Abstract
Spinal reflexes are mediated by synaptic connections between sensory afferents and motor neurons1-3. The organization of these circuits exhibits several levels of specificity. Only certain classes of proprioceptive sensory neurons make direct, monosynaptic, connections with motor neurons4. Those that do are bound by rules of motor pool specificity – they form strong connections with motor neurons supplying the same muscle, but avoid motor pools supplying antagonistic muscles1, 5-7. This pattern of connectivity is initially accurate and is maintained in the absence of activity8, implying that wiring specificity relies on the matching of recognition molecules on the surface of sensory and motor neurons. But determinants of fine synaptic specificity here, as in most regions of the central nervous system (CNS), have yet to be defined. To address the origins of synaptic specificity in these reflex circuits we have used molecular genetic methods to manipulate recognition proteins expressed by subsets of sensory and motor neurons. We show here that a recognition system involving expression of Sema3e by selected motor neuron pools, and its high-affinity receptor PlexinD1 by proprioceptive sensory neurons, is a critical determinant of synaptic specificity in sensory-motor circuits. Changing the profile of Sema3e-PlexinD1 signaling in sensory or motor neurons results in functional and anatomical rewiring of monosynaptic connections, but does not alter motor pool specificity. Our findings indicate that patterns of monosynaptic connectivity in this prototypic CNS circuit are constructed through a recognition program based on repellent signaling.
Several observations led us to focus on the potential contribution of the class 3 semaphorin Sema3e and its receptors as mediators of sensory-motor synaptic specificity. Sema3e is expressed by a restricted set of brachial motor neurons and can serve as a bifunctional ligand, eliciting repellent responses through engagement of PlexinD1 and attractant responses through interactions with a Neuropilin-1/PlexinD1 receptor complex9-12. Moreover, Sema3e expression is lost, and the pattern of monosynaptic sensory-motor connections altered, in mice mutant for Pea3, an ETS transcription factor expressed by several brachial motor neuron pools11, 13.
We analyzed the role of Sema3e and its receptors in two sensory-motor reflex arcs. In one reflex arc that supplies the triceps (Tri) forelimb muscle, motor neurons receive monosynaptic input from Tri sensory afferents13. But in a second, atypical, reflex arc that controls the cutaneous maximus (Cm) muscle, motor neurons fail to receive monosynaptic input from Cm afferents13-15. Cm motor neurons also lack monosynaptic input from Tri proprioceptive neurons, or from any other proprioceptive afferents, and conversely, Tri motor neurons lack monosynaptic input from Cm proprioceptive afferents13. The contrasting circuitry of these two reflex arcs permitted us to examine monosynaptic connectivity between proprioceptive sensory and motor neurons, as well as pool specificity, revealed by the absence of connections between functionally unrelated sensory-motor pairs.
To define the brachial motor neurons that express Sema3e, we identified motor neuron pools by retrograde labeling in Sema3enlz mice in which nuclear LacZ is expressed from the Sema3e locus (Fig 1a, Supplementary Figs 1, 2a). In p0 Sema3enlz/+ mice, all LacZon motor neurons were confined to the Cm pool and ∼85% of all Cm motor neurons expressed LacZ (Fig 1a, b). In contrast, neither Pea3 nor LacZ were expressed in Tri motor neurons (Fig 1a, Supplementary Fig 2b)13. We also determined the profile of expression of Sema3e receptors by sensory neurons. At e16.5, Neuropilin-1 expression was excluded from TrkCon proprioceptive neurons (Supplementary Fig 3a-d). In contrast, ∼90% of PlexinD1on DRG neurons co-expressed TrkC, although only ∼50% of all TrkCon DRG neurons expressed PlexinD1 (Fig 1c, Supplementary Fig 3g). PlexinD1 was expressed by ∼80% of TrkCon proprioceptive neurons labeled retrogradely from the Cm muscle nerve, by ∼50% of TrkCon Tri proprioceptors, but by only ∼5% of TrkCon Ulnaris proprioceptors (Fig 1b, d, data not shown). Thus within the Cm reflex arc, which lacks monosynaptic connectivity, most motor neurons express Sema3e and most proprioceptive sensory neurons express PlexinD1, whereas in the monosynaptically-connected Tri reflex arc the expression of PlexinD1 by proprioceptors is not accompanied by motor neuron Sema3e expression (Fig 1b).
Figure 1. Sema3e marks Cm motor neurons and PlexinD1 proprioceptive neurons.

