Poison frogs contain an alkaloid-based chemical defense that is sequestered directly from a diet of alkaloid-containing arthropods. Geographic and temporal variation in alkaloid defense is common in poison frogs, and is generally attributed to differences in the availability of alkaloid-containing arthropods. Variable chemical defense in poison frogs may have important consequences for predator-prey interactions, requiring a full understanding of the factors involved in explaining such variation. In the present study, we examine alkaloid variation in the dendrobatid poison frog Oophaga pumilio between males and females on Cayo Nancy (Isla Solarte), located in the Bocas del Toro archipelago of Panama. On average, females contained a significantly larger number and quantity of alkaloids when compared to males. Alkaloid composition varied significantly between males and females, illustrating that chemical defense in this population of O. pumilio is sex-dependent. The variation in alkaloids between sexes is attributed to differences in feeding and behavior between males and females. The majority of alkaloids present in the skin of O. pumilio appear to be of oribatid mite origin, supporting the importance of these dietary arthropods in the chemical defense of poison frogs.
Animals that sequester chemical defenses from diet are dependent on specific food sources for protection against predation,1–3 and therefore differences in the chemical composition, availability, or consumption of these dietary sources can result in variable defenses.4–6 Variation in chemical defense is common among animals that sequester defenses, and can have important consequences for predator-prey interactions.7–9 The ecological and evolutionary implications of sequestering defenses have been well studied among some phytophagous arthropods,1,10–11 but are not well understood in vertebrates.
Among vertebrates, the ability to sequester defenses from diet has been proposed in birds of the genera Pitohui and Ifrita from Papua New Guinea12 and experimentally demonstrated in the snake Rhabdophis tigrinus from Japan13 and four lineages of poison frogs found worldwide (dendrobatids of Central and South America, bufonids of South America, mantellids of Madagascar, and myobatrachids of Australia14). Poison frogs, which represent the best studied group, sequester alkaloid-based chemical defenses from dietary arthropods (including mites, ants, beetles, and millipedes15). More than 800 alkaloids have been characterized in poison frog skins, reflecting the large diversity of alkaloids present in arthropods.14 Geographic and temporal variation in alkaloid composition (the number, amount, and type of alkaloids) is common within and among species of poison frogs, and appears to be largely related to the availability of alkaloid-containing arthropods6,15–18 and differences in the amount and type of alkaloids present in arthropods.6,19 Furthermore, alkaloids are accumulated over a lifetime and juvenile dendrobatid poison frogs are known to contain smaller quantities of alkaloids than adults,16,20 suggesting that variation in alkaloid composition is also related to frog age. Differences in alkaloid composition have also been proposed for individuals of different sex.6,21 Recently, differences in the type and amount of certain alkaloids between males and females of the mantellid poison frog Mantella bernhardi, and similar differences in M. aurantiaca and M. milotympanum have been observed;17 however, no studies have been specifically conducted to examine differences in alkaloid composition between sexes.
Alkaloid composition has been well documented in the dendrobatid poison frog Oophaga pumilio, and more than 30 years of research with this species has led to the detection and identification of more than 230 alkaloids from 21 structural classes,6 thus providing a model species for which to examine differences in alkaloid defense between males and females. Here, we quantitatively assess differences in the number, amount, and type of alkaloids between males and females of O. pumilio from Cayo Nancy (Isla Solarte), Bocas del Toro, Panama.
Results and Discussion
GC-MS analyses of 26 individual Oophaga pumilio skin extracts resulted in the detection of 43 alkaloids (including isomers), representing 15 structural classes (Table 1). Most of these alkaloids (67%) have branch points in their carbon skeleton, and are therefore likely derived from oribatid mites.15,22 However, some of these branched-chain alkaloids have been identified in other arthropods as well (e.g., spiropyrrolizidines 236 and 252A, also identified in millipedes23 and pumiliotoxins 307A and 323A, also identified in formicine ants),19 suggesting that in some cases there may be multiple dietary sources for the same alkaloid.15 Although the chemical structures for izidine 211C and unclassified 323I have not yet been determined, they also appear to be derived from oribatid mites.22 A smaller number of alkaloids (26%) do not have branch points in their carbon skeleton, and likely originate from myrmicine ants.15,24–25 The occurrence of all alkaloids and isomers that were detected in frog skins of the present study and their likely dietary sources are presented in Table 1.
Table 1.
