Abstract
Periodontal destruction is initiated by bacteria that stimulate host responses leading to excess production of cytokines. Anticytokine therapy for periodontal diseases especially targets proinflammatory cytokines, that is, TNF-α, IL-1, and IL-6, because these are essential for the initiation of the inflammatory immune reaction and are produced for prolonged periods in periodontitis. This therapy aims to bind the cytokines with the receptors present on target cells such as the fibroblasts. The three basic treatment strategies are: (1) neutralization of cytokines, (2) blockage of cytokine receptors, and (3) activation of anti-inflammatory pathways, such as, immune-suppressive pathways.
This new therapy can act as a host response modulator in the control of inflammatory diseases of gums and may provide the basis for new molecular therapeutic approaches to the treatment of periodontitis.
Keywords: IL-1, IL-6, proinflammatory cytokines, soluble receptors, TNF
INTRODUCTION
Periodontitis is an inflammatory disease fundamentally initiated by chronic bacterial infection.[1,2] Current data suggest that a small group of predominantly gram-negative anaerobic or microaerophilic bacteria within the biofilm are often associated with disease initiation and progression. The microbial challenge consisting of antigens, lipopolysaccharides, and other virulence factors stimulates host responses. Host reactions to these infecting agents result in the release of inflammatory mediators including proinflammatory cytokines (IL-1, IL-6, TNF-α) and prostaglandins (PGE2), which can promote extracellular matrix destruction in the gingiva and stimulate bone resorption[3] [Figure 1].
Figure 1.
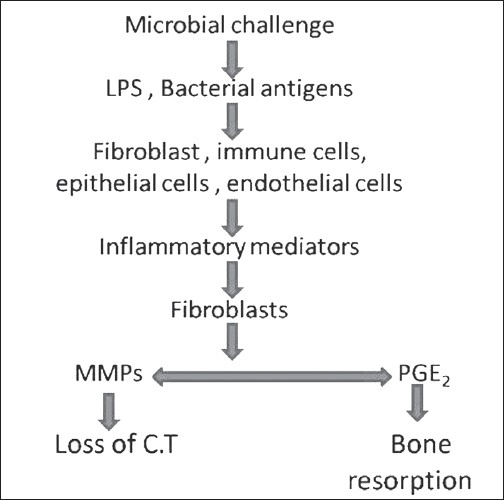
Model for pathogenesis of periodontitis
Although these immune and inflammatory host reactions are essential for host defense against bacterial inflammation, excessive and prolonged reaction is harmful for the functional periodontal tissue. Regulation of the immune and inflammatory reaction, in addition to controlling infection in the periodontal tissue, may be one of the methods to prevent and treat periodontal diseases.
Traditional periodontal treatment includes debridement of infectious matter and inflamed tissue. However, some situations where traditional therapy alone is not successful include:[4]
Patients with non-microbial risk factors, which are difficult to reduce or eliminate, such as, smoking, diabetes, and so on
Factors that are beyond the clinician's ability to control, such as, genetic predisposition
A specific group of periodontal disease susceptible individuals
Therefore, in these instances, the use of the anti-cytokine therapy, in conjunction with anti-biofilm treatments, may prove to be advantageous.
CYTOKINES
Cytokines are a category of signaling proteins and glycoproteins, and are used extensively in cellular communication.
NOMENCLATURE
Early names
Monokines and lymphokines (to describe source)
Now they are called just Cytokines.
Interleukin
A cytokine that is produced and acts on leukocytes. (now used generically)
Chemokine
Cytokine that induces chemotaxis. (Chemotactic cytokines)
CLASSIFICATION
Cytokines are classified as[5]
| FAMILY | MEMBERS |
|---|---|
| Chemotactic | IL-8, MIP-1, MCP-1, RANTES |
| Pro-inflammatory | IL-1α, IL-1β, TNF-α, IL-6 |
| Anti-inflammatory | IL-1Ra, IL-4, IL-10 |
| Growth factor | PDGF, EGF, FGF, IGF, VEGF |
| immunoregulatory | IFN-γ, IL-2, 4, 5, 7 |
MIP - macrophage inflammatory protein, MCP - monocyte chemotactic protein, RANTES - regulated upon activation, normal T cell expressed and secreted, IL-1Ra - interleukin 1 receptor antagonist, PDGF - platelet derived growth factor, EGF - epidermal growth factor, FGF - fibroblast growth factor, IGF - insulin-like growth factor, VEGF - vascular endothelial growth factor, IFN - interferon)
Anticytokine therapy for periodontal diseases mainly targets TNF-α, IL-1β, and IL-6, because they are essential for the initiation of inflammatory immune reactions and are produced for prolonged periods in inflammatory disease.
