Abstract
Iminodiacetic acid (IDAA) forms strong complexes with a wide variety of metal ions. Using self-assembled monolayers in mesoporous supports (SAMMS) to present the IDAA ligand potentially allows for multiple metal-ligand interactions to enhance the metal binding affinity relative to that of randomly oriented polymer-based supports. This manuscript describes the synthesis of a novel nanostructured sorbent material built using self-assembly of a IDAA ligand inside a nanoporous silica, and demonstrates its use for capturing transition metal cations, and anionic metal complexes, such as PdCl4−2.
Ethylenediamine tetraacetic acid (EDTA) is one of the most widely used metal complexants in the world. Iminodiacetic acid (IDAA), effectively half of the EDTA molecule, forms strong complexes with a wide variety of metal ions [1], and polymer-based ion-exchange resins have used the IDAA ligand for chemical separations for many years [2–4]. Silica-based systems containing IDAA ligands have also been described [5, 6], and used for ion chromatography [7–14], as well as for binding a variety of metal ions [15, 16]. Recently, there have been examples of polysiloxanes containing IDAA that have been prepared and described [17, 18]. In many of these syntheses, the IDAA ligand is introduced after polymerization, either by nucleophilic displacement of a halide anion [17, 18], or by nucleophilic ring opening of an epoxide [5].
In recent years, a great deal of effort has been spent developing functional nanomaterials for chemical separations, particularly for environmental clean up efforts [19]. For example, the elegant work of the Clearfield group has shown that it is possible to integrate the IDAA ligand into layered metal phosphonate compounds and to use these materials for ion-exchange processes [20, 21]. In a similar vein, considerable effort has been expended to chemically modify mesoporous ceramic substrates with functional organosilanes, giving rise to sorbents capable of sequestering heavy metals, oxometallate anions, radioiodine, radiocesium, lanthanides, and actinides [22]. Self-assembled monolayers on mesoporous supports (SAMMS®) have been demonstrated to have significantly faster sorption kinetics than polymer-based ion-exchange resins due to the fact that the rigid ceramic backbone of SAMMS® holds the pores open and allows all of the binding sites to be available at all times, whereas the polymer system is subject to solvent swelling, and only a portion of the functionality is kinetically available at any given instant (although there have been efforts to improve these kinetics by integration of these polymers into a fibrous cloth weave [23]). The close proximity of the ligands in the monolayer array also allows for multiple metal ligand interactions [22], something that is not typically possible in disordered polymer systems (although ordered systems have been reported for certain polyamide systems [24]). As a result of these potential advantages, we felt that it would be useful to combine the affinity that IDAA has for transition metal ions with the greater sorbent capabilities of the SAMMS® materials, in order to evaluate their efficacy for chemical separations. We chose to synthesize an IDAA terminated organosilane, purify it by distillation, and then use the purified silane to build a self-assembled monolayer inside a surfactant-templated nanoporous silica. This manuscript describes some of our efforts in this area.
Results and Discussion
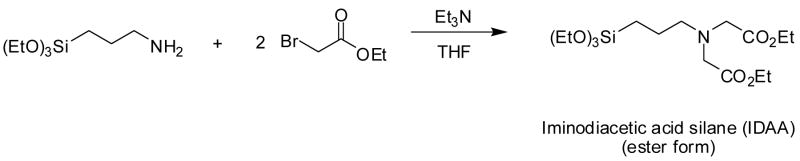
The synthesis of IDAA silane was performed as shown in Figure 1[25]. The silane was purified using vacuum distillation. The synthesis of IDAA SAMMS® was carried out in refluxing toluene [26], with a pre-hydrated sample of MCM-41 [22, 27, 28].
Figure 1.
The synthesis of iminodiacetic acid (IDAA) silane.
The deposition was carried out using the protected ester form of the IDAA silane to avoid any complications from the carboxylic acids interfering with the siloxane hydrolysis/condensation chemistry during the self-assembly process. Thus, the esters needed to be cleaved after the self-assembly process. Trimethylsilyl iodide (TMSI) [29] was used to perform this cleavage at 90–110°C for 12 hours [30].
