Abstract
IL-15 has substantial potential as an immunotherapeutic agent for augmenting immune responses. However, the activity of IL-15 is mediated by a unique mechanism in which the cytokine is transpresented by cell-bound high-affinity IL-15Rα to target cells expressing the IL-15Rβ and the common γ-chain. Thus, the efficacy of administered IL-15 alone may be limited by the availability of free IL-15Rα. We now show that administration of soluble IL-15/IL-15Rα complexes greatly enhanced IL-15 half-life and bioavailability in vivo. Treatment of mice with this complex, but not with IL-15 alone, resulted in robust proliferation of memory CD8 T cells, NK cells, and NK T cells. The activity of the complex required IL-15Rβ, but not IL-15Rα, expression by the responding cells and was IL-7-independent. Interestingly, IL-15/IL-15Rα immunotherapy also caused naive CD8 T cell activation and development into effector cells and long-term memory T cells. Lastly, complexed IL-15, as compared with IL-15 alone, dramatically reduced tumor burden in a model of B16 melanoma. These findings hold significant importance for the use of IL-15 as a potential adjuvant/therapeutic and inducer of homeostatic proliferation, without the necessity for prior immunodepletion.
Interleukin-15 is a member of the four α helix bundle family of cytokines, and IL-15 mRNA can be detected in a wide variety of tissues of both nonhemopoietic and hemopoietic lineages, but is not thought to be produced by T cells (1, 2). In contrast, under normal circumstances, IL-15 is difficult to detect at the protein level in vivo, perhaps due to short protein half-life and tight transcriptional and translational control (1, 3–5). IL-15 was initially discovered in an adult T cell leukemia cell line and a simian kidney epithelial cell line as a 14- to 16-kDa protein able to stimulate CTLL and peripheral blood T cell proliferation and induce peripheral blood mononuclear cell effector function in vitro (1, 2, 6). The analysis of mutant mice lacking IL-15 or the IL-15Rα (7, 8) reveals that IL-15 plays a multifaceted role in the development and control of the immune system. Both IL-15–/– and IL-15Rα–/– mice lack peripheral NK and NK T cell populations, certain intestinal intraepithelial lymphocyte subsets, a portion of the naive CD8 T cell compartment, and most memory phenotype CD8 T cells. In addition, Ag-specific memory CD8 T cells can develop in both types of knockout mice; however, the resulting memory CD8 T cell pool undergoes dramatic erosion over time (9–11).
The IL-15R consists of three polypeptides, the type-specific IL-15Rα, the IL-2/IL-15Rβ, and the common γ-chain (γC),4 which is shared by multiple cytokine receptors (12, 13). Unlike the IL-2Rα-chain, which exhibits low-affinity for IL-2 in the absence of the IL-2Rβ and γC, the IL-15Rα-chain alone binds IL-15 with high-affinity (Kd ~10–11 M). IL-15Rα was originally believed to form a membrane-bound heterotrimeric complex with the shared IL-2Rβ and the γC (13), akin to the structure of the IL-2R. Although this may be the case in certain situations, IL-15Rα is expressed by a wide variety of cell types but not necessarily in conjunction with IL-2Rβ and γC. For example, the IL-15Rα-chain does not coprecipitate with the IL-2/IL-15Rβ/γC in the presence of IL-15, unlike the IL-2Rα bound by IL-2 (14). Moreover, unlike the IL-2Rα-chain, the IL-15Rα-chain mediates signal transduction (15–17). The functional reasons for these apparent discrepancies between the IL-15 and IL-2 systems became apparent when the mechanism of action of IL-15 was shown to occur via transpresentation by IL-15Rα. Thus, IL-15 produced by one cell type is bound to IL-15Rα expressed by the same cell and presented to apposing cells expressing the IL-15Rβ/γC complex (18–25). In vitro experiments demonstrate that the effect is direct, because IL-15 bound to IL-15Rα-Fc supports the survival of IL-15Rα–/– memory CD8 T cells (20), and soluble receptor/cytokine complexes exhibit hyperagonist activity on cell lines in vitro (26, 27). Overall, the results suggest that the stoichiometry of IL-15 production and IL-15Rα expression may serve to regulate IL-15 activity in vivo.
Given the known effects of IL-15 on the immune system, IL-15 has become an immunotherapeutic target. Although IL-15 administration is used to bolster immune responses or augment immune system reconstitution, blockade of IL-15 activity can inhibit immune responses in certain cases. For example, administration of IL-15-blocking agents such as mutant IL-15/Fc proteins or a soluble form of the IL-15Rα has therapeutic potential in mouse models of arthritis and allograft survival (28–30). Conversely, over-expression of IL-15 or administration of IL-15 (as protein or expressed by plasmid DNA) augments protection of mice from a variety of infections and enhances vaccination (31–39). Furthermore, IL-15 therapy stimulates anti-HIV immunity and increases survival of CD4 and CD8 lymphocytes from HIV-infected patients in vitro (40–46). IL-15 can also accelerate immune reconstitution after bone marrow transplantation (47). Lastly, several groups have found that IL-15 therapy, in conjunction with chemotherapy, TLR agonists, or adoptive transfer of tumor-reactive CD8 T cells, results in increased survival or complete tumor regression in mouse tumor models, in contrast to each therapy alone (48–50). Thus, manipulation of IL-15 activity has potential as a therapeutic modality in a number of clinical situations.
Considering these findings and the transpresentation model, we hypothesized that IL-15 action in vivo could be augmented by the administration of soluble IL (sIL)-15Rα and IL-15 complexes. Our results show that forced transpresentation of IL-15 in vivo profoundly enhanced IL-15 activity and drove proliferation and differentiation of IL-15-responsive immune cells. Importantly, complexed IL-15, in contrast to IL-15 alone, reduced B16 tumor burden in a systemic tumor model. These findings hold important ramifications for the future of IL-15-targeted therapy.
Materials and Methods
Mice and cells
C57BL/6-Ly 5.1 mice were purchased from The Jackson Laboratory. C57BL/6-Ly 5.2 mice were purchased from Charles River Laboratories. The OT-I mouse line was provided by Dr. W. R. Heath (Walter and Eliza Hall Institute, Parkville, Australia) and Dr. F. Carbone (University of Melbourne, Parkville, Australia) and was maintained as a C57BL/6-Ly5.2 line on a RAG–/– background. IL-15Rα–/– mice (8) were provided by Dr. A. Ma (University of California San Francisco, CA). Spleen cells from IL-2Rβ–/– mice were provided by Dr. M. Farrar (University of Minnesota, Minneapolis, Minnesota). IL-7–/– mice (51) were originally obtained from DNAX Research Institute and were maintained on a C57BL/6 background. All procedures were conducted under National Institutes of Health guidelines and were approved by the institutional animal care committee. Memory CD8 T cells were generated by adoptive transfer of CD45.1 OT-IRAG–/– cells to CD45.2 C57BL/6 (B6) mice that were then infected with vesicular stomatitis virus (VSV)-OVA. Alternatively, to produce VSV nucleoprotein-specific memory cells, CD45.1 B6 mice were infected i.v. with 1 × 105 PFU VSV-Indiana. In either case, at least 60 days after infection, enriched CD8 T cells containing OT-I or VSV-specific memory cells were used in adoptive transfer studies.
CFSE labeling of cells and adoptive transfer
Single-cell suspensions were created in HBSS by homogenizing spleens or lymph nodes using frosted glass slides. RBC were lysed, and splenocytes were filtered through Nitex. Cells were incubated for 10 min at 37°C with CFSE (2 μM; Molecular Probes), the reaction was squelched with HBSS with 5% FCS (52), and the cells were washed twice. CFSE-labeled cells were resuspended in PBS and injected i.v. into congenic mice.
IL-15 treatment
Human IL-15 was generously provided by Amgen. Recombinant mouse IL-15Rα-Fc chimeric molecule was purchased from R&D Systems. Human IL (hIL)-15 and recombinant murine (rm)IL-15Rα-Fc, both suspended in PBS, were mixed and incubated for 30 min at 37°C. Each mouse, unless specifically noted, received 2.5 μg of IL-15 either alone or precomplexed with 15 μg of rmIL-15Rα-Fc in 200 μl of PBS i.p.
