Abstract
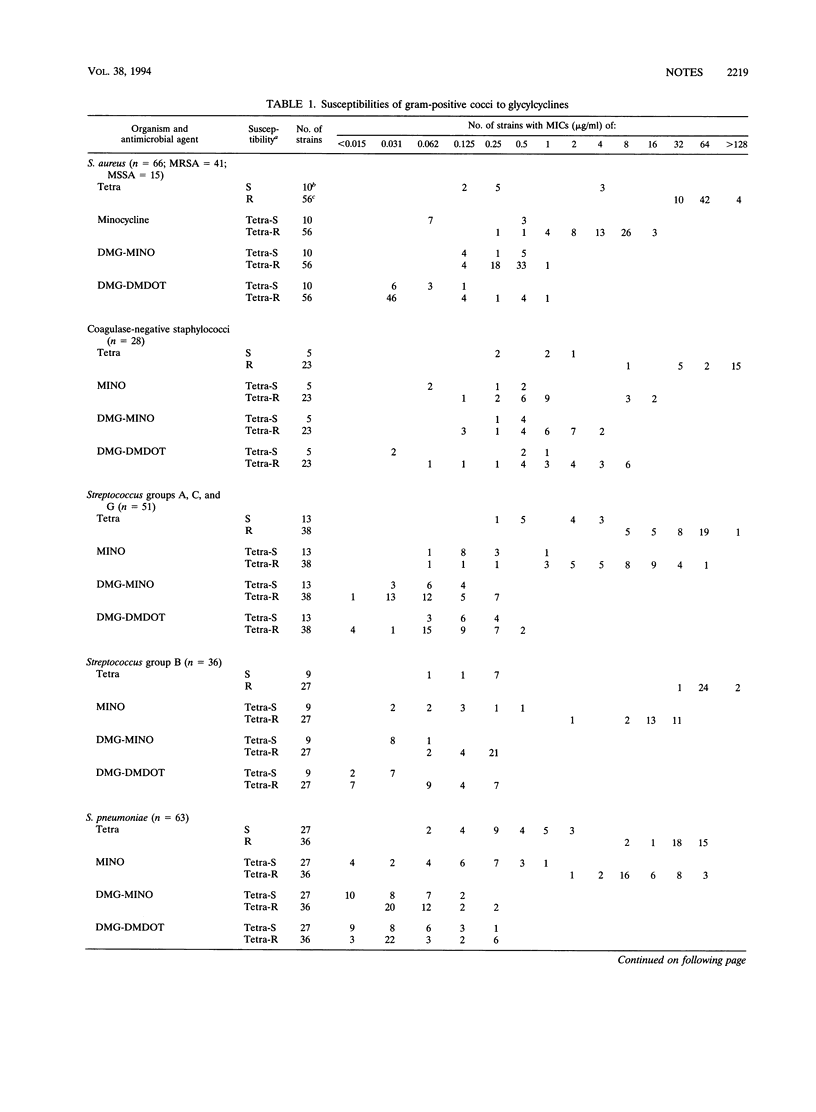
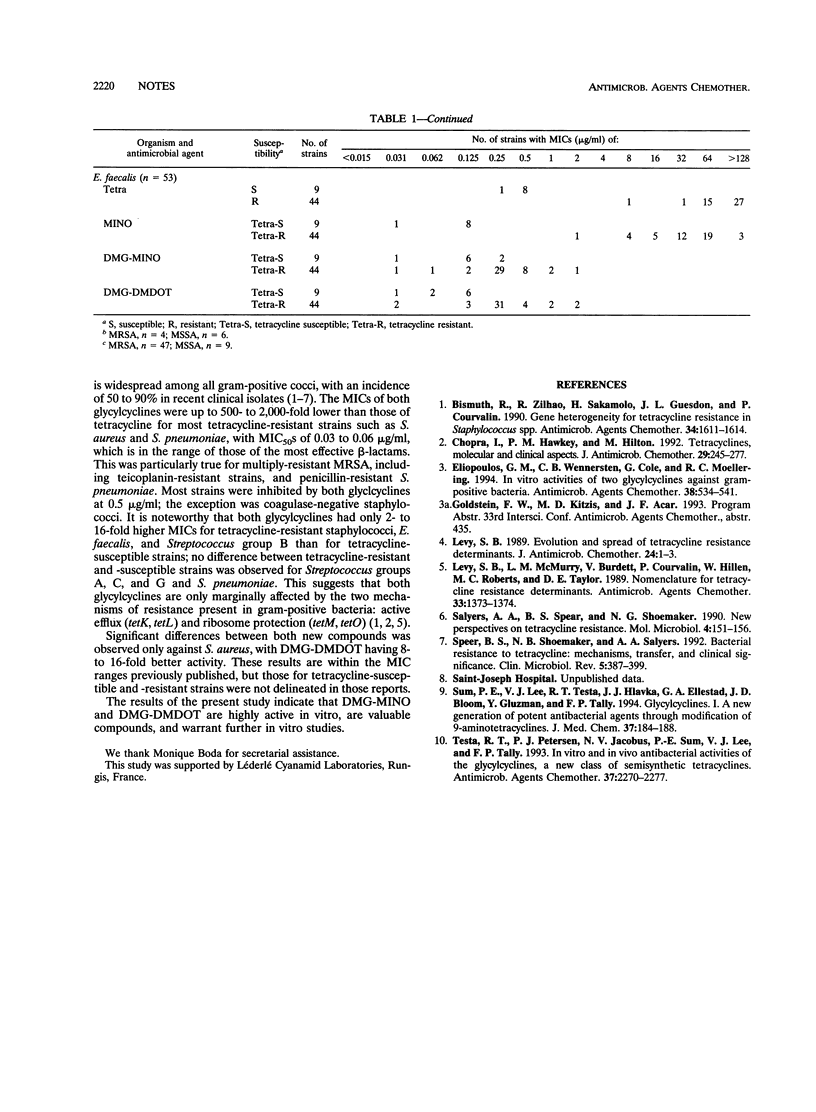
The in vitro activities of the N,N-dimethylglycyl-amino derivative of minocycline (DMG-MINO) and 6-dimethyl-6-dexoxytetracycline (DMG-DMDOT), members of a new generation of tetracyclines, were evaluated by an agar dilution method and were compared with those of tetracycline and minocycline against 224 tetracycline-resistant and 73 tetracycline-susceptible recent clinical isolates of gram-positive cocci, including multiple-antibiotic-resistant methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae. The MICs of DMG-MINO and DMG-DMDOT were up to 500- to 2,000-fold lower than those of tetracycline against methicillin-resistant S. aureus and Streptococcus pneumoniae (MIC for 50% of strains tested [MIC50], < 0.06 microgram/ml). Against Streptococcus groups A, B, C, and G and Enterococcus faecalis, the MIC50 was 0.5 microgram/ml. MIC50s were greater only for coagulase-negative staphylococci (2 micrograms/ml). These data indicate that DMG-MINO and DMG-DMDOT are very potent drugs, and further in vitro and in vivo studies are warranted.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bismuth R., Zilhao R., Sakamoto H., Guesdon J. L., Courvalin P. Gene heterogeneity for tetracycline resistance in Staphylococcus spp. Antimicrob Agents Chemother. 1990 Aug;34(8):1611–1614. doi: 10.1128/aac.34.8.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra I., Hawkey P. M., Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992 Mar;29(3):245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C. B., Cole G., Moellering R. C. In vitro activities of two glycylcyclines against gram-positive bacteria. Antimicrob Agents Chemother. 