Alkaloids detected in male and female Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. Numb. refers to the number of individuals of each sex that contained a specific alkaloid. Quant. refers to the average quantity of alkaloids for individuals of each sex that contained a specific alkaloid. Iso. refers to isomers of an alkaloid. Alkaloid quantities are as follows: +++ > 50 µg per skin; ++ 5–50 µg per skin; + < 5 µg per skin
| Structural Class (Likely Arthropod Source) | Structural Class (Likely Arthropod Source) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaloid | Male | Female | Alkaloid | Male | Female | ||||||
| Numb. | Quant. | Numb. | Quant. | Numb. | Quant. | Numb. | Quant. | ||||
| HTX (Ant) | 5,8-I (Mite) | ||||||||||
| 291A | 0 | − | 3 | + | 233D | 1 | + | 10 | + | ||
| 233D (iso) | 0 | – | 4 | + | |||||||
| DHQ (Ant) | 235B" | 12 | ++ | 12 | ++ | ||||||
| 195A | 13 | ++ | 13 | +++ | 251B | 4 | ++ | 9 | ++ | ||
| 211A | 6 | + | 5 | ++ | 251B (iso) | 2 | + | 8 | + | ||
| 275B | 1 | + | 1 | ++ | 251B (iso2) | 1 | + | 3 | + | ||
| 259B | 0 | – | 3 | ++ | |||||||
| 3,5-P (Ant) | |||||||||||
| cis-223H | 6 | + | 3 | ++ | D-5,8-I (Mite) | ||||||
| trans-223H | 1 | + | 1 | + | 233E | 0 | – | 3 | + | ||
| cis-251K | 1 | + | 1 | ++ | |||||||
| trans-251K | 2 | + | 1 | + | 5,6,8-I (Mite) | ||||||
| 223A | 0 | – | 2 | ++ | |||||||
| 4,6-Q (Ant) | 231B | 0 | – | 2 | + | ||||||
| 237I | 8 | ++ | 7 | + | 253H | 2 | + | 2 | + | ||
| 277E | 1 | + | 3 | + | |||||||
| Pyr (Ant) | |||||||||||
| 197B | 1 | + | 1 | + | Spiro (Millipede/Mite) | ||||||
| 236 | 5 | ++ | 8 | ++ | |||||||
| Pip (Ant) | 252A | 12 | + | 11 | ++ | ||||||
| 241D | 2 | + | 4 | + | |||||||
| Tri (Beetle/Mite) | |||||||||||
| PTX (Mite/Ant) | 191F | 1 | + | 2 | ++ | ||||||
| 251D | 3 | + | 2 | + | 205B | 8 | ++ | 12 | ++ | ||
| 307A | 12 | ++ | 13 | +++ | 223P | 2 | + | 2 | ++ | ||
| 307B | 1 | + | 1 | + | |||||||
| 307F" | 5 | + | 11 | + | Izidine (Unknowna) | ||||||
| 307F"' | 5 | + | 11 | ++ | 211Cb | 0 | – | 4 | ++ | ||
| 309A | 5 | + | 3 | ++ | |||||||
| 321A | 0 | – | 3 | + | Unclass (Unknowna) | ||||||
| 323A | 4 | ++ | 9 | +++ | 323Ib | 1 | ++ | 2 | + | ||
| 323A (iso) | 0 | – | 2 | ++ | 339F | 3 | + | 0 | – | ||
| aPTX (Mite) | |||||||||||
| 323B | 7 | ++ | 9 | ++ | |||||||
| 323B (iso) | 0 | – | 4 | ++ | |||||||
| 325A | 2 | + | 1 | ++ | |||||||
HTX, histrionicotoxins; DHQ, 2,5-disubstituted decahydroquinolines; 3,5-P, 3,5-disubstituted pyrrolizidines; 4,6-Q, 4,6-disubstituted quinolizidines; Pyr, pyrrolidines; Pip, Piperidines; PTX, pumiliotoxins; aPTX, allopumiliotoxins; 5,8-I, 5,8-disubstituted indolizidines; D-5,8-I, Dehydro-5,8-disubstituted indolizidines; 5,6,8-I, 5,6,8-trisubstituted indolizidines; Spiro, spiropyrrolizidines; Tri, tricyclics; Izidine, izidines; Unclass, unclassified.
A probable dietary origin has not been proposed for Izidine or Unclassified alkaloids.
Identified in an oribatid mite.22
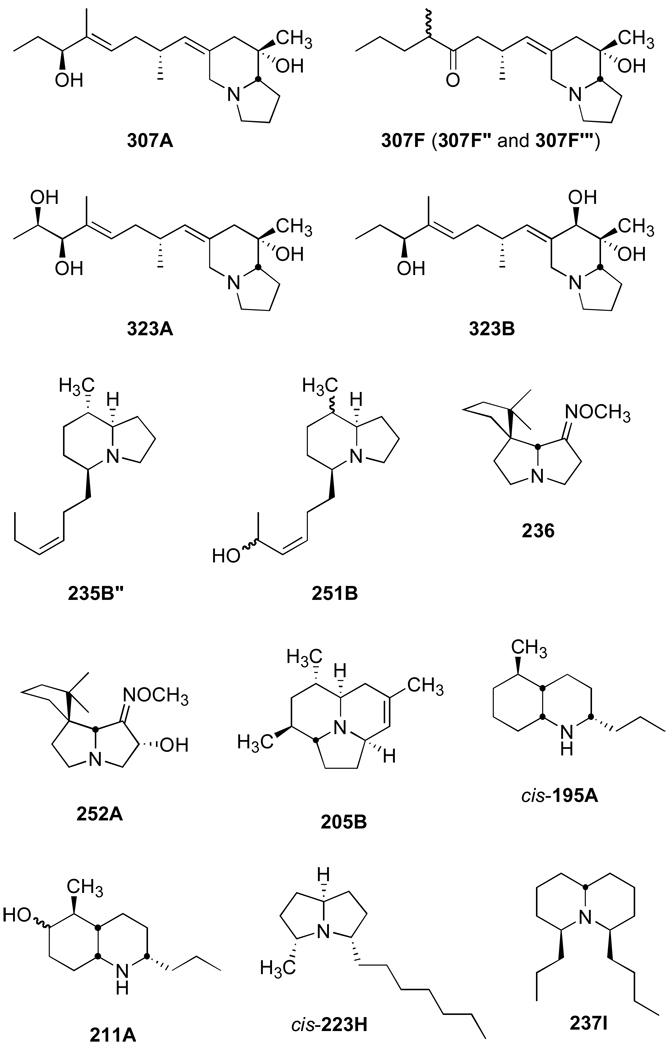
The most common alkaloids (number of times identified and abundance) detected in the Cayo Nancy frogs were the pumiliotoxins (PTX) 307A, 307F”, 307F”’, and 323A, allopumiliotoxin (aPTX) 323B, 5,8-disubstituted indolizidines (5,8-I) 235B” and 251B, spiropyrrolizidines (Spiro) 236 and 252A, and tricyclic (Tri) 205B, all of which have branched carbon skeletons, and the 2,5-disubstituted decahydroquinolines (DHQ) 195A and 211A, 3,5-disubstituted pyrrolizidine cis-223H, and 4,6-disubstituted quinolizidine (4,6-Q) 237I, all of which have unbranched carbon skeletons. The structures of these common alkaloids are presented in Figure 1. The presence of some PTX, 5,8-I, and DHQ alkaloids in Oophaga pumilio from Cayo Nancy appears to have remained relatively constant over the past 30 years. Saporito et al. (2007)6 reported large quantities of PTX 307A, aPTX 323B, and DHQ 195A in frogs from Cayo Nancy sampled in 1981 and 1983, and Mebs et al. (2008)26 reported the presence of 5,8-I 235B” and DHQ 195A in frogs sampled in 2006. However, there have been clear differences also in the presence of some alkaloids over time, particularly the presence of Spiro, Tri, 3,5-P, and 4,6-Q alkaloids in the present study, that were not detected in prior studies.6,26 A change in the presence/absence of alkaloids over time further supports the hypothesis that the availability of alkaloid-containing arthropods changes with time.6,16,18,27
Figure 1.