Flow cytometric analysis
Cells were isolated at the indicated times and analyzed for the presence of donor cells using CD45 allele status and their expression of surface markers and CFSE intensity. The percentage of cells of the original population that had divided (the “responding” population, R) was calculated as described previously (53). VSV nucleoprotein-specific donor cells were detected using H-2Kb tetramers prepared as described previously (54, 55). For staining, lymphocytes were suspended in PBS/0.2% BSA/0.1% NaN3 (FACS buffer) at a concentration of 3–15 × 106/200 μl. For tetramer staining, cells were incubated at room temperature for 1 h with tetramer-APC plus the appropriate dilution of anti-CD8 PerCP. Cells were washed with FACS buffer and stained with Abs specific for either CD44, CD122, NK1.1, CD3, or CD4 (all mAbs from BD Pharmingen) at 4°C for 20 min, washed, and then fixed in PBS with 3% paraformaldehyde. Relative fluorescence intensities were measured with a FACSCalibur (BD Biosciences). Data were analyzed using FlowJo Software (Tree Star).
ELISA for detection of IL-15
Anti-hIL-15 (MAB647; R&D Systems) in PBS (5 μg/ml, 100 μl/well) was added to 96-well high binding plates (3590; Corning) at 37°C for 1 h. The plates were washed and then blocked using PBS/1% BSA/0.2% Tween 20 (200 μl/well) for 1 h at 37°C. Dilutions of serum (in blocking buffer) were incubated for 1 h at 37°C, followed by washing with PBS/0.05% Tween 20 and the addition of biotinylated anti-hIL-15 Ab (BAM247; R&D Systems; 0.2 μg/ml, 100 μl/well) for 1 h at 37°C. Finally, Avidin-HRP (BD Pharmingen) (1/1000 dilution) was added for 1 h at 37°C. After washing, TMB substrate (34021; Pierce) was added, and 1 M phosphoric acid was used to stop the reaction. ODs at 450–570 nm were measured using a microplate reader (Bio-Rad; model 680). The serum half-life of IL-15 was calculated using the medical calculator provided by Cornell University per the given instructions (〈http://www-users.med.cornell.edu/~spon/picu/calc/halfcalc. htm〉).
In vivo cytotoxicity assay
This assay was performed essentially as described previously (56). Normal spleen cells were labeled to low (0.25 μm) or high (2.5 μm) CFSE levels, and CFSElow cells were incubated with 1 μg/ml SIINFEKL peptide for 45 min at 37°C. Equal numbers (10 × 106) of each population were mixed and injected i.v. into OT-I-transferred mice that were either untreated or treated with IL-15/IL-15Rα or were infected with 1 × 105 PFU of VSV-expressing chicken OVA (57) 4 days earlier. Four hours later, spleen cells were analyzed for the presence of CFSEhigh and CFSElow populations. Percentage of lysis = [1 – (ratio unprimed:ratio primed)] × 100. Ratio = percentage of CFSElow:percentage of CFSEhigh.
Intracellular detection of IFN-γ
Lymphocytes were isolated from the spleen and cultured for 5 h with 1 μg/ml Golgistop (BD Pharmingen), with or without 1 μg/ml of the OVA-derived peptide SIINFEKL. After culture, cells were stained for surface molecules then fixed, and cell membranes were permeabilized in Cytofix/Cytoperm solution (BD Pharmingen) and stained with anti-IFN-γ PE or control rat IgG1 PE. Cells were then washed, and the fluorescence intensity was measured on a FACSCalibur (BD Biosciences).
Tumor challenge and treatment
B16-F1 cells were maintained in Advanced DMEM (Invitrogen Life Technologies) supplemented with 10% FCS/100 U/ml penicillin/100 μg/ml streptomycin/430 μg/ml Glut-Max. Cells were harvested using 0.25% trypsin/EDTA (Invitrogen Life Technologies) when 50–80% confluent, and 1 × 105 cells (in PBS) were injected via the lateral tail vein. On day 1 and day 10 post-B16 injection, mice began receiving treatment i.p.: PBS, IL-15 (2.5 μg), or IL-15/R-Fc (2.5 μg/15 μg). Mice were sacrificed on day 21. For tumor examination, mice were randomized and scored in a blinded fashion.
Results
Coadministration of IL-15 and IL-15Rα drives CD8 memory T cell and NK cell proliferation in vivo
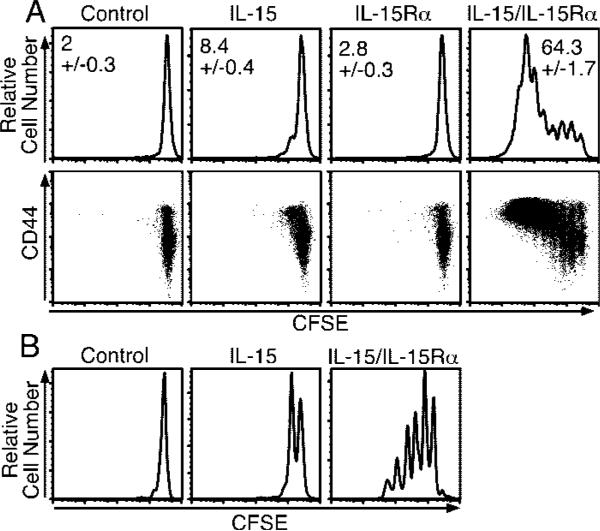
To determine whether precomplexed cytokine and soluble receptor would augment IL-15 activity in vivo, IL-15 and IL-15Rα-Fc (IL-15Rα) were incubated at a 1:1 molar ratio before injection. At this ratio, because rmIL-15Rα-Fc is dimeric, one binding site per rmIL-15Rα-Fc is theoretically filled. Increasing by 2-fold the amount of IL-15 added to the receptor did not increase activity (data not shown). Human and mouse IL-15 provoked similar responses in our model, although hIL-15 exhibited somewhat higher activity than mouse IL-15 (data not shown). To measure IL-15-mediated activity in vivo, we used an adoptive transfer model to gauge the effect of IL-15 on the proliferation of CD8 T cells. CD45.1 CFSE-labeled CD8-enriched splenocytes were transferred to normal CD45.2 mice that were then treated with PBS, IL-15 alone (2.5 μg), IL-15Rα (15 μg), or a mixture of IL-15 (2.5 μg) and IL-15Rα (15 μg). Four days after treatment with IL-15 alone, 8.4% of the donor CD8 T cell population had divided (Fig. 1A, top panels), in agreement with our previous results (23). In dramatic contrast, the coadministration of the same amount of IL-15 bound to IL-15Rα resulted in the proliferation of 64.3% of the donor CD8 T cells (Fig. 1A, top panels). Furthermore, whereas the majority of CD8 T cells responding to IL-15 alone divided once, the cells responding to combination treatment underwent 5–7 divisions, resulting in a substantial increase in donor cell numbers (data not shown). Importantly, administration of IL-15Rα alone did not induce proliferation of CD8 T cells (Fig. 1A, top panels). The bulk of the dividing cells expressed high levels of CD44, suggesting that the responding cells were primarily memory CD8 T cells or that CD44 had been up-regulated (Fig. 1A, bottom panels). To test the action of combined therapy on bona-fide memory CD8 T cells, we adoptively transferred CFSE-labeled nucleoprotein-specific memory CD8 T cells that had been generated by infection with VSV. Similar to the above results, Ag-specific memory CD8 T cells responding to combined IL-15/IL-15Rα treatment proliferated to a much greater extent than those provided IL-15 alone (Fig. 1B) and increased in number (data not shown).
FIGURE 1.
Coadministration of IL-15 and IL-15Rα-Fc enhances CD8 T cell proliferative response to exogenous IL-15. A, On day –1, mice received 1.5 × 107 congenic CFSE-labeled, CD8-enriched lymphocytes i.v. and were treated i.p. on day 0 with PBS, IL-15 (2.5 μg), IL-15Rα-Fc (15 μg), or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg). CD8+ splenocytes were analyzed on day 4 by flow cytometry for CFSE fluorescence and CD45.1 expression (top panels), or CD45.1+CD8+ cells were analyzed for CFSE fluorescence and CD44 expression (bottom panels) (n = 4). Data are representative of three similar experiments. B, On day –1, mice received CFSE-labeled CD8 T cell-enriched splenocytes containing ~6.5 × 105 tetramer+ VSV nucleoprotein-specific memory CD8 T cells and were treated on day 0 with PBS, IL-15 (2.5 μg), or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg). Donor tetramer+ splenocytes were analyzed by flow cytometry on day 4 for CFSE fluorescence. R = the percentage of responding cells.
