Abstract
The transposon-like structure Tn4003 and related elements were found to encode high- and low-level trimethoprim resistance (Tpr) in Staphylococcus aureus and coagulase-negative staphylococci. By using transcriptional fusions in Escherichia coli, the variation in resistance levels was found to correlate with the transcriptional activity of the region presumed to carry the promoter for the operon containing the Tpr dihydrofolate reductase gene, dfrA, encoded by these elements. The reduced transcriptional activities exhibited by elements encoding low-level Tpr appear to be a consequence of deletions adjacent to the copy of IS257 which normally encodes the -35 sequences of these promoters. The data obtained not only support the involvement of IS257 in the transcription of the proposed thyE-dfrA-orf-140 operon of Tn4003 but may also implicate this insertion sequence in the mechanisms resulting in the variation in Tpr levels observed in staphylococci.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur A., Sherratt D. Dissection of the transposition process: a transposon-encoded site-specific recombination system. Mol Gen Genet. 1979 Oct 1;175(3):267–274. doi: 10.1007/BF00397226. [DOI] [PubMed] [Google Scholar]
- Barberis-Maino L., Berger-Bächi B., Weber H., Beck W. D., Kayser F. H. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987;59(1):107–113. doi: 10.1016/0378-1119(87)90271-x. [DOI] [PubMed] [Google Scholar]
- Barker J., Healing D., Hutchison J. G. Characteristics of some co-trimoxazole-resistant Enterobacteriaceae from infected patients. J Clin Pathol. 1972 Dec;25(12):1086–1088. doi: 10.1136/jcp.25.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdeska A., Ott M., Bannwarth W., Then R. L. Identical genes for trimethoprim-resistant dihydrofolate reductase from Staphylococcus aureus in Australia and central Europe. FEBS Lett. 1990 Jun 18;266(1-2):159–162. doi: 10.1016/0014-5793(90)81529-w. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Gillespie M. T., Skurray R. A. 4',4'' adenyltransferase activity on conjugative plasmids isolated from Staphylococcus aureus is encoded on an integrated copy of pUB110. Plasmid. 1991 Jan;25(1):70–75. doi: 10.1016/0147-619x(91)90008-k. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Gillespie M. T., Skurray R. A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990 Nov;34(11):2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughter J. P., Johnston J. L., Archer G. L. Characterization of a staphylococcal trimethoprim resistance gene and its product. Antimicrob Agents Chemother. 1987 Jul;31(7):1027–1032. doi: 10.1128/aac.31.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G. E., Then R. L., Stüber D. Characterization of the gene for chromosomal trimethoprim-sensitive dihydrofolate reductase of Staphylococcus aureus ATCC 25923. Antimicrob Agents Chemother. 1993 Jul;37(7):1400–1405. doi: 10.1128/aac.37.7.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Dyke K. G. Characterization of the conjugation system associated with the Staphylococcus aureus plasmid pJE1. J Gen Microbiol. 1988 Jan;134(1):1–8. doi: 10.1099/00221287-134-1-1. [DOI] [PubMed] [Google Scholar]