Structures of the most common alkaloids detected in Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama.
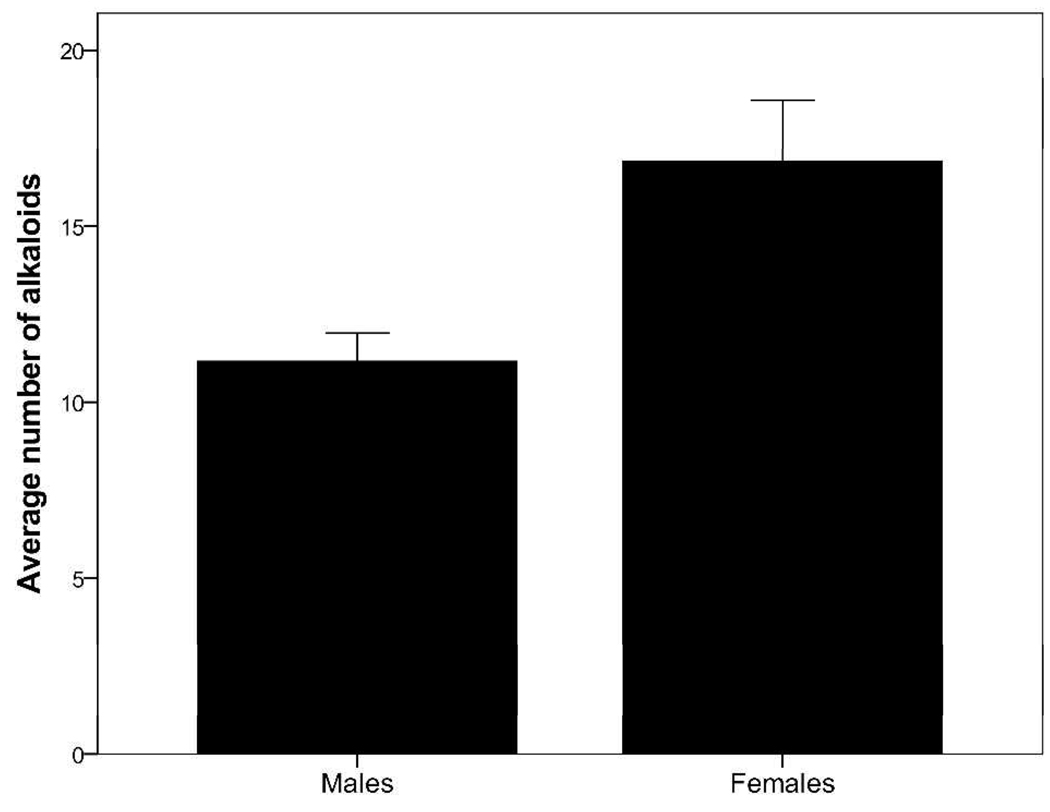
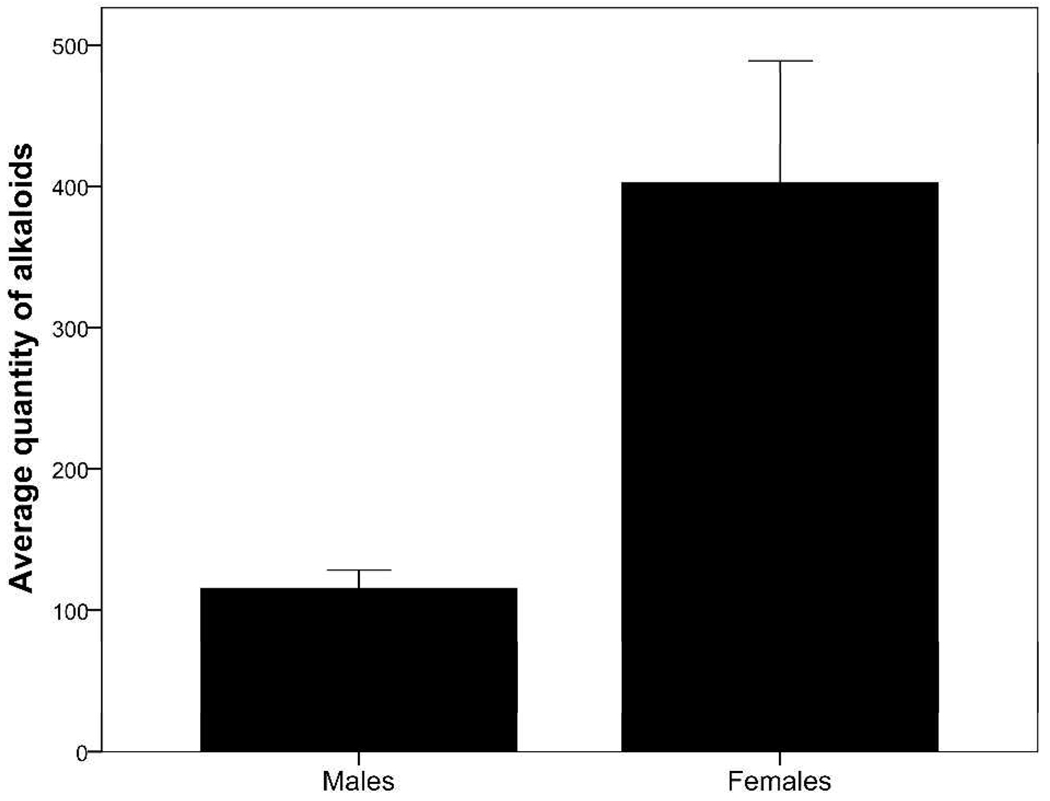
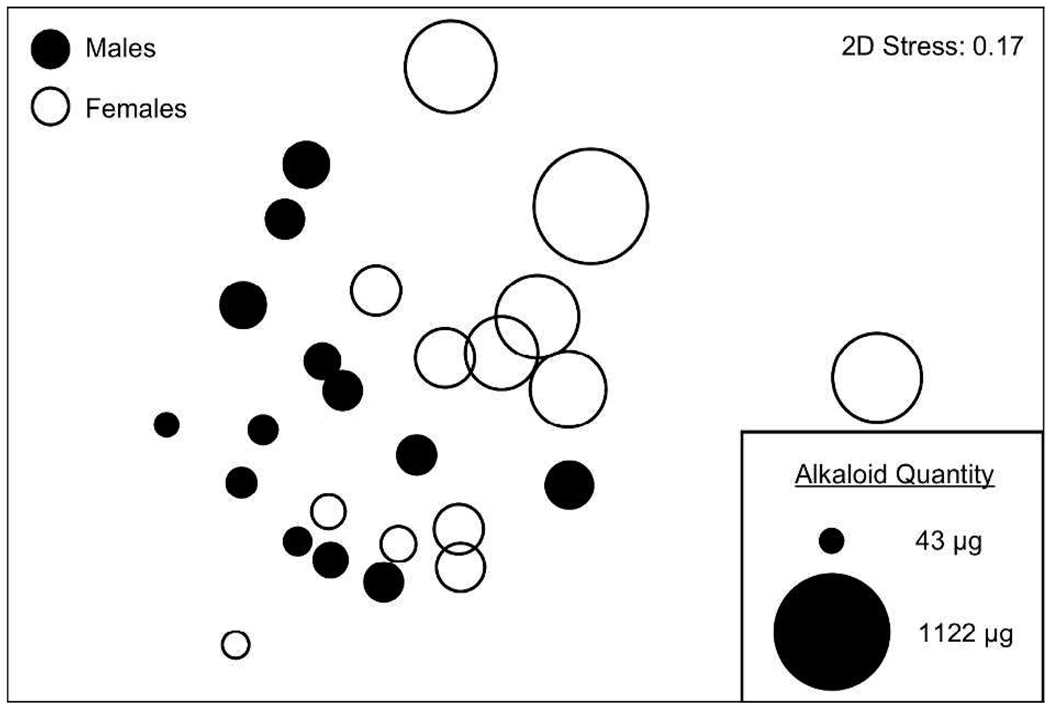
Oophaga pumilio contained an average of 14 alkaloids and an average quantity of 259 µg of alkaloid per skin (Table 2). The number (t24 = 2.89; P = 0.009; Figure 2) and quantity (t24 = 3.60; P = 0.001; Figure 3) of alkaloids differed significantly between males and females, with males containing both a lower number and a lower quantity of alkaloids. On average, males contained 11 alkaloids and 115 µg of alkaloid per skin, whereas females contained 17 alkaloids and 402 µg of alkaloid per skin (Table 2). There was no effect of frog body size (SVL) on the number (F1,23 = 1.62; P = 0.216) or quantity (F1,23 = 0.854; P = 0.365) of alkaloids detected in O. pumilio, suggesting that variation in frog size did not contribute to the differences in alkaloids observed in the present study. It is interesting to note that O. pumilio males and females were, on average, approximately the same size (19.7 and 19.6 mm SVL, respectively). Of the 43 alkaloids detected in frog skins from the present study, 33 (77%) alkaloids were detected in males and 42 (98%) alkaloids were detected in females (see Table 1). Males did not contain alkaloids in the histrionicotoxin (HTX), dehydro-5,8-disubstituted indolizidine (D-5,8-I), or izidine (unknown izidines) structural classes, which are represented in the present study by HTX 291A, D-5,8-I 233E, and Izidine 211C, respectively. Females contained at least one alkaloid in all 15 classes seen here, and the only alkaloid present in males and absent in females was Unclass 339F, which has not been assigned to one of the known structural classes (see Table 1). Overall, 32 (74%) alkaloids were shared between males and females. As a result of the differences in number, quantity, and structural types of alkaloids between sexes, alkaloid composition as depicted in Figure 4 also varied significantly between male and female O. pumilio in the present study (Global R = 0.23; P = 0.002). However, the difference in composition was smaller than that reported among populations of O. pumilio from an adjacent island, Isla Bastimentos,18 indicating that differences in alkaloid composition are generally greater between populations than within populations.
