Abstract
Objective
To test whether the BDNF gene interacts with exercise to predict depressive symptoms. Physical activity is associated with a range of positive health outcomes, including fewer depressive symptoms. One plausible mechanism underlying these findings involves Brain-Derived Neurotrophic Factor (BDNF), a protein hypothesized to limit or repair the damage caused by stress. Physical activity increases expression of BDNF, which may enhance brain health. BDNF expression is controlled by the BDNF gene. Compared with individuals without a BDNF met allele, met-allele carriers have a lower expression of BDNF, which has been associated with Major Depressive Disorder.
Design
Eighty-two healthy adolescent girls were genotyped for the BDNF val66met polymorphism, and their depressive symptoms and physical activity were assessed using questionnaires.
Main Outcome Measures
BDNF genotype, Children's Depression Inventory, and the Physical Activity Questionnaire for Older Children and Adolescents.
Results
The BDNF polymorphism was found to moderate the relation between exercise and depressive symptoms: being physically active was protective for girls with a BDNF met allele (fewer depressive symptoms) but not for girls with the val/val polymorphism.
Conclusion
By integrating psychological and biological factors, the present study enhances our understanding of how physical activity contributes to resilience to psychopathology.
Keywords: BDNF, exercise, depression, adolescents, physical activity
Do our genes influence how much we benefit from exercise? A large literature has documented that physical activity is associated with a range of positive health outcomes, including lower levels of stress, anxiety, and depressive symptoms (e.g., Blumenthal et al., 2007; Byrne & Byrne, 1993; Norris, Carroll, & Cochrane, 1992; Sallis, Prochaska, & Taylor, 2000). One plausible mechanism underlying these effects involves Brain-Derived Neurotrophic Factor (BDNF), a protein that may enhance brain health and plasticity by supporting neuron survival, contributing to growth and differentiation of new neurons and synapses-especially in the hippocampus (e.g., Huang & Reichardt, 2001), and protecting against stress-induced neuronal damage (e.g., Radecki, Brown, Martinez, & Teyler, 2005). Expression of BDNF is controlled by the BDNF gene; individuals who carry a BDNF met allele have a lower regulated expression of BDNF than do those without a met allele (Egan et al., 2003). Egan and colleagues demonstrated that although there is no difference between met BDNF and val BDNF in constitutive secretion of BDNF protein in hippocampal neurons, the regulated or activity-dependent secretion of the BDNF protein is severely reduced in met BDNF (see also Chen et al., 2006). Given that the majority of the BDNF protein is released from the regulated secretory pathway in neurons (Egan et al., 2003; Lu, 2003), impaired or reduced activity-dependent secretion from BDNF met neurons represents a significant decrease in the available BDNF protein in individuals with at least one BDNF met allele. Physical activity also increases levels of BDNF protein (Adlard, Perreau, & Cotman, 2005), as well as BDNF mRNA expression (Adlard et al., 2005; Cotman & Berchtold, 2002; Cotman & Engesser-Cesar, 2002).
Recent research has found that low levels of BDNF are associated with Major Depressive Disorder (MDD; e.g., Duman & Monteggia, 2006; Karege et al., 2002). One recent hypothesis concerning the etiology of MDD is that stress has neurotoxic effects that damage or kill hippocampal cells (Sapolsky, 2000). In turn, this hippocampal damage, including deficient functioning of neuroprotective factors, may underlie symptoms of depression. In this context, the neuroprotective effects of BDNF could help to limit or repair the damage caused by stress and, consequently, reduce risk for depression (see Levinson, 2009, for a review).
Given this literature, the notion that BDNF met-allele carriers, who have a significantly lower activity-dependent expression of BDNF, are at increased risk for developing MDD is plausible. Results of studies examining the association between MDD and the BDNF met allele, however, have been mixed. Whereas some studies have found such an association (e.g., Hwang et al., 2006), others have not (see Chen et al., 2008, and Grataco`s et al., 2007, for meta-analyses). In most cases, genetic polymorphisms have only a small direct effect on phenotypes. This finding is consistent with results of investigations in psychopathology that support the formulation that the likelihood of developing a disorder is a function of the interaction of genotype and other personal or environmental factors. For example, Caspi et al. (2003) demonstrated that a polymorphism in the promoter region of the serotonin transporter gene 5-HTTLPR was associated with risk for depression only in the presence of life stressors. Given findings that exercise influences both levels of BDNF and symptoms of depression (Blumenthal et al., 2007; Gomez-Pinilla, Ying, Roy, Molteni, & Edgerton, 2002), BDNF gene interacting with physical activity in predicting levels of depressive symptoms is plausible (see Post, 2007).
