Abstract
Sonophoresis temporarily increases skin permeability such that medicine can be delivered transdermally. Cavitation is believed to be the predominant mechanism in sonophoresis. In this study, an ultrasound contrast agent (UCA) strategy was adopted instead of low frequency ultrasound to assure that cavitation occurred, and the efficacy of sonophoresis with UCA was quantitatively analyzed by optical measurements. The target drug used in this study was 0.1 % Definity® in 70% glycerol, which was delivered into porcine skin samples. Glycerol was used because it is an optical clearing agent, and the efficiency of glycerol delivery could be analyzed with optical measurements. The applied acoustic pressure was approximately 600 kPa at 1 MHz ultrasound with a 10% duty cycle for 60 minutes. Experimental results indicated that the measured relative contrast (RC) after sonophoresis with UCA was approximately 80% higher than RC after sonophoresis without UCA. In addition, the variance of RC was also reduced by more than 50% with the addition of a UCA. The use of a UCA appeared to increase cavitation, demonstrating that the use of a UCA can be effective in transdermal drug delivery (TDD).
Keywords: Transdermal drug delivery, ultrasound contrast agent, cavitation, sonophoresis, non-invasive drug delivery, optical clearing agent.
INTRODUCTION
Transdermal drug delivery (TDD) has great advantages compared to oral drug delivery in that it avoids gastrointestinal degradation and bypasses first-pass drug metabolism by the liver [1, 2]. However, TDD has been limited in the past due to low skin permeability since the stratum corneum (SC), the outermost layer of the skin, presents a formidable barrier. Hence, the steady delivery of large amounts of medicine is difficult to achieve with TDD.
Numerous innovative technologies have been developed to temporarily increase skin permeability in order to increase the applicability of TDD. Iontophoresis, which utilizes electric current to drive charged molecules through the skin, has been studied for the last 30 years [3-5]. As a result, numerous iontophoretic TDD systems are currently available. Although iontophoresis is quite effective as a TDD method, the great disadvantage is that drugs must be ionized before use. Electroporation uses very short (e.g., msec) high voltage (e.g., 100 – 1000V) pulses to increase skin permeability [1, 6-8]. However, its application can be limited due to pain caused by the electrical pulses [9]. Sonophoresis generally uses relatively low frequency ultrasound (e.g., <100 kHz) compared to ultrasound imaging systems [2], [10], which causes the cavitation of bubbles trapped on the skin. Although cavitation can be a violent event, sonophoresis has not been shown to cause significant skin damage [11, 12] Sonophoresis has also been shown to effectively deliver various types of drugs regardless of their electrical characteristics and can easily be coupled with other TDD methods to enhance the drug delivery rate.
Cavitation is a stochastic event due to random bubble size, but its application has been investigated in many biomedical areas. Actively-induced cavitation was used to lower the energy threshold of tissue damage in ultrasound surgery in the presence of an ultrasound contrast agent (UCA) in the form of engineered microbubbles [13]. UCAs have also been used to temporarily reduce the blood brain barrier (BBB) with low intensity ultrasound [14, 15]. Additionally, UCAs have also been investigated for their use as carriers for targeted drug delivery [16-18].
In the work presented here, sonophoretic TDD was studied in the presence of UCA. The commercially available UCA Definity® was used to enhance the optical clearing agent glycerol for delivery into porcine skin. The efficacy of sonophoresis with UCA was quantitatively and comparatively analyzed. The presented method was expected to be one of the most efficient, non-invasive TDD methods to date.
BACKGROUND
Microbubbles located near a boundary can be excited using an acoustic field to create asymmetric flows [19, 20] While stable cavitation occurs, microstreaming can be induced due to non-symmetric boundary conditions around the microbubbles. Additionally, shear stress can also be induced along the boundary. When combined, these two factors can temporarily increase skin permeability. On the other hand, asymmetric bubble collapse can be induced if the applied acoustic field has a large enough amplitude to cause inertia cavitation [19]. This bubble collapse can cause jet streaming into the boundary such that materials can be actively transported from outside to inside the boundary.
To assure cavitation, the use of low frequency acoustic fields has been adopted [2, 10, 11] because if the insonification frequency is low enough compared to the bubble resonance frequency, the given acoustic field can be assumed to be a quasi-static field, and inertia cavitation can be induced. However, this method may not efficient due to the fact that the existing bubbles are random in size.
