Abstract
Exposure of rats to high strength static magnetic fields of 7 T or above has behavioral effects such as the induction of locomotor circling, the suppression of rearing, and the acquisition of conditioned taste aversion (CTA). To determine if habituation occurs across magnetic field exposures, rats were pre-exposed two times to a 14 T static magnetic field for 30 min on two consecutive days; on the third day, rats were given access to a novel 0.125% saccharin prior to a third 30-min exposure to the 14 T magnetic field. Compared to sham-exposed rats, pre-exposed rats showed less locomotor circling and an attenuated CTA. Rearing was suppressed in all magnet-exposed groups regardless of pre-exposure, suggesting that the suppression of rearing is more sensitive than other behavioral responses to magnet exposure. Habituation was also observed when rats under went pre-exposures at 2–3 hour intervals on a single day. Components of the habituation were also long lasting; a diminished circling response was observed when rats were exposed to magnetic field 36 days after 2 pre-exposures. To control for possible effects of unconditioned stimulus pre-exposure, rats were also tested in a similar experimental design with two injections of LiCl prior to the pairing of saccharin with a third injection of LiCl. Pre-exposure to LiCl did not attenuate the LiCl-induced CTA, suggesting that 2 pre-exposures to an unconditioned stimulus are not sufficient to explain the habituation to magnet exposure. Because the effects of magnetic field exposure are dependent on an intact vestibular apparatus, and because the vestibular system can habituate to many forms of perturbation, habituation to magnetic field exposure is consistent with mediation of magnetic field effects by the vestibular system.
Keywords: vestibular, locomotor circling, rearing, conditioned taste aversion, lithium chloride
Introduction
The magnitude of the static magnetic fields employed in magnetic resonance imaging (MRI) has steadily increased in recent years. Three to four tesla (T) MRI machines are now common, and experimental MRI machines have recently been developed with field strengths of 8 T [1] and 9.4 T [2] for human studies, and 21 T for animal studies [3]. There is increasing evidence that high static magnetic fields interact with the vestibular system of rodents and humans, although the mechanism of interaction is unknown.
Surveys of workers employed and within a 4 T MRI magnet [4] or 9.4 T MRI magnet [2] reported sensations of vertigo, nausea, and illusions of movement that have been attributed to vestibular perturbations. Subjects undergoing MRI scans at 7 T [5] or 8 T [6] reported vertigo while being moved in or out of the MRI machine, but not when positioned in the center of the machine for the duration of the scan. In a thorough psychophysical study, subjects moved slowly into a 7 T MRI experienced sensations of motion while moving but not when stationary [7]. When positioned at the homogeneous center of the magnetic field, movement of the head (e.g. head nodding or rolling) generated mild to severe vertigo [7]. Theoretical models of the mechanisms by which a static magnetic field could interact with the human vestibular system have been proposed [4, 7].
Rodents also show a variety of effects after exposure to high magnetic fields that suggest vestibular perturbation [8]. Following exposure to static magnetic fields of 7 T and above, rats walk in tight head-to-tail circles, and have decreased levels of rearing [9, 10]. The direction of circling is dependent on the orientation of the rat within the magnetic field, i.e., the rat circles counterclockwise if exposed with its head towards B+. If magnetic field exposure is paired with a novel taste solution (e.g. a saccharin or glucose-saccharin solution), the rats acquire a conditioned taste aversion (CTA) [9, 11]. Multiple pairings produce a stronger CTA that extinguishes more slowly than single-pairing CTA [9, 12]. Magnet exposure also activates neurons in the visceral and vestibular relays of the brainstem, as revealed by c-Fos induction [13]. All of these effects are consistent with the consequences of vestibular stimulation or perturbation in rats, e.g., as induced by whole-body rotation or unilateral labyrinthectomy. Furthermore, all of the effects of magnet exposure are abolished in rats after bilateral chemical labyrinthectomy, demonstrating that the peripheral vestibular apparatus of the inner ear is necessary for magnet-induced perturbation [14].
In the course of our experiments on magnet-induced CTA, we have noticed that the acute behavioral response to magnetic field exposure diminished with repeated exposures [9, 10, 12]. In other words, rats were more likely to walk in circles after the first pairing of a glucose-saccharin solution (G+S) and magnetic field exposure than after the third exposure. Conversely, rats were more likely to rear (i.e. raise their forelimbs to the side of a test chamber) after the third pairing of G+S and magnetic field exposure than after the first exposure.
This diminished response could be a result of habituation by the rats to the effects of magnetic field exposure. Habituation is also consistent with magnetic field detection via the vestibular system, because the vestibular system is well-known for its remarkable plasticity and adaptive properties [15, 16]. Therefore, we hypothesized that magnetic field responses are also subject to habituation across repeated exposures.
To explicitly test for habituation, we evaluated the circling, rearing, and CTA induced by a single exposure of 30-min duration to a 14.1 T magnetic field in naive rats, or in rats that had experienced two prior pre-exposures to 14.1 T of 30-min duration each. All rats were videotaped for 2 min immediately after each exposure to quantify locomotor effects. In order to assess CTA acquisition, all rats were given 10-min access to 0.125% saccharin immediately prior to the final test exposure. We evaluated the effects of magnetic field pre-exposure in 3 experiments. 1) To test habituation after magnet exposure at intervals similar to our earlier studies, rats were pre-exposed at 48 h and again at 24 h before the final test exposure. 2) To assess the short-term effects of magnetic field exposure, rats were pre-exposed twice on the same day as the final test exposure 3) To test the long-term effects of magnetic field exposure, rats were pre-exposed on two consecutive days, but received the final test exposure 36 days later. In all cases, the response of the pre-exposed rats was compared to the response of groups that had undergone parallel sham-exposures outside of the magnet.
Pre-exposure to an unconditioned stimulus (US) leading to an attenuated CTA is an alternative mechanism to habituation. For example, repeated injections of LiCl can cause attenuation of a CTA induced by subsequent pairing of a novel taste with the LiCl [17–23]. Thus, it is possible that the reduction in the magnitude of magnet-induced CTA seen after pre-exposures to the magnet is caused by a general US pre-exposure effect, rather than specific vestibular habituation. Therefore, we also evaluated in a comparable experimental design the effects of pre-treatments with LiCl on a CTA induced by the pairing of saccharin and LiCl. Because LiCl pretreatments did not diminish a LiCl-induced CTA, we conclude that the effects of pre-exposure on magnetic field-induced CTA are a specific property of magnetic fields.
Methods
Animals
Adult female Sprague-Dawley rats (175–200 g; Charles River) were housed individually in polycarbonate cages in the temperature-controlled animal facility at the National High Magnetic Field Laboratory at The Florida State University. The light/dark cycle was 12:12 with lights on at 0700 hours. All conditioning trials were conducted during the light cycle. The rats had free access to pelleted Purina Rat Chow 5001 and deionized-distilled water except as specified otherwise. All procedures were approved by the Institutional Animal Care and Use Committee of Florida State University.
Magnet
A 600 MHz Magnex Cryo magnet with an 89 mm bore and a fixed central field strength (B0) of 14.1 T was used; the magnet was located approximately 50 m from the animal facility. Shim magnets extending along the magnet’s bore for approximately ± 15 cm from the magnet core stabilized the magnetic field to give a central core field of uniform strength. The magnetic field was orientated vertically so that the positive pole was at the top of the magnet. The magnet was operated without radiofrequency pulses, so rats were exposed to a static magnetic field only.
Exposure
Rats were placed in restraint tubes for sham- or magnet-exposure. The restraint tubes were 30- cm in length with an inside diameter of 5.6 cm and an outside diameter of 6.4 cm. A plug was inserted into the rostral end of the tube and held in position by nylon screws. The inside of this rostral plug was fabricated in a cone shape to accommodate the head of the rat. A 1- cm hole was bored in this plug at the apex of this cone to allow fresh breathing air. A second plug was inserted into the caudal end of the tube and could be adjusted to restrain the movement of the rat. A hole in the center of this plug accommodated the rat’s tail. When in the tube, the rat was almost completely immobilized.
Restrained rats were transported from the animal facility to the 14.1T magnet in approximately 30 seconds. Rats exposed to the magnetic field were inserted into the center of the bore of the magnet for 30 min at 14.1 T ("magnet exposure"). All rats were inserted into position in less than 10 seconds. As controls for the effects of restraint, some rats were ‘sham-exposed’ by placing them in the restraint tubes and inserting them into an opaque PVC pipe placed in the same room as the magnet but beyond the 5-gauss line of the high magnetic field.
Behavioral Scoring
After 30-min sham-exposure or exposure within the bore of the magnet, the rostral plug of the restraining tube was removed and each rat was released into an open polycarbonate cage (37 cm wide by 47 cm long by 20 cm high) with chip bedding. The locomotor behavior of each rat was recorded on videotape for two minutes after release into the cage. (Most rats exhibited locomotor effects of the magnetic field for less than 1 minute; thus, 2 minutes of recording captured most of the phenomena of interest.) The rat was then returned to its home cage and ad libitum water was returned. The videotapes were scored later by an observer blind to the rats’ treatment. Instances of tight-circling behavior were quantified. Rats were scored as ‘circling’ if they moved continuously around a full circle with diameter less than the length of the rat’s body. Partial circles or circles interrupted by stationary pauses were not counted. Rearing behavior (both forepaws off the floor of the cage and one or both forepaws on the side of the cage) was also scored at this time.
