sections were stained for COX-2 with F4/80, a representative macrophage marker and with vimentin, a fibroblast marker. As expected, double-stained cells were detected both for F4/80 (Figure 2A) and vimentin (Figure 2B). To examine whether COX-2 expression occurs in stromal myofibroblasts, a subclass of fibroblasts, sections were stained for COX-2 and α-smooth muscle actin. Only a very small population of cells is double stained, indicating myofibroblasts are not the fibroblast subclass that expresses COX-2.
Fig. 2.
Immunofluorescent detection of COX-2-expressing cells. Dual-color double-staining immunofluorescent analyses of proteins expressed in AOM–DSS-induced colorectal tumors from wild-type mice, performed with anti-COX-2 and antibody to cell type-specific marker proteins; (A) anti-F4/80 for macrophage, (B) anti-vimentin for fibroblasts, (C) anti-α-smooth muscle actin for myofibroblasts, (D) pan-keratin antibody for epithelial cells and (E) anti-CD34 for endothelial cells. For each row, the two antibodies used for immunofluorescence are named in the right panel; the color of the antibody staining is indicated by the color of the font.
Epithelial cells express COX-2 in many human colon cancers (25). However, double staining with a pan-keratin epithelial cell marker antibody and anti-COX-2 antibody demonstrates that epithelial cells are not the main cell type expressing COX-2 in AOM/DSS-induced tumors (Figure 2D), only a small percentage of pan-keratin staining cells are also stained with anti-COX-2 antibody. Endothelial cells are reported to express COX-2 in Apc+/−-knockout polyps (26) Endothelial cells in AOM/DSS-induced colorectal tumors also express COX-2 (Figure 2E). We find that COX-2-expressing cells in AOM/DSS-induced tumors are mainly macrophages, fibroblasts and endothelial cells, with occasional expression in myofibroblasts and endothelial cells.
AOM/DSS treatment induces tumors in Cox-2−/−-knockout mice
To clarify the role of COX-2 in colitis-associated colon cancer, 10 Cox-2−/−-knockout mice (27) and 10 wild-type controls were treated with AOM and DSS (Figure 3). During the course of AOM and DSS treatment, Cox-2−/− mice lost body weight and 6 mice died (Figure 3A) because of the exacerbated colitis caused by DSS in Cox-2−/− mice (18). Twelve weeks after AOM injection, surviving mice were analyzed for colon tumor formation. All four surviving Cox-2−/− mice, like wild-type mice, develop multiple colon tumors (Figure 3B).
Fig. 3.
AOM/DSS-induced colon tumor formation in Cox-2−/−-knockout mice. (A) Viability of mice following AOM and DSS administration. (B) Numbers of tumors per mouse in Cox-2−/−-knockout and Cox-2+/+ wild-type mice. Vertical bars indicate the mean tumor number and SE. (C) Histological sections of tumors in wild-type Cox-2+/+ (left panels) and Cox-2−/−-knockout (right panels) mice.
Cox-2 plays an important role in colorectal tumor formation in Apc-knockout mice; deleting the Cox-2 gene reduces the number of tumors. In addition, the tumors that do form in Apc+/−/Cox-2−/− mice differ morphologically from the tumors found in control mice (10). To examine the effects of Cox-2 deletion in AOM/DSS tumor histology, hematoxylin and eosin stained histology sections were compared. Tumors induced on Cox-2−/− mice are indistinguishable from tumors induced in control mice (Figure 3C).
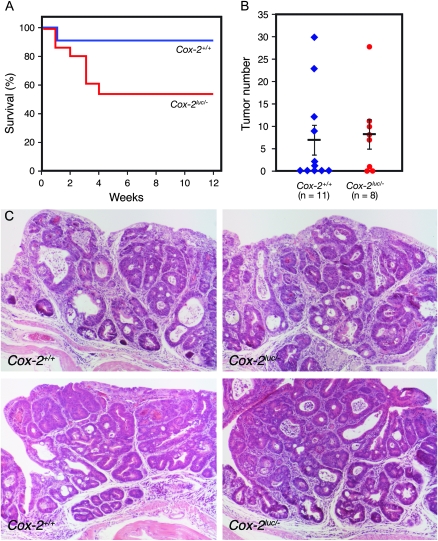
To confirm our observation that Cox-2 deletion does not prevent AOM/DSS induction of colorectal tumors, we increased the number of Cox-2-knockout mice utilized in a second AOM/DSS tumor induction experiment. In this experiment, knockout Cox2luc/− mice were generated from Cox-2luc/+ and Cox-2+/− mice (23). Eight of 15 Cox-2luc/−-knockout mice survived (Figure 4A). Although the number of tumors varied in individual mice (0–30), 6 of 11 (54.5%) Cox-2+/+ control mice developed tumors (Figure 4B). Cox-2luc/− mice developed numbers of tumors (0–28) comparable with Cox-2+/+ mice. The incidence of tumor formation in surviving Cox2luc/− mice was 75.0% (six of eight, Figure 4B). Histological analysis also showed no difference between control Cox-2+/+ and Cox-2luc/− mice (Figure 4C).