- Firth N., Ridgway K. P., Byrne M. E., Fink P. D., Johnson L., Paulsen I. T., Skurray R. A. Analysis of a transfer region from the staphylococcal conjugative plasmid pSK41. Gene. 1993 Dec 22;136(1-2):13–25. doi: 10.1016/0378-1119(93)90442-6. [DOI] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. T., May J. W., Skurray R. A. Antibiotic susceptibilities and plasmid profiles of nosocomial methicillin-resistant Staphylococcus aureus: a retrospective study. J Med Microbiol. 1984 Jun;17(3):295–310. doi: 10.1099/00222615-17-3-295. [DOI] [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Inglis B., Matthews P. R., Stewart P. R. Induced deletions within a cluster of resistance genes in the mec region of the chromosome of Staphylococcus aureus. J Gen Microbiol. 1990 Nov;136(11):2231–2239. doi: 10.1099/00221287-136-11-2231. [DOI] [PubMed] [Google Scholar]
- Jilk R. A., Makris J. C., Borchardt L., Reznikoff W. S. Implications of Tn5-associated adjacent deletions. J Bacteriol. 1993 Mar;175(5):1264–1271. doi: 10.1128/jb.175.5.1264-1271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamond A. I., Travers A. A. Requirement for an upstream element for optimal transcription of a bacterial tRNA gene. Nature. 1983 Sep 15;305(5931):248–250. doi: 10.1038/305248a0. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Hopkins J. D., Syvanen M. Direct involvement of IS26 in an antibiotic resistance operon. J Bacteriol. 1990 Jun;172(6):3229–3236. doi: 10.1128/jb.172.6.3229-3236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichens-Park A., Syvanen M. Cointegrate formation by IS50 requires multiple donor molecules. Mol Gen Genet. 1988 Feb;211(2):244–251. doi: 10.1007/BF00330600. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Gillespie M. T., Skurray R. A. Detection and characterization of IS256, an insertion sequence in Staphylococcus aureus. J Gen Microbiol. 1987 Nov;133(11):3031–3038. doi: 10.1099/00221287-133-11-3031. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., May J. W., Skurray R. A. Analysis of plasmids in nosocomial strains of multiple-antibiotic-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):817–826. doi: 10.1128/aac.23.