Conditioning
Eight days prior to the conditioning day, the rats were placed on a water restriction schedule under which they received daily water access in one drinking session, during which a water bottle was presented simultaneously with an empty bottle to accustom the rats to a 2-bottle choice. The first daily session was 3 h in length and the session times were diminished each day so that for two days before conditioning the rats received water access in a single 10-min session.
On the conditioning day, rats were given access to 0.125% sodium saccharin solution (saccharin) for 10 min. Immediately following saccharin access, rats were placed in restraint tubes for sham- or magnet-exposure for 30 min as described above. After 30-min exposure, the locomotor behavior of the rats was recorded for 2-min as above. Rats were then returned to their home cage and given ad libitum access to water overnight.
The strength of the CTA induced by the magnet was measured with daily 24-h, 2-bottle preference tests that were initiated the day after conditioning. Two bottles were placed on the cages, one containing saccharin and the other distilled water. Fluid consumption was measured every 24 h and a preference score was calculated as the ratio of saccharin to total fluid consumption:
The preference tests were continued for up to 19 post-conditioning test days. The left/right position of saccharin and water bottles on the rats’ cages was reversed each day. Because saccharin access during the preference tests was not paired with any treatment, the preference tests constituted extinction trials. The CTA of an experimental group was considered extinguished when the average saccharin preference was not different from the average preference of sham-exposed rats. Short-term preference for saccharin measured during the first 24-h, 2-bottle test was analyzed as the magnitude of CTA; longer-term changes in preference across repeated 2-bottle tests were analyzed for extinction rate.
Statistics
To detect habituation, responses of pre-exposed rats were compared to the responses of rats after their first exposure to the magnetic field. Comparisons between groups on single-day data were analyzed with appropriate ANOVA’s or t-tests (Statistica). Results collected over multiple 2-bottle preference test days were analyzed by 2-way ANOVA, with groups as one factor and test days as the second factor, which consisted of repeated sampling of the same subjects across test days. Post-hoc comparisons were made with the Tukey test. Data are presented as mean ± standard error of the mean.
Experiment 1. Magnetic field exposures on consecutive days
In previous studies on CTA with multiple pairings of G+S with magnet exposure at 24-h intervals, we observed decreases in circling and increases in rearing with repeated exposures [9, 10, 12]. However, these observations were made in the context of experiments of varying purpose and design. In order to assess explicitly the effects of magnetic field pre-exposure on both locomotor behavior and CTA, rats were exposed within the 14.1T magnet two times prior to the pairing of saccharin and magnet exposure. Rats were placed on a water restriction schedule as above. After their daily ration of water, rats were restrained and exposed either to the 14.1T magnetic field (group MMM, n=8) or sham-exposed (groups SSS and SSM, n=8 per group). Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages. Rats received two pre-exposures (either two magnetic field exposures in group MMM or two sham-exposures in groups SSS and SSM), with exposures administered once per day over two consecutive days.
Approximately 24-h after their second pre-exposure, rats were given 10-min access to 0.125% saccharin. Immediately after saccharin access, the rats were placed in restraint tubes. Sham-pre-exposed rats in group SSM and magnet-pre-exposed rats in group MMM were exposed to the 14.1 T magnetic field for 30 min. As sham-controls, the remaining sham-preexposed rats in group SSS were sham-exposed for 30 min. Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages and given ad libitum access to water overnight. The next day 24-h 2-bottle preference testing was initiated and continued for 11 days.
Experiment 2. Short-term effect of magnetic field pre-exposures
Habitation of behavioral responses to vestibular stimulation can occur rapidly within minutes [24], and habituation after closely-spaced stimulation can be stronger than habituation induced by stimulation repeated across longer intervals [15]. In order to assess the short-term effects of magnetic field pre-exposure on magnetic field-induced locomotor behavior and CTA, rats were exposed within the 14.1T magnet twice on the same day prior to the pairing of saccharin and magnetic field-exposure. Rats were placed on a water restriction schedule as above. On the day of conditioning, rats were restrained and exposed either to the 14.1T magnetic field (n=10, group MMM) or sham-exposed (n=20, groups SSS and SSM). Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages. Rats received two pre-exposures (either two magnetic field exposures or two sham-exposures), with an interval of 2–3 h (mean = 167 ± 6 min) between exposures.
Approximately 2.5 h after the second pre-exposure, all rats were given 10-min access to 0.125% saccharin. Immediately after saccharin access, the rats were placed in restraint tubes. Half of the sham-pre-exposed rats (group SSM, n=10) and all of the magnetic field-pre-exposed rats (group MMM, n=10) were exposed to the 14.1 T magnetic field for 30 min. As sham-controls, the remaining sham-pre-exposed rats (group SSS, n=10) were sham-exposed for 30 min. Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages and given ad libitum access to water overnight. The next day 24-h 2-bottle preference testing was initiated and continued for 13 days.
Experiment 3. Long-term effect of magnetic field pre-exposures
Habituation to vestibular stimulation such as whole-body rotation can persist for weeks or even months [25]. In order to assess the long-term effects of magnetic field pre-exposure on magnetic field-induced locomotor behavior and CTA, rats were exposed within the 14.1T magnet twice either 1 day or 36 days prior to the pairing of saccharin and magnetic field-exposure.
Two groups of rats (MMM36 and SSM36, n= 8 per group) received two pre-exposures (either two magnet exposures or two sham-exposures, respectively), with exposures administered once per day over two consecutive days. Rats were restrained and exposed either to the 14.1T magnetic field or sham-exposed. Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages.
Twenty-eight days after the second pre-exposure, all rats were placed on a water restriction schedule as above. Two other groups (MMM1 and SSM1) were also water restricted and received pre-exposures on the two days preceding conditioning. After their daily ration of water, the rats were restrained and exposed either to the 14.1T magnetic field (MMM1, n=8) or sham-exposed (SSM1, n=8). Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages.
Rats in all 4 groups were conditioned with magnet exposure on the same calendar day. Groups MM36 and SSM36 were exposed thirty-six days after their second pre-exposure; groups MMM1 and SSM1 were exposed one day after their second pre-exposure. (Due to constraints of timing, no sham-conditioned rats, i.e. SSS, were included.) Rats were given 10-min access to 0.125% saccharin. Immediately after saccharin access, the rats were placed in restraint tubes. All rats were exposed to the 14.1 T magnetic field for 30 min. Immediately after each exposure, rats were released into the open field chamber and their locomotor behavior videotaped and scored as above. Rats were then returned to their home cages and given ad libitum access to water overnight. The next day 24-h 2-bottle preference testing was initiated and continued for 7 days.
Experiment 4. Effect of LiCl pre-exposures on LiCl-induced CTA
To determine if US pre-exposure attenuates CTA as measured above, we tested the effects of pre-exposure to LiCl injections to subsequent LiCl-induced CTA against saccharin. Rats were placed on a water restriction schedule as above. Immediately after their daily water access, rats in group LLL (n=8) were injected with LiCl (0.3 M, 5 ml/kg, i.p.), while rats in groups NNL (n=8) and NNN (n=8) were injected with NaCl (0.15M, 5 ml/kg; i.p.). Rats received two injections, with 48 h between each injection to allow for clearance of the lithium. To compensate for the diuretic effect of LiCl, rats were given an extra 30-min access to water late in the lights-on period of the injection day. Forty-eight hours after the second injection, rats were given 10-min access to 0.125% saccharin. Immediately after saccharin access, rats in group LLL and NNL were injected with LiCl (0.3 M, 5 ml/kg). As vehicle controls, rats in group NNN were injected with NaCl (0.15M, 5 ml/kg; n=8). After the injections, rats were given ad libitum access to water overnight. The day after conditioning 24-h 2-bottle preference tests were initiated as above and continued for 19 days.
Results
Experiment 1. Magnetic field exposures on consecutive days
On conditioning day, rats consumed an average of 6.8 ± 0.5 g of saccharin; there was no significant difference among the 3 groups.