MECHANISM OF ACTION
The microbial challenge consisting of antigens, lipopolysaccharides, and other virulence factors, acts as an inducing stimulus. They stimulate cytokine producing cells. Upon stimulation, activation of the cytokine gene occurs and it releases cytokine in the solution. This cytokine binds the receptors present on the target cell. Upon binding, gene activation occurs in the target cell, which releases secondary mediators (i.e., MMPs and PGE2). These mediators are responsible for loss of connective tissue and bone resorption [Figure 2].
Figure 2.
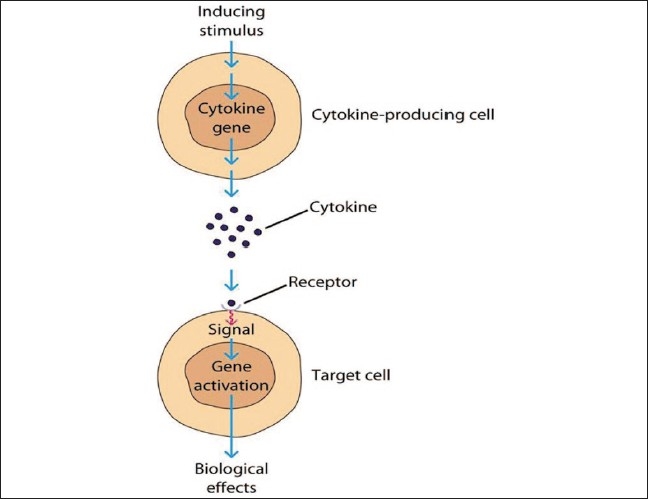
Mechanism of action of cytokines
MECHANISM FOR CYTOKINE-RECEPTOR INTERACTION
Cytokines may react with the receptor present on cell through different mechanisms [Figure 3]
Figure 3.
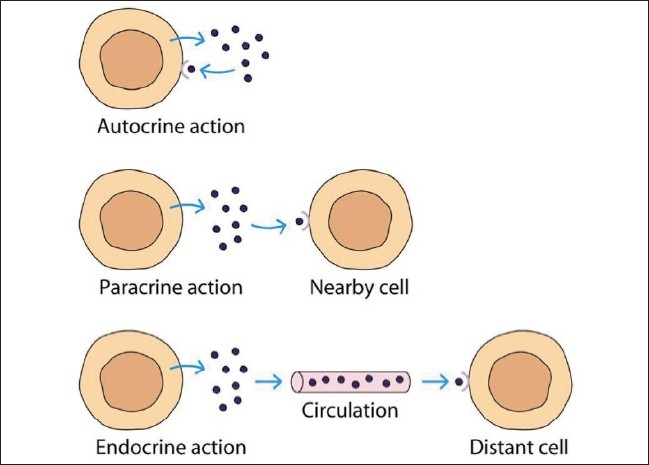
Mechanism for cytokine-receptor interaction
Autocrine — cytokine binds to the receptor on the cell of origin
Paracrine — cytokine binds to the receptor present on the adjacent cells
Endocrine — cytokine is released into circulation and can act at distant sites
Juxtacrine — membrane-bound cytokine that acts on receptors on either the same cell or adjacent cells
CYTOKINE RECEPTORS
The effects of cytokines are mediated by membrane-bound receptors, which are present on the surface of target cells.
| Cytokines | Memberne-bound receptors |
|---|---|
| IL-1β | IL-1 RI |
| IL-1RII | |
| (IL-1RAcP) | |
| TNF-α | TNF-RI |
| TNF-RII | |
| IL-6 | IL-6R |
| (gp130) |
Periodontal therapy for periodontal disease aimed at inhibiting the binding of cytokines to receptors is present on target cells such as fibroblasts.