Fig. 4.
AOM/DSS-induced colon tumor formation in Cox-2luc/−-knockout mice. (A) Viability of mice following AOM and DSS administration. (B) Numbers of tumors per mouse in Cox-2luc/−-knockout and Cox2+/+ wild-type mice. Vertical bars indicate the mean tumor number and SE. (C) Histological sections of tumors in wild-type Cox-2+/+ (left panels) and Cox-2luc/−-knockout (right panels) mice.
AOM/DSS treatment induces tumors in Cox-1−/−-knockout mice
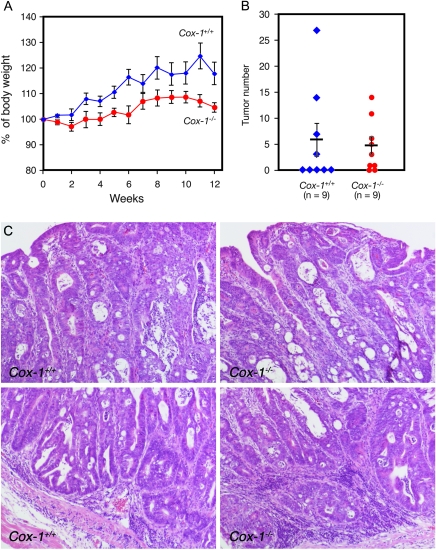
COX-1 is the constitutively expressed cyclooxygenase isozyme. In addition to a homeostatic function for the gastrointestinal mucosa, COX-1 also plays an important role in tumor formation in the ApcMin mouse (28); deletion of the Cox-1 gene also reduces intestinal polyp formation in ApcMin mice. Furthermore, nimesulide, which inhibits both COX-2 and COX-1, suppresses the colitis-associated tumor formation in the AOM/DSS mouse model (21,22), suggesting a possible role of COX-1 in this type of tumor formation. Therefore, we investigated the effects of Cox-1 gene deletion (29) on AOM/DSS-induced tumor formation. Cox-1−/−-knockout mice also show higher susceptibility to DSS-induced colitis (18), reflected in differences of body weight during the 12 weeks of tumor development (Figure 5A). However, all nine Cox-1−/− mice survived. Four of the nine (44.4%) Cox-1+/+ control mice developed (3–28) tumors (Figure 5B). Cox-1−/− mice also developed (1–15) tumors, with 77.7% incidence (seven out of nine mice). The morphology of AOM/DSS-induced tumors in Cox-1−/− mice and AOM/DSS-induced tumors in Cox-1+/+ mice is indistinguishable (Figure 5C).
Fig. 5.
AOM/DSS-induced colon tumor formation in Cox-1−/−-knockout mice. (A) Body weight of mice following AOM and DSS administration. (B) Numbers of tumors per mouse in Cox-1−/−-knockout and Cox1+/+ wild-type mice. Vertical bars indicate the mean tumor number and SE. (C) Histological sections of tumors in wild-type Cox-1+/+ (left panels) and Cox-1−/−-knockout (right panels) mice.
Repeated AOM administration cannot induce tumors in Cox-2−/− mice
The results in the previous sections suggest that inflammation caused by DSS is sufficient to promote tumor formation without COX-2 or COX-1 expression, in contrast to a requirement for COX-1 and COX-2 expression in intestinal tumor formation in Apcmin mice, where much less inflammation occurs. Although AOM is predominantly a tumor-initiating agent, AOM tumor-promoting activity has also been reported (30). Thus, repeated AOM injection causes highly efficient induction of colon tumors in mice; this protocol has been described as a ‘sporadic’ and spontaneous rodent model of colon tumor development (15). Nine Cox-2−/− and nine control Cox-2+/+ mice were injected with AOM every week for 6 weeks. Twenty weeks after the first injection, mice were examined for tumor formation (Figure 6). In wild-type mice, COX-2 expression was observed in the stromal areas of AOM-induced colon tumors (Figure 6A). Six of nine Cox-2+/+ control mice developed tumors; tumor frequency ranged from 1–13 (Figure 6B). In contrast, no tumors were detected in Cox-2−/− mice. These data suggest that COX-2 plays a causal role in colorectal cancer development in the AOM tumor induction protocol.