Importantly, despite documentation that exercise can reduce symptoms of depression (see Barbour, Edenfield, & Blumenthal, 2007, for a review), the effects of exercise on individuals at elevated risk for MDD are not clear. Previous research has demonstrated that adolescent girls at risk for MDD resemble currently depressed adults in their responses to stress (Gotlib, Joormann, Minor, & Hallmayer, 2008) and their attentional biases for negative stimuli (Joormann, Talbot, & Gotlib, 2007). In the present study we also examined adolescent girls because adolescence is a critical developmental period that coincides with the onset of a number of mental disorders, including depression. Approximately twice as many females as males experience depression (Kessler & Wang, 2009), making adolescent girls an especially relevant target population to study in this context. Furthermore, findings from both animal and human studies suggest that levels of BDNF vary with age, with levels increasing until adolescence or young adulthood and being maintained throughout older age (Katoh-Semba, Takeuchi, Semba, & Kato, 1997; Narisawa-Saito & Nawa, 1996; Webster, Weickert, Herman, & Kleinman, 2002). Finally, gene-or environment-related alterations in BDNF levels may lead to behavioral and neuroanatomical changes that vary with age (Casey et al., 2009). Adlard et al. (2005) found that the relation between exercise and BDNF protein content in the mouse hippocampus differs as a function of age. Compared to younger mice, older mice experienced a larger relative increase in BDNF protein levels from less exercise. More specifically, young mice, who ran twice as far as late middle-aged and old mice, experienced only a 25%–33% increase in BDNF protein levels over that exhibited by the older mice, suggesting that exercise in younger samples, compared with older samples, results in a proportionally lower increase in BDNF.
In this study we tested whether physical activity is a protective factor in adolescent girls who carry a BDNF met allele. We predicted that higher levels of physical activity would be associated with lower levels of depressive symptoms for girls with, but not for girls without, a BDNF met allele.
Method
Participants were 82 girls between 10 and 16 years (M = 14.0, SD = 1.8) with no current or past history of Axis I disorder. Fifty-three of the girls identified themselves as white, four as Hispanic, eight as Asian American, and six as biracial; 11 girls did not provide their ethnicity. They were recruited (through their mothers) using advertisements in newspapers and via the Internet, and were paid for participating.
The Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL; Kaufman et al., 2000) was used to confirm that girls had no current or lifetime diagnosable psychopathology. Depressive symptoms were also assessed with the 10-item version of the Children's Depression Inventory (CDI-S; Kovacs, 1985). Physical activity was measured with the 10-item Physical Activity Questionnaire for Older Children and Adolescents (PAQ-CA; Kowalski, Crocker, & Faulkner, 1997a; Kowalski, Crocker, & Kowalski, 1997b). This validated questionnaire uses memory cues such as lunch and evening activity items, days of the week, and elements of the school day, which enhance recall abilities to assess levels of physical activity. Rather than differentiating levels of frequency or intensity of physical activity, which are difficult to reliably assess in children and adolescents, a summary score is computed to represent a general level of activity. Data administration was conducted using dynQuest (Rademacher & Lippke, 2007).
Finally, saliva was collected to genotype participants' BDNF with the Oragene Kit (DNA Genotek, Ottawa, Ontario, Canada). Using the primers 5-ACT CTG GAG AGC GTG AAT GG 3 and 5 ACT ACT GAG CAT CAC CCT GGA-3, a 171 bp product was amplified, followed by digestion with PmaCI restriction enzyme and agarose gel electrophoresis. The following genotypes were assigned: GG two bands 99 bp and 72 bp; GA two bands 171 and 99 bp; AA one band 171 bp. Forty-eight girls had the GG genotype (val/val), 32 had GA (val/met), and 3 had AA (met/met). The allele frequencies of BDNF were in the Hardy-Weinberg equilibrium, χ2(2, 82) = 1.05, p = .59.1
Results
For the full sample, depressive symptoms (as assessed with the CDI-S) ranged from 0 to 9 (M = 1.62, SD = 1.96), the average being well below a score of 10, the recommended cut off for possible depression (Kovacs, 1985). The physical activity scores ranged from 1.02 to 4.48 (M = 2.52, SD = 0.73) on a 5-point scale (1 = no activity, 5 = high activity), indicating that the girls are moderately active on average. The correlation between physical activity and depressive symptoms was not statistically significant (r = −.17, p = .13). Girls with and without a met allele did not differ in age (M = 13.7 vs. M = 14.2 years, respectively, t(80) = 1.12, p = .27), CDI-S scores (M = 1.57 vs. M = 1.66, respectively, t(80) = 0.20, p = .84), or physical activity (M = 2.49 vs. M = 2.53, respectively, t(80) = 0.22, p = .83).
