Abstract
Label retention assays remain the state-of-the-art approach to identify the location of in-traorgan epithelial stem cell niches, in situ and in vivo. They are commonly used in organs with rapid cell turnover but have not been applied to the liver, where cell turnover is very slow. We used a sublethal dose of acetaminophen administered coincident with bromode-oxyuridine to load possible hepatic stem cells in mice with label and then administered a second, sublethal chase of acetaminophen to accomplish “washout” of label from transit amplifying cell populations.
Conclusion
Four possible hepatic stem cell niches are identified by this approach: the canal of Hering (proximal biliary tree), intralobular bile ducts, periductal “null” mononuclear cells, and peribiliary hepatocytes. These results confirm several different and often contradictory lines of investigation regarding the intrahepatic location of stem/progenitor cells and suggest that the liver has a multi-tiered, flexible system of regeneration rather than a single stem/progenitor cell location.
Hepatocytes are normally proliferatively quiescent with 5-bromo-2′-deoxyuridine (BrdU) incorporation studies yielding turnover rates of 1 in 20,000 to 40,000 cells.1 With mild to moderate hepatocellular injury or depletion, hepatocyte replication increases, providing the necessary repopulating cell mass without invoking hepatic stem/progenitor cell (HSPC) responses.1–4 In severe acute injury and in chronic injury, however, HSPCs participate more significantly.4,5 Under such conditions, in which proliferation of mature hepatocytes is inhibited or the parenchyma of the liver is severely injured, in rodent models, proliferating HSPCs, called “oval cells” (OVc) based on their morphology, are observed.6,7 These cells can express markers corresponding to both hepatocyte and cholangiocyte lineages,8,9 and experimentally have been proven to differentiate into either of these cell types.10–12
Historically, 4 anatomic HSPC compartments have been hypothesized1: (1) replicating hepatocytes at the parenchymal– stromal interface (the so-called “streaming liver” hypothesis)13; (2) the most proximal branches of the biliary tree, including canals of Hering (CoH) and perhaps, also, bile ductules14,15; (3) cholangiocytes of the intralobular bile ducts16; and (4) periductular “null” cells (in other words, devoid of hepatocytic and biliary markers) seen in periportal injury, for example, with allyl alcohol toxicity in rats.17
Numerous other antigens have been commonly identified in stem/progenitor cells in many organs, such as c-kit, Sca-1, Thy-1, and CD-34, and have been shown to be present in OVc.1 These markers, however, only highlight cell populations enriching for a subpopulation with stem cell–like functions, but do not exclusively, specifically identify the stem cells themselves. In the current absence of a unique marker for HSPC, it is necessary to turn to functional definitions of stem cells for their identification in living tissues. This functional definition includes slow cycling of asymmetrically dividing stem cells, thereby self-renewing and simultaneously giving rise to a rapidly proliferative, multipotent transit amplifying population. Currently, state-of-the-art experimental approaches based on these functions of stem cells allow in vivo demonstration of epithelial stem cell niches and are called “label retaining cell” (LRC) assays. These assays have been successfully and reproducibly performed in many organs.18–21
A standard LRC assay exploits the fact that on asymmetric stem cell division both daughter cells, one being a new, replacement stem cell and the other the progenitor of transit amplifying cells, are both labeled with tritiated thymidine or BrdU. The new, replacement stem cell becomes quiescent after division and therefore retains its incorporated label. Meanwhile, the other progeny, in other words, rapidly dividing, transit amplifying cells, soon dilute label to undetectable levels in organs with rapid, frequent turnover (such as skin, intestinal tract) or deplete it during a “chase” period of severe organ injury in organs without constant, rapid turnover. LRCs are thus the experimentally marked, in vivo stem cells residing in that tissue’s stem cell niche. Thus, the stem cell niche has been identified in many epithelium-containing organs, for example, skin,22 small intestine,23 kidney,24 and prostate. 25 Given the slow cycling of the liver, however, such LRC experiments are impossible to perform in adulthood without injury, although use of an injury model may stimulate stem cell activation in the liver and make hepatic LRC experiments possible.