A more efficient way to ensure cavitation could be to adopt a method where UCA with ultrasound was used at its resonance frequency. UCAs are engineered microsized bubbles such that they can be stably sustained within the fluid. Albumin, lipid, and polymers have all been used as shell materials to provide a balance force against Laplace pressure due to the curvature of the bubbles [16, 21, 22]. Safe and inert perfluorocarbon gases are generally used to generate the microbubbles. Since the size of the microbubbles is one of the dominant factors in determining the response characteristics to ultrasound stimulation, UCAs are usually filtered at the final stage of production; therefore UCAs have a resonance frequency in the MHz range and the distribution and the size of the microbubbles can be controlled by the addition of UCA.
METHOD
Skin Preparation
Porcine skin samples were used for these experiments as they are easy to access and their anatomical characteristics are similar to those of human skin [23]. Porcine skin samples were obtained and preserved by refrigeration at 5 ºC for no longer than five days. The subcutaneous fat layer was removed before experimentation, and the thickness of tissue samples ranged from 2.0 - 2.1 mm with an average thickness of 2.06 mm. Before the experiment, the prepared sample was kept in room temperature for approximately half an hour, so that the initial temperature was around 20-24°C.
Target Drug & UCA
The optical clearing agent glycerol was used as the target drug since the efficiency of sonophoresis in the presence of UCA can be visualized and quantitatively analyzed. If glycerol is transported into the skin, dehydration and collagen dissociation causes the index of refraction to better match that of air and the skin becomes more transparent [24, 25]. Therefore, the efficiency of TDD can be quantitatively analyzed with optical measurements. For this experiment, 70% glycerol was used.
The UCA Definity® (Bristol-Myers Squibb Medical Imaging, Inc., USA) was used to assure cavitation at high frequency in the MHz range because Definity® has a lipid-based shell and it can be mixed well with glycerol. Definity® was activated by Vialmix® (Lantheus Medical Imaging, Inc., USA) for 45 seconds and then 1 µl Definity® was added into 1 ml glycerol. The mixture was blended again with Vialmix® for uniform distribution of Definity®.
Experiment
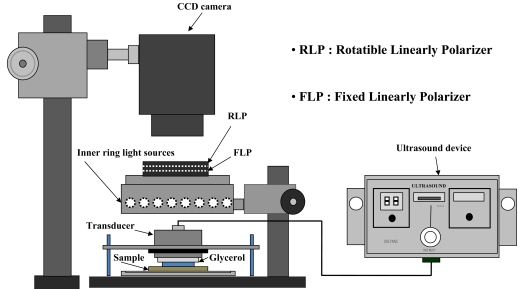
To visualize and analyze the TDD effect, a polarization variable imaging system (MAG Vision OptoBioMed, Korea) was used as shown in Fig. (1). The system consists of a linearly polarized LED ring light source and rotatable linear polarization analyzer. A CCD camera (MAG2000®, Canon, Japan) was located above the prepared skin sample for image acquisition. Images were taken every 20 minutes for 60 minutes using the cross-polarization mode of the imaging system in a dark room to prevent illuminant variation.
Fig. (1).
Experimental Setup. Approximately 1 ml of 70% Glycerol was used as optical clearing agents. If ultrasound used with or without UCA, 1 MHz ultrasound transducer for 20 minutes span. In case UCA was applied to the experiment, 0.1 % concentration was chosen to use according to the guideline in UCA specification. After optical image was taken identical procedure was conducted twice more. Inner ring light sources were used to provide uniform luminance to the porcine skin sample.
TDD efficacy was quantitatively evaluated by calculating the contrast and relative contrast (RC) of the modulation transfer function target (MTFT), which is a simple plate with black and white strips on in order to modulation function on image. A region of interest (ROC) was defined as the central region of glycerol application corresponding to a 1.5 x 1.5 cm2 area extracted from each image. Contrast and RC were calculated as a function of time after glycerol application as shown in equations (1) and (2);
| (1) |
| (2) |
where MC0 and MCt are the mean contrasts of MTFT in the ROC before glycerol application and after the elapsed time (t), respectively.
Six different experimental sets were conducted for comparison as shown in Table 1 and individual set was conducted five times. To provide base control data, experiment I was designed where 70% glycerol was topically applied and no other active TDD method was adopted. In experiment II, 0.1% Definity® was added to the 70% glycerol solution, and the mixture was applied topically without ultrasound insonification, which was conducted to determine if UCA alone could introduce chemically-induced active TDD.