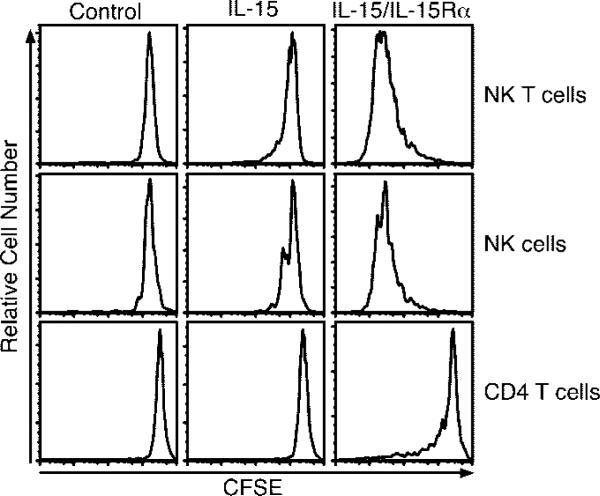
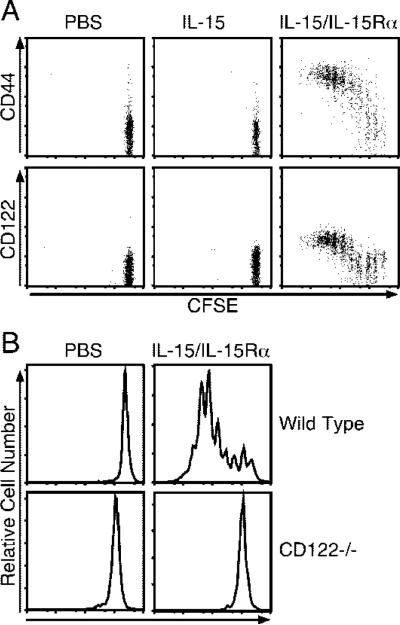
Past studies have implicated IL-15 as an inducer of B cell, NK cell, and NK T cell proliferation (58–63). Therefore, we examined the ability of IL-15 and receptor-complexed IL-15 to induce proliferation of these cell types using the adoptive transfer system. CD4 T cells did not proliferate in response to 2.5 μg of IL-15, whereas NK and NK T cells proliferated very little (Fig. 2). In contrast, coadministration of IL-15Rα with IL-15 induced extensive proliferation of NK and NK T cells (Fig. 2). B cells did not respond to IL-15 alone or complexed IL-15. Interestingly, CD4 T cells responded at an intermediate level to the administered complex (Fig. 2). The responding CD4 T cells tended to express high levels of CD44 (data not shown). Of interest, the polyclonal CD8 T cell population, Ag-specific memory CD8 T cells, and NK cells also exhibited signs of activation 1 day posttreatment in terms of CD69 up-regulation and CD127 down-regulation (data not shown).
FIGURE 2.
NK and NK T cells are highly responsive to IL-15/IL-15Rα-Fc. On day –1, mice received ~1.5 × 107 congenic CFSE-labeled lymphocytes i.v. and on day 0 were treated with PBS, IL-15 (2.5 μg), or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg). Spleen cells were analyzed by flow cytometry on day 4 (n = 3). Samples were gated on the indicated donor population (NK = NK1.1+CD3–; NK T cell = NK1.1+CD3+; CD4 = CD4+ lymphocytes). Data are representative of three similar experiments.
Complexed IL-15/IL-15Rα greatly enhances IL-15 activity in vivo
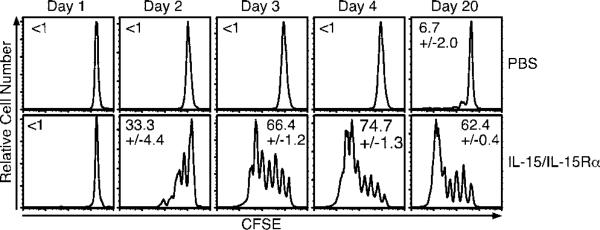
We next examined the early kinetics of the CD8 T cell-proliferative response to the coadministration of IL-15Rα with IL-15. CFSE dilution was negligible 1 day after treatment, but by day 2 33% of the donor CD8 T cell population had divided (Fig. 3). By day 3, 66% of donor CD8 T cells had divided with many cells in divisions 5–6, whereas 74% had divided by day 4 with some cells in their 7th round of division. By day 20 after treatment, many of the cells had completely diluted their CFSE, although cells at intermediate stages of division remained. These results showed that the maximum effect of a single dose of IL-15/IL-15Rα was achieved by ~4 days posttreatment.
FIGURE 3.
CD8 T cells rapidly divide in response to IL-15/IL-15Rα-Fc treatment. On day –1, mice received 1 × 107 congenic CFSE-labeled, CD8-enriched lymphocytes i.v. and were treated with PBS or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) on day 0. Peripheral blood lymphocytes were analyzed by flow cytometry on days 1–4 and day 20. Samples shown are gated on live donor CD8 T cells. Data are representative of two similar experiments. R = the percentage of responding cells.
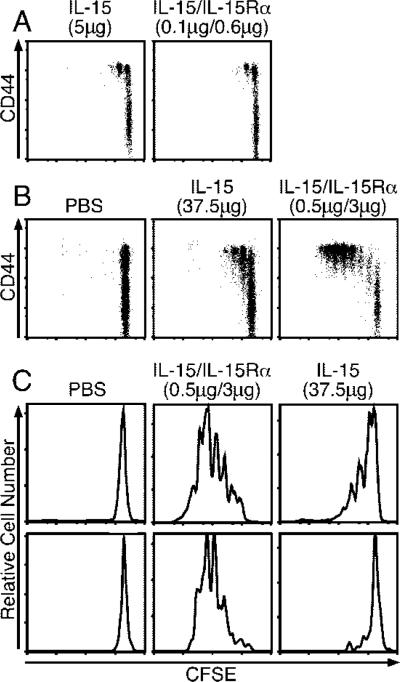
To obtain an approximation of the level of activity enhancement obtained by combined treatment over that of IL-15 alone, we performed titrations of IL-15 and IL-15/IL-15Rα using the adoptive transfer system. Comparisons were based on the extent of donor CD8 T cell proliferation as assessed by CFSE dilution. A dose of 0.1 μg of IL-15 combined with 0.6 μg of IL-15Rα induced a level of proliferation similar to that of 5 μg of IL-15 (Fig. 4A). Thus, in this type of experiment, IL-15 activity was enhanced ~50-fold by coadministration with IL-15Rα. Considering this substantial enhancement, we questioned whether IL-15 alone could achieve a similar level of activity. Even with the administration of 37.5 μg of IL-15, the level of proliferation obtained with 0.5 μg of receptor-complexed IL-15 could not be achieved (Fig. 4B). When examining the NK and NK T cell response to 37.5 μg of IL-15, the proliferation induced was nowhere near the level achieved by 0.5 μg of IL-15 complexed with IL-15Rα (3.0 μg) (Fig. 4C). We also noted that the CD8 T cell proliferation induced by IL-15 alone plateaued at ~12 μg of cytokine and did not increase with increasing dosage (data not shown). These results suggested that IL-15 half-life and/or IL-15Rα availability were limiting in vivo.
FIGURE 4.
Coadministration of IL-15Rα-Fc with IL-15 greatly enhances IL-15 potency. A, On day –1, mice received 1.5 × 106 congenic CFSE-labeled, CD8-enriched lymphocytes i.v. and on day 0 received either PBS (data not shown), IL-15 (5 μg), or varying doses of IL-15/IL-15Rα-Fc (0.5 μg + 3 μg) i.p. (n = 3). Data are representative of two similar experiments. B and C, On day –1, each mouse received 4.5 × 106 congenic CFSE-labeled, CD8-enriched lymphocytes i.v. and on day 0 received either PBS (data not shown), IL-15 (0.5 μg) + IL-15Rα-Fc (3 μg), or IL-15 (37.5 μg) i.p. CD8+ splenocytes were analyzed on day 4 for CFSE dilution by flow cytometry (n = 3). Data are representative of two similar experiments.