Overdose of acetaminophen (N-acetyl-p-aminophenol, APAP) is the most frequent cause of acute fulminant hepatic failure in the United States26,27 and is a pan-species, predictable hepatotoxin. The compound undergoes metabolism by the cytochrome P450 mixed function oxidase to a hepatotoxic metabolite.28,29 Once fulminant hepatic failure has developed, the mortality rate is extremely high.30 Given this clinical importance and the severity of outcomes, we have previously defined a mouse model of APAP toxicity with particular attention to dose relationships to OVc proliferation.31
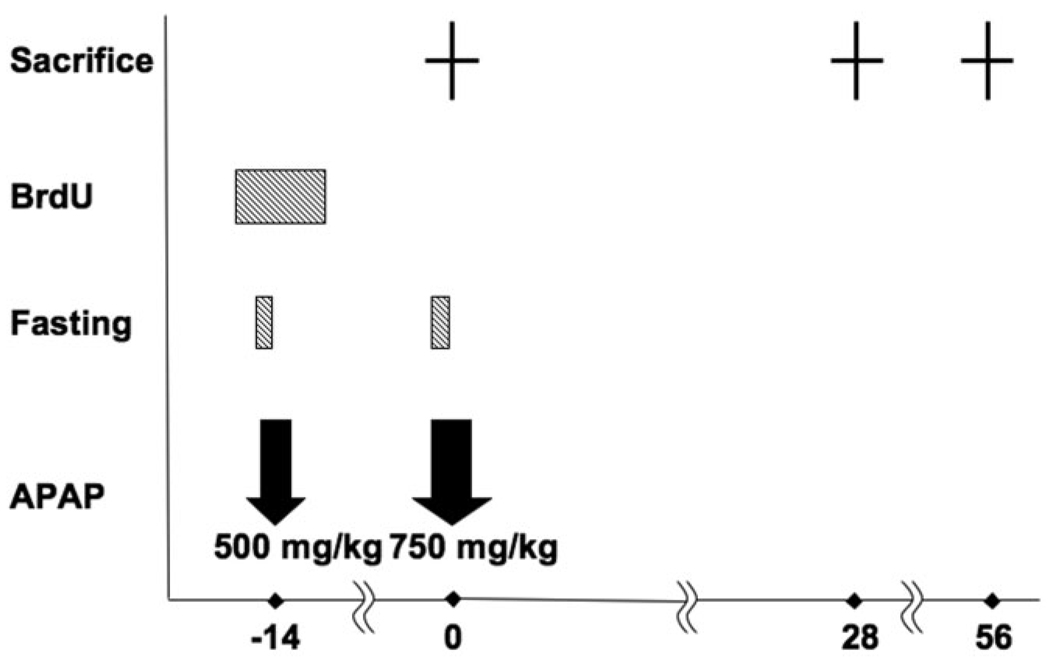
To identify label-retaining cells as a functional assay for HSPC niche, we performed LRC studies with APAP injury providing a loading dose for BrdU incorporation of HSPC and a “chase” for washout of BrdU in transit amplifying OVcs (Fig. 1).
Fig. 1.
Summary of experimental procedures for the “label-retaining” cell assay. Mice were fasted for 8 hours before APAP administration, then received intraperitoneal injection of APAP at the dose of 500 mg/kg at day −14, and 750 mg/kg APAP injection at day 0. Water containing BrdU was given before and after APAP administration. Three mice each were sacrificed at days 0, 28, and 56 to perform experiments. BrdU, bromodeoxyuridine; APAP, acetaminophen.
Materials and Methods
BrdU Loading With APAP Injury
Animal Experiments
Animal experiments were performed with the approval of the Animal Institute Committee, Albert Einstein College of Medicine of Yeshiva University. Protocols were conducted according to the National Institute of Health Guidelines for the Care and Use of Laboratory Animals. Male C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME) for this experiment. Mice were fasted with freely available water for 8 hours before APAP administration to induce severe liver injury. Phosphate-buffered saline (PBS), pH = 7.2, containing 25 mg/mL APAP (Sigma-Aldrich, St. Louis, MO; catalog no. A-5000), was given intraperitoneally at doses of 500, 750, and 1000 mg/kg body weight. Free access to water containing 1 mg/mL BrdU (Sigma-Aldrich, catalog no. 858811) was given from 36 hours before APAP administration to 48 hours after APAP administration. Mice of each group were sacrificed at 0, 4, 12, 48 hours, and 14 days after APAP administration. Liver samples were fixed in 10% neutral buffered formalin for 4 hours and preserved in 70% ethanol. Control mice of the same sex, age, and strain received water containing 1 mg/mL BrdU by the same experimental methods.
Immunohistochemistry
Assessments of LRC
Double immunostaining of BrdU/ Pan-keratin (PanK) was performed for the detection of label-retaining cells. After deparaffinization and rehydration, slides were incubated in target retrieval solution at 99°C for 40 minutes. Quenching of endogenous peroxidase, blocking of nonspecific binding with serum-free proteins, and of endogenous avidin and biotin were carried out by the same methods as previously described. 31 Following the biotinylation of monoclonal mouse anti-bromodeoxyuridine antibody (DAKO; catalog no. M0744) by using the commercially available kit, DakoCytomation ARK (Animal Research Kit) Peroxidase (DAKO; catalog no. K3954) according to manufacturer’s instructions, slides were incubated at 4°C with this biotinylated antibody at a dilution of 1:25 overnight. After washing with PBS, horseradish peroxidase/streptavidin (diluted 1:100) was applied for 30 minutes at room temperature. BrdU was visualized by 3,3′-diaminobenzidine chromogen and substrate buffer. Then, slides were incubated at 4°C with polyclonal rabbit anti-cow keratins antibody (diluted 1:100; DAKO; catalog no. Z0622) overnight. This antibody was raised against cow epidermal cytokeratin units of 58, 56, and 52 kDa, among others, and cross-reacts with a wide range of cytokeratins, including the high-molecular-weight cytokeratins expressed in cholangiocytes. As we reported previously, this antibody detected cells positive for the A6 antigen, which is known as a marker for murine oval cells, with a sensitivity and specificity of greater than 99%.31 Incubation with peroxidase-labeled polymer conjugated to goat antirabbit immunoglobulin (DAKO; catalog no. K4008) was carried out for 30 minutes at room temperature. Counterstaining was performed with 3-amino-9 ethylcarbazole.
Terminology
The terminology assigned to proliferative/regenerative lesions in the rodent liver is difficult, in part because the precise nature of the OVc is not fully understood, and their relationship to the preexisting biliary tree is not necessarily consistent. For the purpose of obtaining counts of labeled cells, we have defined cell populations by morphological appearance, anatomic location, and presence or absence of staining for biliary-type cytokeratins.