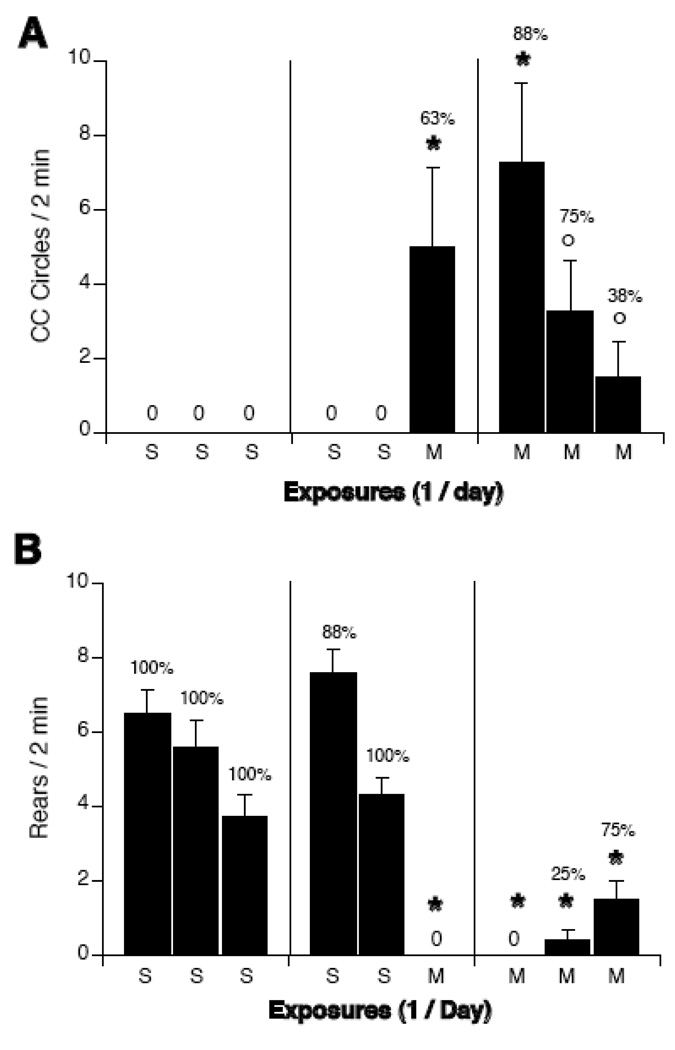
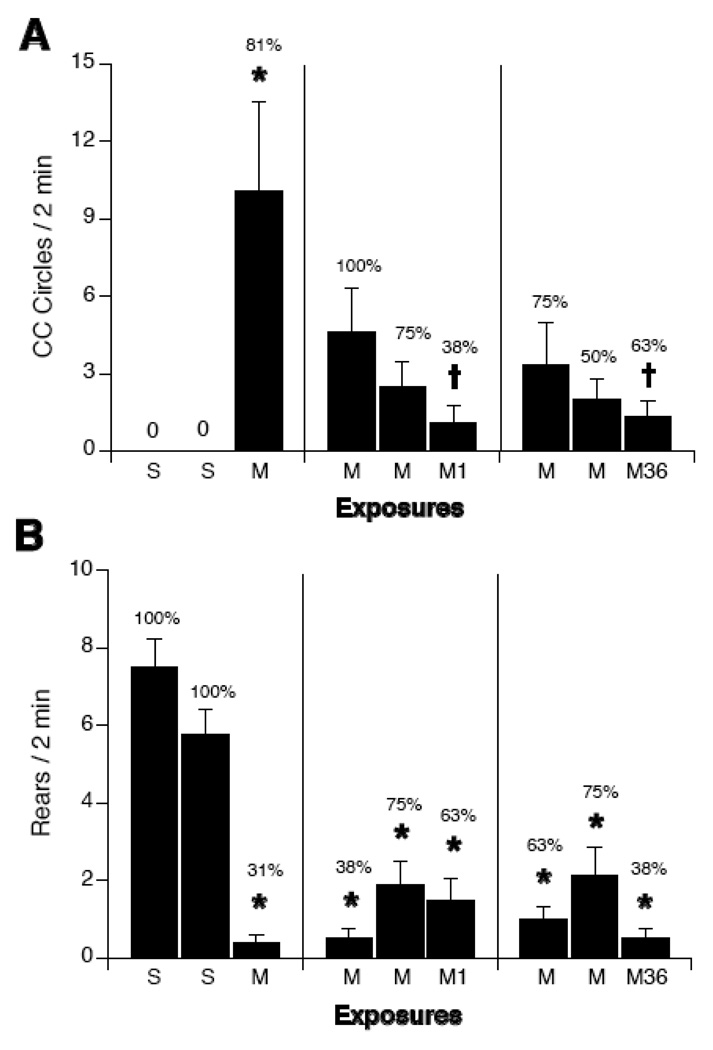
Across repeated exposures to the magnetic field, rats circled less (see Figure 1A). Two-way ANOVAs with group (SSS, SSM, MMM) and exposure days (day 1, day 2, day 3) as factors revealed a significant interaction for circling behavior [F(2,4) = 9.18, p < 0.001] and rearing [F(2,4)=29.28, p < 0.001]. Compared to sham-exposed rats, rats showed significantly greater circling after their first exposure to the magnetic field (i.e. day 3 for group SSM and day 1 for group MMM). After their first exposure to the magnetic field, 5 out of 7 rats in group SSM circled. Circling induced by magnet exposure decreased across days for group MMM, such that circling on days 2 and 3 were significantly lower than circling on day 1. After their first exposure to the magnetic field, 7 out of 8 rats in group MMM circled. After third exposure, only 3 of 8 rats circled.
Figure 1.
Counterclockwise locomotor circling (A) and rearing (B) during a 2-min test after repeated sham (S) or 14T-magnet exposure (M) on 3 consecutive days. Rats were given 3 consecutive sham exposures (SSS), 2 sham exposures and a final magnet exposure (SSM), or 3 consecutive magnet exposures (MMM). Sham-exposed rats did not circle, but consistently reared in the open chamber. First-time magnet exposure of SSM and MMM rats induced circling but suppressed rearing. After the second and third daily exposure to the magnet, however, MMM rats showed decreased circling compared to their first magnet exposure. The increase in rearing in MMM rats was not significant. Percentages above bars indicate the number of rats which circled or reared. *p<0.05 vs. sham-exposure, † p < 0.05 vs. magnet exposure of SSM rats, ∘ p < 0.05 vs. first magnet exposure of MMM rats.
Rearing was significantly suppressed by magnet exposure in both MMM and SSM groups compared to the SSS group (see Figure 1B). The increase in rearing across the 3 days in the MMM group was not significant (Figure 1B right panel).
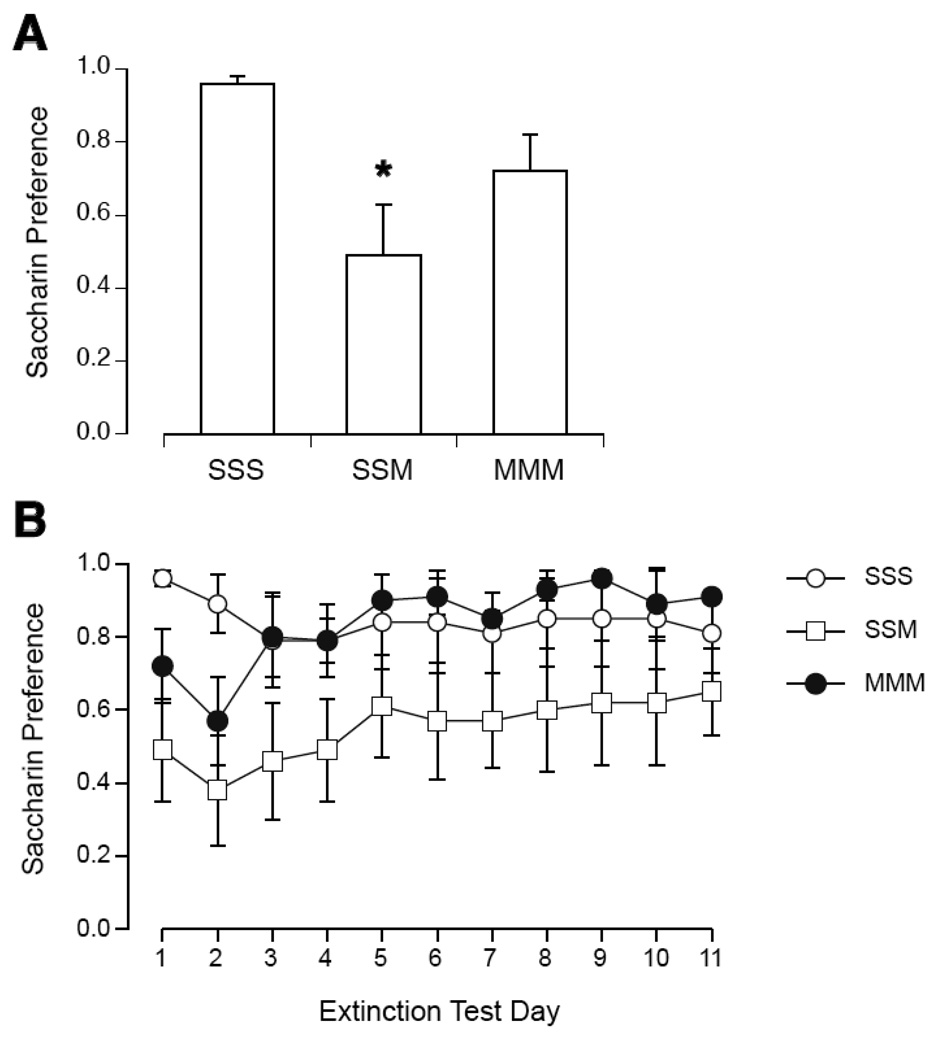
Prior exposure to the magnetic field attenuated the magnitude of CTA induced by pairing saccharin with magnet exposure (see Figure 2). On the first day of preference testing, there was a significant effect of group [F(2,22)=4.6, p < 0.05]. SSM had a significantly lower preference than SSS; MMM was not different from SSS or SSM. Across the 11 days of extinction, 2-way ANOVA showed a significant effect of days [F(10,200)=3.3, p < 0.001] but not group, and the interaction was not significant [F(20,200)=1.6, p = 0.059]. By Tukey's, SSM was different from SSS on days 1–3, MMM was different from SSS only on day 2, and SSM was different from MMM on days 3, 6, 8, and 9.