Table 1.
Experimental Conditions
| Experiment No. | Experiment Condition | Purpose |
|---|---|---|
| Ex 1 | glycerol only | Control |
| Ex 2 | glycerol + UCA | Possible chemical cause |
| Ex 3 | glycerol + ultrasound (US) | Sonophoresis effect |
| Ex 4 | glycerol + microneedling (MN) + US | Air pocket + channel |
| Ex 5 | glycerol + UCA + US | Cavitation control cavitation |
| Ex 6 | glycerol + MN + UCA + US | Cavitation control + channel |
Sonophoresis without UCA was conducted in experiment III. A generic, pain reducing ultrasound system (DM77, Daeyang Inc., Korea) was used in all ultrasound insonification experiments. A single-element, 1 MHz transducer with an approximately 4.7 cm diameter aperture was used in the experiments. After glycerol application, the transducer was kept in good contact with the glycerol but was not allowed to press against the skin sample directly, leaving an approximate 2 mm distance between the transducer and the skin sample. Ten msec long pulses were used every 100 msec (10% duty cycle) for a total of 60 minutes. To ensure skin surface temperature was kept under 40ºC for practical use, the peak pressure was set to approximately 600 kPa. This value was obtained based upon back calculation of the total acoustic power measurement in water using equation (1) under the assumption that the acoustic pressure was evenly generated from the surface of the transducer;
| (3) |
where ρ is water density, C is the speed of sound in water, and I is measured intensity.
In experiment IV, a microneedle roller (Dermaroller, Horst Liebl, France) was used to create microchannels prior to sonophoresis. The needle size used was 70 µm in diameter and 500 µm in height. The roller had 192 needles on the cylindrical surface at a density of 240 channels/cm2. The roller was applied to the skin samples 50 times each in the horizontal, vertical, and diagonal directions. Although microneedling creates artificial transport channels invasively, the trapped air in the indented pockets also act as microbubbles. Glycerol was topically applied after microneedling, and the ultrasound was insonified as in experiment III.
UCA was used in conjunction with sonophoresis in experiment V with the same 0.1% Definity®/70% glycerol mixture as described in the previous section. The ultrasound parameters were identical to those in experiment III. Experiment VI was identical to experiment V except that the microneedling procedure occurred prior to sonophoresis with UCA.
RESULTS
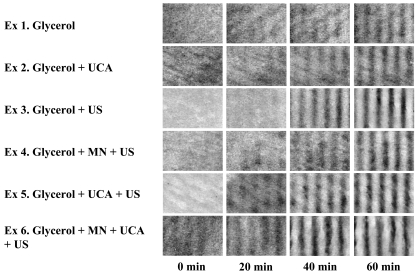
Fig. (2) shows examples of the experimental results. MTFT could be seen more clearly when more glycerol was delivered into the skin, as shown in Fig. (2). Since the initial condition of skin samples varied, the absolute contrast may not be a relevant way to compare the results. Nevertheless, sonophoresis in the presence of UCA still appeared to be the most effective method compared to the other TDD methods (Fig. 2).
Fig. (2).
Representative contrast images. Contrast images indicate that glycerol can be effectively delivered with sonophoresis with UCA. Initial condition of skin samples can be different as shown above pictures, hence relative contrast is required for the quantitative comparison of results.
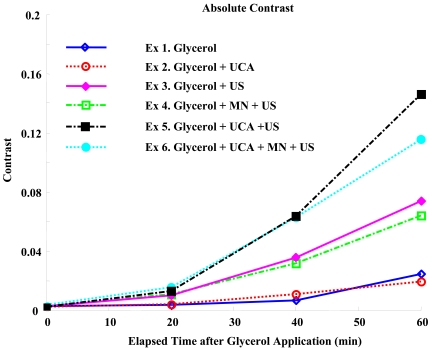
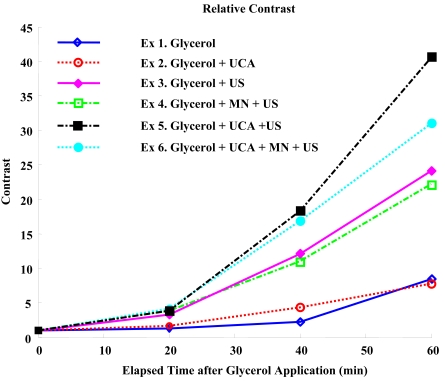
Contrast plots and relative contrast plots in Figs. (3 and 4) show the analytic comparison of individual results. As can be seen in Fig. (3), contrast was improved most by sonophoresis with UCA without advanced microneedling, although the standard deviation overlaps slightly. The relative contrast plot in Fig. (4) indicates that glycerol was delivered most efficiently by sonophoresis with UCA. Based on the experimental setup shown in Fig. (1), it should be noted that light travels through the skin sample twice before image recording, indicating that MTF of the skin will be squared in the current experiment, and an even linear increase will be shown as a second-order polynomial increase. As expected, the data in Figs. (3 and 4) more closely resemble a second-order polynomial than they do a line.