Fig. 6.
Colon tumor formation in Cox-2-knockout mice in response to repeated AOM injection. (A) Immunohistochemical detection of COX-2 expression in a tumor induced by repeated AOM injection. (B) Numbers of tumors per mouse in Cox-2−/−-knockout and Cox-2+/+ control mice. Vertical bars indicate the mean polyp number and SE.
Discussion
Oral DSS administration is a widely used model of human IBD. Although repeated oral DSS ingestion alone can cause colon tumorigenesis, DSS-induced inflammation is suggested to act mainly as a tumor promoter rather than as an initiator (5). AOM is frequently used to initiate tumors by alkylation of DNA. Prior AOM administration accelerates and increases the incidence of DSS-induced tumor formation. To study the consequences of disruption of the Cox-2 and Cox-1 genes on inflammation-mediated colorectal cancer, we utilized a two-stage colon tumor model to mimic colitis-driven tumor development (16). This model utilizes a single initiating AOM injection and a single DSS 7 day cycle to promote colorectal tumor formation.
COX-2 protein is constitutively expressed in a variety of human epithelial cancers and mouse experimental tumors. In the two-stage AOM/DSS model, strong COX-2 expression was found by immunohistochemistry (16). Elevated COX-2 protein could be due to the transcriptional activation of the Cox-2 promoter, posttranscriptional stabilization of the COX-2 message and/or regulation of COX-2 messenger RNA translation (3,4). We previously generated a knock-in Cox-2luc mouse in which the coding region for a luciferase reporter gene is placed at the start site of the protein-coding region of the endogenous Cox-2 gene (23). Luciferase expression in Cox-2luc/+ mice reflects Cox-2 promoter activity; the Cox-2luc allele has an SV40 3′-untranslated regions instead of Cox-2 3′-untranslated regions that controls the posttranscriptional stability of the Cox-2 messenger RNA (31,32). Therefore, elevated luciferase activity in AOM/DSS-induced colon tumors is due to the constitutive activation of the Cox-2 promoter.
Although COX-2 protein is highly expressed in epithelial cells of human colon carcinomas, its expression is found in the stromal cells, rather than the epithelial cells, of benign colon polyps, adenomas and adenocarcinomas (33–35). In AOM/DSS-induced adenomas double immunostaining with marker antibodies revealed that COX-2-expressing cells are vimentin-positive fibroblasts, CD34-positive endothelial cells and F4/80-positive macrophages. In contrast, costaining with either the epithelial cell marker pan-keratin or the myofibroblast marker, α-smooth muscle actin overlapped to only a small degree with COX-2-positive cells. This pattern is similar to COX-2 expression in polyps of Apc-knockout mice, in which (i) epithelial cells generally do not express COX-2 and (ii) the primary COX-2-expressing cells are identified as fibroblasts, endothelial cells and macrophages (26).
COX-2 plays important roles in tumorigenesis of Apc-knockout mice (10). Tumor formation in Apc-knockout mice is suppressed by Cox-2 gene deletion or by the administration of selective COX-2 inhibitors. AOM/DSS-induced tumor formation is also reported to be suppressed by COX-2 preferential NSAIDs; e.g. nimesulide (21). Consequently, we anticipated that deletion of the Cox-2 gene would reduce AOM/DSS-induced colon tumor induction, as it does in the Apc-knockout mouse. However, despite similar COX-2 expression patterns in Apc-knockout tumors and AOM/DSS-induced colon tumors, AOM/DSS tumor formation was not suppressed by deletion of the Cox-2 gene.
Loss of the APC gene leads to the β-catenin stabilization and transcriptional activation of downstream genes. In AOM/DSS tumor formation, cytoplasmic β-catenin accumulation was observed (16), and mutations in the β-catenin gene were detected with high frequency (36). This similarity of what are considered to be cellular initiation events for tumor formation in Apc-knockout tumors and AOM/DSS-induced tumors suggests a difference in tumor promotion mechanisms between these two models. In the Apc-knockout mouse, COX-2 expression is induced locally in the polyp stromal cells, and COX-2-dependent prostaglandins support polyp cell growth through stromal angiogenesis, basement membrane biosynthesis or direct effects on adenoma epithelium (11). The causal role for COX-2 in the tumor promotion process, and not in the initiation step, is also suggested by transgenic mouse studies (37). Mice expressing a Cox-2 transgene specifically in colon epithelial cells do not develop tumors. However, carcinogen-treated Cox-2-transgenic mice have a higher tumor load. In the AOM/DSS model, tumor initiation is undoubtedly due to mutation caused by AOM, and initiated cells are promoted by DSS-induced inflammation. This inflammation step includes COX-2 expression. However, AOM/DSS-induced tumors can fully develop in Cox-2−/−-knockout mice, indicating COX-2—contrary to expectation—does ‘not’ play a crucial role in promoting tumor formation.