DOWNREGULATION OF CYTOKINES
Downregulation of cytokines is mainly brought about by three mechanisms:
i. Cytokine receptor antagonists
Cytokine receptor antagonists bind to the receptor present on the target cell and prevent the cytokine from binding to the target cell. Therefore, there is no activation of the target cell.
Example: IL-1 receptor antagonist. (IL-1Ra) Production of IL-1Ra appears to play a role in regulating the intensity of inflammatory responses.
ii. Soluble cytokine receptors
Soluble cytokine receptors are derived from the proteolytic cleavage of the extracellular domain of cell-bound cytokine receptors. Soluble receptors can be found in blood and extracellular fluid.
The downregulation of cytokine is brought about by mainly two mechanisms
Downregulation — Soluble receptors bind to the cytokine in solution and prevent signaling.
Transactivation — Soluble receptors bind the cytokine and docks on otherwise non-responsive cells and activate them.
| Cytokines | Soluble receptors |
|---|---|
| IL-1β | sIL-1R |
| TNF-α | sTNF-RI |
| sTNF-RII | |
| IL-6 | sIL-6R |
Out of all these soluble receptors only sIL-6R is an agonist in function, the rest are all antagonist in function and bring about the downregulation of cytokines.
Anti-cytokine antibodies
Anti-cytokine antibodies are also antagonist in function and they lower down the levels of cytokines.
| Cytokines | Antibodies |
|---|---|
| TNF-α | Anti-TNF Ab |
| IL-6 | Anti-IL-6 Ab |
IMPLICATION FOR PERIODONTAL DISEASES
Rheumatoid arthritis is one of the best disease models suitable for anti-cytokine therapy. The principles of this strategy have been reviewed previously.[6] There are three basic therapeutic strategies:
Neutralization of cytokines
Blockage of cytokine receptors
Activation of anti-inflammatory pathways such as immunosuppressive pathways
COMMERCIALLY AVAILABLE PREPARATIONS
Infliximab (Remicade)
TNF-α is a special target molecule known for its neutralizing properties, therapeutics. Anti-TNF-α antibodies has effectively attenuated or prevented inflammation of arthritis in experiment models.[7]
Infliximab is a chimeric IgG monoclonal antibody. The term “chimeric” refers to the use of both mouse (murine) and human components of the drug. The drug also has been successfully used in:
Ankylosing spondylitis: 5 mg per kg
Crohn's disease: 5 mg per kg
Psoriatic arthritis: 5 mg per kg
Rheumatoid arthritis: 3 mg per kg
Psoriasis: 5 mg per kg
Etanercept (Enbrel)
TNF-α can also be neutralized with genetically engineered sTNF-α-RII.[8] Etanercept (enbrel) is a fusion protein. It links human soluble TNF receptor to the Fc component of human IgG1. It has been successfully used in some autoimmune diseases:
Ankylosing spondylitis
Juvenile rheumatoid arthritis
Psoriasis
Psoriatic arthritis
Rheumatoid arthritis
Anakinra (Kineret)
It is an interleukin-1 (IL-1) receptor antagonist. It competitively inhibits the binding of IL-1 to the Interleukin-1 type receptor.[9] Anakinra blocks the biological activity of naturally occurring IL-1, including inflammation and cartilage degradation.
It is used for the management of signs and symptoms of rheumatoid arthritis.
Several studies have been carried out for periodontal diseases and have shown the potential of using IL-1β and TNF-α antagonists, to reduce tissue destruction in periodontal diseases.[10–13] These researchers applied exogenous sIL-1RI and sTNF-RII to the gingival tissues of non-human primates with experimental periodontitis and found inhibition of inflammatory cell infiltration, alveolar bone loss, and loss of tissue attachment.
PHARMACOLOGICAL AGENTS STILL UNDER RESEARCH
There are certain pharmacological agents with potential host modulation action, but more studies are yet required toward their therapeutic use in treatment of periodontal diseases.