Table 2.
Sample size, mean number, and mean quantity of alkaloids in Oophaga pumilio
| Sample Size | Number of Alkaloids | Quantity of Alkaloids (µg per frog skin) | |||
|---|---|---|---|---|---|
| (n) | Mean +/− SE | Range | Mean +/− SE | Range | |
| All Individuals | 26 | 14 +/− 1 | 7 – 26 | 259 +/− 51 | 43 – 1122 |
| Males | 13 | 11 +/− 1 | 7 – 16 | 115 +/− 13 | 43 – 183 |
| Females | 13 | 17 +/− 2 | 8 – 26 | 402 +/− 87 | 55 – 1122 |
Figure 2.
Number of alkaloids (+/− 1 SE) between males and females of Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama.
Figure 3.
Average quantity of alkaloids (+/− 1 SE) between males and females of Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. Quantity of alkaloids is reported as µg per frog skin (range: 43 – 1122 µg per frog skin).
Figure 4.
nMDS plot of alkaloid composition between males and females of Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. Each circle represents an individual male or female frog, and the distance between symbols represents the difference in alkaloid composition. The diameter of each circle is directly equivalent to the quantity of alkaloids present in that frog (µg per frog skin).
The presence of alkaloids in Oophaga pumilio (and other poison frogs) is dependent on the sequestration of these compounds from dietary arthropods, including mites, ants, millipedes, and beetles.15 Therefore, differences in the composition of alkaloids between males and females may be related to differences in diet between sexes. In a study of O. pumilio diet from northeastern Costa Rica, Donnelly (1991)28 reported that female frogs consumed significantly more arthropods (including mites and ants) when compared to males. If similar differences in diet exist in O. pumilio from Cayo Nancy, then this could explain the larger amount (number and quantity) of alkaloids observed in females of the present study. Furthermore, Donnelly (1991)28 suggested that differences in diet between sexes might be related to behavioral differences. Male O. pumilio are highly territorial29–31 and have smaller home ranges than females,32–33 which may limit the diversity and number of alkaloid-containing arthropods available to them, and result in a lower amount of alkaloids in males. Likewise, the larger home range of females may allow for them to encounter a more diverse array of alkaloid-containing arthropods, resulting in a larger amount of alkaloids sequestered by females. Additionally, it is possible that differences in the amount of alkaloids between sexes could be the result of differences in natural predation rates on males and females. Male O. pumilio spend a large portion of the day emitting advertisement calls from elevated perches within their territories to maintain boundaries with other males and to attract females,29,31,34 a behavior that may increase the exposure of males to natural predators. If this exposure were to result in more predation attempts on males as compared to females, then it is possible that the lower amount of alkaloids in males is due to a potential loss of alkaloids, due to secretion, during predator attacks.
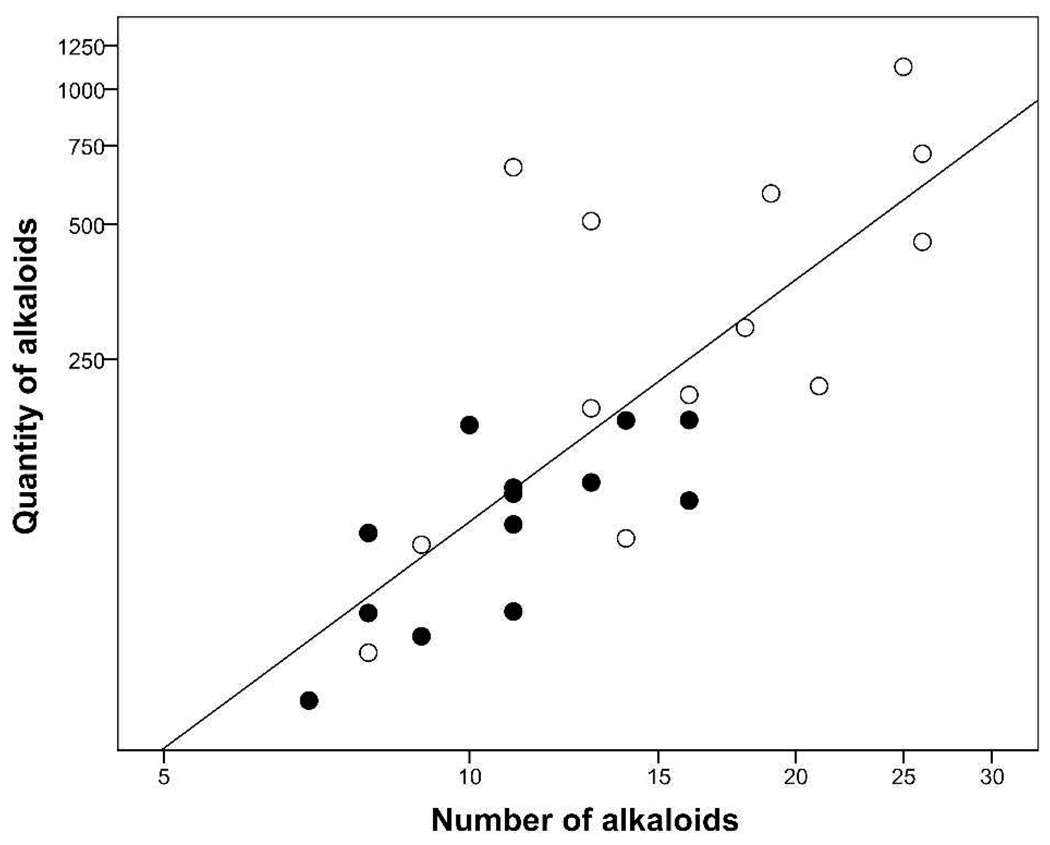
The quantity of alkaloids was positively correlated to the number of alkaloids in males and females combined (F1,24 = 36.41; P ≤ 0.001; R2 = 0.60; Figure 5), and in general, frogs with a larger number of alkaloids tended to also have larger quantities of alkaloids. This relationship remained the same when males (F1,11 = 12.19; P = 0.005; R2 = 0.53; Figure 5) and females (F1,11 = 9.41; P = 0.011; R2 = 0.46; Figure 5) were analyzed separately, demonstrating that this relationship is common to both sexes. Saporito et al. (2007)6 also reported a positive relationship between the number and amount of alkaloids for 53 populations of O. pumilio studied throughout Costa Rica and Panama, indicating that this phenomenon is common to this species, and perhaps to all poison frogs. This observation is not a direct consequence of easier detection when a larger amount of alkaloids is present in an extract since some of the additional alkaloids detected are not present in trace amounts.