Complexing IL-15 to IL-15Rα greatly increases half-life and serum levels of IL-15
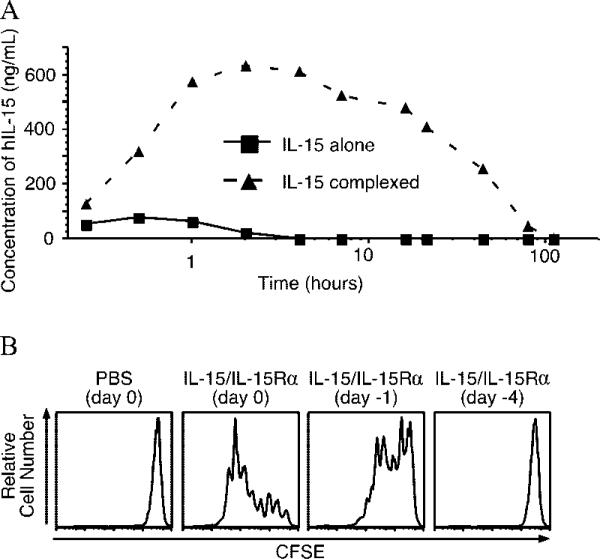
The effect seen by complexing IL-15 and soluble receptor may operate in part by increasing the half-life and bioavailability of exogenously administered IL-15. To test this possibility, we developed an ELISA to detect hIL-15 in mouse serum. Preliminary experiments indicated that complexing hIL-15 with IL-15Rα did not interfere with Ab binding (data not shown). Mice were treated i.p. with hIL-15 (2.5 μg) alone or a mixture of hIL-15 (2.5 μg) and IL-15Rα (15 μg), and serum was obtained at various times after treatment, as well as just before treatment. We noted that the half-life of hIL-15 alone was ~1 h, whereas when complexed to the receptor, IL-15 half-life was extended to ~20 h (Fig. 5A). With regard to maximum serum levels obtained, IL-15 alone peaked at a concentration of ~70 ng/ml 30 min after administration, whereas complexed IL-15 peaked 2 h after administration at a concentration of ~600 ng/ml. Similar results were noted with administration via the i.v. route, indicating that the differences noted were not due to differences in absorption from the peritoneal cavity (data not shown). Using this assay, we also determined that the presence or absence of endogenous IL-15Rα did not affect the serum levels or kinetics of administered hIL-15 (data not shown), suggesting that IL-15 binding to endogenous IL-15Rα did not contribute significantly to the short half-life of IL-15. The serum levels of IL-15 when administered as a receptor complex also correlated with functional activity as measured by CFSE dilution of transferred cells (Fig. 5B). By 24 h after administration, activity had declined substantially and by day 4 after treatment, no activity was detected by this assay.
FIGURE 5.
Complexing IL-15 with IL-15Rα-Fc increases half-life and bioavailability of exogenous IL-15 in the serum. A, Mice were injected with hIL-15 (2.5 μg) i.p. with or without precomplexed IL-15Rα-Fc (15 μg). Mice were bled over time (0.25, 0.5, 1, 2, 4, 7, 16, 21, 44, 79, and 110 h after treatment), and hIL-15 presence in mouse serum was monitored using a hIL-15-specific ELISA. Data are representative of two similar experiments with three mice per group. B, Mice were injected with either PBS on day 0 or IL-15 (2.5 μg) and IL-15Rα-Fc (15 μg) complex on day –4, day –1, or day 0 i.p. On day 0, all mice received 1 × 106 CFSE-labeled CD45.1 CD8 T cells i.v. Splenocytes were examined on day 4 posttransfer for CFSE dilution. Experiment is representative of two similar experiments with three mice each.
Receptor-complexed IL-15 functions through IL-15Rβ
The effects of complexed IL-15/IL-15Rα could either be mediated by direct or indirect effects on the responding cell types. If direct, then it might be expected that the target cells would be required to express IL-15R component(s). To test this, we transferred CFSE-labeled CD45.1 IL-15Rα–/– CD8 T cells into CD45.2 IL-15Rα–/– hosts and treated the mice with either IL-15 or complexed IL-15/IL-15Rα. IL-15 alone could not be transpresented in the absence of endogenous IL-15Rα, and did not induce proliferation (Fig. 6A) (23). In contrast, donor CD8 T cells from IL-15/rmIL-15Rα-treated mice proliferated extensively. Furthermore, the IL-15Rα–/– donor cells, which primarily consisted of naive phenotype CD8 T cells, progressively increased their expression of CD44 and CD122 with division (Fig. 6A).
FIGURE 6.
Activity of complexed IL-15/IL-15Rα-Fc requires IL-2Rβ but not IL-15Rα expression by responding cells. A, On day –1, CD45.2 IL-15Rα–/– mice received 2 × 106 CD45.1 CFSE-labeled, CD8-enriched IL-15Rα–/– lymphocytes i.v. and on day 0 were treated with PBS, IL-15 (2.5 μg), or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) i.p. On day 4, CD8+ donor splenocytes were analyzed for CFSE fluorescence and CD44 and CD122 expression (n = 3). Data are representative of two similar experiments. B, On day –1, CD45.1 B6 mice received 1.7 × 107 CD45.2 CFSE-labeled wild-type or IL-2/IL-15Rβ–/– splenocytes i.v. and on day 0 were treated with either PBS or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) i.p. CD45.2 CD8+ donor splenocytes were analyzed for CFSE dilution on day 4 by flow cytometry.
Because responding T cells did not require IL-15Rα to respond to complexed IL-15/IL-15Rα, we examined the role of IL-15Rβ (CD122) in mediating this effect, the expression of which is required for transpresentation activity of IL-15 (23). To this end, we transferred CFSE-labeled CD45.2 CD122+/+ or CD122–/– CD8 T cells into CD45.1 B6 mice and analyzed the donor cells for CFSE dilution 4 days after treatment. Although control cells proliferated vigorously in response to IL-15/IL-15Rα treatment, CD122–/– donor CD8 T cells did not proliferate in response to coadministration (Fig. 6B). Importantly, endogenous CD8 T cells in both groups expanded in response to treatment. Similar results were obtained when CD122-blocking Ab was used to prevent IL-15 signaling (data not shown). Taken together, the results indicated that IL-15/IL-15Rα operated via direct transpresentation through interaction with the IL-15Rβ likely in conjunction with γC.
Proliferation induced by forced IL-15 transpresentation does not require IL-7
Because IL-7 is essential for the homeostatic proliferation of CD8 T cells in immunodeficient hosts (64), we wished to test the role of IL-7 in proliferation induced by receptor-bound IL-15. Congenic CFSE-labeled CD8 T cells were transferred to control or IL-7–/– mice, and combined IL-15/IL-15Rα was administered. The absence of IL-7 had no effect on CD8 T cell proliferation in response to IL-15 with IL-15Rα (Fig. 7), indicating that IL-7 was not involved in IL-15-mediated proliferation in our system.
FIGURE 7.
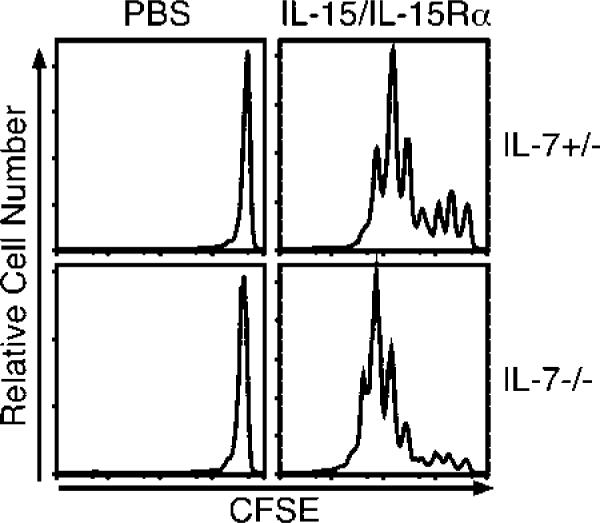
IL-15/IL-15Rα-Fc-driven proliferation of CD8 T cells does not require host IL-7 expression. On day –1, CD45.1 IL-7+/– or IL-7–/– mice received 6 × 106 CD45.2 B6 CFSE-labeled CD8-enriched lymphocytes. On day 0, mice received PBS or IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) i.p. On day 4, CD45.2 CD8+ splenocytes were examined for CFSE dilution (n = 3). Data are representative of two similar experiments.