Figure 2.
Initial magnitude (A) and extinction (B) of CTA expressed as saccharin preference during 2-bottle tests in rats after repeated sham or magnet exposure on 3 consecutive days. On the third day, intake of saccharin was paired with sham exposure (group SSS) or magnet exposure (SSM and MMM). After the first 2-bottle test (A), only SSM rats showed a significant decrease in saccharin preference compared to SSS rats. Across extinction days (B), only on day 2 did MMM rats show a significantly lower preference than SSS rats. *p < 0.05 vs. SSS preference.
Experiment 2. Short-term effect of magnetic field exposures
On conditioning day, rats consumed an average of 7.2 ± 0.7 g of saccharin; there was no significant difference among the 3 groups.
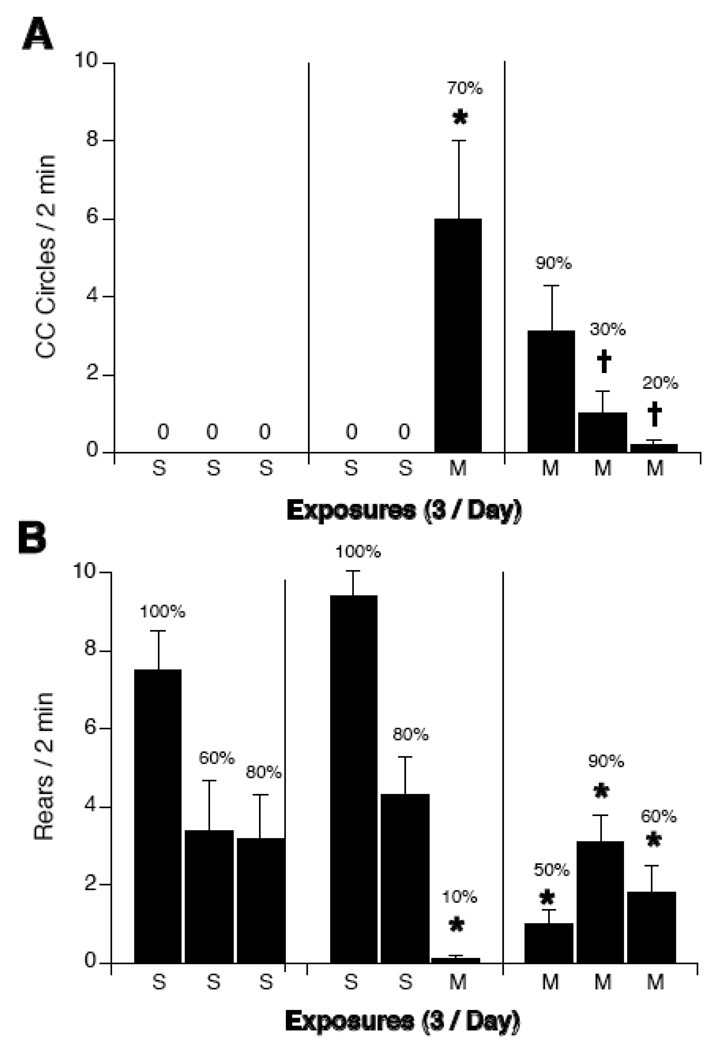
Repeated exposure to the magnetic field attenuated magnet-induced circling and suppression of rearing (see Figure 3). Two-way ANOVAs with group (SSS, SSM, MMM) and exposure (1, 2, or 3) as factors revealed a significant interaction for both circling [F(2,4) =9.95, p < 0.001] and rearing [F(2,4) = 12.1, p < 0.001]. MMM rats circled significantly less after their third magnet exposure compared to SSM rats after their first magnet exposure. In group SSM, 7 out of 10 rats circled after their first exposure to the magnetic field. Similarly, in group MMM, 9 out of 10 rats after their first exposure to the magnetic field but only 2 of the 10 rats circled after their third exposure.
Figure 3.
Counterclockwise locomotor circling (A) and rearing (B) during a 2-min test after repeated sham (S) or 14T-magnet exposure (M), with 3 consecutive exposures on the same day. Rats were given 3 consecutive sham exposures (SSS), 2 sham exposures and a final magnet exposure (SSM), or 3 consecutive magnet exposures (MMM). Sham-exposed rats did not circle, but consistently reared in the open chamber. Magnet exposure of SSM and MMM rats induced circling and suppressed rearing. After the second and third exposure to the magnet, however, MMM rats showed decreased circling compared to the first time magnet exposure of SSM rats. Percentages above bars indicate the number of rats which circled on reared.*p<0.05 vs. sham-exposure, † p < 0.05 vs. magnet exposure of SSM rats.
Rearing was significantly suppressed in all groups after each magnetic field exposure compared to sham-exposed rats.
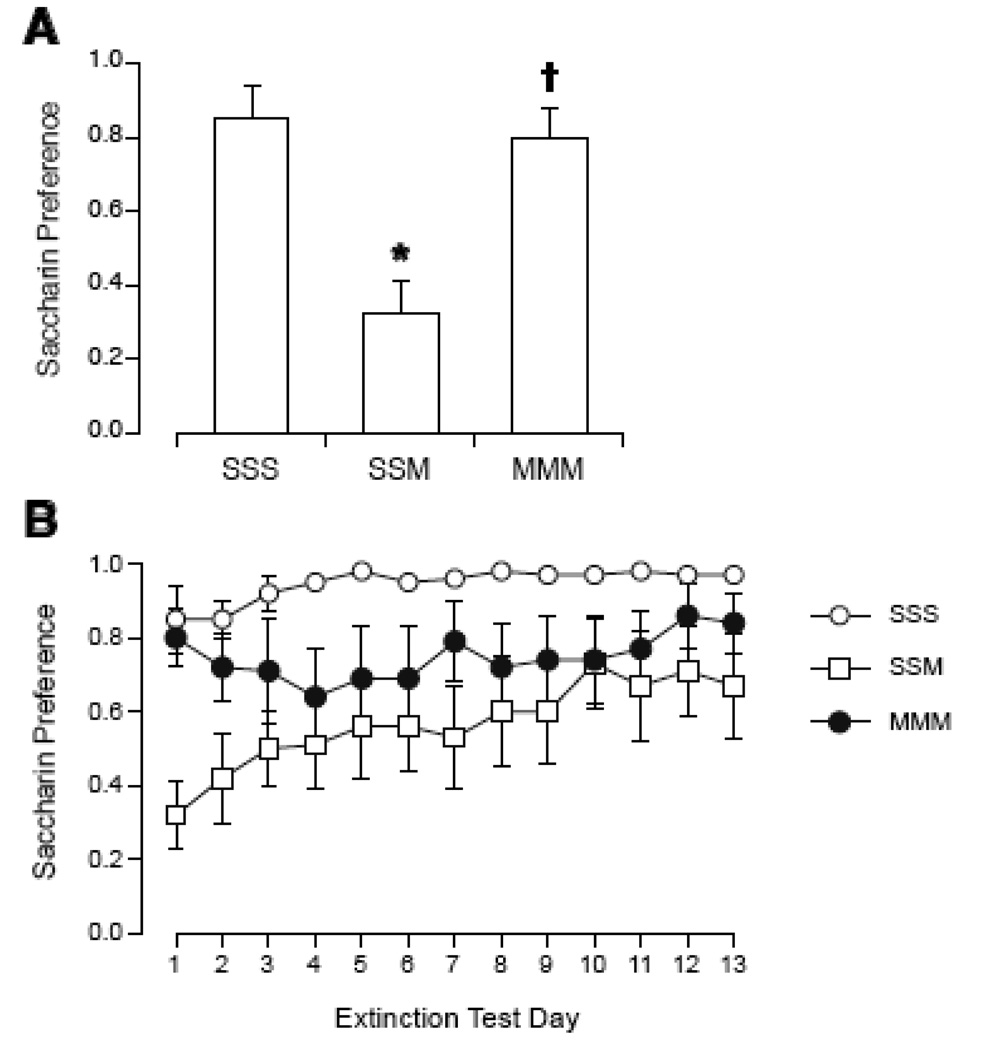
Repeated exposures to the magnetic field within a few hours had a transient effect on the magnitude of CTA (see Figure 4A). On the first day of preference testing, there was a significant effect of group [F(2,26)=11.1, p < 0.0005]. SSM had a significantly lower preference than SSS and MMM; MMM was not different from SSS. Across the 13 days of extinction, however, 2-way ANOVA showed no significant effect of group or days, and no significant interaction (Figure 4B.
Figure 4.
