Abstract
The DNA of patients taking the immunosuppressant and anticancer drugs azathioprine or 6-mercaptopurine contains 6-thioguanine (6-TG). The skin of these patients is selectively sensitive to ultraviolet A radiation (UVA) and they suffer an extremely high incidence of sunlight-induced skin cancer with long-term treatment. DNA 6-TG interacts with UVA to generate reactive oxygen species, which oxidize 6-TG to guanine sulphonate (GSO3). We suggested that GSO3 is formed via the reactive electrophilic intermediates, guanine sulphenate (GSO) and guanine sulphinate (GSO2). Here, GSO2 is identified as a significant and stable UVA photoproduct of free 6-TG, its 2′-deoxyribonucleoside, and DNA 6-TG. Mild chemical oxidation converts 6-TG into GSO2, which can be further oxidized to GSO3—a stable product that resists further reaction. In contrast, GSO2 is converted back to 6-TG under mild conditions. This suggests that cellular antioxidant defences might counteract the UVA-mediated photooxidation of DNA 6-TG at this intermediate step and ameliorate its biological effects. In agreement with this possibility, the antioxidant ascorbate protected DNA 6-TG against UVA oxidation and prevented the formation of GSO3.
INTRODUCTION
Reactive oxygen species (ROS) are hazardous to cells. They cause damage to DNA, proteins and membranes. Cells are well adapted to the levels of ROS produced in the normal course of aerobic metabolism and are equipped with powerful antioxidant defences and repair systems to counteract the effects of inevitable damage to DNA. Exogenous sources of ROS can perturb this homeostasis and excess ROS create a condition of oxidative stress in which cellular defences are overwhelmed and cellular constituents are damaged. Potentially damaging ROS are produced when cells are exposed to ultraviolet A (UVA, wavelengths 320–400 nm) radiation. This reaction, which occurs at high (>100 kJ/m2) UVA doses, involves the absorption of UVA energy by cellular chromophores (1). DNA, a major target for damage by ROS, is a UVC and UVB chromophore but does not absorb the longer wavelength UVA to a significant degree. UVA comprises >90% of incident ultraviolet radiation at the earth’s surface and the inability of DNA to absorb UVA, and thereby generate ROS, is an important factor in minimizing sunlight-induced damage to DNA (2).
The thiopurines azathioprine (Aza), 6-mercaptopurine (6-MP), and 6-thioguanine (6-TG) are immunosuppressant, anti-inflammatory, and anticancer drugs (3). They are all metabolized to 6-TG nucleotides (TGNs) and cause the incorporation of 6-TG into DNA (4). Unlike the canonical DNA bases, 6-TG is a strong UVA chromophore. When 6-TG is irradiated with UVA (340 nm) reactive oxygen species (ROS) and, in particular, singlet oxygen (1O2) are generated (5,6). DNA 6-TG and the TGN pool are both significant sources of ROS when thiopurine-treated cells are UVA irradiated (7). The formation ROS from 6-TG within DNA itself is potentially extremely hazardous and 1O2 is an acknowledged source of oxidized DNA bases, DNA strand breaks (8) and oxidation of DNA associated proteins (9). In addition to this danger to normal cellular constituents, the low oxidation potential of 6-TG makes it a preferred target for oxidation and this compounds the hazard of photochemically generated ROS. We have previously shown that this favoured oxidation of DNA 6-TG generates guanine sulphonate (GSO3) a highly effective block to replication and transcription (5,6,10).
Long-term therapy with thiopurines, for example, in immunosuppressed organ transplant recipients treated with Aza, is associated with a very high incidence of skin cancer for which sunlight exposure is a contributory factor (11). The skin of patients undergoing Aza treatment contains DNA 6-TG and, as a consequence, is selectively sensitive to the induction of erythema by UVA (12). This UVA-selective photosensitivity suggests that photochemically induced DNA damage is physiologically relevant. In view of the huge incidence of skin cancer in this patient group, we have examined in more detail the photochemistry of 6-TG and DNA 6-TG in order to define the DNA lesions likely to be present in the skin of Aza-treated patients exposed to sunlight. The UVA doses we use in these in vitro studies fall well within the range to which patients might be exposed on an averagely sunny summer day in Northern Europe (13). DNA guanine sulphinate (GSO2) as a quantitatively major product of the photochemical oxidation of DNA 6-TG and an intermediate in the formation of the previously described DNA GSO3 (5). We demonstrate that GSO2 is converted back to 6-TG under relatively mild conditions, suggesting that cellular reducing agents may also provide some protection against sunlight by reversing photochemical damage to DNA 6-TG. In view of the involvement of ROS in the formation of these DNA lesions, we also examined the protective potential of the dietary vitamin ascorbate (vitamin C)—an acknowledged antioxidant in skin (14). We report that ascorbate prevents DNA damage and protects 6-TG, and DNA 6-TG, from UVA-induced oxidation.
EXPERIMENTAL PROCEDURES
Chemicals
6-thioguanine (6-TG), deoxyguanosine (dG), ascorbate (vitamin C), sodium sulphide (Na2S·9H2O), hydrogen peroxide 30%, acetic acid, magnesium monoperoxyphthalate (MMPP), Fe(NH4)2(SO4)2, Rose Bengal and iodine (I2) were obtained from Sigma Aldrich (Dorset, UK). Oligodeoxyribonucleotides were from Oligos Etc., Inc. (Wilsonville, USA). GSO3 was prepared as described (5). GSO2 was prepared according to a published protocol (15). Guanine-6-thioguanine (GSG) was prepared as before (6).