It has been recognized that small, biliary-cytokeratin– positive cells at the limiting plate or within the hepatic parenchyma correspond to the normal, intraparenchymal extensions of the proximal biliary tree, namely the canals of Hering and bile ductules. In diseased livers, proliferated cytokeratin- positive cells similar, if not morphologically identical to, cells of these normal structures are referred to as OVc. One difficulty is that in these experiments we are examining normal livers, injured livers containing OVc, and then the restored livers with structures that function as did the original CoH/ductule units, but that may have slightly more complex structures than the undisturbed originals. For the purposes of reporting results of cell counting, however, this paper will use OVc for all extraportal, cytokeratin-positive cells with OVc-type morphology whether in normal, injured, or restored livers.
Analyses of BrdU Incorporation After APAP Injection
BrdU incorporation after APAP administration was evaluated by counting the number of BrdU-labeled intralobular hepatocytes, peribiliary hepatocytes (hepatocytes in direct contiguity to PanK-labeled OVc), and OVc in whole sections of each slide. Other BrdU-positive cells in the injured liver, such as sinusoidal endothelial cells, Kupffer cells, leukocytes, and bile duct epithelial cells in normal interlobular bile ducts, were excluded from the counting.
Post-APAP Chase Label Retaining Cells
Animal Experiments
To detect LRC in the regenerating liver, we established the following experimental protocol (Fig. 1): APAP 500 mg/kg body weight was injected by the same methods as mentioned with 8 hours’ fasting before administration. Mice started receiving water containing 1mg/mL BrdU from 36 hours before to 48 hours after APAP administration. Mice, with an initial dose of 500 mg/kg of APAP, received 750 mg/kg of APAP again at day 14 for acute injury “chase” for label washout and were sacrificed 28 and 56 days after the second APAP injection. A higher dose was selected for the chase because it is recognized that desensitization to APAP effects occur in the weeks after an initial exposure. As a control experiment, some mice of the same sex, age, and strain received the primary injection of APAP but not the secondary administration.
Assessments of LRC
Double immunostaining of BrdU/PanK was performed as described. To evaluate the distribution of LRC, the number of intralobular hepatocytes, peribiliary hepatocytes (hepatocytes in direct contiguity to OVc), and OVc were counted in the sections taken at 28 and 56 days after the second APAP injection. We examined up to 20 of the smallest portal tracts containing visible bile duct and portal vein and counted BrdU-positive OVc, peribiliary hepatocytes, and intralobular hepatocytes.
Cells corresponding to the previously described “null cells,” that is, intraportal, mononuclear cells directly adjacent to interlobular bile ducts that were PanK negative but positive for BrdU, were also investigated. For identification of markers in these cells, additional immunostaining for CD34 (DAKO; catalog no. LS039), Sca-1 (BD Pharmingen, Rockville, MD; catalog no. 557403), vimentin (DAKO; catalog no. M0725), CD45 (BD Pharmingen; catalog no. 550539), c-kit (Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. sc-1494), and alpha-fetoprotein (Santa Cruz Biotechnology; catalog no. sc-8108) was performed. Staining protocols that could accomplish double staining for each of these markers along with BrdU could not be established because antigen retrieval techniques were mutually exclusive. Instead, these antibodies were stained in parallel sections, and examination of periductal mononuclear cells was performed. These periductal, PanK-negative “null” cells could not be reliably quantified as a percentage of a larger population given the absence of a marker distinguishing them from ordinary inflammatory infiltrates.
Data Acquisition and Analysis
Microscopy was performed on the Olympus BX51 microscope equipped with the SensicamQE camera. IPLAB software was used to capture images. Fisher’s exact test and Student t test were used for statistical analyses. A two-tailed probability value of less than 0.05 was considered significant.
Results
BrdU Incorporation After APAP-Induced Liver Injury
BrdU incorporation after one dose of APAP was demonstrated by using double immunostaining of BrdU/PanK (Fig. 2). We evaluated the frequency of BrdU staining in two ways: First, we assessed, where possible, the percentage of a particular cell type that was BrdU positive by counting an average of 3347 ± 1695 BrdU-positive hepatobiliary cells. We were able to adequately quantify intralobular hepatocytes, peribiliary hepatocytes (that is, hepatocytes in direct contiguity with PanK biliary structures in the periportal region), and PanK-positive OVc belonging to the CoH. We could not evaluate periductal “null cell” mononuclear cells in this fashion given that there was no specific morphological or immunophenotypical marker to reliably identify them if they did not take up BrdU.