Initial magnitude (A) and extinction (B) of CTA expressed as saccharin preference during 2-bottle tests in rats after repeated sham or magnet exposure on the same day. Prior to the third exposure, intake of saccharin was paired with sham exposure (group SSS) or magnet exposure (SSM and MMM). After the first 2-bottle test (A), only SSM rats showed a significant decrease in saccharin preference compared to SSS rats. Across extinction days (B), there was no significant effect of group or days. *p < 0.05 vs. SSS preference, † p < 0.05 vs. SSM preference.
Experiment 3. Long-Term effect of magnetic field exposures
On conditioning day, rats consumed an average of 9.0 ± 0.5 g of saccharin; there was no significant difference among the 4 groups.
Repeated exposures to the magnetic field attenuated the circling response even after 36 days (see Figure 5A). There was no significant difference between groups SSM1 and SSM36 after any exposure, in either circling or rearing. Therefore, groups SSM1 and SSM36 were combined into a single SSM group. Two-way ANOVA with group (SSM, MMM1 and MMM36) and exposures as factors revealed a significant interaction for circling behavior [F(4,58)=5.7, p < 0.001]. Rats in SSM did not circle after sham exposures. There was no significant difference in the number of circles induced by the first magnet exposure among the 3 groups. After their only exposure to the magnetic field, 13 out of 16 rats in group SSM circled. After their first exposure to the magnetic field, circling was induced in 8 out of 8 rats in group MMM1 and in 6 out of 8 rats in group MMM36. With repeated magnet exposures, the number of rats circling in groups MMM1 and MMM36 decreased. After their third exposure to the magnetic field, 3 out of 8 rats in group MMM1 and 5 out of 8 rats in group MMM36 circled. There was no significant difference in mean number of circles across exposures within either group, however, nor was there any significant difference between groups MMM1 and MMM36 after any exposure. After their third magnet exposure, however, groups MMM1 and MMM36 circled significantly less than did group SSM after its first magnet exposure.
Figure 5.
Counterclockwise locomotor circling (A) and rearing (B) during a 2-min test after repeated sham (S) or 14T-magnet exposure (M) on 3 consecutive days. SSM rats were given 2 sham exposures on consecutive days, followed by a single magnet exposure on the third consecutive day. MMM1 rats were given 2 magnet exposures on consecutive days, followed by a single magnet exposure on the third consecutive day. MMM36 rats were given 2 magnet exposures on consecutive days, followed by a third magnet exposure 36 days later. Sham-exposed rats did not circle, but consistently reared in the open chamber. Magnet exposure induced circling and suppressed rearing in all groups. After 2 daily exposures to the magnet, however, both MMM1 and MMM36 rats showed decreased circling compared to the first time magnet exposure of SSM rats. Percentages above bars indicate the number of rats which circled on reared. *p<0.05 vs. sham-exposure, † p < 0.05 vs. magnet exposure of SSM rats.
Rearing was significantly suppressed in all groups after every magnet exposure compared to sham-exposure (see Figure 5B). Two-way ANOVA with group (SSM, MMM1 and MMM36) and exposures as factors revealed a significant interaction for rearing [F(4,58)=17.4, p < 0.0001]. There was no significant difference among groups in the suppression of rearing by magnet exposure.
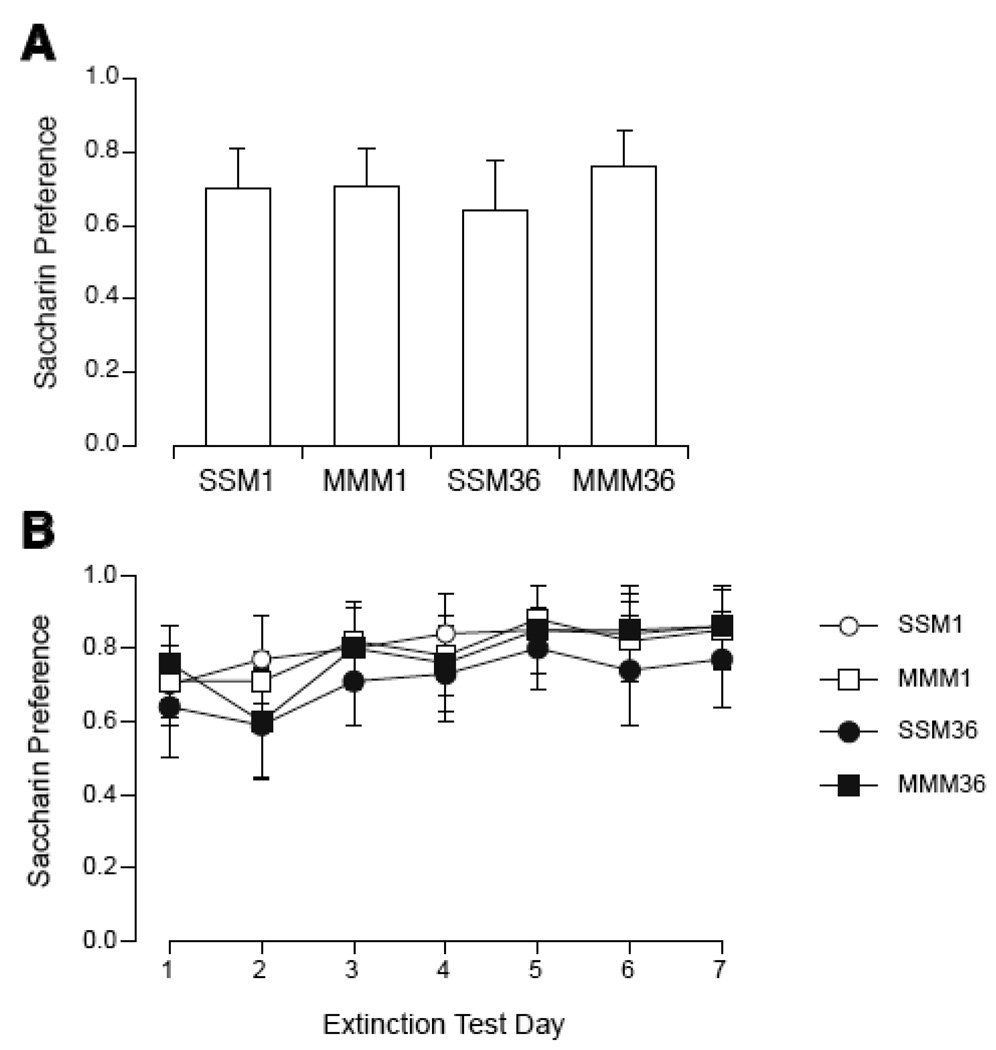
In this experiment repeated magnet exposures had no effect on CTA. On the first day of preference testing, there was no significant difference between the 4 groups. Across the 7 days of extinction, 2-way ANOVA showed a significant effect of days [F(6,168) = 5.6, p < 0.0001) but not group, with no significant interaction. By Tukey's, there was no significant difference between groups on any single day.
Experiment 4. Effect of LiCl pre-exposures on LiCl-induced CTA
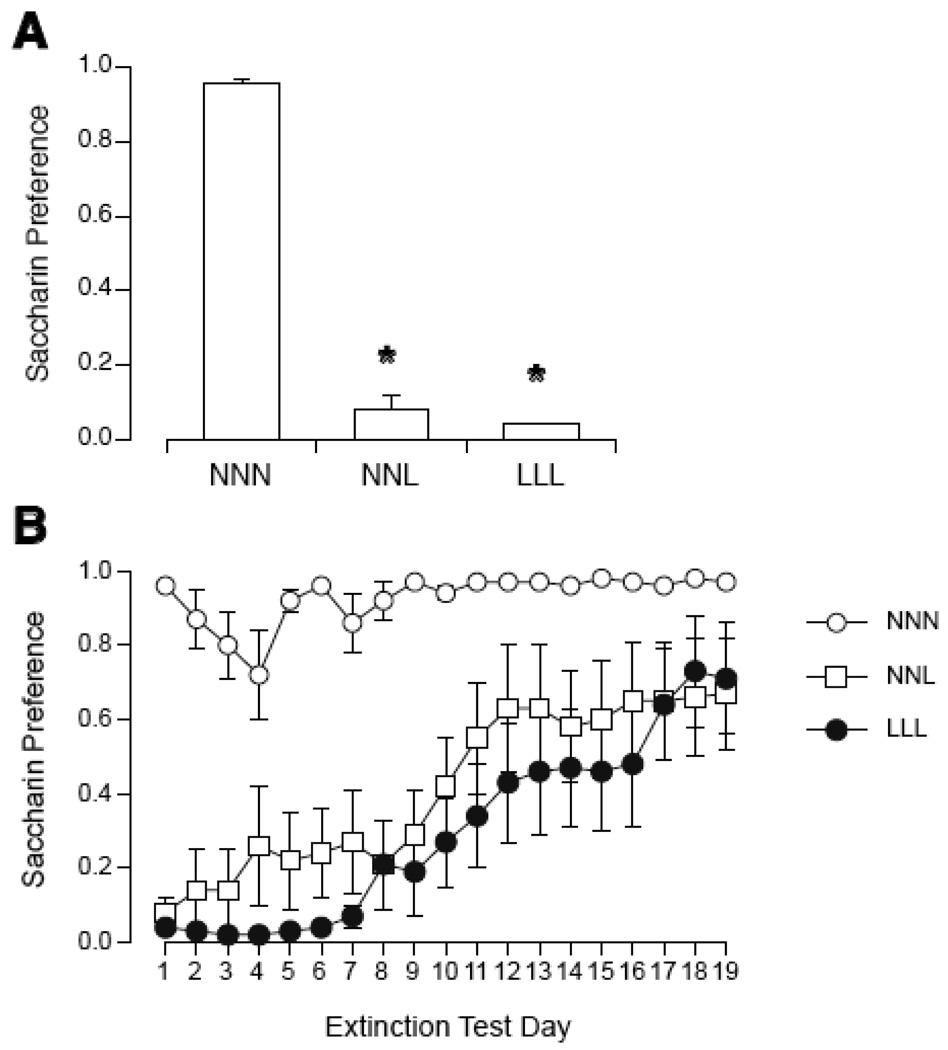
On conditioning day, rats consumed an average of 8.5 ± 0.6 g of saccharin. One-way ANOVA revealed a significant difference among the 3 groups [F(2,23) = 12.7, p < 0.0005)], such that saccharin consumption by the LLL group (5.7 ± 0.3 g) was significantly lower than the intake of the NNL group (8.7 ± 0.9 g) and NNN group (11.0 ± 0.9 g).