Distribution coefficient (Kd) measurements were carried out in 0.01 M HHO3, 0.01 M HCl, filtered Columbia river water (from Richland, WA), or dialysate, spiked with metal ions (Co2+, Ni2+, Ru3+, Rh3+, and Pd2+) to obtain 50 μg/L of each [31]. All batch experiments were performed in triplicates and the averaged values were reported. Kd (in the unit of mL/g throughout) is simply a mass-weighted partition coefficient between the solid phase and the liquid supernatant phase as follows:
| (1) |
where Co and Cf are the initial and final concentrations in the solution of the target species determined by ICP-MS, V is the solution volume in mL, and M is the mass in gram of the sorbent. These results are summarized in Table 1.
Table 1.
Distribution coefficient (Kd) measurements for IDAA SAMMS.
| Sorbent | Matrix | Final pH | Kd (mL/g) |
||||
|---|---|---|---|---|---|---|---|
| Cobalt | Nickel | Ruthenium | Rhodium | Palladium | |||
| IDAA-SAMMS | 0.01 M HCl | 2.536 | 0 | 0 | 6300 | 0 | 190000 |
| 0.01 M HNO3 | 2.511 | 11 | 19 | 9200 | 190 | 140000 | |
| Dialysate | 8.622 | 63000 | 120000 | 470 | 0 | 11000 | |
| Filtered river water | 8.131 | 41000 | 790000 | 8500 | 0 | 9000 | |
|
| |||||||
| Chelex-100 100–200 mesh, Na form | 0.01 M HCl | 2.513 | 0 | 0 | 4600 | 140 | 140000 |
| 0.01 M HNO3 | 2.531 | 0 | 0 | 2500 | 350 | 52000 | |
| Dialysate | 8.619 | 17000 | 15000 | 750 | 0 | 4900 | |
| Filtered river water | 8.272 | 18000 | 13000 | 3300 | 0 | 2500 | |
|
| |||||||
| Darco KB-8 100 mesh | 0.01 M HCl | 2.532 | 0 | 0 | 0 | 0 | 52000 |
| 0.01 M HNO3 | 2.524 | 0 | 0 | 320 | 0 | 41000 | |
| Dialysate | 8.604 | 870 | 1400 | 840 | 0 | 13000 | |
| Filtered river water | 8.039 | 2400 | 4000 | 21000 | 0 | 42000 | |
Initial concentration = 50 ppb of Co, Ni, Ru, Rh, Pd, L/S = 5,000
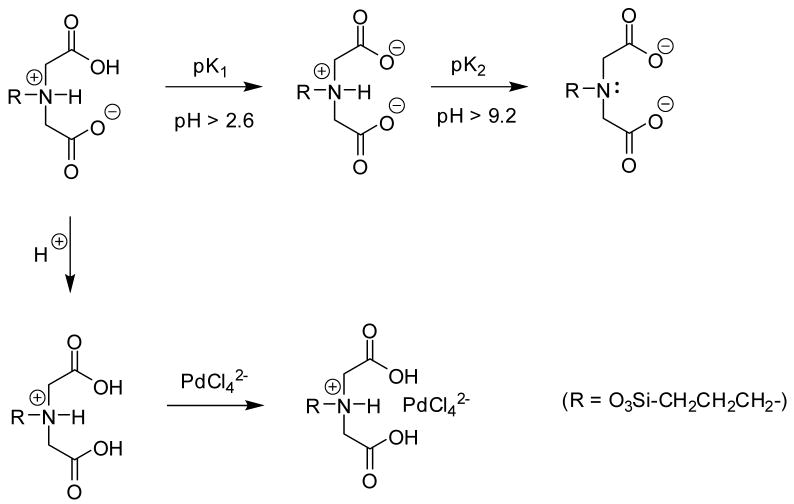
The IDAA ligand is known to have broad binding affinity, especially for the first row transition metals. The first row transition metals chosen for our test matrix are Co(II) and Ni(II), two metal cations that are known to form strong complexes with the IDAA ligand [1]. Looking at the distribution coefficient (Kd) data in Table 1, it is clear that IDAA SAMMS® have a high affinity for both Co(II) and Ni(II) under near neutral, or moderately alkaline conditions. Not surprisingly, under moderately acidic conditions (pH of ~2.5) very little metal cation binding was observed, indicating that once these cations are bound by the IDAA SAMMS® it should be possible to recover them by washing the sorbent with dilute acid. The pK1 of IDAA is 2.54 [1], so the experiments performed at pH ~2.5 will have approximately half of the IDAA sites protonated (i.e. positively charged), and therefore less readily available to complex with a metal cation.