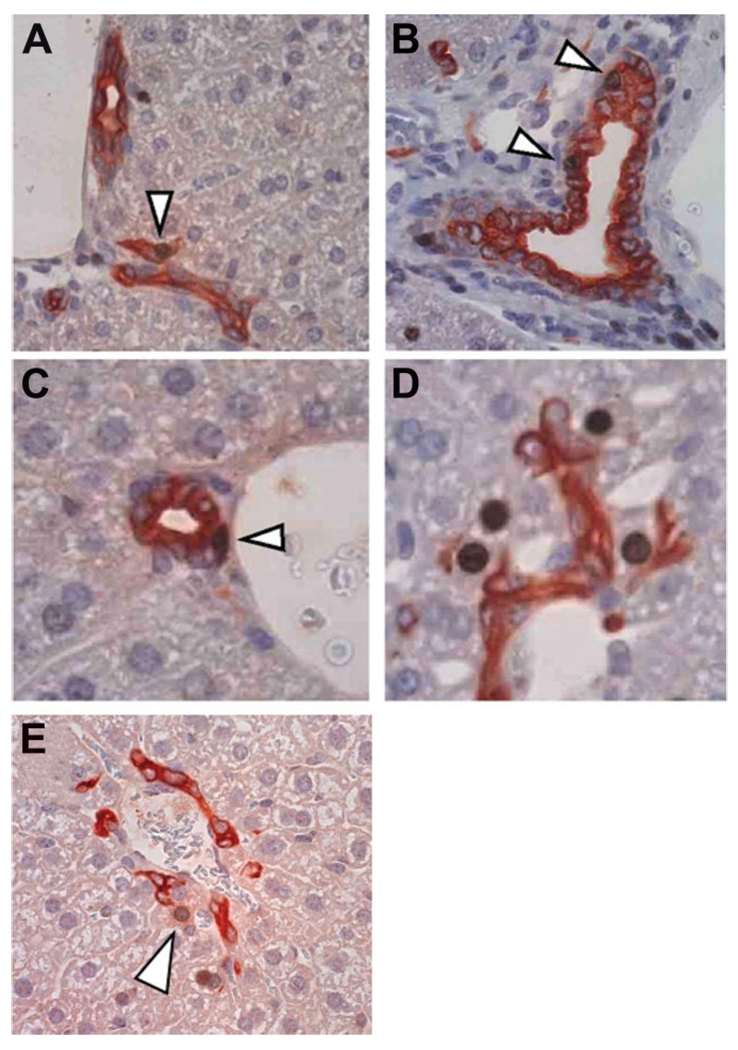
Fig. 2.
Localization of BrdU-labeled cells in APAP-injured liver. APAP-treated BrdU-labeled murine liver was double-immunostained for BrdU (brown) and PanK (red). These images are from 28 days after second (chase) dose of APAP, but are representative of changes seen at later time points. (A) BrdU-labeled OVc; (B) BrdU-labeled cholangiocyte of the intralobular bile duct; (C) BrdU-labeled periductular “null” cell; (D) BrdU-labeled peribiliary hepatocytes; (E) BrdU-labeled peribiliary hepatocyte with faint PanK staining. Original magnification, ×20. OVc, oval cell; BrdU, bromodeoxyuridine; APAP, acetaminophen; PanK, biliary cytokeratins.
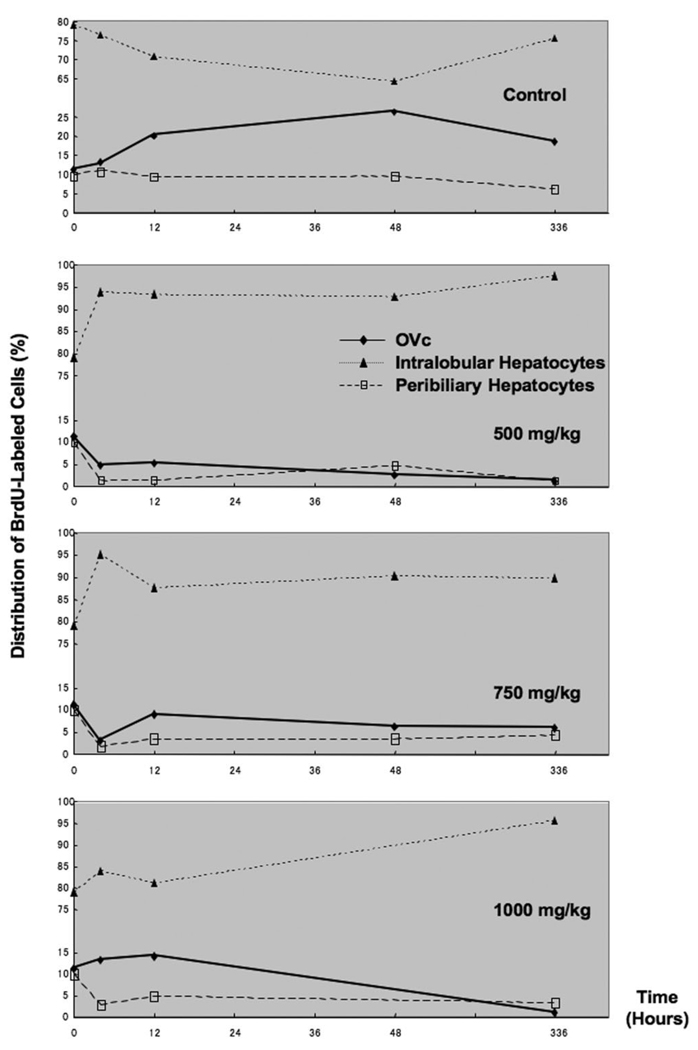
The distribution of particular cell types is shown in Fig. 3. After injection of APAP at 500 and 750 mg/kg, the percentage of BrdU-labeled OVc in total BrdU-labeled cells of all types remained less than the initial levels throughout the observation period. Injection of APAP at the highest (1000 mg/kg) dose, however, instead induced increasing percentages of BrdU-labeled OVc within 12 hours after administration. Conversely, in all doses, the distribution of BrdU-positive peribiliary hepatocytes rapidly decreased and was less than the initial levels during the 2-week observation. This decreasing trend in percentage of peribiliary cells was paralleled by that of OVc after APAP injection at the dose of 500 mg/kg and 750 mg/kg within the early phase. However, there was a discrepancy between OVc and peribiliary hepatocytes in response to the injection of APAP at the dose of 1000 mg/kg.
Fig. 3.