Chromatography
Four different systems of reverse-phase analyses for modified 6-TG and 6-TGdR were used.
System 1
Bases were separated by high-performance liquid chromatography (HPLC) on a Waters dC18 (Atlantis 3 µm, 150 × 2.1 mm) column using a Waters 2695 Alliance system equipped with photodiode array and Waters 474 dual monochromator fluorescence detector. Column eluates were monitored simultaneously by absorbance and fluorescence. Elution was with three solvents: A = methanol; B = water; C = 100 mM KH2PO4, pH 6.7. Flow rate was increased from 0.2 to 0.25 ml/min during the first 23 min, and at 0.25 ml/min thereafter. Solvent C was kept constant at 5% for 23 min and 0% thereafter. A gradient 0–20% Solvent A was applied during the first 10 min. Solvent A was increased to 80% over the next 10 min and to 90% over the next 3 min and maintained at 90% for 7 min.
System 2
Bases were separated by HPLC on a Waters C18 column (Xterra MS 3.5 µm, 150 × 2.1 mm) equipped and monitored as System 1. Solvents A and B were as System 1, Solvent C = 10 mM KH2PO4, pH 6.5. Flow rate was constant at 0.2 ml/min. Solvent C was kept at 20%. A gradient 0–20% Solvent A was applied during the first 10 min. Solvent A was increased to 80% over the next 10 min and maintained at 80% for 5 min.
System 3
Column, solvents A, B and C and eluates monitored were as System 1. Flow rate was 0.2 ml/min. Solvent C was kept constant at 5%. Solvent A was increased from 0% to 20% during the first 10 min, then increased to 80% during the next 10 min and maintained at 80% for 5 min.
System 4
2-deoxyribonucleosides were separated by HPLC on a Waters Symmetry C18 Reversed Phase column as described (5). 6-TGdR and dGSO2 were quantified by A342, dG by A260 and dGSO3 by its fluorescence (Ex = 320 nm; Em = 410 nm). Elution was with two solvents: A = 10 mM KH2PO4 pH 6.7; B = methanol. Flow rate was constant at 0.5 ml/min. A gradient 100–90% Solvent A was applied during the first 10 min, then 90–60% during the next 10 min, maintained at 60% for 2.5 min. This was followed by a sharp gradient 60–100% Solvent A for 0.5 min and constant at 100% for 17 min.
Nuclear magnetic resonance
Nuclear magnetic resonance (NMR) experiments were conducted at 300 MHz from JOEL (JNM-LA 300, FT NMR) and 400 MHz from JOEL (JNM-EX 400, FT NMR). Samples were dissolved in DMSO-d6 and chemical shifts were adjusted with reference to an internal standard tetramethylsilane (TMS).
Mass spectroscopy
Mass spectroscopy (MS) analysis was performed on a Fisons Instruments (VG BioTech, Altrincham, UK) Quattro Electrospray ionization mass spectrometer. Samples were dissolved in 50:50 MeOH:Water (ESI negative mode). High-resolution mass spectra (ESI negative mode) were obtained by the EPSRC Mass Spectrometry Service Centre, University of Swansea.
Fluorescence spectrometry
Fluorescence emission and excitation spectra were obtained using a FLUOROMAX-P spectrofluorometer, Jobin Yvon Horiba Inc. UK.
UVA irradiation
6-TG, 6-TGdR, oligonucleotides and genomic DNA were irradiated at a dose rate of 0.07 kJ/m2/s (for 6-TG) or 0.1 kJ/m2/s (for all other samples) using a UVH 254 lamp (UV Light Technology, wavelength range 320–400).
Digestion of DNA to 2′-deoxyribonucleosides
Genomic DNA was extracted using the Wizard Genomic DNA purification kit (Promega, Madison, WI, USA) from cells grown in medium containing 1 µM 6-TG for 24 h. Standard digestion to nucleosides for single-stranded oligonucleotides and genomic DNA was with nuclease P1 at pH 4.7 followed by shrimp alkaline phosphatase as described (10).
RESULTS
6-TG photoproducts
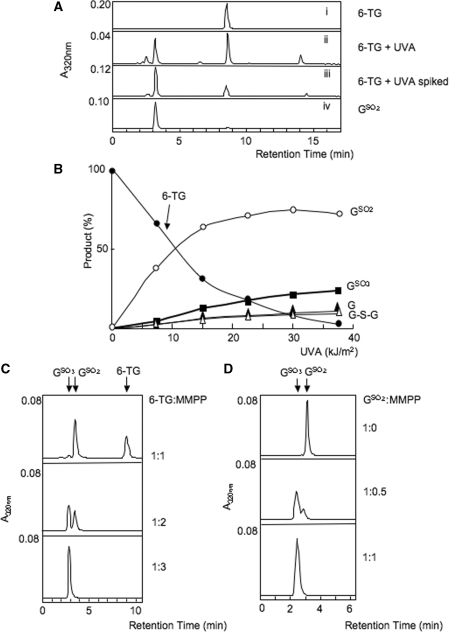
UVA irradiation of 6-TG in 0.1 mM aqueous solution causes a rapid loss of its characteristic A342 (5). HPLC separation of the 6-TG photoproducts after 21 kJ/m2 revealed four novel peaks with absorbance at 320 nm eluting at 2.8, 3.5, 6.5 and 14.8 min (Figure 1A, ii). The small amount of unchanged 6-TG eluted at 9 min. The compounds eluting at 2.8 and 14.8 min were the previously identified as the highly fluorescent (λex = 324 nm, λem = 408 nm) GSO3 and the 6-TG dimer, G-S-G, respectively. Their identities were confirmed by co-elution with the authentic compounds. A small peak at 6.5 min was identified as guanine by its UV spectrum.