Figure 5.
Relationship between quantity of alkaloids (µg per frog skin) and the number of alkaloids in males and females of Oophaga pumilio from Cayo Nancy, Bocas del Toro, Panama. Filled circles indicate males and open circles indicate females. Graph axes are log10-scaled.
The large difference in chemical defense observed between males and females of Oophaga pumilio may have important implications with regard to predator-prey interactions. On average, female O. pumilio contained a larger number (i.e., diversity) and approximately 3.5 times more alkaloid than males, suggesting that females may be better defended against predation. Although it is not currently known whether or not larger amounts of alkaloids in O. pumilio will translate into increased avoidance by natural predators, Daly and Myers (1967)35 reported that differences in the amount of alkaloids among adults correspond to differences in ‘toxicity’ when extracts were injected sub-cutaneously into laboratory mice. However, not all poison frog alkaloids are considered ‘toxic’,20,36–37 and it may be generally more appropriate to consider alkaloids as ‘unpalatable’ because of their unpleasant and/or bitter taste.6,38–42 The bitter nature of alkaloids appears to act as a warning of potential toxicity to predators before ingestion,11,43–44 and it is therefore reasonable to expect that increased amounts of alkaloids in female O. pumilio will result in increased predator avoidance. Whether or not natural predators can detect differences in the amount of alkaloids between males and females of O. pumilio will require future study. Additional research is clearly needed to understand the complex relationship among arthropod diet, natural predation, and alkaloid-based chemical defense in O. pumilio.
Experimental Section
General Experimental Procedures
GC-MS analysis was performed on a Thermo-Electron Polaris-Q instrument coupled to a Focus GC with a 30 m × 0.25 mm i.d. Restek-5MS (Bellefonte, PA, USA) fused silica column. GC separation of alkaloids was achieved using a temperature program from 100 to 280 °C at a rate of 10 °C per minute with He as the carrier gas (flow rate: 1 mL/min.). Each alkaloid fraction was analyzed with both electron impact-mass spectrometry (EI-MS) and chemical ionization-mass spectrometry (CI-MS) with NH3 as the reagent gas.
Individual alkaloid fractions were prepared from methanol extracts of skin for each specimen of Oophaga pumilio. Individual frogs were skinned and the skins were stored in methanol (see below for details). This methanol extract was treated as follows: For each individual, 10 µg of nicotine ((-)-nicotine ≥99%, Sigma-Aldrich, Milwaukee, Wisconsin) in a methanol solution (internal standard) and 50 µL of 1N HCl were added to 1 mL of the original MeOH extract. This combined MeOH extract was concentrated with N2 to 100 µL, and then diluted with 200 µL of water. This solution was then extracted 4 times, each time with 300 µL of hexane. The HCl fraction was then basified with saturated NaHCO3, followed by extraction 3 times, each time with 300 µL of ethyl acetate. The combined ethyl acetate fractions were dried with anhydrous Na2SO4 and evaporated to 100 µL. The wet skins were estimated to weigh 100 mg, and therefore in the final volume of the ethyl acetate alkaloid extract, 1 µL is equivalent to about 1 mg of wet skin.
Identification of individual alkaloids was based on comparison of mass spectrometry properties and GC retention times with those of previously reported anuran alkaloids.14 Anuran alkaloids have been assigned code names, consisting of a bold-faced number corresponding to the nominal mass and a bold-faced letter to distinguish of alkaloids with the same nominal mass.14 All alkaloids within each fraction were assessed quantitatively by comparison of the alkaloid peak area to the peak area of the nicotine internal standard, using the ICIS peak detection function in Xcalibur © version 1.4 SR1 (Thermo Electron Corporation, 1998–2003).
Frog Collections
A total of 26 adult Oophaga pumilio (13 males & 13 females; average size: 19.7 mm snout-to-vent length (SVL); size range: 19.0–21.0 mm SVL) were collected from Cayo Nancy (Isla Solarte), Bocas del Toro Province, Panama on August 17, 2005 (GPS coordinates: 09°19’59.06” N, 82°13’09.18” W). All frogs were collected within a single 45 × 45 m plot. Individual frogs were measured for SVL, euthanized by freezing, skinned, and frog skins were stored in glass vials with Teflon lined caps, containing 4 mL of 100% methanol. Voucher specimens are deposited in the herpetological collection at Florida International University.