The pairing of saccharin and LiCl induced a robust CTA in rats regardless of pretreatment with NaCl or LiCl (see Figure 7). On the first day of preference testing, there was a significant effect of group [F(2,23)=442.8, p < 0.0001]. Both NNL and LLL had a significantly lower preference than NNN; NNL was not different from LLL. Across the 19 days of extinction, 2-way ANOVA revealed a significant interaction of group and days [F(26,378)=2.57, p < 0.0001]. By Tukey's, LLL was different from NNN on days 1–16; NNL was different from NNN on days 1–10. NNL and LLL groups were not different on any day.
Figure 7.
Initial magnitude (A) and extinction (B) of CTA expressed as saccharin preference during 2-bottle tests in rats after repeated injections of NaCl or LiCl (0.15 M, 12 ml/kg, i.p.) at 48-h intervals. NNN rats received 2 injections of NaCl, and a third injection of NaCl paired with saccharin. NNL rats received 2 injections of NaCl, and a third injection of LiCl paired with saccharin. LLL rats received 2 injections of LiCl, and a third injection of LiCl paired with saccharin. NNN rats showed a consistently high preference for saccharin. Both NNL and LLL groups showed a robust initial CTA to saccharin with slow and incomplete extinction over 19 days. * p < 0.05 vs. NNN.
Discussion
As in previous studies, exposure to a high magnetic field induced counter-clockwise locomotor circling, suppressed rearing, and induced a CTA against saccharin paired with the magnet exposure. We found, however, that repeated exposure to the high magnetic field of a 14.1 T magnet caused a decrease in the response of rats to a subsequent exposure within the magnet. Rats were twice exposed to 14.1 T for 30 min, and then received a novel saccharin solution paired with a third exposure to the magnetic field. Compared to the behavior of rats after their first exposure to the magnet, pre-exposed rats showed a smaller number of counterclockwise circles and a decreased magnitude of CTA. Similar results were found when magnet exposures were presented 24 h apart on consecutive days (Experiment 1) or hours apart on the same day (Experiment 2). Furthermore, when the first two magnet exposures and the third exposure to the magnet were separated by 36 days, a decrease in locomotor circling was still observed (although no decrease in CTA magnitude was observed). These results demonstrate that repeated exposure to high magnetic fields results in reduced behavioral responsiveness, perhaps due to habituation.
Exposure to high magnetic fields also reduces locomotor rearing when measured in the open field immediately after exposure. Although the average amount of rearing increased after repeated exposures, the increase in rearing was not significant in any experiments. This may reflect the sensitivity of rearing to the effects of magnetic field exposure. As with vestibular perturbation, magnetic field exposure causes transient postural changes [26]. As a manifestation of magnet field effects, a significant decrease in spontaneous rearing is seen after exposure to field strengths (4 T; [10]) and durations of exposure (1 min at 14.1 T; [9]) that do not induce locomotor circling or CTA. Because of the greater sensitivity of rearing to magnetic field exposure, habituation of rearing might only be apparent after a larger number of pre-exposures or after a lower magnitude test stimulus.
There are at least three possible explanations for the attenuation of magnet-induced circling and CTA after repeated exposures: 1) adaptation to repeated stress of restraint; 2) an effect of US pre-exposure; or 3) sensory habituation to the magnetic field.
Adaptation to Stress
Adaptation to repeated stress is well known [27], as seen in decreased sympathetic nervous system and adrenocortical activation, e.g. after repeated restraint stress [28]. Thus it is possible that rats habituated to the stress associated with magnet exposure, rather than to the effects of the magnetic field per se. Both sham- and magnet-exposed rats were tightly restrained in Plexiglas tubes during the pre-exposures, however, so that any specific habituation in the magnet-exposed rats was not related to adaptation to restraint stress. Furthermore, while adaptation to repeated stress might result in a decrease responsiveness of some variables, it is unclear how stress adaptation would result in a decrease in counterclockwise circling.
Acute stress can also interact with the acquisition of CTA. An unconditioned stimulus such as LiCl can act a stressor, i.e., the injection of LiCl causes activation of the hypothalamic-pituitary-adrenal axis, adrenocortical activation, and elevated plasma levels of ACTH and corticosterone [29, 30]. Although under some conditions exogenous corticosterone can enhance CTA learning[31, 32], in general exteroceptive stress attenuates the magnitude of CTA. For example, a swim test [33], tail pinch [34], inescapable shock [35], or restraint stress [35] interposed between presentation of a saccharin CS and a LiCl US results in a diminished CTA. Therefore, adaptation to a repeated stress would be predicted to enhance CTA, rather than diminish CTA as observed after repeated magnet exposure.
US Pre-Exposure
Pre-exposures to a drug can attenuate a CTA induced by pairing a novel flavor with the drug as US. This US pre-exposure effect has been reported for a variety of drugs [36], including LiCl [18, 19, 21, 22], cyclophosphamide [17], apomorphine [20], and morphine [23]. Generally, a relatively large number of pre-exposures is required to cause attenuation of a subsequent CTA, e.g. 5–8 injections of the drug across multiple days. The effect of US pre-exposure to attenuate subsequent induction of a CTA by the US is variable and dependent upon the class of drug and the experimental protocol [23]. Two mechanisms have been proposed to explain the US pre-exposure effect: i) the development of physiological tolerance or habituation to the effects of the US, and ii) blocking of the CTA by the association of the US with ambient environmental cues during pre-exposure trials in the absence of the conditioned taste stimulus.
In the case of LiCl, physiological tolerance or habituation does not appear to occur. Across repeated injections of LiCl, there is no attenuation of unconditioned responses such as decreased locomotor activity and hypothermia (e.g. across 8 injections [37]) or enhanced neophobia (e.g. after 3 injections [38]). However, there is evidence that injections of LiCl become associated with the cues of a test environment [23] or injection procedure [39, 40], so associative blocking may contribute more to any LiCl pre-exposure effect.
In the present study, there was no evidence that LiCl pre-exposure attenuated a subsequent CTA induced by pairing saccharin with LiCl. Rats in both the magnet experiments and the LiCl experiments received only 2 pre-exposures, and this number of US pre-exposures is likely too low to attenuate the subsequent CTA. Furthermore, blocking by association of the pre-exposures with ambient environmental cues may have been minimized, because rats were housed in their home cages in the time surrounding their pre-exposures and during presentation of the saccharin CS.
Vestibular Habituation
It is well known that the vestibular system shows rapid and persistent habituation to variety of perturbations across a range of behavioral and physiological responses [16]. For example, habituation can be seen in humans as a decreased response in measures such as nystagmus, nausea and motion sickness after stimuli such as optokinetic stimulation [41], caloric stimulation [42], galvanic stimulation of the inner ear [43], whole-body rotation[44], and space flight [25]. In rats, habituation to whole-body rotation has been observed in nystagmus [45], defecation [46], pica[47], and c-Fos expression in the amygdala [48].
Of particular relevance to this present study, repeated episodes of whole-body rotation cause habituation of rotation-induced CTA. Braveman [18] found that daily rotation at 60 rpm for 15 min for five days blocked a subsequent CTA induced by pairing saccharin paired with a 15 minute rotation. More recently Li et al. [49] found that 15 daily exposures to 30-min rotation in swinging cages with alternating acceleration and deceleration almost completely blocked a subsequent CTA induced by rotation.
We presume that habituation to magnetic field exposure, if it involves the vestibular system, occurs centrally, as is the case for most forms of vestibular habituation and compensation. An alternative, however, is that responses are dampened after repeated exposure because the high magnetic fields persistently alter or damage the peripheral vestibular apparatus. Prolonged or intense stimuli, such as chronic weightlessness [50, 51], rotation [52], changes in atmospheric pressure [53], or even a percussive auditory stimulus [54] can cause long-lasting damage to the semicircular canals or otolith organs. In Experiment 3 we observed a long-term decrement (after 36 days) in the induction of locomotor circling, although not in CTA acquisition. Additional studies will be required to distinguish whether damage contributes to this long-term decrement.