Figure 1.
6-TG/UVA photoproducts. (A) Identification of GSO2 as a major photoproduct. 6-TG (0.1 mM in aqueous solution) was irradiated with 21 kJ/m2 UVA. Products were separated by HPLC System 1 as described in ‘Experimental Procedures’ section and the eluate monitored at 320 nm. (Top panel) Unirradiated 6-TG. (Second panel) 6-TG after 21 kJ/m2 UVA. (Third panel) As second panel, with authentic GSO2 added before HPLC. (Bottom panel) Authentic GSO2 (prepared by mild I2 oxidation of 6-TG) alone. (B) Quantitation of photoproducts. 6-TG [as in (A)] was irradiated with UVA at a dose rate of 0.07 kJ/m2/s. Samples were removed and analysed on HPLC. The four peaks of 320 mn absorbance with retention times of 2.8 min (filled square), 3.5 min (open circle), 6.5 min (filled triangle) and 14.8 min (open triangle) were identified as GSO3, GSO2, G and G-S-G as described in the text. Unaltered 6-TG (filled circle) eluted at 9 min. Quantitation was at the absorbance maximum for each product and by comparison to authentic standard compounds. GSO3 (A325), GSO2 (A320), G (A273), 6-TG (A340) and G-S-G (A331). Products are expressed mole % of unirradiated 6-TG. A representative of three independent experiments is shown. (C) 6-TG oxidation by MMPP. 6-TG (0.1 mM) was treated for 10 min at 20°C with MMPP at the final molar ratio indicated. Products were separated by HPLC System 2 and eluates monitored at 320 nm. Unchanged 6-TG elutes at 9 min, GSO2 at 3.5 min and GSO3 at 3.0 min. (D) GSO2 oxidation by MMPP. GSO2 (0.1 mM) was treated for 10 min at 20° with MMPP at the molar ratios shown. Products were separated by HPLC System 3.
The formation of these photoproducts was examined over a range of UVA doses. HPLC analysis indicated that the photoproduct eluting at 3.5 min was generated rapidly and that its formation preceded that of detectable amounts of GSO3 and G-S-G (Figure 1B). Comparison of its absorbance spectrum with published data (15) suggested that this initial product was guanine-6-sulphinate, GSO2. Authentic GSO2 was prepared by I2-mediated oxidation of 6-TG according to a published protocol (15) and crystallized. The product was not fluorescent, as expected, and its absorbance spectrum together with mass and NMR spectrometry confirmed that it was GSO2 (Supplementary Figure S1). Authentic GSO2 co-eluted from HPLC exactly coincident with the 3.5-min 6-TG photoproduct (Figure 1A, iii and iv). Together, the four identifiable UVA photoproducts: GSO2, GSO3, G, and G-S-G accounted for >90% of the 6-TG that was destroyed by UVA. GSO2 was the most abundant initial product. These findings indicate that GSO2 is a relatively stable intermediate in UVA-mediated 6-TG oxidation. It can be further oxidized to GSO3. Guanine and the G-S-G dimer are also generated as minor photoproducts.
Sequential oxidation of 6-TG was investigated further using MMPP which oxidizes 6-TG to GSO3 (6). Figure 1C shows that when 6-TG was treated for 10 min at room temperature (RT) with equimolar MMPP, some 6-TG remained unchanged and GSO2 was the only product detectable by A320. When MMPP was in 2-fold molar excess over 6-TG, approximately equal amounts of GSO2 and GSO3 were produced. Under stronger oxidising conditions (6-TG:MMPP = 1:3), 6-TG was stoichiometrically converted to GSO3. Purified GSO2 was quite stable and remained unchanged by overnight incubation at 20° in aqueous solution (data not shown). It could be, however, oxidized by MMPP. A 10-min treatment at RT with a limiting MMPP concentration (GSO2:MMPP = 1: 0.5) converted about 80% to GSO3. No other oxidation products were detected and higher MMPP concentrations (GSO2:MMPP = 1: 1), induced a stoichiometric conversion to GSO3 (Figure 1D).
We conclude that GSO2—which has absorbance at 320 nm but is not fluorescent (Supplementary Figure S1)—is the first stable product of 6-TG photooxidation. GSO2, GSO3, guanine and G-S-G together account quantitatively for all the 6-TG destroyed by UVA. GSO2 is produced by chemical oxidation under relatively mild conditions and, although stable, it easily undergoes further oxidation to GSO3.
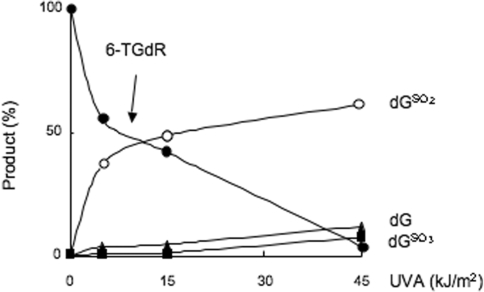
The stoichiometry of oxidation of the 6-TG 2′-deoxyribonucleoside (6-TGdR) was similar and dGSO2 was the major UVA photoproduct when an aqueous solution of 6-TGdR (20 µM) was irradiated. Approximately 50% was destroyed by 15 kJ/m2 and 90% by 45 kJ/m2. Figure 2 shows that at 15 kJ/m2, dGSO2 accounted for around 90% of the destroyed 6-TGdR. At higher doses, both dGSO3 and dG were formed. These minor products were present in similar amounts and each accounted for 10–20% of the destroyed 6-TGdR at 45 kJ/m2 UVA. There was no indication of the formation of other significant photoproducts at least up to 135 kJ/m2, the highest dose used (data not shown). dGSO3, dGSO2 and dG together accounted for ≥90% of the 6-TGdR destroyed by UVA. The minor fraction of input 6-TGdR that was unaccounted for was most likely converted to the nucleoside dimer analogous to G-S-G as we also observed a late-eluting product with A342 which might be consistent with this compound (Supplementary Figure S2). A final confirmation and quantification of this structure awaits synthesis of the authentic nucleoside dimer.