(a) Sema3e directed LacZ in p0 Sema3enlz spinal cords after retrograde f-dextran tracing from Cm or Tri muscle nerves and summary of Cm, Ld, Tri motor pool position.
(b) PlexinD1 expression in TrkCon proprioceptive afferents (top) and Sema3e expression in motor neurons (bottom) projecting to Cm, Tri or Uln muscles.
(c) PlexinD1 expression in e16.5 c7 DRG, matched with TrkC and TrkA.
(d) PlexinD1 (left) and TrkC (right) expression in e16.5 DRG neurons after retrograde f-dextran tracing from Cm or Tri muscle nerves.
We used gene targeting to assess the contribution of Sema3e and PlexinD1 to the formation and specificity of monosynaptic connections in the Cm and Tri reflex arcs. We analyzed homozygous null Sema3enlz/nlz mice (Supplementary Fig 1b-d) and conditional PlexinD1flox mice in which PlexinD1 function in DRG neurons was eliminated by intercrossing with Wnt1Cre mice16, 17. Sema3enlz/nlz and PlexinD1flox/-Wnt1Cre (PlexinD1cond) mice are viable and fertile, permitting studies of sensory-motor connectivity at post-natal stages. Analysis of LacZon, f-dex labeled motor neurons in p1 Sema3enlz/+ and Sema3enlz/nlz mice revealed no difference in the positions of the cell bodies and dendrites of Cm or Tri motor neurons (Supplementary Fig 4). Pea3 mutant mice exhibit pronounced defects in Cm motor neuron positioning and dendrite patterning11, 13, but our findings indicate that Sema3e does not mediate these aspects of Pea3's influence on motor neuron differentiation.
To assess the contribution of Sema3e signaling to the functional specificity of monosynaptic connections in sensory-motor reflex arcs we stimulated, separately, the Cm and Tri muscle nerves in spinal cord preparations isolated from p5 to p7 mice and recorded intracellular responses from identified motor neurons (Supplementary Figs 5, 6)13. In wild-type mice, Cm motor neurons lacked monosynaptic input after stimulation of Cm afferents (Fig 2a)13. In contrast in Sema3enlz/nlz mice, 45% of Cm motor neurons received monosynaptic input after stimulation of Cm afferents (mean latency 2.9 ± 0.2ms; 21/47 cells in 10 mice) (Fig 2b). Cm motor neurons in Sema3enlz/nlz mice still lacked monosynaptic input from Tri afferents (mean latency 8.8±0.8ms; n=1/35 cells in 6 mice; Fig 2h). Moreover, the pattern of monosynaptic connectivity in the Tri arc was unaltered in Sema3enlz/nlz mice: Tri motor neurons received monosynaptic input from Tri but not Cm sensory afferents (Fig 2e, g, h). We conclude that the loss of Sema3e expression by Cm motor neurons permits monosynaptic input from Cm but not from Tri sensory afferents. The loss of PlexinD1 expression from proprioceptive neurons elicited a similar change in the pattern of monosynaptic connectivity within the Cm and Tri reflex arcs. In PlexinD1cond mice, 43% of Cm motor neurons received monosynaptic input from Cm sensory afferents (mean onset latency 3.3 ± 0.2ms; 13/30 cells in 7 mice; Fig 2c, g), but they still lacked monosynaptic input from Tri sensory afferents (Fig 2h). Tri motor neurons received monosynaptic input from Tri but not Cm sensory afferents in PlexinD1cond mice (Fig 2f-h). These findings provide functional evidence that Sema3e and PlexinD1 interact during the formation of proprioceptive sensory-motor reflex circuits.
Figure 2. Loss of Sema3e or PlexinD1 function perturbs monosynaptic specificity.
(a-f) Cm and Tri onset latency histograms upon homonymous muscle nerve stimulation (0-8ms in 0.15ms bins). Blue box: monosynaptic latency. Right of two lines: # neurons with onset latencies >8ms. Right of each histogram: Summary diagram # neurons with monosynaptic input/ #cells assayed. Sema3e or PlexinD1 mutation lead to monosynaptic Cm-Cm connections (light green), absent in wild-type (no presynaptic terminal).
(g) Mean onset latencies (ms ± SEM) of homonymous pairs. Cm: data within (green) and outside (white) monosynaptic window. Significant differences: Cm; Tri in wild-type, Cm(no mono); Tri and Cm(no mono); Cm(mono) in Sema3e-/- and PlexinD1cond (p≤0.001; Student's t-test).