In addition to Cox-2, nuclear factor-kappaB (NF-κB) is another key gene in the inflammatory process and cancer development. NF-κB regulates the expression of a large number of cytokines and modulates the inflammatory processes in IBD. NF-κB also plays a procarcinogenic role in both myeloid cells and epithelial cells in the AOM/DSS model (38). NF-κB activation in intestinal epithelial cells promotes the survival of premalignant cells, and NF-κB-driven cytokine production by myeloid cells is important in tumor growth. IL-6, a multifunctional NF-κB-regulated cytokine, and its downstream effector STAT3 are important regulators of AOM/DSS-induced murine colon cancer (39,40). tumor necrosis factor-α is another NF-κB-regulated cytokine essential for AOM/DSS colitis-associated cancer (41). We suggest that, in AOM/DSS tumor promotion, the absence of Cox-2 in Cox-2−/− mice can be overridden by those proinflammatory cytokines driven by NF-κB. We suggest that, in contrast, COX-2-derived prostaglandins are essential for colorectal tumor promotion in inherited and sporadic colon cancer in which proinflammatory cytokines are not strongly expressed.
Toll-like receptors (TLRs) play an important role in host defense against microbial infection. TLRs also control intestinal epithelial homeostasis and protection from injury (42). TLR4 is overexpressed in colitis-associated cancer of human patients, and TLR4-deficient mice exhibit a significantly decreased incidence and size of colorectal tumors in the AOM/DSS model (24). COX-2, one of the downstream molecules induced by the activation of TLR4 (24,43), is proposed to mediate the proliferation of colitis-associated tumors (44). However, our results indicate other TLR4-downstream factors promote colorectal cancer formation. Activation of TLR4, transduced by an intracellular receptor complex that includes MyD88, activates a variety of signaling pathways including NF-κB. MyD88-knockout mice produce very low level of DSS-induced cytokines, such as tumor necrosis factor, IL-6 and KC-1 (42). These studies of TLR4 signaling also suggest that NF-κB-regulated cytokines can promote colitis-associated tumor formation.
Repeated AOM injection is presumed to lead to colorectal cancer by a pathway that employs the same (AOM) initiating event as AOM/DSS-induced colorectal cancer but is dependent on a distinct—and less florid inflammatory—promotion mechanism. In keeping with our hypothesis that COX-2-dependent prostanoid production plays a causal role in colon cancer progression when expression of other inflammatory mediators is less florid, induction of colorectal cancers by repeated AOM administration is suppressed in Cox-2−/−-knockout mice, indicating COX-2 plays an essential role in this sporadic spontaneous (15) model of colon tumor formation.
COX-1, the constitutive cyclooxygenase isoform, also plays a significant role in colon tumor formation. Like deletion of the Cox-2 gene, deletion of the Cox-1 gene in Apc-mutant mice reduces the number and size of intestinal polyps (28). In contrast to COX-2, which is induced only in polyps larger than 1 mm, COX-1 is found in polyps of all sizes, suggesting that COX-1 expression in stromal cells secures the basal levels of PGE2 required to support polyp growth to 1 mm in size (45). In the AOM/DSS colon cancer model, Cox-1 deletion (like Cox-2 deletion) did not reduce either the frequency of tumor induction or the size of tumors. These data support the suggestion that that cyclooxygenase dependent prostanoids are not major factors in promoting tumorigenesis in the AOM/DSS model.
In conclusion, COX-2 is strongly expressed in stromal cells in the AOM/DSS of mouse model of colitis-associated tumor; however, AOM/DSS tumor formation is neither COX-1 nor COX-2 dependent. Our results suggest that the stromal factors responsible for tumor promotion in colitis-associated colorectal cancer differ from the stromal factors that promote both inherited and sporadic colorectal cancer.
Funding
Crohn's and Colitis Foundation of America; Stop Cancer; National Institutes of Health/National Cancer Institute (R01 CA084572, R01 CA123055, P50 CA086306). H.H. is the P.I. for these awards.