Figure 2.
UVA oxidation of 6-TG 2′-deoxyribonucleoside. 6-TGdR (20 µM aqueous solution) was irradiated with UVA in neutral conditions (10 mM Tris–HCl pH 7.5). Products were immediately separated by HPLC System 4. Quantitation of unaltered 6-TGdR (filled circle) and dGSO2 (open circle) by their A342, dGSO3 (filled square) by its fluorescence at 410 nm, dG (filled triangle) by its A260 were by comparison to authentic standard compounds. Products are expressed mole % of unirradiated 6-TG. A representative of three independent experiments is shown.
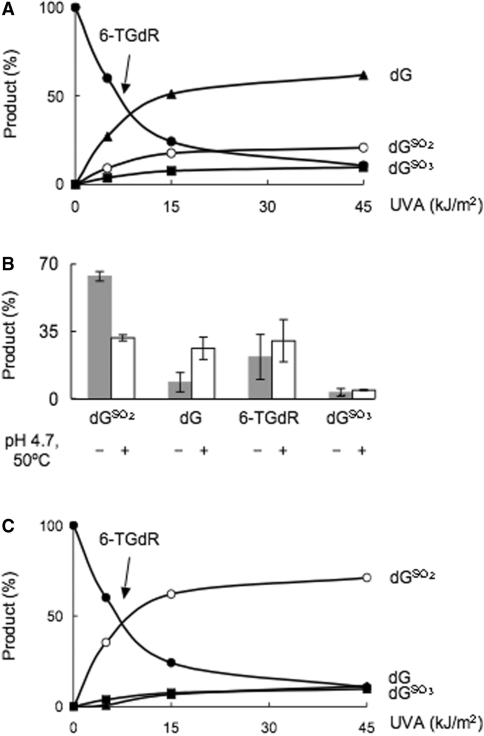
To approach the identification of DNA 6-TG photoproducts, we first examined the effect of UVA on single-stranded oligonucleotides containing a single 6-TG. Since our previous findings indicated that dG was a potential photoproduct, we first examined the effect of UVA on an 8-mer comprising 7As and a single 6-TG (A7TG1). This permitted the accurate quantitation of any dG formed. Irradiated single-stranded oligos were digested with nuclease P1 followed by alkaline phosphatase and the 2′-deoxyribonucleosides were quantified as above. In an attempt to minimize losses due to the known acid lability of the photoproducts (15), nuclease digestion of irradiated 8-mers was first carried out at pH 7. Under these conditions, photoproduct recovery was poor and over a range of UVA doses (5–45 kJ/m2) only around 30% of destroyed 6-TG could be accounted for by dGSO3, dGSO2 and dG. Previously unidentified photoproducts were generated in UVA-dependent fashion (Supplementary Figure S3). This appeared to be the product of incomplete P1 nuclease digestion under these sub-optimal conditions. Digestion was also incomplete when the irradiated oligo was incubated under optimal conditions (6 h at pH 8.0) for benzonase (Sigma), phosphodiesterase I and alkaline phosphatase (data not shown). These findings suggest that some 6-TG photoproducts are somewhat resistant to enzymatic digestion.
Under conditions that were optimal for nuclease P1, pH 4.7 and 50°C, digestion of irradiated (A7TG1) was complete and all of the destroyed 6-TG was accounted for by dGSO3, dGSO2 and dG. Under these conditions, dG appeared to be the major product (Figure 3A). In view of the known lability of GSO2 under acid conditions, it seemed likely that some of the dG had been generated from dGSO2 during DNA digestion at pH 4.7. To investigate this, UVA-irradiated 6-TGdR was subjected to the same conditions of temperature and pH. Figure 3B shows that this treatment resulted in loss of a significant fraction of dGSO2. The major part of the missing dGSO2 was recovered as dG with a small amount converted to 6-TGdR. dGSO2 formation in UVA irradiated oligos was corrected for this artefactual destruction of dGSO2 (see legend to Figure 3C). When this correction for artefactual conversion of dGSO2 to dG was applied, dGSO2 and dGSO3 together accounted for ≥95% of the destroyed 6-TG. dGSO2 was the major photoproduct (75–90% of the total) (Figure 3C).
Figure 3.