(h) Summary of heteronymous recordings (Tri–Cm and Cm–Tri).
The functional changes in Cm connectivity observed in Sema3enlz/nlz and PlexinD1cond mice were accompanied by a localized reorganization of proprioceptive sensory terminals, revealed by analysis of the pattern of expression of the vesicular glutamate transporter vGlut118. In wild-type mice, vGlut1on proprioceptive terminals are distributed sparsely within the Cm domain (Fig 3a, Supplementary Figs 7, 8), and anatomically identified Cm motor neurons were contacted only by very few proprioceptive terminals (Fig 3c, d). In contrast, in the Cm domain of Sema3enlz/nlz and PlexinD1cond mice we detected a ∼5-fold increase in the density of vGlut1on proprioceptive terminals within the Cm domain (Fig 3a, Supplementary Fig 8) and a ∼3-fold increase in the number of proprioceptive synaptic contacts with identified Cm motor neurons (Fig 3d). Thus, monosynaptic sensory input to Cm motor neurons in the absence of Sema3e-PlexinD1 signaling appears to result from the formation of new proprioceptive sensory contacts with Cm motor neurons.
Figure 3. Increase in vGlut1 sensory contacts with Cm motor neurons in Sema3e mutant mice.
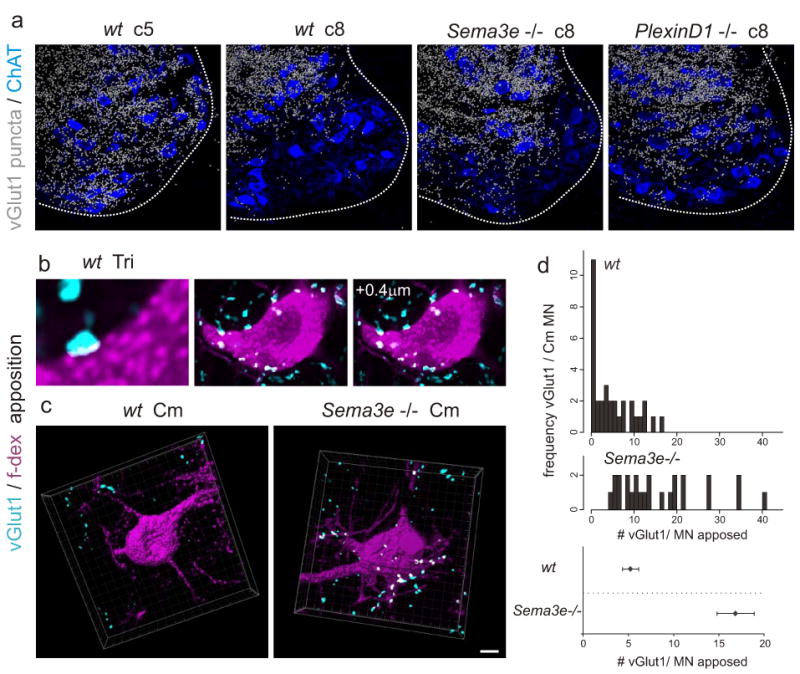
(a) Digitalized vGlut1on proprioceptive terminals overlaid with ChATon motor neuron cell bodies in wild-type (c5 and c8), Sema3e-/- (c8) and PlexinD1cond (c8) mice.
(b-d) vGlut1 apposition with f-dex labeled Tri (b) or Cm (c) motor neuron in wild-type (b, c) and Sema3e-/- (c) mice. Single confocal planes (b) or 3-dimensional view (c). White indicates contact site. (d) Frequency histograms of vGlut1 input to Cm motor neurons. (Bottom) average vGlut1 number in contact with Cm motor neurons (± SEM; p≤0.001; Student's t-test). Scale bar = (a): 30μm; (c):10μm.
