Abstract
Nonalcoholic fatty liver disease (NAFLD) is characterized by insulin resistance, elevated serum levels of free fatty acids (FFAs) and fatty infiltration of the liver. Accumulation of triglycerides in the hepatocyte results from the uptake and esterification of circulating FFAs by the liver. Contrary to current theory, hepatic steatosis appears to be a detoxification process, as FFAs are directly cytotoxic for the hepatocyte and inhibition of triglyceride formation enhances FFAs toxicity. Hepatocyte apoptosis is a key feature of NAFLD and correlates with disease severity. Since FFA-induced toxicity, or lipoapoptosis, represents a mechanism for the pathogenesis of NAFLD, this article will highlight the cellular pathways contributing to hepatocyte lipoapoptosis. To date, there is no proven effective therapy for patients with NAFLD and insights into the molecular mediators of lipoapoptosis should help promote effective therapeutic strategies for this disease.
Keywords: BH3-only proteins, CCAAT/enhancer binding homologous protein, c-Jun N-terminal kinase, death receptor, endoplasmic reticulum stress, hepatic steatosis, nonalcoholic steatohepatitis
Nonalcoholic steatohepatitis & lipoapoptosis
Nonalcoholic fatty liver disease (NAFLD) is highly prevalent in Western countries and is commonly associated with clinical features of metabolic syndrome, such as Type 2 diabetes, obesity and dyslipidemia [1]. This disease is characterized by an accumulation of fat in the liver and encompasses a wide spectrum of liver disorders, ranging from benign simple steatosis to steatohepatitis. Nonalcoholic steatohepatitis (NASH), which represents 5–10% of NAFLDs, is associated with hepatic inflammation as well as the presence of steatosis, hepatocyte damage and various degrees of fibrosis [2]. NASH represents a potentially progressive liver disease, as it can ultimately lead to cirrhosis, chronic liver disease with portal hypertension, liver failure and hepatocellular carcinoma [3,4].
A major risk factor for NASH is insulin resistance, which occurs within the context of metabolic syndrome [1,5,6]. Indeed, impaired insulin suppression of lipolysis within peripheral adipose tissue leads to increased plasma levels of free fatty acids (FFAs) [7]. As a consequence of this, increased delivery of adipose-derived FFAs to the liver directly contributes to the development of hepatic steatosis in patients with Type 2 diabetes [8]. Abnormal and excessive accumulation of lipids in nonadipose tissues, with limited capacity for storage of lipids, results in cellular dysfunction or cell death, a phenomenon termed lipoapoptosis [9,10]. Athough steatosis characterizes NAFLD, lipoapoptosis appears to be mediated by FFAs rather than by their esterified products (triglycerides). Indeed, numerous studies have demonstrated that nonesterified FFAs are inherently toxic to liver cells [11–17], whereas the esterification of FFAs to form neutral triglycerides appears to be a detoxification process [18,19]. Hepatic FFA levels are increased during murine experimental steatohepatitis [20,21], and NASH is characterized by both increased serum FFA levels and hepatocyte apoptosis, with the magnitude of circulating FFA correlating with disease severity [22,23]. Consistent with an increase in hepatocyte apoptosis, elevated serum caspase-cleaved cytokeratin 18 fragments distinguish simple human hepatic steatosis from NASH [24]. Therefore, FFA-induced toxicity or lipoapoptosis could represent a potential mechanism that relates apoptosis to NASH.
Hepatic lipids
Increased availability of circulating FFAs and the augmented delivery of FFAs to the liver most probably play a key role in the development of hepatic steatosis in NAFLD. Throughout this article, the term FFAs will refer to the saturated and unsaturated long-chain FFAs with aliphatic tails longer than 16 carbons atoms.
Hepatic free fatty acid
Free fatty acids entering the liver are derived from either hydrolysis of dietary triglycerides or from lipolysis of adipose tissue triglycerides in the fasting state. Excessive fat accumulation in the liver can also result from increased fat synthesis, reduced fat oxidation (β-oxidation) or reduced capacity to export fat in the form of VLDL. However, studies performed in humans and rodents have demonstrated that excessive accumulation of hepatic triglycerides is principally the result of increased delivery of adipose-derived FFAs to the liver and enhanced de novo lipid synthesis in the liver, and was only modestly affected by lipid disposal via β-oxidation or VLDL export [25]. In addition, dietary lipids contribute minimally to hepatic steatosis [26].