Fig. (3).
Absolute Contrasts. Sonophoresis with UCA shows most improvement in absolute contrast.
Fig. (4).
Relative Contrasts. Sonophoresis with UCA increases the relative contrast approximately 40 times. Additionally, RC is 80% higher compared to sonophoresis without UCA.
Average RC, the square root of the average RC, and standard deviations (STD) of RC were summarized in Tables 2 and 3. As demonstrated by the data in Fig. (4), microneedling did not seem to have a positive effect on sonophoresis, even though micro-sized channels were formed in advance. This may have occurred if the created channels acted as air pockets instead of as transporting channels in sonophoresis. Since the needle size was 70 µm in diameter and 500 µm in height, the created pocket size was expected to be on the order of 10 µm in diameter, at the least. Hence, the resonance frequency will be much lower than the 1 MHz insonification frequency.
Table 2.
Average Relative Contrasts (Standard Deviation)
| After 0 min | After 20 min | After 40 min | After 60 min | |
|---|---|---|---|---|
| Ex 1 | 1 | 1.29(0.34) | 2.26(1.01) | 8.51(4.19) |
| Ex 2 | 1 | 1.63(0.58) | 4.36(2.50) | 7.78(3.16) |
| Ex 3 | 1 | 3.30(4.24) | 12.09(4.01) | 24.20(8.05) |
| Ex 4 | 1 | 3.81(1.97) | 10.94(4.22) | 22.13(7.45) |
| Ex 5 | 1 | 3.87(1.68) | 18.30(5.59) | 40.66(3.93) |
| Ex 6 | 1 | 4.12(2.58) | 16.87(4.72) | 31.05(5.14) |
Table 3.
Square Root of Average Relative Contrast (RC)
| After 0 min | After 20 min | After 40 min | After 60 min | |
|---|---|---|---|---|
| Ex 1 | 1 | 1.14 | 1.50 | 2.92 |
| Ex 2 | 1 | 1.28 | 2.50 | 2.79 |
| Ex 3 | 1 | 1.81 | 3.48 | 4.92 |
| Ex 4 | 1 | 1.95 | 3.31 | 4.70 |
| Ex 5 | 1 | 1.97 | 4.28 | 6.38 |
| Ex 6 | 1 | 2.03 | 4.11 | 5.57 |
Another interesting observation of Fig. (4) and Table 4 was that the variance was small if ultrasound was applied in the presence of UCA without microneedling. This result may indicate that cavitation may be more controllable by regulating the bubbles, as in UCA.
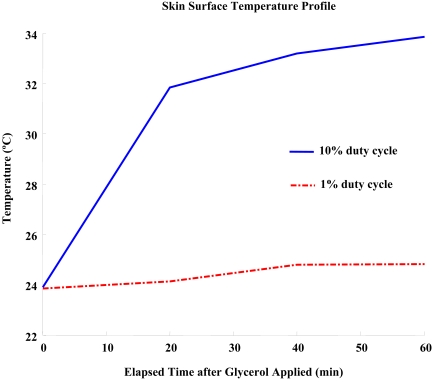
During the 10% duty cycle insonification, the temperature at the tissue surface increased by approximately 10ºC from 24ºC to 33ºC (see Fig. 5). Although the increase in temperature would be reduced in practical applications due to blood circulation, the effect of temperature in the current results should be isolated with cavitation. Therefore, a 1% duty cycle insonification was conducted and the results were compared to experiment V. The 1% duty cycle insonification still delivered glycerol effectively, although the used energy was only 10% of that in experiment V and the temperature increase was nominal (Table 4). Therefore, cavitation was the predominant mechanism over a thermal mechanism, and UCA could provide an affirmative environment for sonophoresis.