To test whether the BDNF polymorphism moderated the relation between physical activity and depressive symptoms, we conducted a hierarchical linear regression using centered variables. The results are presented in Table 1. Neither physical activity score nor BDNF polymorphism individually predicted CDI-S scores. The interaction of physical activity and BDNF polymorphism, however, significantly predicted depressive symptoms, Fchange(1, 78) = 5.33, ΔR = .06, p = .02, indicating that physical activity had differential effects on depressive symptoms for the two genotype groups. Follow-up analyses indicated that, for girls who were homozygous for the val allele, physical activity was not associated with depressive symptoms (r = .04, p = .79). In contrast, for girls with at least one met allele, higher physical activity was associated with lower CDI-S scores (r = −.44, p = .01).2,3 Figure 1 presents CDI-S scores as a function of BDNF polymorphism and a median split on physical activity.
Table 1. Results of the Hierarchical Regression Analysis.
| B | SE B | β | p | |
|---|---|---|---|---|
| Step 1 constant | 1.62 | 0.22 | <.001 | |
| Physical activity | −0.33 | 0.22 | −.17 | .14 |
| BDNF-genotype | −0.05 | 0.22 | −.03 | .81 |
| Step 2 constant | 1.61 | 0.21 | <.001 | |
| Physical activity | −0.33 | 0.21 | −.17 | .12 |
| BDNF-genotype | −0.06 | 0.21 | −.03 | .77 |
| Physical activity × BDNF genotype | −0.49 | 0.21 | −.25 | .02 |
Figure 1.
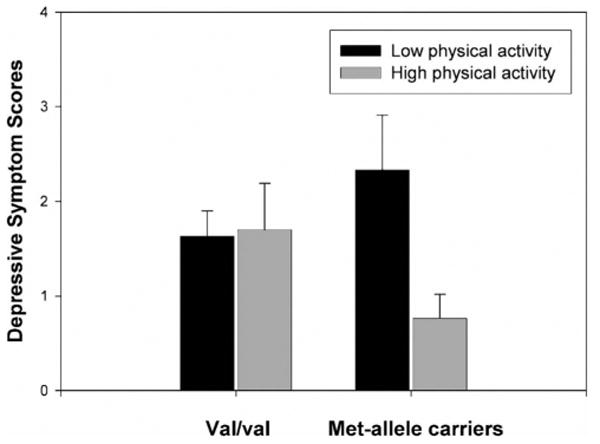
CDI-S scores by BDNF polymorphism and level of physical activity (separated in low and high physical activity based on a median split). Note: CDI-S = Child Depression Inventory-Short Form; Val/val = girls with two BDNF val-alleles; met-carriers = girls with a least one BDNF met-allele; error bars are standard errors of the mean. Group sizes for val/val homozygotes are n = 24 for low physical activity and n = 23 for high physical activity; for met-allele carriers n = 18 for low physical activity and n = 17 for high physical activity.
Discussion
This study was designed to examine the interaction of BDNF polymorphism and physical activity in affecting level of depressive symptoms. Physical activity was conceptualized as a protective factor; the interaction between BDNF and physical activity was hypothesized to be associated with lower levels of depressive symptoms. The results indicate that BDNF genotype moderates the protective effect of physical activity on depressive symptoms in adolescent girls. Physical activity was beneficial for girls at increased genetic risk for depression (i.e., those who carry a BDNF met allele), but not for low-risk girls (i.e., those homozygous for the BDNF val allele). Previous research has found that exercise is more beneficial for individuals with higher levels of depression than it is for less severely depressed persons (e.g., Browman, 1981). The present findings parallel these data, demonstrating that physical activity is particularly beneficial for girls at higher genetic risk for depression.