Data Analysis
Alkaloid composition is a combined measure of the number, type, and quantity of alkaloids present within an individual frog skin. Non-metric multidimensional scaling (nMDS) was used to graphically visualize patterns of alkaloid composition between males and females. In nMDS plots, the distance between any two points is directly equivalent to the dissimilarity in alkaloid composition between these same two points. A one-way analysis of similarity (ANOSIM) was used to detect differences in alkaloid composition between males and females. See Saporito et al. (2006, 2007)6,18 and Daly et al. (2008)17 for additional examples and discussion on the use of nMDS and ANOSIM in the study of poison frog alkaloids. nMDS and ANOSIM results are based on Bray-Curtis dissimilarity matrices. All multivariate statistical analyses were performed using PRIMER-E version 5.
In order to meet the assumptions of normality (i.e., normally distributed data) and homoscedasticity (i.e., equality of variances between variables), all of our raw data was log10 transformed. Differences between males and females in the number and quantity of alkaloids were determined using t-tests. To control for differences in the number/quantity of alkaloids in males and females as a function of size (SVL), a one-way analysis of covariance (ANCOVA) with SVL as a covariate was used. The number of alkaloids present in an individual frog skin is a measure of alkaloid diversity, whereas the quantity of alkaloids is a measure of the total amount of alkaloid and may be independent of diversity. Linear regression was used to determine if the quantity of alkaloids is related to the number of alkaloids for males and females. All statistical analyses were performed using SPSS version 17.0 for Mac (SPSS, Inc., Chicago, IL).
Acknowledgments
The authors dedicate this paper to our friend and colleague, the late John W. Daly, who continues to inspire research on the chemical ecology of poison frogs. We thank the Republic de Panama and Autoridad Nacional del Ambiente for permission to conduct this research (permit no. SE/A-23-06; CITES permit no. SEX/A-129-06). We also thank the Smithsonian Tropical Research Institute (STRI) Bocas del Toro Research Station, Gabriel Jacome, and Orelis Arosemena for their assistance with the logistics of this study. We would like to especially thank Clyde S. Stephens and Phyllis Stephens for permission to work on their private property and for their informative historical perspective on Bocas del Toro. Jenise M. Snyder provided valuable comments on this manuscript. The Institutional Animal Care and Use Committee of Florida International University approved the methods used in this study. An Environmental Protection Agency Fellowship and a Smithsonian Tropical Research Institute Fellowship awarded to R.A.S funded the field component of this research. An NIH Courtesy Appointment and an NSF Postdoctoral Fellowship supported R.A.S. The work at NIH was funded by intramural funds of NIDDK.
Footnotes
Dedicated to the late Dr. John W. Daly of NIDDK, NIH, Bethesda, Maryland for his pioneering work on bioactive natural products.
References and Notes
- 1.Bowers MD. In: Insect Chemical Ecology: An Evolutionary Approach. Roitberg BD, Isman MB, editors. London: Chapman & Hall; 1992. pp. 216–244. [Google Scholar]
- 2.Termonia A, Pasteels JM, Windsor DM, Milinkovitch MC. Proc. R. Soc. Lond. B. 2002;269:1–6. doi: 10.1098/rspb.2001.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mebs D. Toxicon. 2001;39:87–96. doi: 10.1016/s0041-0101(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 4.Bowers MD, Williams EH. Ecol. Entom. 1995;20:208–212. [Google Scholar]
- 5.Fahey SJ, Garson MJ. J. Chem. Ecol. 2002;28:1773–1785. doi: 10.1023/a:1020509117545. [DOI] [PubMed] [Google Scholar]
- 6.Saporito RA, Donnelly MA, Jain P, Garraffo HM, Spande TF, Daly JW. Toxicon. 2007;50:757–778. doi: 10.1016/j.toxicon.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Brower LP, Ryerson WN, Coppinger LL, Glazier SC. Science. 1968;161:1349–1351. doi: 10.1126/science.161.3848.1349. [DOI] [PubMed] [Google Scholar]
- 8.Moranz R, Brower LP. J. Chem. Ecol. 1998;24:905–932. [Google Scholar]
- 9.Fordyce JA. Entomol. Exp. Appl. 2001;100:339–346. [Google Scholar]
- 10.Rowell-Rahier M, Pasteels JM. In: Herbivores: Their Interactions with Secondary Plant Metabolites: Ecological and Evolutionary Processes. Rosenthal GA, Berenbaum MR, editors. New York: Academic Press; 1992. pp. 243–277. [Google Scholar]