Thus, if the magnetic field induces circling and CTA by stimulation of the vestibular system, the decreased responsiveness after repeated exposures to the magnet may reflect vestibular habituation. We have collected significant evidence that high strength static magnetic fields perturb the vestibular system. In addition to suppression of rearing, induction of locomotor circling, and acquisition of CTA [9], magnetic field exposure also causes c-Fos induction in vestibular relays of the brainstem [13]. The effects of the magnet are largest when the head of the rat is exposed to the homogenous peak of the magnetic field [55]. Chemical labyrinthectomy blocks all the observed effects of magnet exposure, so an intact inner ear is required for full interaction with high strength magnetic fields [14]. Theoretical models [4, 7] have been proposed that suggest mechanisms by which a high magnetic field might interact with the inner ear (e.g., by inducing a magnetohydrodynamic force upon the endolymph within the semicircular canals [4]). The precise substrate and mechanism for magnetic field effects is still unknown, however.
Most models of magnetic field effects posit a requirement for movement of a person or animal through the static magnetic field, in order to generate either electrical currents in endogenous conductive tissue [7, 56], or to induce magnetohydrodynamic forces on conductive fluids, e.g. the endolymph of the semicircular canals [4]. In practice, patients are moved in and out of MRI machines very slowly, in order to minimize potential side effects caused by movement within the high magnetic fields [5–7].
In preliminary experiments, we have explicitly tested the role of speed of entry and exit from the 14.1 T magnet using an adjustable motor to raise the rats into the magnet’s core at variable speeds. We have found that fast movement (1 m/s) entry and exit induced more post-exposure circling than slow entry and exit (0.01 m/s). However, the magnitude of CTA acquired after magnet exposure is not different between rats placed quickly or slowly in and out of the magnet. Therefore, it is possible that the acute effects of magnet exposure are due to a transient perturbation of the vestibular system during rapid movement through the field, while static exposure within the magnet has an aversive effect distinguishable from the acute effects.
Another possibility is that vestibular perturbation occurs if the rats move their heads after being raised to the core of the magnet during their 30-min exposure. This would be consistent with reports from humans that head motion within high magnetic fields generates vertigo [4, 5, 7]. Head movements of our rats are probably minimal, however, because the conical cap of the restraint tube holds the head snugly. Furthermore, we have made preliminary observations of head movements while rats are held in a pillory-like restraint tube, i.e. the rat’s body is restrained in a tube with an adjustable neck collar, which leaves the head of the rat unrestrained outside of the tube. After being placed in the center of the 14.1 T magnet, rats immediately tilt their heads to one side; however, they maintain this posture throughout the 30-min exposure, with little or no subsequent movement of the head until they are removed. Thus, while the high magnetic field causes rats to tilt their heads, otherwise the rats do not display large head movements, and therefore gross head movements are not required for the magnetic field effects.
Figure 6.
Initial magnitude (A) and extinction (B) of CTA expressed as saccharin preference during 2-bottle tests in rats after repeated sham or magnet exposure separated by 1 (SSM, MMM1) or 36 days (MMM36). Prior to the third exposure to the magnet, intake of saccharin was paired with magnet exposure (SSM and MMM) in all 3 groups. No significant difference was found among the groups in either initial magnitude or extinction of CTA.
Acknowledgements
Supported by National Institute on Deafness and Other Communication Disorders Grant RO1DC4607. We thank Drs. Timothy Cross and Zhehong Gan of the United States National Magnetic Field Laboratory for providing access to the magnet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chakeres DW, Kangarlu A, Boudoulas H, Young DC. Effect of static magnetic field exposure of up to 8 Tesla on sequential human vital sign measurements. J. Magn. Reson. Imaging. 2003:18. doi: 10.1002/jmri.10367. [DOI] [PubMed] [Google Scholar]
- 2.Patel M, Williamsom RA, Dorevitch S, Buchanan S. Pilot study investigating the effect of the static magnetic field from a 9.4-T MRI on the vestibular system. J. Occup. Environ. Med. 2008;50:576–583. doi: 10.1097/JOM.0b013e318162f5d6. [DOI] [PubMed] [Google Scholar]
- 3.Fu R, Brey WW, Shetty K, Gor'kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Brüschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD, Cross TA. Ultra-wide bore 900 MHz high-resolution NMR at the National High Magnetic Field Laboratory. J Magn Reson. 2005;177:1–8. doi: 10.1016/j.jmr.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Schenck JF. Health and physiological effects of human exposure to whole-body four-tesla magnetic fields during MRI. Ann. NY Acad. Sci. 1992;649:285–301. doi: 10.1111/j.1749-6632.1992.tb49617.x. [DOI] [PubMed] [Google Scholar]
- 5.Theysohn JM, Maderwald S, Kraff O, Moenninghoff C, Ladd ME, Ladd SC. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA. 2008;21:63–72. doi: 10.1007/s10334-007-0095-x. [DOI] [PubMed] [Google Scholar]
- 6.Kangarlu A, Burgess RE, Zhu H, Nakayama T, Hamlin RL, Abduljalil AM, Robitaille PML. Cognitive, cardiac, and physiological safety studies in ultra high field magnetic resonance imaging. Magn. Reson. Imag. 1999;17:1407–1416. doi: 10.1016/s0730-725x(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 7.Glover PM, Cavin I, Qian W, Bowtell R, Gowland PA. Magnetic-field-induced vertigo: a theoretical and experimental investigation. Bioelectromagnetics. 2007:28. doi: 10.1002/bem.20316. [DOI] [PubMed] [Google Scholar]
- 8.Houpt TA, Smith JC. In: Conditioned taste aversion induced by exposure to high-strength static magnetic fields, in Conditioned Taste Aversion: Behavioral and Neural Processes. Reilly S, Schachtman TR, editors. NY: Oxford University Press; 2009. pp. 422–441. [Google Scholar]
- 9.Houpt TA, Pittman DM, Barranco JM, Brooks EH, Smith JC. Behavioral effects of high strength magnetic fields on rats. J. Neurosci. 2003;23:1498–1505. doi: 10.1523/JNEUROSCI.23-04-01498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houpt TA, Pittman DW, Riccardi C, Cassell JA, Lockwood DR, Barranco JM, Kwon BS, Smith JC. Behavioral effects on rats of high strength magnetic fields generated by a resistive electromagnet. Physiol. Behav. 2005;86:379–389. doi: 10.1016/j.physbeh.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Nolte CM, Pittman DW, Kalevitch B, Henderson R, Smith JC. Magnetic field conditioned taste aversion in rats. Physiol. Behav. 1998;63:683–688. doi: 10.1016/s0031-9384(97)00526-x. [DOI] [PubMed] [Google Scholar]
- 12.Cason AM, DenBleyker MD, Ferrance K, Smith JC, Houpt TA. Sex and estrous cycle differences in the behavioral effects of high-strength static magnetic fields: role of ovarian steriods. Am. J. Physiol. 2006;290:R659–R667. doi: 10.1152/ajpregu.00305.2005. [DOI] [PubMed] [Google Scholar]
- 13.Snyder D, Jahng JW, Smith JC, Houpt TA. c-Fos induction in visceral and vestibular nuclei of the rat brainstem by a 9.4 T magnetic field. NeuroReport. 2000;11:1681–1685. doi: 10.1097/00001756-200008210-00015. [DOI] [PubMed] [Google Scholar]
- 14.Cason AM, Kwon BS, Smith JC, Houpt TA. Labyrinthectomy abolishes the behavioral and neural response of rats to a high-strength static magnetic field. Physiol. Behav. 2009;97:36–43. doi: 10.1016/j.physbeh.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Guedry FE., Jr . In: Psychophysiological Studies of Vestibular Function, in Contributions to Sensory Physiology. Neff WD, editor. New York: Academic Press; 1965. pp. 63–135. [DOI] [PubMed] [Google Scholar]
- 16.Collins WF. In: Habituation of vestibular responses with and without visual stimulation, in Vestibular System Part 2: Psychophysics, applied aspects and general interpretations. Kornhuber HH, editor. New York: Springer-Verlag; 1974. pp. 369–388. [Google Scholar]
- 17.Elkins RL. Bait shyness acquisition and resistance to extinction as functions of US exposure prior to conditioning. Physiol. Psychol. 1974;2:341–343. [Google Scholar]
- 18.Braveman NS. Formation of taste aversions in rats following prior exposure to sickness. Learn. Motiv. 1975;6:512–534. [Google Scholar]