Free fatty acids enter the cells by both passive diffusion and facilitated transport involving specific fatty acid transporters such as the fatty acid transport protein (FATP) [27] and the fatty acid translocase FAT/CD36 [28]. Modifications in the expression of these fatty acid transporters directly contribute to fat-induced triacylglycerol accumulation in the cells. In particular, FATP2 and FATP5 are highly expressed in the liver and are involved in the uptake of long-chain FFAs [29]. Loss of hepatic FATP5 reduces dietary lipid deposition in murine hepatocytes by redirecting lipid fluxes toward peripheral tissues; and small hairpin RNA-targeted knockdown of FATP5 can decrease established hepatic lipid accumulation in animal models of diet-induced hepatic steatosis [30]. Furthermore, hepatic expression of FAT/CD36 is increased in mice fed a highfat diet [31,32] and enforced expression of FAT/CD36 in hepatocytes markedly increases hepatic fatty acid uptake [31]. To date, genetic polymorphisms of FATP5 or FAT/CD36 have not yet been reported, but it is possible that alterations in the expression of these proteins play an important role in susceptibility to and/or progression of NAFLD. Hepatic expression of FAT/CD36 and FATP5 are also augmented in the livers of patients with NAFLD and correlate with liver fat content [33–35]. Finally, genetic deletion of the cytosolic liver-specific fatty acid-binding protein (L-FABP) decreases incorporation of unsaturated FFAs into cellular triglycerides [36,37], and L-FABP hepatic expression is increased in early stages of human NAFLD [38].
Hepatic lipids in steatosis & nonalcoholic steatohepatitis
Circulating levels of FFAs are increased in NASH patients and correlate with disease severity [23,39,40]. Although de novo lipogenesis is increased in NAFLD patients [40], 60–80% of the circulating FFAs in these individuals are derived from adipocyte lipolysis and provide approximatively 60% of FFAs in the liver [26]. More precisely, de Almeida et al. studied the exact composition of the circulating long-chain FFAs increased in NASH patients. NASH was demonstrated to be predominantly associated with elevated levels of the saturated FFA, palmitic acid (C16:0), and the monounsaturated FFAs, oleic acid (C18:1) and palmitoleic acid (C16:1) [39]. Despite an increase in total (esterified and nonesterified) hepatic saturated and unsaturated fatty acids, no or minimal increase of hepatic saturated or unsaturated FFAs was observed in liver biopsies of NAFLD and NASH patients when compared with normal patients [41,42]. Therefore, there is evidence to suggest that excess circulating saturated and unsaturated FFAs are rapidly esterified within the hepatocytes to form diacylglycerides and triacylglycerides [41].
It is interesting to note that the greatest increase of total hepatic lipid content, diacylglycerides or triacylglycerides, was observed in NAFLD patients with benign simple steatosis rather than in NASH patients with a more advanced form of NAFLD [41]. Along with this observation, a new emerging concept suggests that cellular triglyceride accumulation is not toxic per se. Rather, accumulation of excess nonesterified saturated FFAs or their metabolic products mediate lipotoxicity [43]. Indeed, considerable data indicate that saturated FFAs are more toxic to liver cells than unsaturated FFAs [11–17]. Some recent studies have reported that this difference in toxicity between saturated and unsaturated FFAs relies on their ability to be rapidly esterified and incorporated as triglycerides [18,44,45]. Furthermore, esterified unsaturated fatty acids are the most abundant in various kinds of lipids, including phospholipids and triglycerides. Therefore, exposure of liver cells to nontoxic unsaturated FFAs (e.g., oleic acid and palmitoleic acid) results in a significant accumulation of triglycerides, whereas exposure to the toxic saturated FFA (e.g., palmitic acid) minimally increases hepatic lipid droplets [18,44,45].