- 11.Nishida R. Annu. Rev. Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- 12.Dumbacher JP, Beehler BM, Spande TF, Garraffo HM. Science. 1992;258:799–801. doi: 10.1126/science.1439786. [DOI] [PubMed] [Google Scholar]; Dumbacher JP, Spande TF, Daly JW. Proc. Nat. Acad. Sci. U.S.A. 2000;97:12970–12975. doi: 10.1073/pnas.200346897. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dumbacher JP, Wako A, Derrickson SR, Samuelson A, Spande TF, Daly JW. Proc. Nat. Acad. Sci. U.S.A. 2004;101:15857–15860. doi: 10.1073/pnas.0407197101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson DA, Mori A, Savitzky AH, Burghardt GM, Wu XG, Meinwald J, Schroeder FC. Proc. Nat. Acad. Sci. U.S.A. 2007;104:2265–2270. doi: 10.1073/pnas.0610785104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hutchinson DA, Savitzky AH, Mori A, Meinwald J, Schroeder FC. Chemoecology. 2008;18:181–190. [Google Scholar]
- 14.Daly JW, Spande TF, Garraffo HM. J. Nat. Prod. 2005;68:1556–1575. doi: 10.1021/np0580560. [DOI] [PubMed] [Google Scholar]
- 15.Saporito RA, Spande TF, Garraffo HM, Donnelly MA. Heterocycles. 2009;79:277–297. [Google Scholar]
- 16.Daly JW, Kaneko T, Wilham J, Garraffo HM, Spande TF, Espinosa A, Donnelly MA. Proc. Nat. Acad. Sci. U.S.A. 2002;99:13996–14001. doi: 10.1073/pnas.222551599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daly JW, Garraffo HM, Spande TF, Giddings LA, Saporito RA, Vieites DR, Vences M. J. Chem. Ecol. 2008;34:252–279. doi: 10.1007/s10886-007-9396-9. [DOI] [PubMed] [Google Scholar]; Andriamaharavo NR, Garraffo HM, Spande TF, Giddings L, Saporito RA, Vieites DR, Vences M. doi: 10.1007/s10886-015-0616-4. unpublished data. [DOI] [PubMed] [Google Scholar]
- 18.Saporito RA, Donnelly MA, Garraffo HM, Spande TF, Daly JW. J. Chem. Ecol. 2006;32:795–814. doi: 10.1007/s10886-006-9034-y. [DOI] [PubMed] [Google Scholar]
- 19.Saporito RA, Garraffo HM, Donnelly MA, Edwards AL, Longino JT, Daly JW. Proc. Nat. Acad. Sci. U.S.A. 2004;101:8045–8050. doi: 10.1073/pnas.0402365101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers CW, Daly JW, Malkin B. Am. Mus. Nov. 1978;161:307–366. [Google Scholar]
- 21.Myers CW, Daly JW, Garraffo HM, Wisnieski A, Cover JF., Jr Am. Mus. Nov. 1995;3144:1–21. [Google Scholar]
- 22.Saporito RA, Donnelly MA, Norton RA, Garraffo HM, Spande TF, Daly JW. Proc. Nat. Acad. Sci. U.S.A. 2007;104:8885–8890. doi: 10.1073/pnas.0702851104. [DOI] [PMC free article] [PubMed] [Google Scholar]; Saporito RA, Grant T, Colombo P, Norton RA, Andriamaharavo NR, Garraffo HM, Spande TF. unpublished data. [Google Scholar]
- 23.Saporito RA, Donnelly MA, Hoffman RL, Garraffo HM, Daly JW. J. Chem. Ecol. 2003;29:2781–2786. doi: 10.1023/b:joec.0000008065.28364.a0. [DOI] [PubMed] [Google Scholar]
- 24.Jones TH, Gorman JST, Snelling RR, Delabie JHC, Blum MS, Garraffo HM, Jain P, Daly JW, Spande TF. J. Chem. Ecol. 1999;25:1179–1193. [Google Scholar]
- 25.Daly JW, Garraffo HM, Jain P, Spande TF, Snelling RR, Jaramillo C, Rand SA. J. Chem. Ecol. 2000;26:73–85. [Google Scholar]
- 26.Mebs D, Pogoda W, Batista A, Ponce M, Köhler G, Kauert G. Salamandra. 2008;44:241–247. [Google Scholar]
- 27.Daly JW, Myers CW, Whittaker N. Toxicon. 1987;25:1023–1095. doi: 10.1016/0041-0101(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 28.Donnelly MA. Copeia. 1991;3:723–730. [Google Scholar]
- 29.Bunnell P. Copeia. 1973;2:277–284. [Google Scholar]
- 30.McVey ME, Zahary RG, Perry D, MacDougal J. Copeia. 1981;1:1–8. [Google Scholar]
- 31.Pröhl H. Amphib-Reptil. 1997;18:437–442. [Google Scholar]
- 32.Donnelly MA. Oecologia. 1989;81:212–218. doi: 10.1007/BF00379808. [DOI] [PubMed] [Google Scholar]
- 33.Pröhl H, Berke O. Oecologia. 2001;129:534–542. doi: 10.1007/s004420100751. [DOI] [PubMed] [Google Scholar]
- 34.Pröhl H, Hagemann S, Karsch J, Höbel G. Ethology. 2007;113:825–837. [Google Scholar]
- 35.Daly JW, Myers CW. Science. 1967;156:970–973. doi: 10.1126/science.156.3777.970. [DOI] [PubMed] [Google Scholar]
- 36.Daly JW, Brown GB, Mensahdwumah M, Myers CW. Toxicon. 1978;16:163–188. doi: 10.1016/0041-0101(78)90036-3. [DOI] [PubMed] [Google Scholar]
- 37.Daly JW, Garraffo HM, Spande TF, Clark VC, Ma JY, Ziffer H, Cover JF., Jr Proc. Nat. Acad. Sci. U.S.A. 2003;100:11092–11097. doi: 10.1073/pnas.1834430100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverstone PA. Nat. Hist. Mus. L. A. 1975;21:1–55. [Google Scholar]
- 39.Myers CW, Daly JW. Am. Mus. Nov. 1976;157:173–262. [Google Scholar]
- 40.Fritz G, Rand SA, Depamphilis CW. Biotropica. 1981;13:158–159. [Google Scholar]
- 41.Szelistowski WA. Biotropica. 1985;17:345–346. [Google Scholar]
- 42.Hagman M. Herp. Rev. 2006;37:73–74. [Google Scholar]
- 43.Brower LP. In: The Biology of Butterflies. Vane-Wright RI, Ackery PR, editors. New York: Academic Press; 1984. pp. 109–134. [Google Scholar]
- 44.Hartmann T. In: Herbivores: Their Interactions with Secondary Plant Metabolites: The Chemical Participants. Rosenthal GA, Berenbaum MR, editors. San Diego: Academic Press; 1991. pp. 79–121. [Google Scholar]