Another ramification of this protonation is the fact that such a positively charged ammonium ion is capable of undergoing anion exchange chemistry (see Figure 2). Under these acidic conditions we observed significant binding of a Pd(II) complex. Pd(II) is known to form anionic complexes with Cl− anion [32], suggesting that perhaps this binding by our IDAA SAMMS® may be driven by such an anion exchange process, and not necessarily the typical chelation. Since the pK2 for the IDAA ligand is 9.12 [1], this ammonium ion is still protonated at all of the pHs we studied. Similar Pd(II) binding was observed in HNO3. Since the Pd was added as an ICP standard in HCl solution there was an excess of Cl− present, making the anion exchange mechanism a likely explanation once again.
Figure 2.
Scheme showing the structures of IDAA ligand as a function of pH, as well as how the cationic form can undergo anion exchange.
Poor to modest binding of the second row transition metal cations Ru and Rh was observed in all 4 matrices (Rh and Ru were added in their +2 oxidation states, but were likely air-oxidized to the +3 oxidation state during the course of these experiments). Presumably, the less effective binding of Ru and Rh is a reflection of the fact that first row transition metal cations generally have higher binding constants with the IDAA ligand than do metal cations farther down in the periodic table [1, 3, 33].
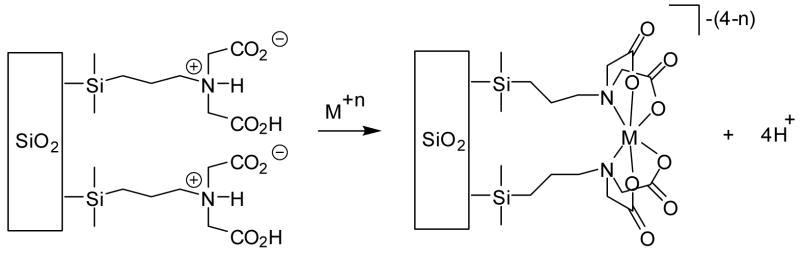
When comparing the performance of IDAA SAMMS® to Chelex 100 (a polymer-based ion-exchange resin that also contains the IDAA ligand), similar qualitative trends were observed at all pHs. However, the affinity the Chelex showed for the first row transition metal cations was notably weaker than that observed for the IDAA SAMMS®. One possible explanation for this might be found in the close proximity of the IDAA groups at the monolayer interface in SAMMS®. At a loading density of 0.91 silanes/nm2, the average distance between the silane anchors (and hence the IDAA ligands) is only about ~10Å, meaning that each IDAA ligand only needs to “lean over” ~5Å to achieve such a complex. This is easily within the flexibility provided by the alkyl silane. It is not unreasonable to suggest that if two adjacent IDAA groups were to chelate the same metal ion (as shown in Figure 3), that this would result in a higher net binding affinity for that metal ion than would be observed with a single IDAA ligand. In a disordered, globular polymer ion-exchange resin such multiple ligand chelation is less likely, and hence the overall binding affinity is lower.
Figure 3.
Possible chelation of a metal ion by adjacent IDAA groups on the silica surface.
The same affinity for Pd(II) under acidic conditions was noted with Chelex, lending support to the conclusion that it is an anion exchange process with PdCl4−2 taking place at the protonated ammonium sites.
Historically speaking, one of the more common sorbent materials used has been activated carbon, so it seemed logical for us to compare the performance of IDAA SAMMS® against this baseline. Given that the functionality of activated carbon is a randomly oriented mixture of carboxylic acids, phenols, lactones, etc. [34] it is not surprising that the affinity for the first and second row transition metals in these experiments was significantly lower than observed with either the IDAA SAMMS® or the Chelex 100 ion-exchange resin. As a general statement, single coordination sites simply cannot compete with a chelation effect, especially in an ordered monolayer system where multiple metal-ligand interactions are possible. There were two exceptions to this trend – the binding of Ru in filtered river water, and the binding of Pd(II). The uniqueness of the Ru bonding suggests that it may be some sort of speciation issue arising from some complexant in the filtered river water matrix (the Columbia River is known to have several ppm of chlorophyll, dissolved organic carbon, etc. in it [35] that may be responsible for this observation). As for the moderately good binding of Pd(II) by activated carbon observed in these experiments, several observations can be made. The Pd(II) speciation hasn’t changed relative to the IDAA SAMMS® and Chelex 100 experiments (discussed above), so we are most likely dealing with the same anionic chloride complex (PdCl4−2). The similar Pd(II) binding by activated carbon over a wide pH range suggests that there is no significant chemical change in the binding site from a pH of 2.5 to 8, so the binding site most likely is not a carboxylic acid (a “typical” ion exchange site for activated carbon). Phenols are excellent hydrogen bond donors and are capable of hydrogen bonding to anions, so we postulate that the observed affinity of activated carbon for Pd(II) is due to hydrogen bonding between the native phenols on the activated carbon and the anionic PdCl4−2 complex (we have observed such anion H-bonding to phenols in activated carbon previously [36]).