Fig. (5).
Temperature profile during ultrasound insonification.
Table 4.
RC of Duty Cycle 10% and 1% Insonification (Square Root of RC)
| After 0 min | After 20 min | After 40 min | After 60 min | |
|---|---|---|---|---|
| 10%(Ex 5) | 1 | 3.87(1.97) | 18.30(4.28) | 40.66(6.38) |
| 1% | 1 | 2.95(1.72) | 13.86(3.72) | 30.35(5.50) |
DISCUSSION
Since skin is not perfectly smooth, air pockets will always be formed on the skin surface. However, the size and the distribution of air pockets cannot be controlled. Traditionally, sonophoresis has been adopted low frequency to insure the caviation under this circumstance [2, 3, 10]. If the applied frequency is low enough compared to resonance frequency of the formed bubble, then it can be modeled as quasi pressure. In quasi-static low pressure, bubble can grow up to breaking stage. However, the efficiency will be low. This problem may be overcome by introducing UCA since UCA has specific size distribution fit to MHz range resonance frequency. Preliminary experimental results seem to support this hypothesis.
Although cavitation is a matter of probability, the results indicate that cavitation seems to be controllable and useful in TDD. To maximize cavitation in the presence of UCA, ultrasound parameters such as frequency, intensity, duty cycle, pulse repetition rate and pulse duration need to be optimized. In addition, the viscosity of the fluid and the type of UCA used need to be considered for individual drugs to be delivered. The viscosity of the fluid can be a deterministic factor for resonance frequency, and the surface materials of individual UCAs have different hydrophilic characteristics, which can be a determining factor in mixing drugs with UCA.
Although the efficacy of sonophoresis in the presence of UCA was shown using an in vitro system, in vivo animal model experiments need to be conducted for deterministic verification. In addition, drugs that are clinically relevant need to be studied with the suggested TDD method. Future work will include parameter optimization and the use of various animal models investigating insulin delivery by sonophoresis with UCA. Additionally, low frequency can be still used in the presence of UCA even though the resonance frequency does not match. Hence, low frequency driving system and equipment are being prepared for the further study.
CONCLUSIONS
The efficacy of sonophoresis with 0.1% UCA was quantitatively analyzed using optical measurements. Glycerol was used as the target drug for the purpose of optical clearance. Experimental results indicated that the proposed TDD method improved RC by approximately 80% compared to traditional sonophoresis at 1 MHz. In addition, the variance of RC was reduced by more than 50%. Based on these results, cavitation, which is believed to be the main mechanism in TDD, seems to be more predictable due to the small amount of UCA used. The proposed TDD method is expected to be useful for the transdermal delivery of various drugs.
ACKNOWLEDGEMENTS
The Ministry of Knowledge and Economy, Republic of South Korea supported this work.
REFERENCES
- 1.Prausnitz M R. “A practical assessment of transdermal drug delivery by skin electroporation”. Adv. Drug Deliv. Rev. 1999;35:61–76. doi: 10.1016/s0169-409x(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 2.Lavon I, Kost J. “Ultrasound and transdermal drug delivery”. DDT. 2004;9:670–676. doi: 10.1016/S1359-6446(04)03170-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh P, Maibach H I. “Iontophoresis in drug delivery: basic principles and applications”. Crit. Rev. Ther. Drug Carrier Syst. 1994;11:161–213. [PubMed] [Google Scholar]
- 4.Li G L, van der Geest R, Chanet L, van Zanten E, Danhof M, Bouwastra J. A. “In vitro iontophoresis of R–apomorphine across human stratum corneum Structure-transport relationship of penetration enhancement”. J. Controlled Release. 2002;84:49–57. doi: 10.1016/s0168-3659(02)00259-6. [DOI] [PubMed] [Google Scholar]