UVA oxidation of 6-TG in oligonucleotide. (A) Single-stranded 8-mer oligo A7TG1 (AAAAXAAA where X = 6-TG; 50-µM aqueous solution) was irradiated with UVA, then digested to 2′-deoxyribonucleosides by nuclease P1 (1 h, 50°, pH 4.7) followed by alkaline phosphatase as described in ‘Experimental Procedures’ section. Products were separated and quantitated as described in legend to Figure 2. Products are expressed mole % of unirradiated 6-TG. A representative of three independent experiments is shown. (B) 6-TGdR (20 µM aqueous solution) was irradiated with 15 kJ/m2 UVA, half the sample was immediately analysed by HPLC. The other half was subjected to the same conditions of temperature and pH as for nuclease P1 (pH 4.7, 1 h at 50°C) and alkaline phosphatase digestion before HPLC analysis. The means and SD of three independent experiments are shown. (C) Corrected photoproduct yield. The amount of dGSO2 destroyed under acidic conditions was calculated based on yield of dG from the irradiated A7TG1 and the known dG formation after UVA irradiation of 6-TGdR at pH 7. The total yield of dGSO2 = dGSO2 measured + dGSO2 calculated from (dG measured in A7TG1 oligo digested at pH 4.7—dG measured from irradiated 6-TGdR at neutral pH from Figure 2).
dGSO2 was also the major photoproduct in double-stranded DNA (Table 1). DNA uniformly substituted with 6-TG to ∼2% of G, was purified from cells that had been grown for 24 h in the presence of 6-TG. After UVA irradiation, heat denaturation, digestion with nuclease P1 under standard conditions (pH 4.7, 1 h, 50°) and treatment with alkaline phosphatase, dGSO3 appeared to be the predominant photoproduct detected by HPLC (it was not possible to quantify any increase in dG). The two oxidized forms, dGSO3 and dGSO2, together accounted for ∼20% of the starting DNA 6-TG and no other significant peaks of absorbance at 260, 320 or 342 nm were observed. When corrected for losses of labile dGSO2 under the conditions of enzyme digest, however, around 90% of input DNA 6-TGdR was accounted for and dGSO2 was again the major photoproduct. These data indicate that dGSO3 and dGSO2 comprise almost all of the UVA photoproducts of DNA 6-TGdR. At the relatively low doses of UVA that we use (5–15 kJ/m2), dGSO2 accounts for essentially all of the destroyed 6-TGdR and that dGSO3 and dG are formed to a minor extent. At higher doses, a measurable fraction (10–20% of the total) is oxidized to dGSO3 and a similar fraction of dG is formed. These photochemical reactions are largely independent of the 6-TG context and the proportions of photoproducts are similar for the base, the 2′-deoxyribonucleoside and for 6-TG in single- or double-stranded DNA.
Table 1.
6-TG/UVA photoproducts in genomic DNA
| UVA (kJ/m2) | Measured 6-TGdR (pmole) | Total dGSO2 (pmole) | Measured dGSO3 (pmole) | Estimated dG (pmole) | Recovery (%) |
|---|---|---|---|---|---|
| 0 | 510 | 0 | 0 | 0 | 100 |
| 5 | 282 | 158 | 30 | 7 | 94 |
| 15 | 158 | 230 | 53 | 26 | 92 |
| 45 | 65 | 271 | 78 | 54 | 92 |
DNA containing 6-TG (2% of guanine) was irradiated with UVA, and digested to nucleosides which were separated and quantified. Data are corrected for destruction of dGSO2 as in the legend to Figure 3.
Mechanism of 6-TG photooxidation
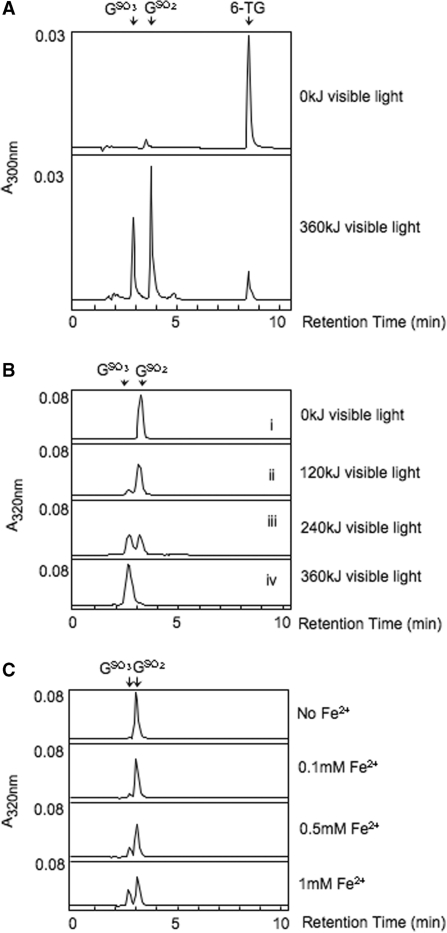
6-TG is a Type II UVA photosensitizer (6). We therefore investigated whether 1O2 generated photochemically by Rose Bengal and visible light mediated the stepwise conversion of 6-TG to GSO3 via GSO2. When a mixture of 6-TG (0.1 mM) with Rose Bengal (0.5 mM) was irradiated with 360-kJ visible light, ≥90% of the 6-TG was oxidized. Both GSO2 and GSO3 were produced (Figure 4A).
Figure 4.
Chemical oxidation of 6-TG, GSO2 and GSO3. (A) Rose Bengal and light treatment of 6-TG. Aqueous 6-TG (0.1 mM) was irradiated with visible light (200 W) at a dose of 360 kJ in the presence of 0.5 mM Rose Bengal. Products were separated by HPLC System 1. The relevant part of the absorbance (300 nm) trace is shown. (B) Rose Bengal and light treatment of GSO2 and GSO3. Aqueous GSO2 (upper panels) or GSO3 (lowest panel) (both 0.1 mM) were irradiated with visible light for the indicated times in the presence of 0.5 mM Rose Bengal. Products were separated by HPLC System 3. (C) Oxidation of GSO2. Aqueous GSO2 (0.1 mM) was incubated with the indicated concentrations of Fe(NH4)2SO4, and products analysed immediately by HPLC System 3.