The distribution of BrdU-labeled cells after single “loading” dose of APAP-induced injured liver through 14 days (336 hours) after injury as a percentage of total BrdU-labeled extraportal hepatobiliary cells. Note that the high percentage of oval cells in the control, PBS-only injected mice reflect the increase in these cells seen in response to abdominal fluid injection without any hepatocyte injury; thus, only their relative number is quite high compared with APAP-injected mice. OVc, oval cell; APAP, acetaminophen; PanK, biliary cytokeratins.
It should be noted, also, that the high percentage of oval cells in the control, PBS-only injected mice reflect the increase in these cells seen in response to abdominal fluid injection without any hepatocyte injury, as we reported in our earlier study of murine APAP injury31; thus, only their relative number is quite high compared with APAP-injected mice.
The second approach to quantification of BrdU incorporation was to evaluate BrdU-positive cells in these three readily identifiable cell compartments and present each cell compartment’s data in terms of percentage of total BrdU-positive cells. For this an average of 1935 ± 909 OVc were counted to determine the percentage of BrdU-labeled OVc in total OVc. The expansion of OVc was reflected by increasing percentages of BrdU-positive OVc in all doses. The range of BrdU-labeled OVc ranged from approximately 2%–14% during observation periods. The incidence of BrdU-labeled OVc after APAP injection in all doses were increased approximately three to seven times more than uninjected control livers (data not shown).
LRC Assay
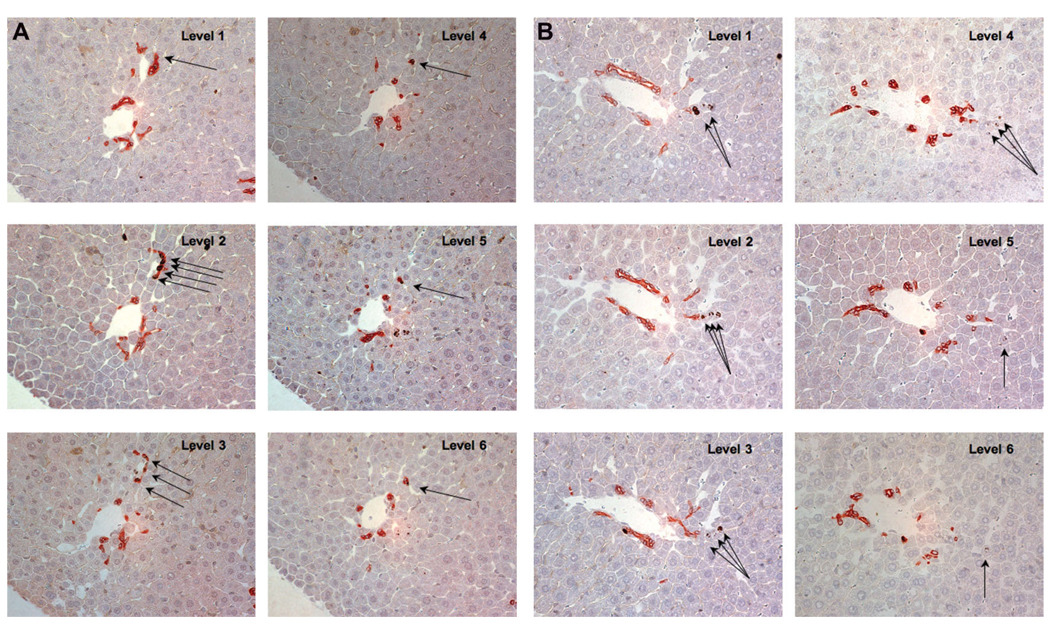
LRCs were confirmed and quantified by using double immunostaining of BrdU/PanK (Fig. 4A, B). Double-immunostaining of BrdU/PanK was performed in 20 serial sections taken at day 28. An example is shown in Fig.4A and 4B. The strings of label-retaining OVc in ductular reaction seemed to be connected with a small bile duct in level 1 (Fig. 4A). Six sequential sections tracking label-retaining hepatocytes are shown in Fig. 4B. This analysis in three dimensions is required to reveal that individual label-retaining hepatocytes, which are located away from the portal tract (levels 5 and 6), are often lining up in strings (Levels 1–4) in contiguity to OVc (Levels 1 and 2).
Fig. 4.
Immunohistochemistry for BrdU (brown) and PanK (red) in serial section. (A) Sample sequential images of label-retaining cells in ductular reaction. On each level, label-retaining OVc are marked by arrowhead. These cells in ductular reactions are connected with a small bile duct in level 1 (arrows). (B) Six sequential sections tracking label-retaining hepatocytes. Double immunostaining for BrdU and PanK revealed that an individual label-retaining cell, which locates away from the portal tract (levels 5 and 6), are lining in the label-retaining hepatocytes (levels 2–4) and connected with OVc (levels 1 and 2). Original magnification, ×40. OVc, oval cell; BrdU, bromodeoxyuridine; PanK, biliary cytokeratins.