IL-15/IL-15Rα immunotherapy induces naive T cell activation and effector function
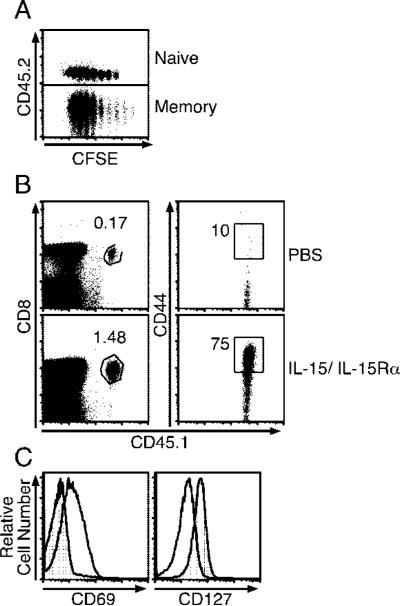
In previous experiments, we noted that CD44low polyclonal CD8 T cells as well as naive TCR transgenic T cells responded to IL-15 when coadministered with IL-15Rα (Figs. 1 and 6). Considering that under homeostatic conditions CD8 memory T cells exhibit much greater responsiveness to IL-15 than do naive CD8 T cells, we wished to directly compare the responsiveness of these two subsets to complexed IL-15/rmIL-15Rα. To do so, CFSE-labeled memory OT-I and naive OT-I CD8 T cells were adoptively transferred into the same congenic C57BL/6 hosts, and proliferation was analyzed 4 days after treatment with IL-15/IL-15Rα. Surprisingly, naive OT-I CD8 T cells proliferated almost as well as memory OT-I CD8 T cells (Fig. 8A). The naive OT-I cells also expanded ~10-fold in response to the complex as compared with controls and up-regulated CD44 (Fig. 8B). Similar results were seen with FACS-purified CD44low OT-I cells (data not shown); thus, any contaminating CD44high naive OT-I cells cannot account for the naive CD8 T cell proliferation seen. We also examined the activation status of naive OT-I cells 1 day after treatment by measuring the expression level of CD69, which is up-regulated after T cell activation, and IL-7Rα (CD127), which is down-regulated after T cell activation (64). Interestingly, CD69 was increased and CD127 was decreased on OT-I cells 1 day after treatment with IL-15/IL-15Rα (Fig. 8C).
FIGURE 8.
Naive CD8 T cells acquire effector phenotype in response to IL-15/IL-15Rα-Fc treatment. A, On day –1, CD45.2 B6 mice received a mixture of CD45.1/2 naive and CD45.1 memory OT-I-RAG–/– cells and were treated with either PBS or rmIL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) on day 0. Four days later, splenocytes were examined for CFSE intensity of each CD8 + donor population (n = 2). B, On day –1, CD45.2 B6 mice received 3 × 106 CD45.1 OT-I RAG–/– cells i.v. and were treated with either PBS or rmIL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) on day 0. The donor OT-I population was examined for frequency and CD44 expression by flow cytometry (n = 3). Percentage shown is for percentage of OT-I per total splenocytes and is taken from one mouse representative of each population. Data are representative of two similar experiments. C, Mice received 6 × 106 naive CD45.1 OT-I-RAG–/– cells i.v. and either received PBS or IL-15 (2.5 μg) and IL-15Rα-Fc (15 μg) i.p. on day 0. Mice were sacrificed, and the expression of CD69 and CD127 by transferred OT-I population was examined by flow cytometry (n = 3).
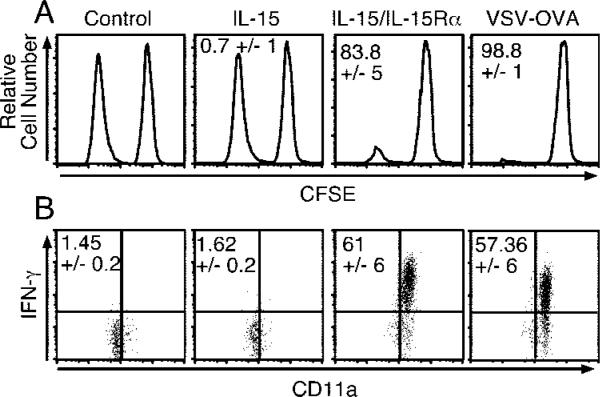
In light of the robust proliferation induced in naive T cells, it was of interest to establish whether effector function was concomitantly induced. To test this possibility, we adoptively transferred naive OT-I CD8 T cells into congenic C57BL/6 hosts and, using an in vivo cytotoxicity assay, measured Ag-specific lytic activity 4 days after treatment with IL-15/rmIL-15Rα or after infection with recombinant VSV-expressing OVA (VSV-OVA) for comparison. Interestingly, IL-15/rmIL-15Rα treatment resulted in induction of robust Ag-specific lytic activity, similar to the level obtained with virus infection (Fig. 9A). In addition to lytic activity, the majority of naive OT-I CD8 T cells activated by IL-15/IL-15Rα or VSV-OVA infection produced high levels of IFN-γ following in vitro restimulation with peptide (Fig. 9B). This result was in contrast to the negligible frequency of OT-I cells producing IFN-γ from control (PBS) and IL-15-treated mice (Fig. 9B). Thus, the induction of effector function in naive CD8 T cells by coadministration of IL-15Rα with IL-15 paralleled the activation obtained by infection.
FIGURE 9.
Receptor-complexed IL-15 induces effector function in naive CD8 T cells. A, On day –1, mice received 2.5 × 106 naive OT-I-RAG–/– cells and were treated with PBS, IL-15 (2.5 μg), IL-15Rα-Fc (15 μg) with IL-15 (2.5 μg) i.p., or 1 × 105 PFU of VSV-OVA i.v. On day 4 posttreatment, each mouse received a mixture of CD45.1/2 CFSE-labeled (1.5 μM) nonpeptide-pulsed splenocytes and CFSE-labeled (0.0015 μM) SIINFEKL peptide-pulsed splenocytes. Four hours later, splenocytes were analyzed for the presence of the CFSE-labeled target populations (data shown) (n = 4). Data are representative of two experiments. Percentage shown is percentage of Ag-specific killing per group ± SD. B, On day –1, CD45.2 mice received ~2 × 106 naive CD45.1 OT-I-RAG–/– cells and on day 0 were treated with PBS, IL-15 (2.5 μg), IL-15Rα-Fc (15 μg), and IL-15 (2.5 μg), or 1 × 105 PFU VSV-OVA i.v. On day 4, splenocytes were incubated in vitro with or without SIINFEKL peptide for 5 h, and the production of IFN-γ OT-I (gated on CD45.1 donor cells) was analyzed by intracellular staining (n = 3). Data are representative of two similar experiments. Percentage shown is percentage of the gated OT-I donor population staining for intracellular IFN-γ.
Treatment of naive T cells with complexed IL-15/IL-15Rα generates memory CD8 T cells
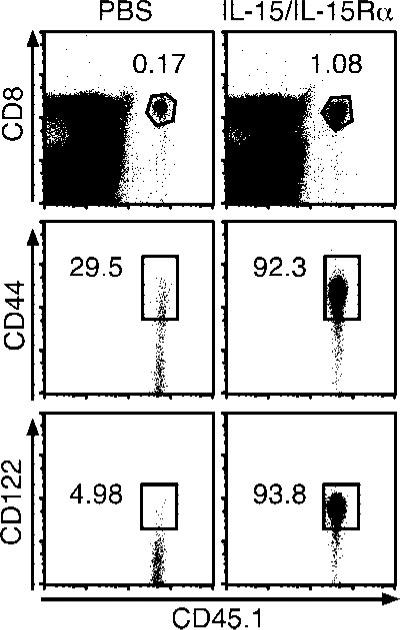
Although naive T cells developed into effector cells in response to transpresented IL-15, it remained to be seen whether this was a transient effect or resulted in memory T cell development. Therefore, we analyzed the number and phenotype of OT-I T cells 44 days after naive OT-I T cell transfer and IL-15/IL-15Rα treatment. At this time point, a ~5-fold higher percentage of OT-I cells was present following IL-15/IL-15Rα administration as compared with untreated mice, as well as greater OT-I numbers (Fig. 10, top panels; data not shown). Moreover, nearly all of these cells expressed high levels of CD44 and CD122 (Fig. 10, middle and bottom panels). We have also noted that memory OT-I cells induced by receptor-complexed IL-15 were able to produce IFN-γ upon peptide restimulation to a similar extent as bona-fide Ag-experienced OT-I cells (data not shown). Thus, in the absence of Ag, IL-15/IL-15Rα treatment was able to induce the development of memory CD8 T cells.