We also examined whether Sema3e signaling by motor neurons is sufficient to repel proprioceptive synaptic inputs. To test this, we directed Sema3e expression to Tri (and all other spinal) motor neurons by crossing a Cre-inducible TauSema3e.ires.nlz mouse line with a ChATcre line, to generate MN∷Sema3e mice (Supplementary Fig 9). In MN∷Sema3e mice, > 95% of brachial motor neurons now expressed Sema3e at levels equal or greater than that of endogenous Sema3e (Supplementary Fig 9b-d). In MN∷Sema3e mice, only 23% of Tri motor neurons received monosynaptic input from Tri afferents, compared to >95% in wild-type (Fig 4a-c, Supplementary Fig 10). Yet, Tri and Cm motor neurons still lacked monosynaptic input from Cm afferents (Fig 4d, e). In addition, we detected a ∼50% reduction in the number of vGlut1on terminal contacts with the cell bodies and proximal dendrites of Tri motor neurons in MN∷Sema3e mice, compared to wild-type (Fig 4f, g). The persistence of some vGlut1on afferent terminal contacts with Tri motor neurons that express Sema3e, and the corresponding preservation of physiological inputs, may have its basis in the expression of PlexinD1 expression by only about half of all Tri proprioceptive neurons, and could also reflect the existence of synaptic inputs from PlexinD1off proprioceptive neurons that supply synergistic muscles. We conclude that Sema3e expression by Tri motor neurons is sufficient to reduce the incidence of monosynaptic input from Tri sensory afferents.
Figure 4. Ectopic expression of Sema3e in motor neurons prevents monosynaptic connectivity.
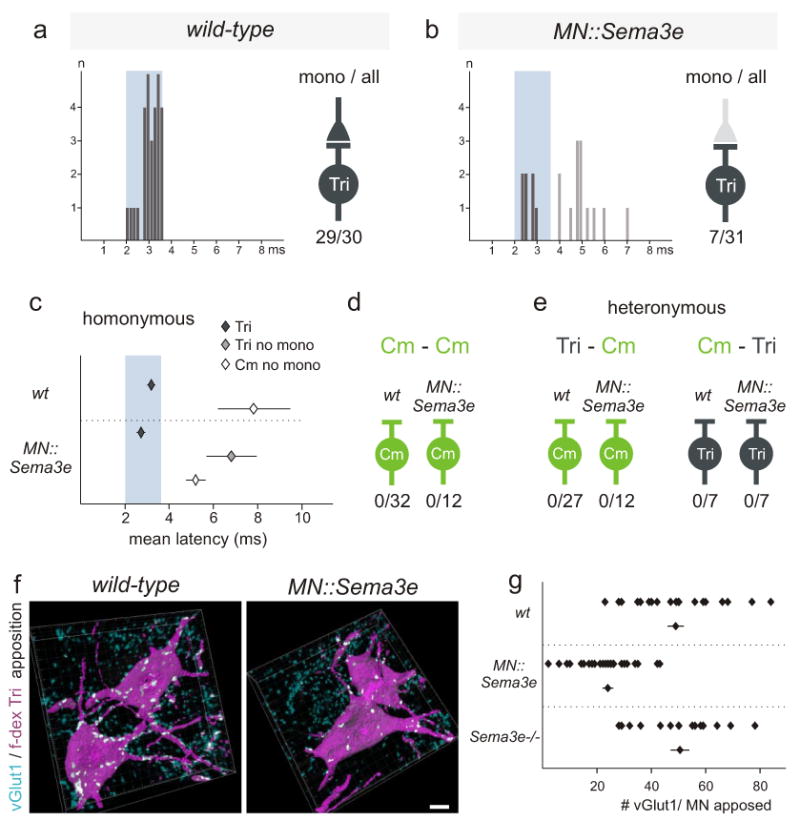
(a, b) Tri onset latency histograms upon Tri muscle nerve stimulation (0-8ms in 0.15ms bins). Blue box: monosynaptic latency. Right of each histogram: Summary diagram #neurons with monosynaptic input/ #cells assayed. Light grey color of presynaptic terminal (b): reduction in Tri motor neurons with monosynaptic Tri input.
(c) Mean onset latencies (ms ± SEM) measured from homonymous pairs. Tri: data within (dark grey) and outside (light grey) monosynaptic window. Significant differences: Cm;Tri in wild-type mice (p≤0.001; Student's t-test) and Tri(mono);Tri(no mono) (p≤0.01; Student's t-test) and Tri(mono);Cm (p≤0.001; Student's t-test) in MN∷Sema3e mice.
(d, e) Summary of Cm-Cm, Tri-Cm and Cm-Tri recordings.
(f, g) Analysis of vGlut1 apposition with f-dex labeled Tri motor neurons (contact sites: white). (g) (top rows) values from individual motor neurons; (bottom) mean of analyzed cells (± SEM). Significant differences: wild-type; MN∷Sema3e and Sema3e-/-; MN∷Sema3e (p≤0.001; Student's t-test). Scale bar=10μm.