Acknowledgments
We thank Art Catapang for technical assistance.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AOM
azoxymethane
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- DSS
dextran sulfate sodium
- IBD
inflammatory bowel disease
- NF-κB
nuclear factor-kappaB
- NSAID
non-steroidal anti-inflammatory drug
- TLR
toll-like receptor
References
- 1.Farrow DC, et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol. Biomarkers Prev. 1998;7:97–102. [PubMed] [Google Scholar]
- 2.Thun MJ, et al. Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 3.Herschman HR. Historical aspects of COX-2: cloning and characterization of the cDNA, protein and gene. In: Harris RE, editor. COX-2 Blockade in Cancer Prevention and Therapy. Clifton, NJ: Jumana Press; 2003. pp. 13–32. [Google Scholar]
- 4.Herschman HR. Regulation and function of prostaglandin synthase 2/cyclooxygenase. In: Curtis-Prior P, editor. The Eicosanoids. Vol. 4. Chichester, West Sussex, England: John Wiley & Sons, Ltd; 2004. pp. 43–52. [Google Scholar]
- 5.Karin M, et al. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 7.Eaden JA, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jess T, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am. J. Gastroenterol. 2007;102:829–836. doi: 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 9.Gupta RA, et al. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 10.Oshima M, et al. Suppression of intestinal polyposis in ApcD716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 11.Oshima M, et al. COX selectivity and animal models for colon cancer. Curr. Pharm. Des. 2002;8:1021–1034. doi: 10.2174/1381612023394953. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach G, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 13.Wirtz S, et al. Chemically induced mouse models of intestinal inflammation. Nat. Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 14.Meira LB, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J. Clin. Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neufert C, et al. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, et al. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter BK, et al. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J. Clin. Invest. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morteau O, et al. Impaired mucosal defense to acute colonic injury in mice lacking cyclooxygenase-1 or cyclooxygenase-2. J. Clin. Invest. 2000;105:469–478. doi: 10.1172/JCI6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guslandi M. Exacerbation of inflammatory bowel disease by nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors: fact or fiction? World J. Gastroenterol. 2006;12:1509–1510. doi: 10.3748/wjg.v12.i10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agoff SN, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. Am. J. Pathol. 2000;157:737–745. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohno H, et al. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T, et al. Therapeutic effect of nimesulide on colorectal carcinogenesis in experimental murine ulcerative colitis. J. Gastroenterol. Hepatol. 2007;22:1474–81. doi: 10.1111/j.1440-1746.2007.04866.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa T, et al. Imaging cyclooxygenase-2 (Cox-2) gene expression in living animals with a luciferase knock-in reporter gene. Mol. Imaging Biol. 2006;8:171–187. doi: 10.1007/s11307-006-0034-7. [DOI] [PubMed] [Google Scholar]
- 24.Fukata M, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sano H, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 26.Sonoshita M, et al. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res. 2002;62:6846–6849. [PubMed] [Google Scholar]
- 27.Morham SG, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 28.Chulada PC, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 29.Langenbach R, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 30.Bissahoyo A, et al. Azoxymethane is a genetic background-dependent colorectal tumor initiator and promoter in mice: effects of dose, route, and diet. Toxicol. Sci. 2005;88:340–345. doi: 10.1093/toxsci/kfi313. [DOI] [PubMed] [Google Scholar]
- 31.Dean JL, et al. The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell. Signal. 2004;16:1113–1121. doi: 10.1016/j.cellsig.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Espel E. The role of the AU-rich elements of mRNAs in controlling translation. Semin. Cell Dev. Biol. 2005;16:59–67. doi: 10.1016/j.semcdb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Adegboyega PA, et al. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin. Cancer Res. 2004;10:5870–5879. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 34.Chapple KS, et al. Localization of cyclooxygenase-2 in human sporadic colorectal adenomas. Am. J. Pathol. 2000;156:545–553. doi: 10.1016/S0002-9440(10)64759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bamba H, et al. High expression of cyclooxygenase-2 in macrophages of human colonic adenoma. Int. J. Cancer. 1999;83:470–475. doi: 10.1002/(sici)1097-0215(19991112)83:4<470::aid-ijc6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, et al. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26:229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 37.Al-Salihi MA, et al. Transgenic expression of cyclooxygenase-2 in mouse intestine epithelium is insufficient to initiate tumorigenesis but promotes tumor progression. Cancer Lett. 2009;273:225–232. doi: 10.1016/j.canlet.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greten FR, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bollrath J, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Popivanova BK, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J. Clin. Invest. 2008;118:560–570. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakoff-Nahoum S, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Fukata M, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–877. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukata M, et al. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takeda H, et al. Cooperation of cyclooxygenase 1 and cyclooxygenase 2 in intestinal polyposis. Cancer Res. 2003;63:4872–4877. [PubMed] [Google Scholar]