FIGURE 10.
IL-15/IL-15Rα-Fc treatment generates memory cells from naive CD8 T cells. On day –1, B6 mice received ~6 × 106 CD45.1 CFSE-labeled naive OT-I-RAG–/– cells and on day 0 were treated i.p. with PBS or IL-15 (2.5 μg) and IL-15Rα-Fc (15 μg). Forty-four days later, splenocytes were analyzed for percentage of donor OT-I CD8 T cells (top panels) and OT-I expression of CD44 and CD122 (middle and bottom panels). Percentages shown are from individual mice (n = 2). Data are representative of two experiments.
IL-15/IL-15Rα acts as an antitumor immunotherapeutic agent
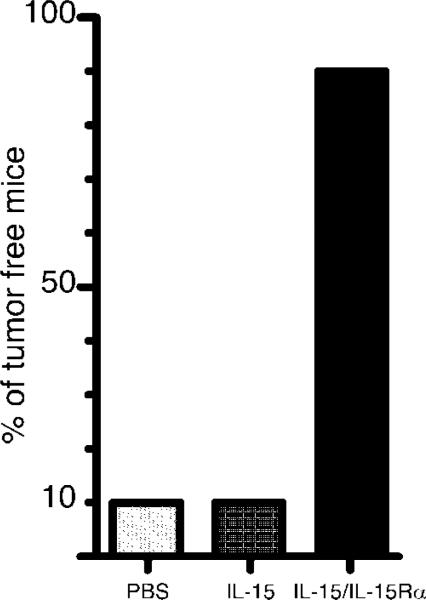
Because IL-15/IL-15Rα leads to the expansion and activation of CD8 T cells and NK cells, two populations known to be involved in tumor surveillance, we wished to compare the ability of IL-15 vs IL-15/IL-15Rα in enhancing tumor immunity. To this end, we injected 1 × 105 B16-F1 melanoma cells i.v. on day 0 and treated mice with either PBS, IL-15 (2.5 μg), or IL-15/IL-15Rα (2.5 μg/15 μg) i.p. on day 1 and day 10. This tumor protocol leads to the establishment of tumors in the lung and liver. Mice were examined and scored for the presence of tumor nodules. We found that 90% of the PBS and IL-15-treated mice (compiled from two separate experiments) were tumor positive (Fig. 11) and exhibited a similar tumor burden between groups (multiple tumors >5 mm in diameter), whereas only one of the IL-15/IL-15Rα-treated mice was tumor positive and contained only a single 2-mm lung tumor. These results indicated a potential therapeutic value of combining IL-15 and IL-15Rα to prevent tumor engraftment.
FIGURE 11.
IL-15/IL-15Rα complex is an effective antitumor immunotherapy agent. Mice were given 1 × 105 B16-F1 cells i.v. (day 0), followed by two doses of either PBS, IL-15 (2.5 μg), or IL-15 (2.5 μg) with IL-15Rα-Fc (15 μg) i.p. on day 1 and day 10. On day 21 mice were sacrificed, and tumor (black nodules) burden in lung and liver were noted. Data shown are the compilation of two separate experiments (n = 10).
Discussion
Recent findings support the use of IL-15 as an adjuvant for vaccination, tumor immunotherapy, and immune system reconstitution in immunodeficiency (50, 65–67). In the case of cancer treatment, induction of lymphopenia is now being used to enhance the functional activity of adoptively transferred lymphocytes (68–72). This modality is based on the finding that CD8 T cells undergoing lymphopenia-driven homeostatic proliferation differentiate into effector cells with lytic and cytokine-producing activities (73, 74). Thus, the proliferation and functional activities induced by the IL-15/IL-15Rα complex in intact hosts mimicked homeostatic proliferation triggered by lymphopenia.
Noteworthy, the level of proliferation obtained by treatment with the complex could not be achieved by high doses of IL-15 alone. Because the same cell producing IL-15 may also transpre-sent the cytokine (23–25, 75), the availability of free IL-15Rα may be limited thus restricting the effectiveness of treating with IL-15 alone. The fact that the serum half-life or level of exogenous IL-15 was not significantly altered by the presence or absence of endogenous IL-15Rα (data not shown) further supports the theory that free IL-15Rα is limiting in vivo. Our results demonstrated that IL-15/IL-15Rα recapitulated IL-15 responsiveness in an IL-15Rα–/– host. This was illustrated by the proliferation of IL-15Rα–/– donor CD8 T cells in an IL-15Rα–/– host, as well as the induction of memory phenotype CD8 T cells and NK cells (data not shown), that are normally lacking in IL-15Rα–/– mice. This effect highlighted the potential of receptor-complexed IL-15 to reestablish the cytolytic arm of the immune system during states of lymphopenia. In addition, our studies showed that the short half-life of IL-15 was extended ~20-fold when complexed to the receptor. Equally impressive was the notable increase in the serum availability of IL-15 when administered bound to IL-15Rα. Indeed, when complex was delivered i.v., nearly all of the administered IL-15 was accounted for in the serum some 15 min later, whereas when uncomplexed, only 4% of the IL-15 dose could be detected in the serum (data not shown). Therefore, treatment with IL-15 alone is unlikely to achieve the full therapeutic potential of the cytokine. The combined administration of IL-15/IL-15Rα may provide improved efficacy by driving transpresentation through available IL-15Rβ/γC.
The activity of complexed IL-15/IL-15Rα appears to be mediated at multiple levels. Mortier et al. (26) recently demonstrated that IL-15 bound to sIL-15Rα has a higher affinity for IL-15Rβ/γC than does free IL-15. Thus, from our data and that of others, binding of IL-15 by IL-15Rα-chain can increase IL-15 potency by 1) increasing IL-15 half-life (Fig. 4), 2) increasing IL-15 affinity for IL-15Rβ/γC (26), and 3) providing a platform for transpresentation. Future work will examine the mechanisms of the IL-15Rα-mediated increase in IL-15 half-life. Possible mechanisms involve 1) protection of IL-15 from degradation by proteases, 2) inhibition of clearance via receptor binding or other mechanisms, or 3) FcR-mediated binding/recycling of complex. In regard to the latter, although the presence of the Ig Fc portion in the complex could augment activity through FcR-mediated signaling, IL-15 bound to monomeric sIL-15Rα lacking a Fc, retained function in vivo, although a quantitative comparison with the Fc-containing molecule was not performed (data not shown).
The ability of IL-15/IL-15Rα to drive T cell activation was of particular interest given the current paradigm regarding the requirements for naive and memory T cell homeostatic survival and proliferation. Under normal conditions, survival of both naive and memory CD8 T cells requires IL-7 (64), whereas IL-15 is essential for homeostatic proliferation of memory CD8 T cells (9) and NK cell survival (24, 76) in normal hosts. In a lymphopenic environment, IL-7 is required for homeostatic proliferation of naive CD8 and CD4 T cells (64), and plays a role, along with IL-15, in mediating CD8 memory T cell homeostatic proliferation (62). Thus, it was unexpected that naive CD8 T cells responded vigorously to the IL-15/IL-15Rα complex. It should be noted, however, that in IL-15–/– mice, the naive CD8 T cell pool is decreased ~50%, suggesting that either naive CD8 T cell development and/or survival requires IL-15 (7). In any case, proliferation of naive, as well as memory phenotype, CD8 T cells driven by receptor-bound IL-15 was IL-7 independent and required IL-15Rβ signaling. This result indicated that naive CD8 T cells expressed sufficient levels of IL-15Rβ to respond to IL-15/IL-15Rα but not to sIL-15 alone. Previous reports also show that IL-15 activates naive human CD8 T cells in vitro (77, 78). Interestingly, the naive CD8 T cell response to cytokine paralleled that of an Ag-specific response, although the response was driven by unphysiological levels of IL-15 activity. Nevertheless, clonal expansion occurred, and effector function was induced, followed by contraction of the responding population and generation of memory CD8 T cells. Phenotypic changes similar to those observed following TCR triggering also accompanied activation via receptor-complexed IL-15, with CD69 levels up-regulated and IL-7Rα down-regulated early after treatment. This process occurred in the absence of Ag, although whether MHC is necessary for these events is currently under investigation. A previous in vitro study showed that IL-15 induced a similar activation and genetic profile in human CD8 memory T cells as did TCR cross-linking (79), suggesting some overlap in the signaling pathways activated by these receptors. Additional experimentation will be needed to decipher the underlying mechanisms of naive CD8 T cell activation mediated by IL-15.