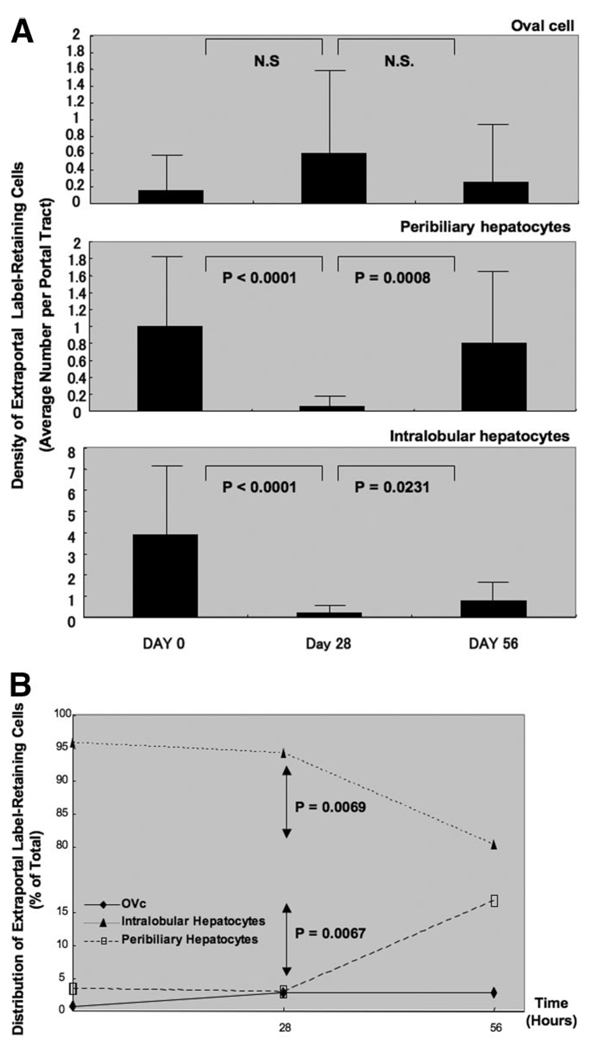
We examined the change in the cell numbers of LRC in the 20 smallest portal tracts. The density of BRDU-positive peribiliary hepatocytes and lobular hepatocytes were statistically decreasing between day 0 and day 28 (P < 0.0001 for both populations). The number of these cells increased statistically between day 28 and day 56 (P < 0.0008, and P < 0.0231, respectively). Meanwhile, there was no significant change in the density of OVc during 56 days after the second APAP injection for acute injury “chase” for label washout.
To investigate the distribution of BrdU-labeled cells, 3550 ± 2894 LRC were counted in each case, classifying them into three cell types: OVc, peribiliary hepatocytes, and lobular hepatocytes (Fig. 5B).
Fig. 5.
Distribution of “label-retaining” cells at various periods after APAP “chase.” (A) The density of label-retaining cells in the smallest portal tract after APAP “chase” at days 0, 28, and 56. Label-retaining peribiliary hepatocytes and intralobular hepatocytes were decreasing for the first 4 weeks, then increasing during the next 4 weeks. During the 8-week follow-up, the density of label-retaining OVc was not changing statistically. (B) The distribution of label-retaining cells after APAP “chase” at days 0, 28, and 56. The percentage of label-retaining intralobular hepatocytes decreased between day 28 and day 56 statistically, whereas the percentage of label-retaining peribiliary hepatocytes increased during this same period. There was no statistical difference in the percentage of label-retaining OVc. These data suggest that intralobular hepatocytes are not “true” label retaining cells, losing label with time and normal hepatocyte turnover.
The distribution of “label-retaining” OVc, peribiliary hepatocytes, and lobular hepatocytes were not different significantly between day 0 and day 28. From day 28 to day 56, however, the percentage of peribiliary hepatocytes increased significantly [P = 0.0067; 95% confidence interval (CI), −18.80 to −8.97), whereas the distribution of lobular hepatocytes decreased significantly (P = 0.0069; 95% CI, 8.93–18.94). During this later period, there was no significant change in the distribution of BrdU-labeled OVc (1635 ± 1111 counted per specimen).
Discussion
Acute liver failure in humans is most commonly caused by intentional or accidental APAP injury. Although there are many chemical models of injury in rodents, none shows similar histological or physiological features to APAP toxicity. Thus, we have begun examining similarities between murine and human APAP injury, to shed light on human conditions.31
Because normal cell turnover in the liver is relatively low, LRC assay must be investigated by inducing uptake of label (loading dose) by injury and then a second injury to provide a “chase.” Having established the utility of APAP injury for production of OVc in a manner similar to that seen in human liver, we used that model to accomplish both uptake of BrdU label and the chase injury to reveal label retention. Four cell compartments were identified as containing LRC.
LRC: Canals of Hering
As predicted from prior studies, BrdU-positive/PanK-positive cells within the juxtaportal, proximal biliary tree were found to be BrdU label-retaining as far out as 56 days after APAP administration. Thus, these represent true LRCs, sufficiently slow cycling that they persist at least 8 weeks after initial, injury-related cell divisions. Given the rarity with which the intrahepatic HSPC compartment probably contributes to normal hepatocyte turnover in the absence of overt injury, these data support the canal of Hering as being an intrahepatic stem cell niche.
LRC: Peribiliary Hepatocytes
The finding of these BrdU hepatocytes, always periportal, directly contiguous with PanK-positive cells, was the most unexpected and intriguing finding of this segment of the study. At the earliest time points postinjury, these cells were the most frequently identified as BrdU positive, other than the mid-acinar to central-acinar hepatocytes, which were turning over rapidly, representing the predominant regenerative response to parenchymal loss. But over time, these BrdU intraparenchymal hepatocytes slowly diminished, whereas the BrdU-positive, peribiliary hepatocytes had a relative increase in number (Fig. 5B), suggesting that whereas the intraparenchymal hepatocytes lose BrdU by division, the BrdU-positive, peribiliary hepatocytes remain; in other words, they also represent a true LRC population. On its face, this finding would seem to support the old “streaming liver” hypothesis, slightly modified to reflect our current understanding that the hepatocyte acinus begins not at the limiting plate but at the interface between the hepatocyte canaliculi and the biliary tree, at the CoH.