- 19.Revusky SH, Taukulis H. Effects of alcohol and lithium habituation on the development of alcohol aversions through contingent lithium injection. Behaviour research and therapy. 1975;13:163–166. doi: 10.1016/0005-7967(75)90010-8. [DOI] [PubMed] [Google Scholar]
- 20.Brookshire KHBR, BR Formation and retention of conditioned taste aversion and UCS habituation. Bull. Psychon. Soc. 1976;7:125–128. [Google Scholar]
- 21.Riley AL, Jacobs WJ, LoLordo VM. Drug exposure and the acquisition and retention of a conditioned taste aversion. J. Comp. Physiol. Psychol. 1976;90:799–807. doi: 10.1037/h0077251. [DOI] [PubMed] [Google Scholar]
- 22.Suarez EM, Barker LM. Effects of water deprivation and prior LiCl exposure in conditioning taste aversions. Physiol. Behav. 1976;17:555–559. doi: 10.1016/0031-9384(76)90150-5. [DOI] [PubMed] [Google Scholar]
- 23.Dacanay RJ, Riley AL. The UCS preexposure effect in taste aversion learning: tolerance and blocking are drug specific. Animal Learn. Behav. 1982;10:91–96. [Google Scholar]
- 24.Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Expt. Brain Res. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- 25.Clément G, Deguine O, Parant M, Costes-Salon MC, Vasseur-Clausen P, Pavy-LeTraon A. Effects of cosmonaut vestibular training on vestibular function prior to spaceflight. Eur. J. Appl. Physiol. 2001;85:539–545. doi: 10.1007/s004210100494. [DOI] [PubMed] [Google Scholar]
- 26.Houpt TA, Cassell JA, Riccardi C, Kwon BS, Smith JC. Suppression of drinking by exposure to a high-strength static magnetic field. Physiol. Behav. 2007;90:59–65. doi: 10.1016/j.physbeh.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 28.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress--comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 29.Smotherman WP. Glucocorticoid and other hormonal substrates of conditioned taste aversion. Ann. N.Y. Acad. Sci. 1985;443:126–144. doi: 10.1111/j.1749-6632.1985.tb27068.x. [DOI] [PubMed] [Google Scholar]
- 30.Spencer CM, Jahng JW, Ryu V, Houpt TA. Lithium-induced gene expression of inducible cyclic adenosine monophosphate early repressor in the rat adrenal gland. J. Neurosci. Res. 2005;82:273–282. doi: 10.1002/jnr.20617. [DOI] [PubMed] [Google Scholar]
- 31.Gorzalka B, Hanson L, Harrington J, Killam S, Campbell-Meiklejohn D. Conditioned taste aversion: modulation by 5-HT receptor activity and corticosterone. Eur. J. Pharmacol. 2003;471:129–134. doi: 10.1016/s0014-2999(03)01790-4. [DOI] [PubMed] [Google Scholar]
- 32.Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learn. Mem. 2008;15:468–476. doi: 10.1101/lm.964708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourne MJ, Calton JL, Gustavson KK, Schachtman TR. Effects of acute swim stress on LiCl-induced conditioned taste aversions. Physiol. Behav. 1992;51:1227–1234. doi: 10.1016/0031-9384(92)90313-q. [DOI] [PubMed] [Google Scholar]
- 34.Misanin JR, Kaufhold SE, Paul RL, Hinderliter CF, Anderson MJ. A time contraction effect of acute tail-pinch stress on the associative learning of rats. Behav. Processes. 2006;71:16–20. doi: 10.1016/j.beproc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Dess NK, Raizer J, Chapman CD, Garcia J. Stressors in the learned helplessness paradigm: effects on body weight and conditioned taste aversion in rats. Physiol. Behav. 1988;44:483–490. doi: 10.1016/0031-9384(88)90309-5. [DOI] [PubMed] [Google Scholar]
- 36.Gamzu E. In: The multifaceted nature of taste-aversion-inducing agents: is there a single common feature, in Learning mechanisms in food selection. Barker LM, Best MR, Domjon M, editors. Houston: Baylor University Press; 1977. pp. 477–502. [Google Scholar]
- 37.Batson JD. Effects of repeated lithium injections on temperature, activity, and flavor conditioning in rats. Animal Learn. Behav. 1983;11:199–204. [Google Scholar]
- 38.de Brugada I, González F, Cándido A. Repeated administration of LiCl produces an unconditioned stimulus preexposure effect in backward excitatory CTA but not habituation of the unconditioned increment in neophobia. Behav. Processes. 2003;60:227–233. doi: 10.1016/s0376-6357(02)00125-0. [DOI] [PubMed] [Google Scholar]
- 39.de Brugada I, González F, Gil M, Hall G. The role of habituation of the response to LiCl in the US-preexposure effect. Learning & behavior. 2005;33:363–370. doi: 10.3758/bf03192864. [DOI] [PubMed] [Google Scholar]
- 40.de Brugada I, Hall G, Symonds M. The US-preexposure effect in lithium-induced flavor-aversion conditioning is a consequence of blocking by injection cues. Animal behavior processes. 2004;30:58–66. doi: 10.1037/0097-7403.30.1.58. [DOI] [PubMed] [Google Scholar]
- 41.Hu S, Hui L. Adaptation to optokinetic rotation-induced motion sickness without experiencing nausea. Percept. Motor Skills. 1997;84:1235–1240. doi: 10.2466/pms.1997.84.3c.1235. [DOI] [PubMed] [Google Scholar]
- 42.Davey PG, Harpur ES, Jabeen F, Shannon D, Shenoi PM. Variability and habituation of nystagmic responses to hot caloric stimulation of normal subjects. Evidence that this test may be inapplicable to monitoring drug-induced vestibular toxicity. J. Laryngol. Otol. 1982;96:599–612. doi: 10.1017/s0022215100092896. [DOI] [PubMed] [Google Scholar]
- 43.Balter SG, Stokroos RJ, Eterman RM, Paredis SA, Orbons J, H K. Habituation to galvanic vestibular stimulation. Acta Otolaryngol. 2004;124:941–945. doi: 10.1080/00016480410017350. [DOI] [PubMed] [Google Scholar]
- 44.Rohleder N, Otto B, Wolf JM, Klose J, Kirschbaum C, Enck P, Klosterhalfen S. Sex-specific adaptation of endocrine and inflammatory responses to repeated nauseogenic body rotation. Psychoneuroendocrinol. 2006;31:226–236. doi: 10.1016/j.psyneuen.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Griffith CR. The effect upon the white rat of continued bodily rotation. Amer. Naturalist. 1920;54:524–534. [Google Scholar]
- 46.Ossenkopp K-P, Frisken NL. Defecation as an index of motion sickness in the rat. Physiol. Psychol. 1982;10:355–360. [Google Scholar]
- 47.McCaffrey RJ. Appropriateness of kaolin consumption as an index of motion sickness in the rat. Physiol. Behav. 1985;35:151–156. doi: 10.1016/0031-9384(85)90329-4. [DOI] [PubMed] [Google Scholar]
- 48.Nakagawa A, Uno A, Horii A, Kitahara T, Kawamoto M, Uno Y, Fukushima M, Nishiike S, Takeda N, Kubo T. Fos induction in the amygdala by vestibular information during hypergravity stimulation. Brain Res. 2003;986:114–123. doi: 10.1016/s0006-8993(03)03220-7. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Jiang ZL, Wang GH, Fan JW. Plasma vasopressin, an etiologic factor of motion sickness in rat and human? Neuroendocrinology. 2005;81:351–359. doi: 10.1159/000088991. [DOI] [PubMed] [Google Scholar]
- 50.Vinnikov YA, Gazenko OG, Titova LK, Bronstein AA, Govardovskii VI, Gribakin FG, Pevzner RA, Aronova MZ, Kharkeevich TA, Tsirulis TP, Pyatkina GA, Lichako DV, Pal'mbach LP, Anichin VF. The structural and functional organization of the vestibular apparatus of rats exposed to weightlessness for 20 days on board the Sputnik "Kosmos-782". Acta Otolaryngol. 1979;87:90–96. doi: 10.3109/00016487909126392. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Peng Z, Yang M, Zhang X, Wei J, Xu M, Zheng QY. Observation of the morphology and calcium content of vestibular otoconia in rats after simulated weightlessness. 2005;125:1039–1042. doi: 10.1080/00016480510037915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hozawa J, Kimura N, Kamata S, Ishida T, Usami S, Kamimura T. Influence of long-term repetitive rotatostimulations on lateral semicircular canals. Acta otolaryngolog. 1984;406:S245–S250. doi: 10.3109/00016488309123044. [DOI] [PubMed] [Google Scholar]
- 53.Nakashima T, Itoh M, Watanabe Y, Sato M, Yanagita N. Auditory and vestibular disorders due to barotrauma. Ann. Otol. Rhinol. Laryngol. 1988;97:146–152. doi: 10.1177/000348948809700211. [DOI] [PubMed] [Google Scholar]
- 54.Perez R, Freeman S, Cohen D, Sohmer H. Functional impairment of the vestibular end organ resulting from impulse noise exposure. Laryngoscope. 2002;112:1110–1114. doi: 10.1097/00005537-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 55.Houpt TA, Cassell JA, Cason AM, Reidell A, GJ G, Riccardi C, Smith JC. Evidence for a cephalic site of action of high magnetic fields on the behavioral responses of rats. Physiol. Behav. 2007;92:665–674. doi: 10.1016/j.physbeh.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glover PM, Bowtell R. Measurement of electric fields induced in a human subject due to natural movements in static magnetic fields or exposure to alternating magnetic field gradients. Phys. Med. Biol. 2008;53:361–373. doi: 10.1088/0031-9155/53/2/005. [DOI] [PubMed] [Google Scholar]