Conclusions
Iminodiacetic acid (IDAA) SAMMS® has been shown to be an easily made, useful sorbent for separating metal cations under a variety of conditions. IDAA SAMMS® have been shown to have good affinity for transition metal cations, especially first row transition metal cations. As has been observed with IDAA containing polymer ion-exchange resins, IDAA SAMMS® can also undergo anion exchange processes if the pH is < 9 (so the N atom is still protonated). The protonated ammonium salt allows for the binding of anionic metal complexes, like the PdCl4−2 complex. The close proximity (~10Å) of the IDAA ligands in the monolayer environment allows the IDAA SAMMS® to have a higher binding affinity than similar polymer-based ion exchange resins (e.g. Chelex 100), possibly as a result of multiple metal-ligand interactions. Such macromolecular chelation results in significantly higher binding affinity for transition metal species than is possible with simpler sorbent materials, like activated carbon.
Acknowledgments
This work was performed at Pacific Northwest National Laboratories, which is operated for the US-DOE by Battelle Memorial Institute under contract DE AC06-76RLO 1830. This research was supported by the Laboratory Directed Research and Development Program, and the National Institute of Environmental Health Sciences (NIEHS), grant# R21ES015620.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Cited Literature
- 1.Chaberek S, Jr, Martell AE. J Am Chem Soc. 1952;74:5052–5056. [Google Scholar]
- 2.Krasner J, Marinsky Jacob A. J Phys Chem. 1963;67:2559–2561. [Google Scholar]
- 3.Yuchi A, Sato T, Morimoto Y, Mizuno H, Wada H. Anal Chem. 1997;69:2941–2944. doi: 10.1021/ac9612685. [DOI] [PubMed] [Google Scholar]
- 4.Oleinikova M, Muraviev D, Valiente M. Anal Chem. 1999;71:4866–4873. doi: 10.1021/ac9902345. [DOI] [PubMed] [Google Scholar]
- 5.Anspach FB. Journal of Chromatography A. 1994;672:35–49. [Google Scholar]
- 6.Tikhomirova TI, Lukyanova MV, Fadeeva VI, Kudryavtsev GV, Shpigun OA. J Anal Chem. 1993;48:52–55. [Google Scholar]
- 7.Sugrue E, Nesterenko P, Paull B. J Sep Sci. 2004;27:921–930. doi: 10.1002/jssc.200401794. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue E, Nesterenko P, Paull B. Analyst. 2003;128:417–420. doi: 10.1039/b303467d. [DOI] [PubMed] [Google Scholar]
- 9.Nesterenko PN, Jones P. Journal of Chromatography A. 1998;804:223–231. [Google Scholar]
- 10.Nesterenko PN, Jones P. Analytical Communications. 1997;34:7–8. [Google Scholar]
- 11.Nesterenko P, Jones P. Journal of Liquid Chromatography & Related Technologies. 1996;19:1033–1045. [Google Scholar]
- 12.Nesterenko PN, Jones P. J Sep Sci. 2007;30:1773–1793. doi: 10.1002/jssc.200700126. [DOI] [PubMed] [Google Scholar]
- 13.Bashir W, Paull B. Journal of Chromatography A. 2001;907:191–200. doi: 10.1016/s0021-9673(00)01027-x. [DOI] [PubMed] [Google Scholar]
- 14.Bashir W, Paull B. Journal of Chromatography A. 2002;942:73–82. doi: 10.1016/s0021-9673(01)01358-9. [DOI] [PubMed] [Google Scholar]
- 15.Sadikova ZA, Tikhomirova TI, Lapuk AV, Fadeeva VI. J Anal Chem. 1997;52:206–208. [Google Scholar]
- 16.Gao ZF, Wang L, Qi T, Chu J, Zhang Y. Colloids and Surfaces A- Physicochem and Eng Aspects. 2007;304:77–81. [Google Scholar]
- 17.El-Ashgar NM, El-Nahhal IM, Chehimi MM, Babonneau F, Livage J. Materials Letters. 2007;61:4553–4558. [Google Scholar]