However, examination of serial sections and relative proportions of CoH LRC to peribiliary hepatocyte LRC suggests that the peribiliary hepatocyte LRC actually derive from the COH-LRC through a differentiative process. First, occasional peribiliary hepatocytes-LRC show faint, apparently retained PanK staining (Fig. 2E). Second, serial section examination (Fig. 5A, B) shows linear arrays of BrdU-positive hepatocytes trailing off from the ends of strings of PanK-positive cells, that is, the CoH, suggesting derivation from those structures. Third, the data at 28 days and at 56 days show that while parenchymal hepatocyte BrdU staining declines the combined number of CoH-LRC and peribiliary hepatocyte-LRC remains stable, supporting a possible differentiative shift of the former population into the latter. Thus, CoH-LRC and peribiliary hepatocyte-LRC may represent the same population of cells, but in shifting differentiative states.
LRC: Intraductal Cholangiocytes
Prior studies indicate HSPCs within interlobular bile ducts of human livers on the basis of c-kit expression.16 In our own studies of human intraseptal hepatocyte “buds” in cirrhosis caused by diverse diseases we also showed that although most such regenerating hepatocyte nodules were linked to the CoH, some appeared to bud directly from interlobular bile ducts.8 Thus, our current finding of cholangiocyte-LRC in the bile ducts is not necessarily surprising; however, in the absence of direct communication of these structures with the hepatic parenchyma itself, it is difficult to see how these might function as HSPC for hepatocyte regeneration in APAP injury. Instead, these could represent labeled cells in the stream of repopulating a normal biliary system or a response to physical injury secondary to the APAP-induced parenchymal collapse that then have little reason to divide in the face of complete restitution.
LRC: Peribiliary“Null”Cells
These cells are particularly difficult to study and have not disclosed their nature to us with this set of investigations. These cells were first described as representing a subpopulation of OVcs in a periportal model of rat hepatic injury induced by allyl alcohol toxicity.17 These were small, periductal cells that were negative for typical OVc markers such as alpha-fetoprotein, biliary-type cytokeratins (as stained in that experiment with antibody OV-6), albumin, and also negative for leukocyte common antigen (CD45) and desmin. The origin of these null cells remains unclear, though the possibility that they derived from extrabiliary, perhaps even extrahepatic, sources was the clue that initiated our own studies of marrow-to-liver engraftment. Considering that possible source for these cells, we not only evaluated alpha-fetoprotein and biliary-cytokeratin staining, but also hematopoietic-type markers that have been previously identified on OVc, including CD34, Sca-1, Thy-1, and CD45. We also evaluated staining for vimentin and desmin. The successful double staining protocol for PanK/BrdU allowed us to conclusively identify these small, periductal cells as negative for PanK. We were not able to establish reliable double staining for the other markers, however. In single marker staining of APAP-injured livers, the only marker that was ever seen to stain any periductal monocytes was CD45, which did so quite rarely.
Thus, we can conclude that these periductal monocytic LRC are “null” for the tested hepatobiliary markers as well as for hematopoietic markers described, and for other possible stromal cells that might be labeled by desmin or vimentin. Their more striking appearance in models of periportal injury, rather than the more typical centrilobular APAP injury, suggests that they may play a more important role in hepatobiliary regeneration when the canal of Hering stem cell niche is disrupted or obliterated along with destruction of the periportal hepatocytes.
Thus, in summary, using the APAP injury model, we have applied standard approaches for identifying in vivo functional stem cell niche locations. We find evidence that supports partly or wholly the four previously postulated niches: the hepatocytes at the beginning of the hepatic acinus (though now redefined as those hepatocytes that meet the CoH rather than those sitting directly on the portal tract stroma in the limiting plate), PanK-positive cells in the CoH, intraductal cholangiocytes, and, possibly, periductal “null cells.” That our data suggest that the first of these two are linked physiologically, as CoH-LRC differentiate into hepatocytes, thereby becoming peribiliary hepatocyte-LRC, is not surprising. The absence of clear participation of the other two cell compartments in hepatobiliary regeneration may reflect the nature of the hepatic injury.
It is clear, however, that HSPC participation in hepatic repair is probably more complicated than in organs with normally rapid cell turnover and that multiple sites may, depending on severity of injury, location of injury, and chronicity of injury, function as an HSPC niche. Further LRC studies with other injury models and following LRC through normal development from fetal through adult postnatal life will be necessary to understand HSPC location and functioning. Moreover, more sophisticated, transgenic approaches to LRC identification, not relying on cell cycle progression for initial labeling, needs to be pursued such as those already reported for skin LRC by Tumbar et al.32 Such models are currently under development in our laboratory and may shed further light on these important questions.
Acknowledgments
Supported by National Institutes of Health grant R01 DK58559 and a generous grant from the John J. Gerard Foundation. (N.D.T.).