Our findings also highlight the potential of receptor-complexed IL-15 as a cancer therapeutic. Two doses of complexed IL-15 were able to lead to the rejection (or prevent the establishment) of B16 melanoma tumors, whereas IL-15 alone was not able to diminish tumor burden. Future studies will examine the effect of IL-15/IL-15Rα on more established tumors using a variety of doses and treatment schedules, as well as determining the mechanism and cell types involved in tumor rejection.
These findings present a conundrum, considering that sIL-15Rα has been used to inhibit collagen-induced arthritis (29), cardiac allograft rejection (30), delayed type hypersensitivity (75), and allergic airway disease (80). Interestingly, initiation of all of these conditions is dependent, either solely or in part, on CD4 T cells, suggesting that CD4 T cells may respond to IL-15 indirectly. Thus, IL-15 may activate dendritic cells during certain immune responses (75) leading to CD4 T cell activation, and this event could hypothetically occur via direct IL-15 action through IL-15Rα in the absence of IL-15Rβ, rather than through transpresentation. In this scenario, activation of CD4 T cells, and in some cases CD8 T cells, could be inhibited by administration of sIL-15Rα if levels of free IL-15 are elevated, as is known to occur in inflammatory diseases. Recent data (81) also show that a cell surface form of IL-15 exists whose function in vivo remains obscure, but binding of sIL-15Rα to such a molecule could also exert potential inhibitory effects. Some diversion of the response toward CD8 and NK/NK T cell activation through treatment with sIL-15Rα may also result in inhibition of certain immune responses. Further studies are needed to determine the parameters that determine whether inhibition or augmentation of immune responses is the outcome of manipulation of the IL-15 system.
During the course of our studies, we became aware of the work of Rubinstein et al. (82), whose study also shows that complexing sIL-15Rα to IL-15 results in hyperagonist activity toward CD8 memory T cells and NK cells in vivo, although the mechanisms of action based on transpresentation and increasing half-life and bioavailability were not examined in detail in that study. Our studies also go beyond their findings by showing that naive CD8 T cells respond to IL-15/IL-15Rα, resulting in effector cell induction and memory T cell generation. In addition, our B16 tumor findings highlight the potential of complexed IL-15 as a tumor immunotherapy agent. Together, these findings illustrate the potential power of IL-15 in driving robust NK/NK T and CD8 T cell expansion and effector differentiation in intact hosts, which may have important immunotherapeutic applications.
Footnotes
This work was supported by National Institutes of Health Grants AI51583 and P01 AI56172 (to L.L.) and U.S. Public Health Service Training Grant T32 AI07080 (to T.A.S.).
Abbreviations used in this paper: γC, common γ-chain; sIL, soluble IL; VSV, vesicular stomatitis virus; hIL, human IL; rm, recombinant murine.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M. Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 2.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. The interleukin (IL)-2 receptor-β chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer-cells. Proc. Natl. Acad. Sci. USA. 1994;91:4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region/IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc. Natl. Acad. Sci. USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura H, Fujimoto A, Tamura N, Yajima T, Wajjwalku W, Yoshikai Y. A novel autoregulatory mechanism for transcriptional activation of the IL-15 gene by a nonsecretable isoform of IL-15 generated by alternative splicing. FASEB J. 2005;19:19–28. doi: 10.1096/fj.04-2633com. [DOI] [PubMed] [Google Scholar]
- 5.Villinger F, Miller R, Mori K, Mayne AE, Bostik P, Sundstrom JB, Sugimoto C, Ansari AA. IL-15 is superior to IL-2 in the generation of long-lived antigen specific memory CD4 and CD8 T cells in rhesus macaques. Vaccine. 2004;22:3510–3521. doi: 10.1016/j.vaccine.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Burton JD, Bamford RN, Peters C, Grant AJ, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. A lymphokine, provisionally designated interleukin-T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer-cells. Proc. Natl. Acad. Sci. USA. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin-15-deficient mice. J. Exp. Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 9.Schluns KS, Williams K, Ma A, Zheng XX, Lefrançois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 10.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM. Identification and cloning of a novel IL-15 binding protein that is structurally related to the α chain of the IL-2 receptor. EMBO J. 1995;15:3654–3633. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong JL, Farner NL, Widmer MB, Giri JG, Sondel PM. Interaction of IL-15 with the shared IL-2 receptor β and γ c subunits: the IL-15/β/γ c receptor-ligand complex is less stable than the IL-2/β/γ c receptor-ligand complex. J. Immunol. 1996;156:1339–1348. [PubMed] [Google Scholar]
- 15.Bulfone-Paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-α-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Rα chain. FASEB J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 16.Bulanova E, Budagian V, Pohl T, Krause H, Durkop H, Paus R, Bulfone-Paus S. The IL-15Rα chain signals through association with Syk in human B cells. J. Immunol. 2001;167:6292–6302. doi: 10.4049/jimmunol.167.11.6292. [DOI] [PubMed] [Google Scholar]
- 17.Bulfone-Paus S, Bulanova E, Budagian V, Paus R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. BioEssays. 2006;28:362–377. doi: 10.1002/bies.20380. [DOI] [PubMed] [Google Scholar]
- 18.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Rα signals are required for bystander proliferation. J. Exp. Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 20.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15Rα expression on CD8+ T cells is dispensable for T cell memory. Proc. Natl. Acad. Sci. USA. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15Rα-deficient natural killer cells survive in normal but not IL-15Rα-deficient mice. J. Exp. Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor α expression. Proc. Natl. Acad. Sci. USA. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schluns KS, Klonowski KD, Lefrançois L. Transregulation of memory CD8 T cell proliferation by IL-15Rα+ bone marrow-derived cells. Blood. 2004;103:988–994. doi: 10.1182/blood-2003-08-2814. [DOI] [PubMed] [Google Scholar]
- 24.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Rα and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J. Exp. Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15Rα by the same cells. J. Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 26.Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, Plet A, Jacques Y. Soluble interleukin-15 receptor α (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rβ/γ: hyperagonist IL-15 × IL-15Rα fusion proteins. J. Biol. Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- 27.Giron-Michel J, Giuliani M, Fogli M, Brouty-Boye D, Ferrini S, Baychelier F, Eid P, Lebousse-Kerdiles C, Durali D, Biassoni R, et al. Membrane-bound and soluble IL-15/IL-15Rα complexes display differential signaling and functions on human hematopoietic progenitors. Blood. 2005;106:2302–2310. doi: 10.1182/blood-2005-01-0064. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari-Lacraz S, Zanelli E, Neuberg M, Donskoy E, Kim YS, Zheng XX, Hancock WW, Maslinski W, Li XC, Strom TB, Moll T. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J. Immunol. 2004;173:5818–5826. doi: 10.4049/jimmunol.173.9.5818. [DOI] [PubMed] [Google Scholar]
- 29.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor α-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J. Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 30.Smith XG, Bolton EM, Ruchatz H, Wei X, Liew FY, Bradley JA. Selective blockade of IL-15 by soluble IL-15 receptor α-chain enhances cardiac allograft survival. J. Immunol. 2000;165:3444–3450. doi: 10.4049/jimmunol.165.6.3444. [DOI] [PubMed] [Google Scholar]
- 31.Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J. Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- 32.Tsunobuchi H, Nishimura H, Goshima F, Daikoku T, Suzuki H, Nakashima I, Nishiyama Y, Yoshikai Y. A protective role of inter-leukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology. 2000;275:57–66. doi: 10.1006/viro.2000.0455. [DOI] [PubMed] [Google Scholar]