Together, our findings show that the specificity of monosynaptic sensory-motor connections in the mammalian spinal cord depends on a recognition system in which the matching expression of a Sema ligand and its Plexin receptor prevents synapse formation. Yet even for these relatively simple reflex circuits, an additional layer of specificity is evident (Fig 5a). Elimination of Sema3e-PlexinD1 signaling uncovers a latent propensity of Cm sensory afferents to form monosynaptic connections with Cm motor neurons, but Tri motor neurons remain off-limits (Fig 5a). Similarly, Tri sensory afferents stripped of PlexinD1 still ignore Cm motor neurons. These observations argue that a recognition system independent of Sema3e-PlexinD1 signaling underlies motor pool specificity (Fig 5a). Other Sema and Plexin proteins are expressed by motor and sensory neurons12, 16 and thus pool specificity could involve a more elaborate matrix of Sema-Plexin recognition. More generally, our findings extend the influence of repellant Sema signaling from the guidance and long-range pruning of axons19, 20 (see Supplementary Material) to fine synaptic specificity.
Figure 5. Regulation of synaptic specificity by repellant Sema3e-PlexinD1 signaling.
(a) Connectivity patterns in Cm and Tri reflex arcs in wild-type mice (top) and with altered Sema3e-PlexinD1 signaling (bottom). Top: In wild-type, Tri but not Cm motor neurons receive direct homonymous proprioceptive inputs. Bottom left: changing the profile of Sema3e-PlexinD1 expression rewires homonymous connectivity. Loss of Sema3e-PlexinD1 signaling results in monosynaptic connections between Cm afferents and Cm motor neurons; Ectopic Sema3e expression reduces monosynaptic connections between Tri afferents and motor neurons. Bottom right: changing Sema3e-PlexinD1 signaling does not erode pool-specificity: no aberrant heteronymous sensory-motor connections are observed (red cross).
(b) Genetic program for Sema3e expression in Cm motor neurons. Hox transcription factors program Cm motor pool identity28, conferring Cm motor neuron sensitivity to the retrograde inductive influence of Gdnf29. Pea3 expression by Cm motor neurons controls cell position, dendrite pattern and the selectivity of proprioceptive inputs through distinct downstream pathways11, 13, 30.
The recruitment of a repellant recognition system to the cause of synaptic specificity in spinal sensory-motor circuits provides an intriguing contrast with recent studies of connectivity in the nematode motor system and vertebrate retina, where specificity appears to depend on adhesive interactions between Ig superfamily proteins expressed by pre- and post-synaptic partners21, 22. Whether the intricate patterns of sensory-motor connectivity in the mammalian spinal cord emerge solely through layers of repellent filtering or also involve adhesive recognition remains to be determined. Nevertheless, a prominent role for repellent recognition in synaptic specificity seems likely, given recent evidence that Wnt-mediated repellent signaling controls the position and selectivity of synaptic contacts in invertebrate neural circuits23, 24.
Methods Summary
Sema3enlz mice were generated using a targeting strategy similar to that described10 but by the integration of a CreERT2-IRES-Nls-LacZ-pA cassette into the endogenous start codon of the Sema3e locus. For the generation of TauSema3e mice, a lox-STOP-lox-Sema3e-IRES-Nls-LacZ-pA targeting cassette was integrated into exon 2 of the Tau locus25, 26. PlexinD1flox mice were generated by insertion of LoxP sites flanking the first coding exon of the PlexinD1 locus as described17. Electrophysiological recordings from p5 to p7 animals and analysis were carried out as described13. Retrograde tracing experiments from Cm and Tri muscles were carried out by f-dex injection at p12 and followed by analysis of vGlut1 input to f-dex labeled motor neurons at p14. Cryostat sections were processed for immunohistochemistry by sequential application of primary antibodies and fluorophore-conjugated secondary antibodies (Invitrogen and Jackson Laboratories)27. For in situ hybridization experiments on cryostat sections, PlexinD1 (Accession number BC19530) and Sema3e11 plasmids were used to generate probes. Images were collected on an Olympus confocal or dissection microscope. For quantitative analysis of synaptic input to Cm and Tri motor neurons, IMARIS colocalization tool (version 6.1.5.) was used to assess proprioceptive input contacting motor neurons retrogradely labeled by f-dex, using images acquired at 0.2μm confocal steps and 150× magnification.