Abbreviations
- APAP
acetaminophen (N-acetyl-p-aminophenol)
- BrdU
5-bromo-2′-deoxyuridine
- CoH
Canal of Hering
- HSPC
hepatic stem/progenitor cells
- LRC
label-retaining cell
- OVc
oval cells
- panK
pan-keratin
- PBS
phosphate-buffered saline
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. HEPATOLOGY. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- 2.Rabes HM, Wirshing R, Ruxzek HV, Iseler G. Analysis of cell cycle compartments of hepatocytes after partial hepatectomy. Cell Tissue Kinet. 1976;9:517–532. doi: 10.1111/j.1365-2184.1976.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 3.Grisham JW, Thorgeirsson SS. Liver stem cells. In: Potten CS, editor. Stem cells. New York: Academic Press; 1997. pp. 233–282. [Google Scholar]
- 4.Forbes S, Vig P, Poulsom R, Thomas H, Alison M. Hepatic stem cells. J Pathol. 2002;197:510–518. doi: 10.1002/path.1163. [DOI] [PubMed] [Google Scholar]
- 5.Newsome PN, Hussain MA, Theise ND. Hepatic oval cells: helping redefine a paradigm in stem cell biology. Curr Top Dev Biol. 2004;61:1–28. doi: 10.1016/S0070-2153(04)61001-5. [DOI] [PubMed] [Google Scholar]
- 6.Farber E. Similarities of the sequence of the early histological changes induced in the liver of the rat by 2-acetylaminofluorine, and 3′-methyl-4-dimethylaminoazo- benzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- 7.Factor VM, Radaeva SA, Thorgeirsson SS. Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am J Pathol. 1994;145:409–422. [PMC free article] [PubMed] [Google Scholar]
- 8.Falkowski O, An HJ, Ianus IA, Chiriboga L, Yee H, West AB, et al. Regeneration of hepatocytes 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 9.Lowes KN, Brennan BA, Yeah GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to diseases severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debeva MD, Shafritz DA. Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993;143:1606–1620. [PMC free article] [PubMed] [Google Scholar]
- 11.Lenzi R, Liu MH, Tarsetti F, Slott PA, Alpini G, Zhai WR, et al. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis: evidence for a biliary epithelial nature of oval cells. Lab Invest. 1992;66:390–402. [PubMed] [Google Scholar]
- 12.Fougere-Deschatrette C, Imaizumi-Scherrer T, Strick-Marchand H, Morosan S, Charneau P, Kremsdorf D, et al. Plasticity of hepatic cell differentiation: bipotential adult mouse liver clonal cell lines competent to differentiate in vitro and in vivo. Stem Cells. 2006;24:2098–2109. doi: 10.1634/stemcells.2006-0009. [DOI] [PubMed] [Google Scholar]
- 13.Zajicek G, Oren R, Weinreb N., Jr The streaming liver. Liver. 1985;5:293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 14.Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, et al. The canal of Hering and hepatic stem cells in humans. HEPATOLOGY. 1999;30:1425–1433. doi: 10.1002/hep.510300614. [DOI] [PubMed] [Google Scholar]
- 15.Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver-exploring the hidden interfaces. HEPATOLOGY. 1999;30:1339–1346. doi: 10.1002/hep.510300607. [DOI] [PubMed] [Google Scholar]
- 16.Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human progenitor hepatic cell. HEPATOLOGY. 1999;30:112–117. doi: 10.1002/hep.510300140. [DOI] [PubMed] [Google Scholar]
- 17.Yavorkovsky L, Lai E, Ilic Z, Sell S. Participation of small intraportal stem cells in the restitutive response of the liver to periportal necrosis induced by allyl alcohol. HEPATOLOGY. 1995;21:1702–1712. [PubMed] [Google Scholar]
- 18.Morris RJ, Potten CS. Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol. 1999;112:470–475. doi: 10.1046/j.1523-1747.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 19.Bickenbach JR, Chism E. Selection and extended growth of murine epidermal stem cells in culture. Exp Cell Res. 1998;244:184–195. doi: 10.1006/excr.1998.4163. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the hematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 21.Albert MR, Foster RA, Vogel JC. Murine epidermal label-retaining cells isolated by flow cytometry do not express the stem cell markers CD34, Sca-1, or Flk-1. J Invest Dermatol. 2001;117:943–948. doi: 10.1046/j.0022-202x.2001.01517.x. [DOI] [PubMed] [Google Scholar]
- 22.Morris RJ, Potten CS. Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif. 1994;27:279–289. doi: 10.1111/j.1365-2184.1994.tb01425.x. [DOI] [PubMed] [Google Scholar]
- 23.Potten CS, Morris RJ. Epithelial stem cells in vivo. J Cell Sci Suppl. 1988;10:45–62. doi: 10.1242/jcs.1988.supplement_10.4. [DOI] [PubMed] [Google Scholar]
- 24.Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q. The renal papilla is a niche for adult kidney stem cells. J Clin Invest. 2004;114:795–804. doi: 10.1172/JCI20921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WM. Acute liver failure in the United States. Semin Liver Dis. 2003;23:217–226. doi: 10.1055/s-2003-42641. [DOI] [PubMed] [Google Scholar]
- 27.Lee WM. Acetaminophen and the U.S. Acute Liver Failure Study Group: lowering the risks of hepatic failure. HEPATOLOGY. 2004;40:6–9. doi: 10.1002/hep.20293. [DOI] [PubMed] [Google Scholar]
- 28.Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SD, Khairallah EA. Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab Rev. 1997;29:59–77. doi: 10.3109/03602539709037573. [DOI] [PubMed] [Google Scholar]
- 30.Lee WM. Drug-induced hepatotoxicity. N Engl J Med. 1995;333:1118–1127. doi: 10.1056/NEJM199510263331706. [DOI] [PubMed] [Google Scholar]
- 31.Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, et al. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. HEPATOLOGY. 2005;41:1252–1261. doi: 10.1002/hep.20696. [DOI] [PubMed] [Google Scholar]
- 32.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]