Diversion of saturated FFAs into triglycerides provides hepatocytes protection against saturated FFA-induced toxicity (Figure 1). In fact, monounsaturated FFAs, such as oleic acid and palmitoleic acid, inhibit liver toxicity induced by saturated FFAs [15–18] and the protective effects of the unsaturated FFAs is suggested to be due to their ability to redirect saturated FFAs into triglyceride storage [18]. For example, the enzyme stearoyl CoA desaturase (SCD) 1, which catalyzes the desaturation of palmitic acid and stearic acid to palmitoleic acid and oleic acid, respectively, plays an important role in hepatic triglyceride accumulation; and SCD1 overexpression increases triglyceride synthesis and protects against lipoapoptosis [18]. By contrast, genetic deletion of SCD1 decreases hepatic steatosis and aggravates hepatocellular apoptosis and liver injury during murine experimental steatohepatitis [44]. In a similar manner, antisense oligonucleotide-mediated silencing of diacylglycerol acyltransferase 2, a key enzyme in the esterification of FFAs to triglyceride, enhances liver injury and fibrosis despite reducing hepatic triglyceride content in a murine model of NASH [19].
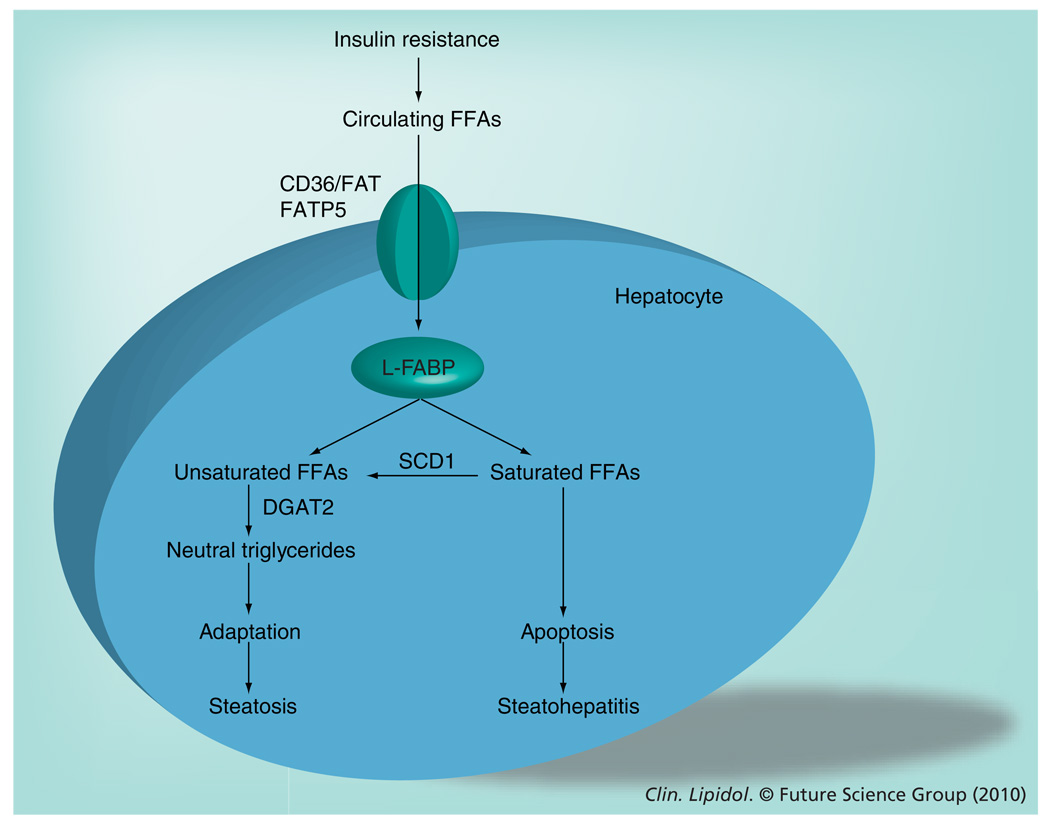
Figure 1. Diversion of saturated free fatty acids to neutral triglyceride formation as a protective mechanism against lipoapoptosis in the liver.