- 18.El-Nahhal IM, Zaggout FR, Nassar MA, El-Ashgar NM, Maquet J, Babonneau F, Chehimi MM. J Sol–Gel Sci Tech. 2003;28:255. [Google Scholar]
- 19.Fryxell GE, Cao G, editors. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors. 2007. published by Imperial College Press. [Google Scholar]
- 20.Zhang B, Poojary DM, Clearfield A, Peng G. Chem Mater. 1996;8:1333–1340. [Google Scholar]
- 21.Mao JG, Clearfield A. Inorg Chem. 2002;41:2319–2324. doi: 10.1021/ic011094w. [DOI] [PubMed] [Google Scholar]
- 22.Fryxell GE, Mattigod SV, Lin Y, Wu H, Fiskum S, Parker K, Zheng F, Yantasee W, Zemanian TS, Addleman RS, Liu J, Kemner K, Kelly S, Feng X. J Mater Chem. 2007;17:2863 – 2874. [Google Scholar]
- 23.Jyo A, Kugara J, Trobradovic H, Yamabe K, Sugo T, Tamada M, Kume T. Ind Eng Chem Res. 2004;43:1599–1607. [Google Scholar]
- 24.Woods CR, Ishii T, Boger DL. J Am Chem Soc. 2002;124:10676–10682. doi: 10.1021/ja026588m. [DOI] [PubMed] [Google Scholar]
- 25.Synthesis of IDAA silane. In a 1-L round bottomed flask, 30.5 mL (0.22 mole) of triethylamine was dissolved in 500 mL of dry tetrahydrofuran. The flask was then stoppered with a rubber septum and 24.4 mL (0.104 mole) of 3-aminopropyltriethoxysilane and 23.0 mL (0.208 mole) of ethyl bromoacetate (Caution! Lachrymator!) were added sequentially via syringe. The mixture was stirred overnight under N2, then septum was removed and the flask fitted with a reflux condenser and mixture was heated to reflux for 5 hours. At the end of this reflux period, it was cooled to room temperature and filtered to separate the precipitated Et3N-HBr. The solvent was removed by distillation and the crude product subjected to vacuum distillation to afford 35.76 g (87.0 mmole; 84% yield) of a pale yellow liquid (bp 160–170°C at 0.375 torr). 1H NMR (CDCl3) 4.15 (q, 4H, J = 7.2 Hz), 3.81 (q, 6H, J = 7.2 Hz), 3.55 (s, 4H), 2.71 (pt, 2H, J = 7.5 Hz), 1.59 (quintet, 2H, J = 8.1 Hz), 1.27 (t, 6H, J = 7.2 Hz), 1.22 (t, 9H, J = 7.0 Hz), and 0.61 (m, 2H). 13C NMR (CDCl3) 171.4, 60.4, 58.3, 57.3, 54.9, 21.2, 18.3, 14.2, and 7.6.
- 26.Deposition into MCM-41. 10.017 g of MCM-41 (920 m2/g, 35Å pores)[27, 28] was suspended in 200 mL of toluene and treated with 3.2 mL of water to hydrate the silica surface [22]. This slurry was stirred at room temperature overnight to insure uniform distribution of water throughout the sample. To this slurry, 11.0 g of the IDAA silane was added, and the mixture was heated to reflux for 4 hours. After this reflux period, the reflux condenser was replaced with a short path distillation head and the ethanol and water azeotropes were removed via distillation. The product was then collected by vacuum filtration, washed copiously with 2-propanol and air-dried to give 13.752 g of a free-flowing white powder. Given that the original silica sample had a surface area of ~9215 m2, and we observed a mass increase is 3.735g, if we assume a MW for the hydrolyzed silane of 267.05 g/mole (the “HO-Si(O)-R assumption”, see ref. 9), this corresponds to approximately 14 mmole of IDAA silane being deposited onto the MCM-41. Converting these results to molecules per square nanometer, reveals a surface population density of 0.91 silanes/nm2. For this silane, this corresponds to approximately 1.0 mmole/g.