Rose Bengal plus visible light treatment of authentic GSO2 provided further evidence for stepwise oxidation from 6-TG to GSO3. When combined with a 5-fold molar excess of Rose Bengal and irradiated with different doses of visible light, GSO2 was oxidized to GSO3 (Figure 4B, i-iii). In contrast, GSO3 was stable and remained unaltered when it was irradiated with 360-kJ visible light (Figure 4B, iv). Thus, oxidation of 6-TG to GSO2 in a Type II photochemical reaction can be recapitulated using Rose Bengal and visible light as a source of 1O2. GSO3, in which the S atom is in the highest oxidation state, is refractory to further oxidation by 1O2.
Direct chemical oxidation of 6-TG also proceeded via a reactive GSO2 intermediate to the ultimate product, GSO3, which resisted further oxidation. When 6-TG was treated under mild oxidizing conditions, GSO2 was formed as a stable product. Thus, oxidation with I2 is the synthetic route for GSO2 (15) (Figure 1A, iv). Under more stringent conditions, GSO2 was susceptible to oxidation and the pure compound was converted to GSO3 in the presence of Fe2+ in the form of Fe(NH4)2SO4 in a concentration-dependent manner (Figure 4C). These findings are consistent with the favourable oxidation of 6-TG to GSO2 as the first stable reaction product. GSO2 is further oxidized to GSO3 in a reaction that requires more rigorous conditions. GSO3 is refractory to further oxidation.
Ascorbate protects 6-TG against UVA-mediated oxidation
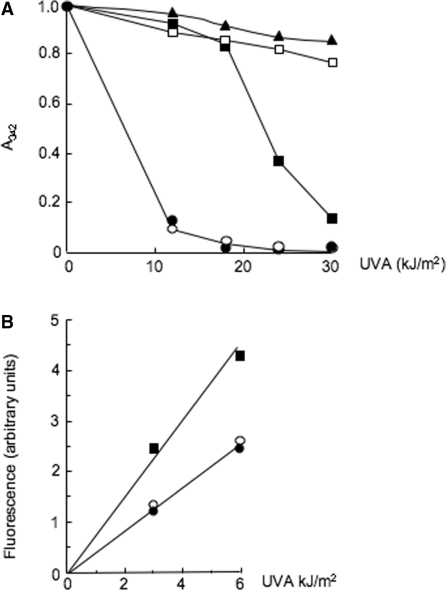
Ascorbate (vitamin C, Vc), an acknowledged antioxidant, prevented the UVA-mediated destruction of 6-TG. Solutions of 6-TG (0.1 mM) and ascorbate were UVA irradiated and the loss of A342 was monitored spectrophotometrically. In the absence of ascorbate, a dose of 11 kJ/m2 UVA destroyed >80% of the 6-TG (Figure 5A). A 12-fold molar excess of ascorbate during irradiation conferred significant protection and >80% and >30% of the 6-TG remained unchanged after UVA doses of 11 and 23 kJ/m2, respectively (Figure 5A). Higher ascorbate concentrations were even more protective and approached completion at Vc:6-TG ratios of ≥25, at which <20% of the 6-TG was destroyed by 30 kJ/m2 UVA. Experiments conducted in parallel confirmed that neither GSO2 nor GSO3 reacted with ascorbate (data not shown). Ascorbate only protected against 6-TG oxidation if it was present during the irradiation. When a 100-fold excess of ascorbate was added to 6-TG that had been previously irradiated with 21 kJ/m2, there was no change in the amounts of photoproducts and 6-TG was not regenerated (data not shown).
Figure 5.
Ascorbate protects against oxidation. (A) Protection of 6-TG against photochemical destruction. The 6-TG (0.1 mM in aqueous solution) was irradiated with the doses shown in the presence of different concentrations of ascorbate. Photochemical destruction of 6-TG was monitored by the reduction in A342 spectrophotometrically. (open circle) no ascorbate (filled circle) ascorbate:6-TG 3: 1 (filled square) 12: 1 (open square) 25: 1 (filled triangle) 100: 1. (B) Protection of DNA 6-TG. A 11-mer oligonucleotide (1 µM) (CAGXAATTCGC where X = 6-TG) was UVA irradiated in the presence of ascorbate as indicated; 0 µM ascorbate (filled square), 10 µM (open circle) or 20 µM (filled circle). Conversion of 6-TG to GSO3 in the intact oligonucleotide was monitored fluorimetrically (λex 320 nm; λem410 nm).
Ascorbate also prevented oxidation of 6-TG in an oligodeoxynucleotide. A 10- or 20-fold molar excess of ascorbate reduced by ∼2-fold the rate of UVA-induced conversion from 6-TG to GSO3 as monitored by spectrophotofluorimetry (Figure 5B).
Reversal of 6-TG oxidation by Na2S
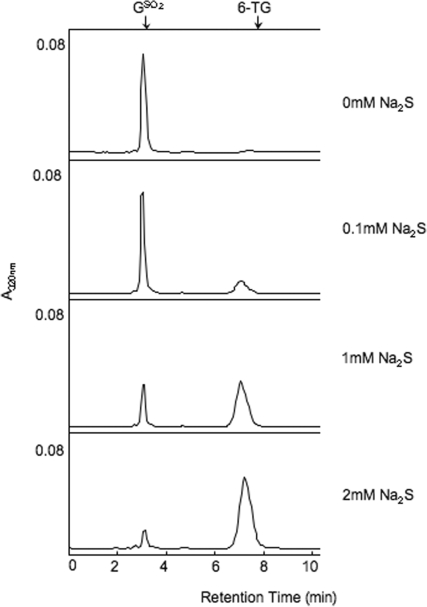
Na2S converts GSO3 to 6-TG in a slow reaction that takes several days at RT (6). When GSO2 (0.1 mM aqueous solution) was combined with Na2S at RT and the mixture analysed immediately by HPLC, there was a significant Na2S concentration-dependent conversion of GSO2 to 6-TG (Figure 6). A 20-fold excess of Na2S, converted ∼90% of the input GSO2 to 6-TG. This rapid and efficient reaction is in contrast to the slow generation of 6-TG from GSO3.