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Morton T. M., Eaton D. M., Johnston J. L., Archer G. L. DNA sequence and units of transcription of the conjugative transfer gene complex (trs) of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1993 Jul;175(14):4436–4447. doi: 10.1128/jb.175.14.4436-4447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J., Archer G. L. Mobilization of the relaxable Staphylococcus aureus plasmid pC221 by the conjugative plasmid pGO1 involves three pC221 loci. J Bacteriol. 1989 Apr;171(4):1841–1845. doi: 10.1128/jb.171.4.1841-1845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Skurray R. Cloning and polypeptide analysis of the leading region in F plasmid DNA transfer. Plasmid. 1983 May;9(3):262–272. doi: 10.1016/0147-619x(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Reddy P., Peterkofsky A., McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5656–5660. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. E., Ascherman D., Kleckner N. IS10 promotes adjacent deletions at low frequency. Genetics. 1991 May;128(1):37–43. doi: 10.1093/genetics/128.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
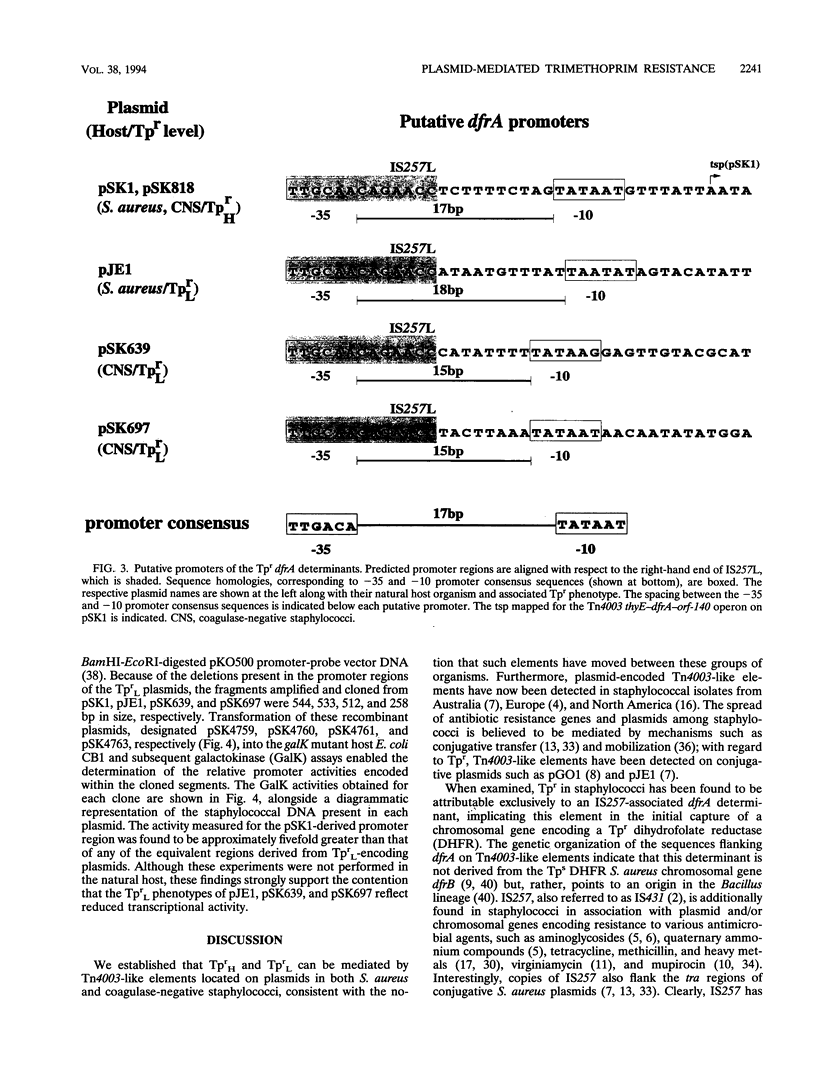
- Rouch D. A., Messerotti L. J., Loo L. S., Jackson C. A., Skurray R. A. Trimethoprim resistance transposon Tn4003 from Staphylococcus aureus encodes genes for a dihydrofolate reductase and thymidylate synthetase flanked by three copies of IS257. Mol Microbiol. 1989 Feb;3(2):161–175. doi: 10.1111/j.1365-2958.1989.tb01805.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Tucker J. F. The virulence of trimethoprim-resistant thymine-requiring strains of Salmonella. J Hyg (Lond) 1976 Feb;76(1):97–108. doi: 10.1017/s0022172400054991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. M., Lyon B. R., Gillespie M. T., May J. W., Skurray R. A. Cloning and expression of Staphylococcus aureus plasmid-mediated quaternary ammonium resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Jan;27(1):79–83. doi: 10.1128/aac.27.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennent J. M., May J. W., Skurray R. A. Multiple antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis: plasmids in strains associated with nosocomial infection. Pathology. 1984 Jul;16(3):250–255. doi: 10.3109/00313028409068532. [DOI] [PubMed] [Google Scholar]
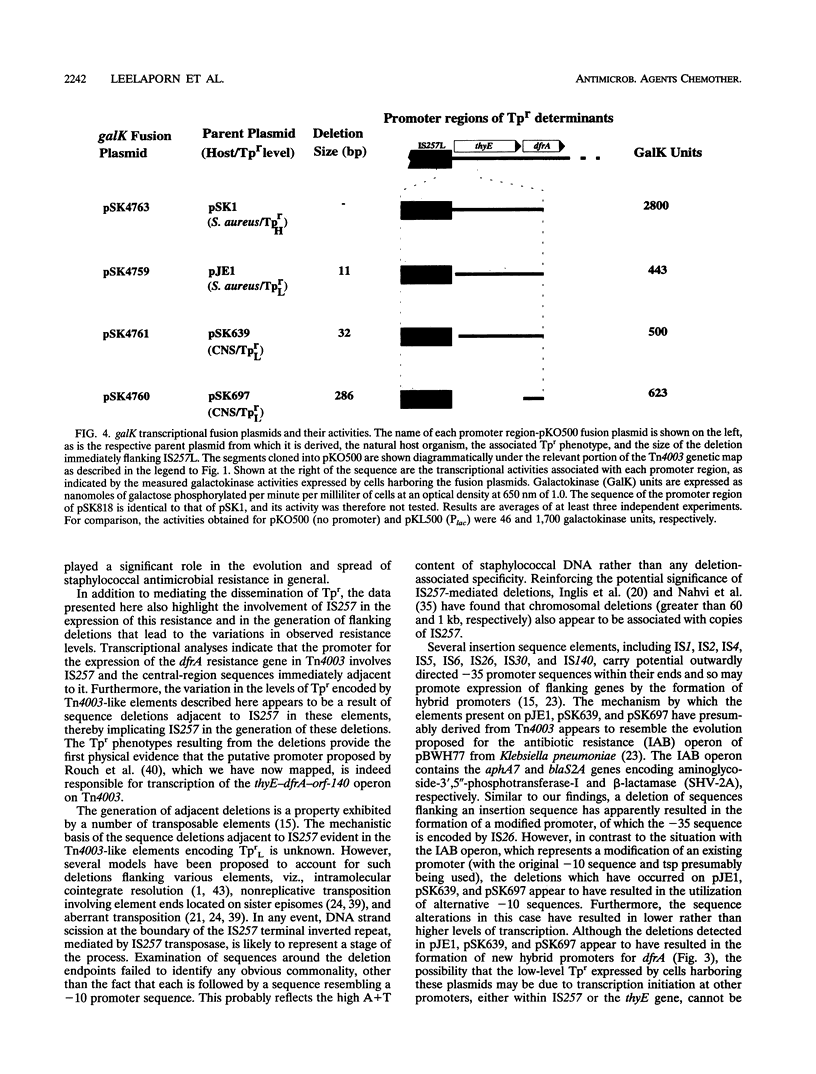
- Tennent J. M., Young H. K., Lyon B. R., Amyes S. G., Skurray R. A. Trimethoprim resistance determinants encoding a dihydrofolate reductase in clinical isolates of Staphylococcus aureus and coagulase-negative staphylococci. J Med Microbiol. 1988 May;26(1):67–73. doi: 10.1099/00222615-26-1-67. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Young H. K., Skurray R. A., Amyes S. G. Plasmid-mediated trimethoprim-resistance in Staphylococcus aureus. Characterization of the first gram-positive plasmid dihydrofolate reductase (type S1). Biochem J. 1987 Apr 1;243(1):309–312. doi: 10.1042/bj2430309. [DOI] [PMC free article] [PubMed] [Google Scholar]