1. Recombinant human interleukin-11 (rhIL-11)
Interleukin 11 has been shown to have anti-inflammatory effects by inhibition of TNF-α and other proinflammatory cytokines.[14] IL-11 directly minimizes tissue injury through the stimulation of a tissue inhibitor of metalloproteinases-1 (TIMP-1).[15]
Based on these previous studies, Martuscelli et al. carried out a study using recombinant human interleukin-11 (rhIL-11) in the treatment of ligature-induced periodontitis, in dogs.[16] They found a significant reduction in the rate of clinical attachment loss and radiographic bone loss after an eight-week period of rhIL-11 administration, twice a week.
2. Disruption of cell signaling pathways
Strategies for preventing cell activation seek to inhibit the intracellular transduction of signals produced when ligands bind to their membrane receptors. Signal transduction pathways are mainly activated by cytokines, but also by other factors, such as, bacterial proteins, lipoproteins or environmental stress. Mitogen-activated protein kinase (MAPK) pathway is one of the signal transduction pathways closely involved in inflammation. MAPKs are divided into three families — the extracellular signal-regulated kinases (ERK1/2), c-jun N-terminal kinases (JNKs), and p38.
In recent years, the identification of proinflammatory signal transduction pathways has suggested new therapeutic targets. As these are shared by several cytokines, their inhibition will probably prove more powerful than the current treatment strategies.
i. Cytokine suppressive anti-inflammatory drugs (CSAIDS) / p38 inhibitors
The role for p38 MAPK, in various stages of inflammation, has prompted the production of several imidazole compounds capable of inhibiting p38 (RWJ 67657, VX- 745, and others). These inhibitors are called CSAIDs and are responsible for the in vitro and in vivo inhibition of LPS-induced TNF-α expression.[17]
In the experimental arthritis models, p38 inhibitors prevent the development of arthritis and bone erosions. Parasrampuria DA et al. tested RWJ 67657 in human volunteers.[18] After a single dose of RWJ 67657, the serum levels of the proinflammatory cytokines TNF-α, IL-6, and IL-8 were decreased by 90% compared with their plasma peak.
Kirkwood et al. showed that p38α selective mitogen activated the protein kinase inhibitor, which prevents periodontal bone loss in rats.[19]
ii. JNK inhibitors
The specific JNK inhibitor, SP600125, not only diminishes the production of TNF-α, interferon-γ, IL-6, COX-2, and matrix metalloproteinases, but also decreases the joint destruction in the adjuvant arthritis model.[20]
To date, no human trials have been initiated with these inhibitors. With JNK, it seems that both isoforms (JNK1 and JNK2) must be inhibited to produce an anti-inflammatory effect.
Resolvins
They are compounds that are made by the human body from the omega-3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Compounds derived from EPA are designated as Resolvins of the E series (RvE1), and those biosynthesized from DHA are denoted as Resolvins of the D series (RvD).
Resolvins stimulate the resolution of inflammation through multiple mechanisms, including preventing neutrophil penetration, phagocytosing apoptotic neutrophils to clear the lesion, and enhancing clearance of inflammation within the lesion to promote tissue regeneration.[21,22,23]
Hasturk et al.[24] showed that, in a rabbit model of human periodontal disease, RvE1 prevents the initiation and progression of tissue destruction.
These results support the hypothesis that both EPA- and DHA-derived resolvins have therapeutic potential in resolving periodontal inflammation and restoring the tissues' health.
DRAWBACKS
Periodontitis is an inflammatory disease fundamentally initiated by chronic infection. When inflammation is inhibited, the immune system is also downregulated. This increases the risk of microbial infection.
Opportunistic infection has been reported when TNF-α was neutralized for rheumatoid arthritis therapy.[25]
The screening of latent infectious diseases, such as tuberculosis, should be performed before using this type of anti-cytokine therapeutic.
With antimicrobials, caution must be taken to prevent inapparent infection, without inflammatory symptoms, when anti-cytokine therapy is performed. If anti-cytokine therapy is applied to periodontal treatment, we may use chemical plaque control reagents such as chlorhexidine gluconate in addition to mechanical control.
CONCLUSION
In this era of molecular biology where research has been focused on the genetic level of analysis, treatment should be focused on eliminating the root cause.