Figure 6.
Reversion of GSO2 by Na2S. Aqueous GSO2 (0.1 mM) was mixed with Na2S at RT to the final concentration indicated and the sample was immediately analysed by HPLC System 3. Products were detected by A320 nm. The known position of elution of 6-TG is shown arrowed.
DISCUSSION
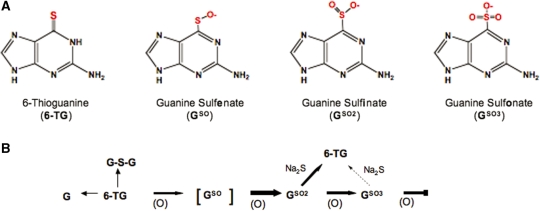
We previously reported that 6-TG is labile to UVA irradiation both as a free base and when incorporated into DNA (5,6). Exposure of 6-TG to UVA in the presence of oxygen causes the formation of 1O2. 6-TG is particularly vulnerable to oxidation and is easily oxidized by Rose Bengal plus visible light, an acknowledged 1O2 source. The highly fluorescent DNA GSO3 was previously identified as a significant product of 1O2-mediated oxidation (5,6). 6-TG has a low oxidation potential and under conditions in which normal DNA bases are invulnerable, 6-TG is quantitatively oxidized to GSO3 by treatment with the mild oxidizing agent MMPP. We proposed that, on UVA exposure, GSO3 was formed via the partially oxidized intermediates guanine sulfenate (GSO) and GSO2, both of which are reported to be unstable (16). Figure 7 summarizes these reactions. In this report, we identify GSO2 as a major stable photoproduct of the free 6-TG base, its 2′-deoxyribonucleoside, and of 6-TG in DNA. Under mild conditions, GSO2 is also the major stable product of MMPP oxidation of 6-TG. The initial oxidation of 6-TG to GSO2 is more favourable than the subsequent oxidation to GSO3. GSO3 itself is refractory to further oxidation. UVA irradiation of 6-TG or its 2′-deoxyribonucleoside also generated small amounts of G or dG. We did not observe any photoproduct, either from the free base or from DNA 6-TG, with properties expected of guanine sulfenate (GSO). This likely intermediate is possibly too unstable to withstand our analysis procedure. In a description of the properties of sulfenate, Abraham et al. (16) reported that it was not possible to isolate free sulphenic acids, and instead purified the silver salt of purine-6-sulfenate from which purine sulphenic acid could be released by acidification. The free acid had a relatively short half-life in aqueous solution, particularly at neutral pH. Under acidic conditions, purine-6-sulphenic acid was shown to break down to 6-thiopurine and hypoxanthine via purine-6-sulphenic acid. In the case of GSO, the analogous products would be 6-TG and G produced via GSO2.
Figure 7.
The 6-TG Oxidation: products and reactions. (A) Structures of 6-TG and oxidation products. (B) Reaction scheme for 6-TG. (O) represents oxidizing treatment and, in particular the 1O2 that is generated by the interaction of 6-TG with UVA. The more favourable reactions are shown with bold arrows.
This skin of patients taking thiopurines contains DNA 6-TG and is selectively hypersensitive to erythema induction by UVA (5,12). This is consistent with the formation of replication- and transcription-blocking DNA lesions (17). UVA treatment of cells containing DNA 6-TG generates 1O2 in DNA and this is also associated with severe inhibition of transcription and replication (6,10). The formation of replication- and transcription-blocking DNA lesions can be demonstrated in biochemical assays using purified enzymes and UVA- or MMPP-treated synthetic DNA substrates containing 6-TG. One aim of this study was to characterize the 6-TG photoproducts in DNA. In general, the DNA photoproducts qualitatively and quantitatively mirrored those formed by irradiation of free 6-TG or 6-TGdR in solution. The major exception is formation of the G-S-G dimer. This would be unlikely in DNA with a relatively low level of 6-TG substitution. We confirmed the formation of GSO3 and showed further that GSO2 is the major stable DNA photoproduct. Since together these two lesions account for >90% of the destroyed DNA 6-TG, at least at lower doses (45 kJ/m2), they are likely to be the ones with the biggest impact on biological processes.
In biochemical assays of DNA replication and transcription using template oligos containing a single 6-TG, the inhibition caused by a quantitative destruction of template 6-TG by UVA irradiation or by MMPP are closely similar (6,10). Our measurements reported here indicate that in the former case, >80% of the photoproducts will be GSO2, whereas MMPP treatment results in 100% conversion to GSO3. The similar effects of these DNA lesions on DNA and RNA polymerases indicated that DNA GSO2 and GSO3, which contain large, negatively charged substituent groups, are both very effective inhibitors of these enzymes.