- 27.Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL. J Am Chem Soc. 1992;114:10834–10842. [Google Scholar]
- 28.Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS. Nature. 1992;359:710–712. [Google Scholar]
- 29.Ho TL, Olah GA. Ang Chem Int Eng Ed. 1976;15:774–775. [Google Scholar]
- 30.Ester cleavage. The deposition was carried out using the protected ester form of the IDAA silane to avoid any complications from the carboxylic acids interfering with the siloxane hydrolysis/condensation chemistry during the self-assembly process. Thus, the esters needed to be cleaved after the self-assembly process. Trimethylsilyl iodide (TMSI) (Caution! Lachrymator!) [29] was used to perform this cleavage. Thus, a portion of the IDAA ester SAMMS® was placed in a pressure tube, along with an excess of TMSI, then the tube was sealed and then heated to 90–110°C for approximately 12 hours (this cleavage reaction was also carried out in benzene and CCl4 solution, with equal success). After cooling to room temperature, the product was subjected to hydrolysis in aqueous alcohol for 2 hours, then collected by vacuum filtration, washed with water, then with 5% NaHCO3 solution. If the sample retained any color (due to residual I2) then it was also washed with dilute aqueous NaHSO3 solution to bleach out the color. At this point the sample was once again washed with water, and 2-propanol, then air-dried.
-
31.Distribution coefficient measurements. For distribution coefficient (Kd) measurements, 0.01 M HHO3, 0.01 M HCl, filtered river water (from Columbia River, Richland, WA), or dialysate was spiked with metal ions (Co2+, Ni2+, Ru3+, Rh3+, and Pd2+) to obtain 50 μg/L each. The dialysate (PrismaSate® BGK4/2.5, Gambo Inc., Lakewood, CO) consisted of 2.5 mEq/L of Ca2+, 1.5 mEq/L of Mg2+, 140 mEq/L of Na+, 4 mEq/L of K+, 113 mEq/L of Cl−, 3 mEq/L of lactate, 32 mEq/L of HCO3−, 110 mg/dL of glucose, and osmolarity of 300 mOSm/L. After 30 min of incubation, 4.9 mL aliquots were added to a 20 mL polypropylene vial. The solution was then spiked with 0.1 mL of a suspension of solid sorbent and deionized distilled (DI) water at liquid per solid ratio of 100 mL/g. This resulted in a final L/S of 5,000. The control was performed in the same fashion but without solid sorbent. The samples were then shaken for 2 hrs at 160 rpm on an orbital shaker. Next, the solution was filtered thru 0.45-μm syringe Nylon-membrane filters and the filtrate was kept in 2 vol. % HNO3 prior to metal analysis. The metal concentrations were then analyzed using an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7500ce, Agilent Technologies, CA). All batch experiments were performed in triplicates and the averaged values were reported. Kd (in mL/g) is simply a mass-weighted partition coefficient between the solid phase and the liquid supernatant phase as follows:
where Co and Cf are the initial and final concentrations in the solution of the target species determined by ICP-MS, V is the solution volume in mL, and M is the mass in gram of the sorbent.(1) - 32.Cotton FA, Wilkinson G, editors. Advanced Inorganic Chemistry. 4. Wiley-Interscience; New York: 1980. p. 951. [Google Scholar]
- 33.Thompson LC. Inorg Chem. 1962;1:490–493. [Google Scholar]
- 34.For an excellent summary of the structure and properties of activated carbons, see: “Activated Carbon Adsorption” by R.C Bansal and M. Goyal; Taylor & Francis; New York; 2005; pp.1–51.
- 35.http://water.usgs.gov/nasqan/data/statsum/beaver.html
- 36.“Novel Sorbents for Removal of Gadolinium-Based Contrast Agents in Sorbent Dialysis: A Preventive Approach to Nephrogenic Systemic Fibrosis (NSF)” by Wassana Yantasee, Glen E. Fryxell, George A. Porter, Kanda Pattamakomsan, View Koonsiripaiboon, Vichaya Sukwarotwat, Jide Xu, and Kenneth N. Raymond, submitted for publication.