1994 Mar;38(3):534–541. doi: 10.1128/aac.38.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B. Evolution and spread of tetracycline resistance determinants. J Antimicrob Chemother. 1989 Jul;24(1):1–3. doi: 10.1093/jac/24.1.1. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. M., Burdett V., Courvalin P., Hillen W., Roberts M. C., Taylor D. E. Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother. 1989 Aug;33(8):1373–1374. doi: 10.1128/aac.33.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Speer B. S., Shoemaker N. B. New perspectives in tetracycline resistance. Mol Microbiol. 1990 Jan;4(1):151–156. doi: 10.1111/j.1365-2958.1990.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Speer B. S., Shoemaker N. B., Salyers A. A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992 Oct;5(4):387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum P. E., Lee V. J., Testa R. T., Hlavka J. J., Ellestad G. A., Bloom J. D., Gluzman Y., Tally F. P. Glycylcyclines. 1. A new generation of potent antibacterial agents through modification of 9-aminotetracyclines. J Med Chem. 1994 Jan 7;37(1):184–188. doi: 10.1021/jm00027a023. [DOI] [PubMed] [Google Scholar]
- Testa R. T., Petersen P. J., Jacobus N. V., Sum P. E., Lee V. J., Tally F. P. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob Agents Chemother. 1993 Nov;37(11):2270–2277. doi: 10.1128/aac.37.11.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]