Previous health behavior research has shown that interactions between genotype and depression predict negative health behaviors, such as smoking (Lerman et al., 1998), and has underscored new possibilities for health psychology research using genetic information, including tests of biological mechanisms intervening between genes and behavior (e.g., Plomin, 1998). By integrating psychological and biological factors, the present study contributes to our understanding of the role of exercise for resilience to psychopathology. It also adds to a growing literature investigating genetic aspects of risk for depression (e.g., Monroe & Reid, 2008) and highlights a specific health behavior, physical activity, which may protect individuals who are at an increased risk for psychopathology.
These findings are exciting, particularly given that the protective effects of the BDNF met allele were evident even at relatively low levels of depressive symptoms and in an age group in which more exercise might lead to relative lower BDNF levels, compared with older groups (cf. Adlard et al., 2005). Future research is needed to replicate these results in larger and more diverse samples in order to control for effects of gender, age, or psychopathology, as well as to examine longitudinally whether physical activity acts as a protective factor for individuals at genetic risk for the development of MDD. We should note here that even though we obtained statistically significant findings, our study was slightly underpowered; future research should attempt to recruit samples of about 130 participants, especially when examining groups of participants that are not as homogeneous as the group of healthy adolescent females studied here.
It is critical that future investigations further test mechanisms that might underlie the association of physical activity with depressive symptoms by comparing biological (e.g., BDNF level) and psychological factors (e.g., self-concept, self-esteem; cf. Dishman et al., 2006), as well as by examining the efficacy of interventions designed to affect specific changes in health behaviors in children at risk for depression.
Acknowledgments
This research was supported by Grants SFRH/BPD/35953/2007 from Fundação para a Ciência e a Tecnologia and Wi3496/41 from the Deutsche Forschungsgemeinschaft awarded to JM, NIMH Grant Supplement MH74849 awarded to RJT, and NIMH Grant MH74849 awarded to IHG. We thank Kirsten Gilbert, Yamanda Wright, Jutta Joormann, and Joachim Hallmayer for their help with this study and Rui Mata, Christian Waugh, and Michael Chen for their comments on previous versions of this article.
Footnotes
Because of the small number of met/met girls, in subsequent analyses we combined girls with val/met and met/met alleles to compare met-allele carriers with homozygous valallele participants (see also e.g., Lang et al., 2005).
These results replicated even when the three girls with met/met alleles were excluded and when CDI-S scores were transformed to yield a normal distribution.
The physical activity questionnaire used in this study provides only a general physical activity score. To more precisely understand the role of frequency of physical activity in predicting depressive symptoms, we calculated a subscore of the items based on the number of sports, dance, or play in which participants reported being physically active in the afternoons and evenings after school, as well as on the weekend, ranging from 1 (physically active once last week) to 5 (physically active 7 or more times). This score correlated highly with the original measure (r = .81, p < .001). Using this variable in our hierarchical regression, the results replicate the findings we obtained with the full scale. Moreover, follow-up regressions showed that whereas in girls with a BDNF val/val polymorphism the frequency of being physically active after school was not associated with level of depressive symptoms (B = 0.27, SE B = 0.28, β = .14, p = .34), in girls with at least one BDNF met-allele being physically active between one and two times per week decreased the level of depression by 0.86 units (B = −0.86, SE B = 0.33, β = −.42, p = .01).