The generation of damaging ROS is one of the inescapable hazards of aerobic metabolism and oxidation is a constant threat to DNA. Cellular antioxidant defences protect key cellular macromolecules from damage by normal levels of ROS. In addition to being a photochemical source of 1O2, DNA 6-TG is an important target for damage by cellular ROS, including H2O2 (18). We observed that ascorbate, an acknowledged dietary antioxidant and a significant intracellular consumer of 1O2, protected 6-TG and DNA 6-TG against photooxidation. These findings suggest that antioxidant defences may protect thiopurine-treated patients by preventing the oxidation of DNA 6-TG. Additional protection might arise via reversion of the major photoproduct, GSO2, back to 6-TG. Although GSO3 is stable and its reversion requires quite stringent conditions, the reaction of GSO2 is relatively favourable. Conversion of this potentially replication- and transcription-blocking DNA lesion back to the relatively innocuous 6-TG would ameliorate the UVA-mediated biological effects of DNA 6-TG. Overall, the oxidation of 6-TG via the reactive intermediates guanine sulfenate (GSO) and GSO2, and the persistence of the relatively reactive GSO2 is, however, likely to be hazardous. Both GSO and GSO2 are much more reactive towards nucleophiles than GSO3 the final oxidation product. Their reactivity raises the possibility that intermediate DNA GSO or accumulated GSO2, which forms in the skin of UVA-exposed patients taking thiopurines, may interact with cellular nucleophiles to form complex addition products that might compromise cellular DNA transactions and contribute to the extremely high incidence of sun-exposure-related skin cancer in this patient group. The possible reactions of GSO2 (and the highly unstable guanine sulfenate, GSO) with cellular reducing agents and nucleophiles are currently under investigation.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Funding for open access charge: Cancer Research UK.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to members of the EPSRC Mass Spectrometry Service Centre, University of Swansea for recording some of the mass spectra.
REFERENCES
- 1.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.WHO. International Programme on Chemical Safety, Ultraviolet Radiation. Geneva: World Health Organization; 1994. [Google Scholar]
- 3.Elion GB. The purine path to chemotherapy. Science. 1989;244:41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- 4.Weinshilboum R. Thiopurine pharmacogenetics: clinical and molecular studies of thiopurine metabolism. Drug Metab. Dispos. 2001;29:601–605. [PubMed] [Google Scholar]
- 5.O'D;onovan P, Perrett C, Zhang X, Montaner B, Xu Y.-Z, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science. 2005;309:1871–1874. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Jeffs G, Ren X, O'D;onovan P, Montaner B, Perrett CM, Karran P, Xu Y.-Z. Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light. DNA Repair. 2006;6:344–354. doi: 10.1016/j.dnarep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Cooke MS, Duarte TL, Cooper D, Chen J, Nandagopal S, Evans MD. Combination of azathioprine and UVA irradiation is a major source of cellular 8-oxo-7,8-dihydro-2′-deoxyguanosine. DNA Repair. 2008;7:1982–1989. doi: 10.1016/j.dnarep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Sies H. Damage to plasmid DNA by singlet oxygen and its protection. Mutat. Res. 1993;299:183–191. doi: 10.1016/0165-1218(93)90095-u. [DOI] [PubMed] [Google Scholar]
- 9.Montaner B, O’Donovan P, Reelfs O, Perrett CM, Zhang X, Xu Y.-Z, Ren X, Macpherson P, Frith D, Karran P. Reactive oxygen-mediated damage to a human DNA replication and repair protein. EMBO Rep. 2007;8:1074–1079. doi: 10.1038/sj.embor.7401084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brem R, Li F, Karran P. Reactive oxygen species generated by thiopurine/UVA cause irreparable transcription-blocking DNA lesions. Nucleic Acids Res. 2008;37:1951–1961. doi: 10.1093/nar/gkp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penn I. The problem of cancer in transplant patients: an overview. Transplant. Sci. 1994;4:23–32. [PubMed] [Google Scholar]
- 12.Perrett CM, Walker SL, O'D;onovan P, Warwick J, Harwood CA, Karran P, McGregor J. Azathioprine treatment sensitizes human skin to ultraviolet A radiation. Br. J. Dermatol. 2008;159:198–204. doi: 10.1111/j.1365-2133.2008.08610.x. [DOI] [PubMed] [Google Scholar]
- 13.Driscoll CMH, Campbell JI, Pearson AJ, Grainger K.J.-L, Dean SF, Clark IE. 2002. National Radiological Protection Board, Chilton, Didcot, Oxon. OX11 0RQ, pp. 1–14. [Google Scholar]
- 14.Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr. 2004;24:173–200. doi: 10.1146/annurev.nutr.24.012003.132320. [DOI] [PubMed] [Google Scholar]
- 15.Doerr IL, Wempen I, Clarke DA, Fox JJ. Thiation of nucleosides III. Oxidation of 6-mercaptopurines. J. Org. Chem. 1961;26:3401–3409. [Google Scholar]
- 16.Abraham RT, Benson LM, Jardine I. Synthesis and pH-dependent stability of purine-6-sulfenic acid, a putative reactive metabolite of 6-thiopurine. J. Med. Chem. 1983;26:1523–1526. doi: 10.1021/jm00364a031. [DOI] [PubMed] [Google Scholar]
- 17.Garssen J, van Steeg H, de Gruijl F, de Boer J, van der Horst GTJ, van Kranen H, van Loveren H, van Dijk M, Fluitman A, Weeda G, et al. Transcription-coupled and global genome repair differentially influence UV-B-induced acute skin effects and systemic immunosuppression. J. Immunol. 2000;164:6199–6205. doi: 10.4049/jimmunol.164.12.6199. [DOI] [PubMed] [Google Scholar]
- 18.Daehn I, Karran P. Immune effector cells produce lethal DNA damage in cells treated with a thiopurine. Cancer Res. 2008;69:2393–2399. doi: 10.1158/0008-5472.CAN-08-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.