Periodontal advancement should be diverted toward the use of anti-cytokine therapy in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Listgarten MA. Pathogenesis of periodontitis. J Clin Periodontal. 1986;13:418–30. doi: 10.1111/j.1600-051x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 2.Jotwani R, Cutler CW. Adult periodontitis-specific bacterial infection or chronic inflammation? J Med Microbiol. 1998;47:187–8. doi: 10.1099/00222615-47-3-187. [DOI] [PubMed] [Google Scholar]
- 3.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumour necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 4.Academy report. Modulation of the host response in periodontal therapy. J Periodontol. 2002;73:460–70. doi: 10.1902/jop.2002.73.4.460. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP. Physiology of cytokine pathways in rheumatoid arthritis. Arthritis Rheum. 2001;45:101–6. doi: 10.1002/1529-0131(200102)45:1<101::AID-ANR90>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 6.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 7.Elliott MJ, Feldmann M, Maini RN. TNF-α blockade in rheumatoid arthritis: Rationale, clinical outcomes and mechanisms of action. Int J Immunopharmacol. 1995;17:141–5. doi: 10.1016/0192-0561(94)00092-3. [DOI] [PubMed] [Google Scholar]
- 8.Wooley PH, Dutcher J, Widmer MB, Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen induced arthritis in mice. J Immunol. 1993;151:6602–7. [PubMed] [Google Scholar]
- 9.Dashash M, Blinkhorn A, Drucker DB, Hutchinson IV, Glenny AM. Interleukin-1 receptor antagonist for treating periodontitis. Cochrane Database Syst Rev. 2004 Oct;(Issue 4) [Google Scholar]
- 10.Assuma R, Oates T, Cochrn D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory responses and bone loss in experimental periodontitis. J Immunol. 1998;160:403–9. [PubMed] [Google Scholar]
- 11.Graves DT, Delima AJ, Assuma R, Amar S, Oates T, Cochran D. IL-1 TNF antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J Periodontal 198. 69:1419–25. doi: 10.1902/jop.1998.69.12.1419. [DOI] [PubMed] [Google Scholar]
- 12.Chiang CY, Ktritsis G, Graves DT, Amar S. IL-1 and TNF activities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immune. 1999;67:4231–6. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, et al. Soluble antagonist to IL-1 and TNF inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontal. 2001;28:233–40. doi: 10.1034/j.1600-051x.2001.028003233.x. [DOI] [PubMed] [Google Scholar]
- 14.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–34. [PubMed] [Google Scholar]
- 15.Leng SX, Elias JA. Interleukin 11 inhibits macrophage IL-12 production. J Immunol. 1997;159:2161–8. [PubMed] [Google Scholar]
- 16.Martuscelli G, Fiorellini JP, Crohin CC, Howell TH. The effect of IL-11 on the progression of ligature induced periodontal disease in the beagle dog. J Periodontal. 2000;71:573–8. doi: 10.1902/jop.2000.71.4.573. [DOI] [PubMed] [Google Scholar]
- 17.Adams JL, Badger AM, Kumar S, Lee JC. p38 MAP kinase: Molecular target for the inhibition of proinflammatory cytokines. Prog Med Chem. 2001;38:1–60. doi: 10.1016/s0079-6468(08)70091-2. [DOI] [PubMed] [Google Scholar]
- 18.Parasrampuria DA, de Boer P, Desai-Krieger D, Chow AT, Jones CR. Single-dose pharmacokinetics and pharmacodynamics of RWJ 67657: A specific p38 mitogen-activated protein kinase inhibitor: A first-in-human study. J Clin Pharmacol. 2003;43:406–13. doi: 10.1177/0091270002250615. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood KL, Li F, Rogers JE, Otremba J, Coatney DD, Krieder JM, et al. A p38 alpha selective mitogen activated protein kinase inhibitor prevents periodontal bone loss. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.106.112466. in press. [DOI] [PubMed] [Google Scholar]
- 20.Han Z, Boyle DL, Chang L, Bennet B, Karin M, Yang L, et al. c-jun N-Terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 22.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–55. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 23.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, et al. RvE1 protects from local inflammation and osteoclast-mediated bone destruction in periodontitis. FASEB J. 2006;20:401–3. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 25.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with Infliximab: A TNF-α neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]