Supplementary Material
Acknowledgments
We thank D. Ladle for help in analysis of electrophysiological data, J. Livet and C. Henderson for discussions, and P. Schwarb, A. Ponti, and M. Stadler for image and statistical analysis. M. Mendelsohn, J. Kirkland and B. Han helped in the generation of PlexinD1cond mice and J.F. Spetz, P. Kopp and B. Kuchemann in the generation of Sema3e mutant and MN∷Sema3e mice. R. Axel, P. Caroni, E. Frank, A. Hantman, C. Henderson, D. Ladle and A. Luethi provided comments on the manuscript. E.P.V., M.S. and S.A. were supported by the Swiss National Science Foundation, NCCR Frontiers in Genetics, the Kanton Basel-Stadt, EU Framework Program 7 and the Novartis Research Foundation. T.M.J. is an HHMI Investigator, and is supported by grants from NINDS, EU Framework Program 7, Project ALS, The Harold and Leila Mathers Foundation, and The Wellcome Trust.
Footnotes
Author contributions: E.P.V., M.S. and Y. Y. made critical primary contributions to this study. E.P.V. performed physiological analysis of sensory-motor connectivity and tracing experiments in wild-type and mutant mice. M.S. participated in Sema3e and PlexinD1 subpopulation assignment, anatomical analyses of sensory-motor organization, and generated Sema3enlz/nlz and MN∷Sema3e mice. Y.Y. found that PlexinD1 is expressed by subsets of proprioceptive sensory neurons and generated PlexinD1cond mice. T.M.J and S.A. initiated complementary aspects of this project, analyzed data, and wrote the manuscript.
References
- 1.Eccles JC, Eccles RM, Lundberg A. The convergence of monosynaptic excitatory afferents on to many different species of alpha motoneurones. J Physiol. 1957;137:22–50. doi: 10.1113/jphysiol.1957.sp005794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiological reviews. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 3.Windhorst U. Muscle proprioceptive feedback and spinal networks. Brain research bulletin. 2007;73:155–202. doi: 10.1016/j.brainresbull.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Brown A. Organization in the spinal cord. Springer; New York: 1981. [Google Scholar]
- 5.Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. The Journal of comparative neurology. 1996;372:465–485. doi: 10.1002/(SICI)1096-9861(19960826)372:3<465::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Mears SC, Frank E. Formation of specific monosynaptic connections between muscle spindle afferents and motoneurons in the mouse. J Neurosci. 1997;17:3128–3135. doi: 10.1523/JNEUROSCI.17-09-03128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldissera F, Hultborn H, Ilert M. Handbook of Physiology: Integration in spinal neuronal systems. American Physiological Society; Bethesda, MD, USA: 1981. pp. 509–595. [Google Scholar]
- 8.Frank E. The formation of specific synaptic connections between muscle sensory and motor neurons in the absence of coordinated patterns of muscle activity. J Neurosci. 1990;10:2250–2260. doi: 10.1523/JNEUROSCI.10-07-02250.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauvet S, et al. Gating of Sema3E/PlexinD1 signaling by neuropilin-1 switches axonal repulsion to attraction during brain development. Neuron. 2007;56:807–822. doi: 10.1016/j.neuron.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu C, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science (New York, N.Y. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 11.Livet J, et al. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35:877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, et al. A semaphorin code defines subpopulations of spinal motor neurons during mouse development. The European journal of neuroscience. 2005;21:1767–1776. doi: 10.1111/j.1460-9568.2005.04021.x. [DOI] [PubMed] [Google Scholar]
- 13.Vrieseling E, Arber S. Target-induced transcriptional control of dendritic patterning and connectivity in motor neurons by the ETS gene Pea3. Cell. 2006;127:1439–1452. doi: 10.1016/j.cell.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 14.Theriault E, Diamond J. Intrinsic organization of the rat cutaneus trunci motor nucleus. Journal of neurophysiology. 1988;60:463–477. doi: 10.1152/jn.1988.60.2.463. [DOI] [PubMed] [Google Scholar]
- 15.Holstege G, van Neerven J, Evertse F. Spinal cord location of the motoneurons innervating the abdominal, cutaneous maximus, latissimus dorsi and longissimus dorsi muscles in the cat. Experimental brain research. 1987;67:179–194. doi: 10.1007/BF00269465. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, et al. Tie2Cre-mediated inactivation of plexinD1 results in congenital heart, vascular and skeletal defects. Dev Biol. 2009;325:82–93. doi: 10.1016/j.ydbio.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira AL, et al. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- 19.Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 20.Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 22.Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- 23.Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 25.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS biology. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramer I, et al. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 28.Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Haase G, et al. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35:893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 30.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