- 33.Maeurer MJ, Trinder P, Hommel G, Walter W, Freitag K, Atkins D, Storkel S. Interleukin-7 or interleukin-15 enhances survival of Mycobacterium tuberculosis-infected mice. Infect. Immun. 2000;68:2962–2970. doi: 10.1128/iai.68.5.2962-2970.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umemura M, Nishimura H, Hirose K, Matsuguchi T, Yoshikai Y. Overexpression of IL-15 in vivo enhances protection against Mycobacterium bovis bacillus Calmette-Guerin infection via augmentation of NK and T cytotoxic 1 responses. J. Immunol. 2001;167:946–956. doi: 10.4049/jimmunol.167.2.946. [DOI] [PubMed] [Google Scholar]
- 35.Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J. Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- 36.Oh S, Berzofsky JA, Burke DS, Waldmann TA, Perera LP. Coadministration of HIV vaccine vectors with vaccinia viruses expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl. Acad. Sci. USA. 2003;100:3392–3397. doi: 10.1073/pnas.0630592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiromatsu T, Yajima T, Matsuguchi T, Nishimura H, Wajjwalku W, Arai T, Nimura Y, Yoshikai Y. Overexpression of interleukin-15 protects against Escherichia coli-induced shock accompanied by inhibition of tumor necrosis factor-α-induced apoptosis. J. Infect. Dis. 2003;187:1442–1451. doi: 10.1086/374643. [DOI] [PubMed] [Google Scholar]
- 38.Toka FN, Rouse BT. Mucosal application of plasmid-encoded IL-15 sustains a highly protective anti-Herpes simplex virus immunity. J. Leukocyte Biol. 2005;78:178–186. doi: 10.1189/jlb.1004621. [DOI] [PubMed] [Google Scholar]
- 39.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Invest. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J. Immunol. 1998;160:4418–4426. [PubMed] [Google Scholar]
- 41.Mastroianni CM, d'Ettorre G, Forcina G, Lichtner M, Mengoni F, D'Agostino C, Corpolongo A, Massetti AP, Vullo V. Interleukin-15 enhances neutrophil functional activity in patients with human immunodeficiency virus infection. Blood. 2000;96:1979–1984. [PubMed] [Google Scholar]
- 42.Chitnis V, Pahwa R, Pahwa S. Determinants of HIV-specific CD8 T-cell responses in HIV-infected pediatric patients and enhancement of HIV-gag-specific responses with exogenous IL-15. Clin. Immunol. 2003;107:36–45. doi: 10.1016/s1521-6616(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 43.Mueller YM, Bojczuk PM, Halstead ES, Kim AHJ, Witek J, Altman JD, Katsikis PD. IL-15 enhances survival and function of HIV-specific CD8+ T cells. Blood. 2003;101:1024–1029. doi: 10.1182/blood-2002-07-1957. [DOI] [PubMed] [Google Scholar]
- 44.Castelli J, Thomas EK, Gilliet M, Liu YJ, Levy JA. Mature dendritic cells can enhance CD8+ cell noncytotoxic anti-HIV responses: the role of IL-15. Blood. 2004;103:2699–2704. doi: 10.1182/blood-2003-06-1913. [DOI] [PubMed] [Google Scholar]
- 45.Lum JJ, Schnepple DJ, Nie Z, Sanchez-Dardon J, Mbisa GL, Mihowich J, Hawley N, Narayan S, Kim JE, Lynch DH, Badley AD. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J. Virol. 2004;78:6033–6042. doi: 10.1128/JVI.78.11.6033-6042.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forcina G, d'Ettorre G, Mastroianni CM, Carnevalini M, Scorzolini L, Ceccarelli G, D'Agostino C, Lichtner M, Massetti AP, Vullo V. Interleukin-15 modulates interferon-γ and β-chemokine production in patients with HIV infection: implications for immune-based therapy. Cytokine. 2004;25:283–290. doi: 10.1016/j.cyto.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Alpdogan O, Eng JM, Muriglan SJ, Willis LM, Hubbard VM, Tjoe KH, Terwey TH, Kochman A, van den Brink MRM. Interleukin-15 enhances immune reconstitution after allogenic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 48.Chapoval AI, Fuller JA, Kremlev SG, Kamdar SJ, Evans R. Combination chemotherapy and IL-15 administration induce permanent tumor regression in a mouse lung tumor model: NK and T cell-mediated effects antagonized by B cells. J. Immunol. 1998;161:6977–6984. [PubMed] [Google Scholar]
- 49.Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104:4142–4149. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- 50.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, Grewal N, Spiess PJ, Antony PA, Palmer DC, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc. Natl. Acad. Sci. USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 53.Gudmundsdottir H, Wells AD, Turka LA. Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J. Immunol. 1999;162:5212–5223. [PubMed] [Google Scholar]
- 54.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 55.Masopust D, Jiang J, Shen H, Lefrançois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J. Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- 56.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 57.Kim SK, Reed DS, Olson S, Schnell MJ, Rose JK, Morton PA, Lefrançois L. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carson WE, Giri JG, Lindemann MJ, Linett ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K, Caligiuri MA. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunne J, Lynch S, O'Farrelly C, Todryk S, Hegarty JE, Feighery C, Doherty DG. Selective expansion and partial activation of human NK cells and NK receptor-positive T cells by IL-2 and IL-15. J. Immunol. 2001;167:3129–3138. doi: 10.4049/jimmunol.167.6.3129. [DOI] [PubMed] [Google Scholar]
- 60.Warren HS, Kinnear BF, Kastelein RL, Lanier LL. Analysis of the costimulatory role of IL-2 and IL-15 in initiating proliferation of resting (CD56dim) human NK cells. J. Immunol. 1996;156:3254–3259. [PubMed] [Google Scholar]
- 61.Armitage RJ, Macduff BM, Eisenman J, Paxton R, Grabstein KH. IL-15 has stimulatory activity for the induction of B cell proliferation and differentiation. J. Immunol. 1995;154:483–490. [PubMed] [Google Scholar]
- 62.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park CS, Yoon SO, Armitage RJ, Choi YS. Follicular dendritic cells produce IL-15 that enhances germinal center B cell proliferation in membrane-bound form. J. Immunol. 2004;173:6676–6683. doi: 10.4049/jimmunol.173.11.6676. [DOI] [PubMed] [Google Scholar]
- 64.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 65.Roychowdhury S, May KF, Jr., Tzou KS, Lin T, Bhatt D, Freud AG, Guimond M, Ferketich AK, Liu Y, Caligiuri MA. Failed adoptive immunotherapy with tumor-specific T cells: reversal with low-dose interleukin 15 but not low-dose interleukin 2. Cancer Res. 2004;64:8062–8067. doi: 10.1158/0008-5472.CAN-04-1860. [DOI] [PubMed] [Google Scholar]
- 66.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinricjs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alpdogan O, van den Brink MRM. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J. Clin. Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baccala R, Gonzalez-Quintial R, Dummer W, Theofilopoulos AN. Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin. Immunopathol. 2005;27:75–85. doi: 10.1007/s00281-004-0196-9. [DOI] [PubMed] [Google Scholar]
- 70.Wrzesinski C, Restifo NP. Less is more: lymphodepletion followed by hematopoietic stem cell transplant augments adoptive T-cell-based anti-tumor immunotherapy. Curr. Opin. Immunol. 2005;17:195–201. doi: 10.1016/j.coi.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porter DL, June CH. T-cell reconstitution and expansion after hematopoietic stem cell transplantation: ‘T’ it up! Bone Marrow Transplant. 2005;35:935–942. doi: 10.1038/sj.bmt.1704953. [DOI] [PubMed] [Google Scholar]
- 73.Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J. Exp. Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kieper WC, Jameson SC. Homeostatic expansion and phenotypic conversion of naive T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA. 2000;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruckert R, Brandt K, Bulanova E, Mirghomizadeh F, Paus R, Bulfone-Paus S. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 2003;33:3493–3503. doi: 10.1002/eji.200324545. [DOI] [PubMed] [Google Scholar]
- 76.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J. Exp. Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195–2197. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 78.Alves NL, Hooibrink B, Arosa FA, van Lier RA. IL-15 induces antigen-independent expansion and differentiation of human naive CD8+ T cells in vitro. Blood. 2003;102:2541–2546. doi: 10.1182/blood-2003-01-0183. [DOI] [PubMed] [Google Scholar]
- 79.Liu K, Catalfamo M, Li Y, Henkart PA, Weng NP. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl. Acad. Sci. USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruckert R, Brandt K, Braun A, Hoymann HG, Herz U, Budagian V, Durkop H, Renz H, Bulfone-Paus S. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J. Immunol. 2005;174:5507–5515. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]
- 81.Budagian V, Bulanova E, Orinska Z, Pohl T, Borden EC, Silverman R, Bulfone-Paus S. Reverse signaling through membrane-bound interleukin-15. J. Biol. Chem. 2004;279:42192–42201. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- 82.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc. Natl. Acad. Sci. USA. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]