- 5.Guy R H, Kaliaa Y N, Delgado-Charroa M B, Merinoa V, Lopeza A, Marroa D. “Iontophoresis: electrorepulsion and electroosmosis”. J. Controlled Release. 2000;64:129–32. doi: 10.1016/s0168-3659(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Vanbever and R, Preat V. “In vivo efficacy and safety of skin electroporation”. Adv. Drug Deliv. Rev. 1999;35:77–88. doi: 10.1016/s0169-409x(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Hasegawa T, Sato S, Sugibayshi K. “Effect of electric field on the enhanced skin permeation of drugs by electroporation”. J. Controlled Release. 2003;90:171–9. doi: 10.1016/s0168-3659(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 8.Denet A R, Vanbever R, Préat V. “Skin electroporation for transdermal and topical delivery”. Adv. Drug Deliv. Rev. 2004;56:659–74. doi: 10.1016/j.addr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Wonga T W, Chena C H, Huangb C C, Linb C D, Hui S W. “Painless electroporation with a new needle-free microelectrode array to enhance transdermal drug delivery”. J. Controlled Release. 2006;110:557–65. doi: 10.1016/j.jconrel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Langer R. “New methods of drug delivery”. Science. 1990;249:1527–33. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 11.Mitragotri S, Blankschtein D, Langer R. “Transdermal Drug Delivery Using Low-Frequency Sonophoresis”. Pharm. Res. 1996;13:411–20. doi: 10.1023/a:1016096626810. [DOI] [PubMed] [Google Scholar]
- 12.Mitragotri S, Kost J. “Low-frequency sonophoresis: A review”. Adv. Drug Deliv. Rev. 2004;56:589–601. doi: 10.1016/j.addr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Tran B C, Seo J, Hal T L, Fowlkes J B, Cain C A. “Microbubble-enhanced cavitation for noninvasive ultrasound surgery”. IEEE Trans. UFFC. 2003;50:1296–304. doi: 10.1109/tuffc.2003.1244746. [DOI] [PubMed] [Google Scholar]
- 14.Hynynen K, McDannold N, Vykhodtseva N, Jolesz F A. “Noninvasive MR Imaging–guided Focal Opening of the Blood-Brain Barrier in Rabbits”. Radiology. 2001;220:640–6. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Pernot M, Small S, Konofagou E E. “Noninvasive, transcranial and localized opening of the blood-brain barrier using focused ultrasound in mice”. Ultrasound Med. Biol. 2007;33:95–104. doi: 10.1016/j.ultrasmedbio.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Klibanov A L. “Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging”. Adv. Drug Deliv. Rev. 1999;37:139–57. doi: 10.1016/s0169-409x(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 17.Huang S L, MacDonald R C. “Acoustically active liposomes for drug encapsulation and ultrasound-triggered release”. BBA. 2004;1665:134–41. doi: 10.1016/j.bbamem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara K, Pollard R, Borden M. “Ultrasound Microbubble Contrast Agents: Fundamentals and Application to Gene and Drug Delivery”. Ann. Rev. Biomed. Eng. 2007;9:415–47. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 19.Crum L A. “Sonoluminescence, sonochemistry, and sonophysics”. J. Acoust. Soc. Am. 1994;95:559–62. [Google Scholar]
- 20.Leighton T.G. The Acoustic Bubble: Academic Press; 1994. [Google Scholar]
- 21.GE Healthcare “OPT-1B”. Available: http://www.amershamhealth-us.com . 2006.
- 22.Waqqas s. “Full Prescribing Information for DEFINITY”. Available at: http://www.definityimaging.com/prescribing_info.html . 2007.
- 23.Netzlaff F, Schaefer U F, Lehr C M, Meiers P, Stahl J, Kietzmann M, Niedorf F. “Comparison of Bovine Udder Skin with Human and Porcine Skin in Percutaneous Permeation Experiments”. ATLA. 2006;34:499–513. [PubMed] [Google Scholar]
- 24.Moulton K, Lovell F, Williams E, Ryan P, Lay DC, Jansen D, Willard S. “Use of glycerol as an optical clearing agent for enhancing photonic transference and detection of Salmonella typhimurium through porcine skin”. J. Biomed. Optics. 2006;11:054027. doi: 10.1117/1.2363366. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Boese M, Turner P H, Wang R. “Penetration kinetics of dimethyl sulphoxide and glycerol in dynamic optical clearing of porcine skin tissue in vitro studied by Fourier transform infrared spectroscopic imaging”. J. Biomed. Optics. 2008;13:021105. doi: 10.1117/1.2899153. [DOI] [PubMed] [Google Scholar]
- 26.Yoon J, Park D, Son D, Seo J J, Nelson J S, Jung B. “A physical method to enhance transdermal delivery of tissue optical clearing agent: combination of microneedling and sonophoresis”. Lasers Surg. Med. Submitted. 2009 doi: 10.1002/lsm.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]