References
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across the life-span. Neurobiology of Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Barbour KA, Edenfield TM, Blumenthal JA. Exercise as a treatment for depression and other psychiatric disorders: A review. Journal of Cardiopulmonary Rehabilitation and Prevention. 2007;27:359–367. doi: 10.1097/01.HCR.0000300262.69645.95. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic Medicine. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman CP. Physical activity as a therapy for psychopathology: A reappraisal. Journal of Sports Medicine and Physical Fitness. 1981;21:192–197. [PubMed] [Google Scholar]
- Byrne A, Byrne DG. The effect of exercise on depression, anxiety and other mood states: A review. Journal of Psychosomatic Research. 1993;37:565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Glatt CE, Tottenham N, Soliman F, Bath K, Amso D, Lee FS. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen L, Lawlor DA, Lewis SJ, Yuan W, Abdollahi MR, Timpson NJ, Shugart YY. Genetic association study of BDNF in depression: Finding from two cohort studies and a meta-analysis. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2008;147B:814–821. doi: 10.1002/ajmg.b.30686. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Engesser-Cesar C. Exercise enhances and protects brain function. Exercise and Sport Sciences Reviews. 2002;30:75–79. doi: 10.1097/00003677-200204000-00006. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Hales DP, Pfeiffer KA, Felton GA, Saunders R, Dowda M, Felton GA. Physical self-concept and self-esteem mediate cross-sectional relations of physical activity and sport participation with depression symptoms among adolescent girls. Health Psychology. 2006;25:396–407. doi: 10.1037/0278-6133.25.3.396. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Egan M, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of Neurophysiology. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA Axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grataco`s M, González JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor val66met and psychiatric disorders: Meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biological Psychiatry. 2007;61:911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annual Review of Neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JP, Tsai SJ, Hong CJ, Yang CH, Lirng JF, Yang YM. The val66met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiology of Aging. 2006;27:1834–1837. doi: 10.1016/j.neurobiolaging.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116:135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrhopic factor levels in major depressed patients. Psychiatry Research. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain- derived neurotrophic factor in rats and its changes with development in the brain. Journal of Neurochemistry. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. K-SADSPL: Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Wang PS. The epidemiology of depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd. New York: Guilford Press; 2009. pp. 5–22. [Google Scholar]
- Kovacs M. The Children's Depression Inventory (CDI) Psychopharmacology Bulletin. 1985;21:995–1124. [PubMed] [Google Scholar]
- Kowalski KC, Crocker PRE, Faulkner RA. Validation of the Physical Activity Questionnaire for Older Children. Pediatric Exercise Science. 1997a;9:174–186. [Google Scholar]
- Kowalski KC, Crocker PRE, Kowalski NP. Convergent validity of the Physical Activity Questionnaire for Adolescents. Pediatric Exercise Science. 1997b;9:342–352. [Google Scholar]
- Lang UE, Hellweg R, Kalus P, Bajbouj M, Lenzen KP, Sander T, Gallinat J. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology. 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, Shields PG. Depression and self-medication with nicotine: The modifying influence of the dopamine D4 receptor gene. Health Psychology. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- Levinson DF. Genetics of major depression. In: Gotlib IH, Hammen CL, editors. Handbook of depression. 2nd. New York: Guilford Press; 2009. pp. 165–186. [Google Scholar]
- Lu B. Pro-region of neurotrophins: Role in synaptic modulation. Neuron. 2003;39:735–738. doi: 10.1016/s0896-6273(03)00538-5. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression research: Genetic polymorphisms and life-stress polyprocedures. Psychological Science. 2008;19:947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Nawa H. Differential regulation of hippocampal neurotrophins during aging in rats. Journal of Neurochemistry. 1996;67:1124–1131. doi: 10.1046/j.1471-4159.1996.67031124.x. [DOI] [PubMed] [Google Scholar]
- Norris R, Carroll D, Cochrane R. The effects of physical activity and exercise training on psychological stress and well-being in an adolescent population. Journal of Psychosomatic Research. 1992;36:55–65. doi: 10.1016/0022-3999(92)90114-h. [DOI] [PubMed] [Google Scholar]
- Plomin R. Using DNA in health psychology. Health Psychology. 1998;17:53–55. doi: 10.1037//0278-6133.17.1.53. [DOI] [PubMed] [Google Scholar]
- Post RM. Role of BDNF in bipolar and unipolar disorder: Clinical and theoretical implications. Journal of Psychiatric Research. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress- induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:256–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Rademacher JDM, Lippke S. Dynamic online surveys and experiments with the free open source software dynQuest. Behavior Research. 2007;39:415–426. doi: 10.3758/bf03193011. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Prochaska JJ, Taylor WC. A review of correlates of physical activity of children and adolescents. Medicine & Science in Sports & Exercise. 2000;32:963–975. doi: 10.1097/00005768-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. The possibility of neurotoxicity in the hippocampus in major depression: A primer on neuron death. Biological Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weickert CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Developmental Brain Research. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]