Insulin resistance, a hallmark of NAFLD, leads to an increase in serum concentration of circulating FFAs. These circulating FFAs are transported into hepatocytes by specific fatty acid transporters (CD36/FAT and FATP5) and binding proteins (L-FABP). Within the hepatocytes, these FFAs can be esterified to neutral triglycerides, resulting in hepatic steatosis. Esterification of FFAs acts as a buffering mechanism, allowing cells to maintain viability in the face of excess nonesterified FFA exposure. Saturated and unsaturated FFAs differ with regard to their potential for lipoapoptosis. Unsaturated FFAs are less toxic than saturated FFAs and are rapidly esterified by the enzyme DGAT2 and incorporated as triglycerides. By contrast, conversion of the saturated FFAs into unsaturated FFAs by the enzyme SCD1 seems to be necessary in the diversion of saturated FFAs to triglyceride formation. Failure to partition nonesterified FFAs as triglycerides, by inhibiting SCD1 or DGAT2 activities, results in hepatocellular apoptosis and liver damage, which further leads to the development of steatohepatitis.
DGAT: Diacylglycerol acyltransferase; FATP: Fatty acid transport protein; FFA: Free fatty acid; L-FABP: Liver-specific fatty acid-binding protein; NAFLD: Nonalcoholic fatty liver disease; SCD: Stearoyl CoA desaturase.
Different patterns of L-FABP expression have been observed in human NAFLD progression, from simple steatosis to NASH [38]. L-FABP is overexpressed in the liver of patients with simple steatosis, but as the stage of NAFLD progresses to NASH, L-FABP expression dramatically decreases [38]. Similarly, feeding mice a high-fat diet, which results in extensive steatosis without hepatocellular injury or fibrosis, increases hepatic SCD1 expression, whereas feeding mice a methionine-and choline-deficient diet, which reproduces the fibrosing steatohepatitis observed in patients with severe NASH, markedly decreases SCD1 expression [44]. Hence, a potential pathogenic mechanism accounting for the development of liver injury in NASH may involve impaired cellular capacity to incorporate toxic FFAs into neutral triglycerides.
Hepatocyte cell death in steatosis & steatohepatitis
Failure of the hepatocyte to dispose of excess FFAs by converting them into triglyceride is associated with increased risk for hepatocyte lipoapoptosis, a cardinal pathogenic feature of NASH [22]. The mechanisms involved in FFA-induced toxicity have yet to be completely defined, but recent compelling data suggest that hepatocyte lipoapoptosis mainly arises from FFA-induced lipotoxic stress of intracellular organelles, in particular the endoplasmic reticulum (ER) and mitochondria.
Endoplasmic reticulum stress
Disturbances in the ER seem to be implicated in lipoapoptosis pathways and may even be an important causative factor in mediating cell death. The ER is a critical organelle responsible, among other functions, for the synthesis, maturation, folding and transport of proteins, as well as lipid synthesis and packaging, and the regulation of cellular calcium homeostasis. Perturbation of these processes creates a condition referred to as ER stress. As a primary event, ER stress leads to the activation of an adaptive and protective signaling network termed the unfolded protein response (UPR), which serves to overcome the stress stimulus and re-establish ER homeostasis [46]. However, if the cell fails to adapt, a prolonged and persistent ER stress will trigger proapoptotic signals that will indirectly cause cell death by activating downstream molecules, mainly c-Jun N-terminal kinase (JNK) and the transcription factor CCAAT/enhancer binding homologous protein (CHOP).
Endoplasmic reticulum stress responses are induced by three distinct ER membrane-spanning signaling molecules, namely, activating transcription factor 6 (ATF6), PKR-like ER kinase (PERK) and inositol-requiring enzyme (IRE) 1α, which can be held inactive by the chaperone glucose-regulated protein (GRP) 78. As an initial event, the activation of these three kinases will induce several mechanisms to reduce the burden of unfolded proteins in the ER. However, when these adaptive mechanisms fail to re-establish ER homeostasis, excessive activation of the resident ER kinases will induce proapoptotic signals. When activated, PERK phosphorylates and inactivates eukaryotic translation initiation factor (eIF) 2α, reducing mRNA translation and decreasing protein load in the ER. Paradoxically, phosphorylation of eIF2α selectively benefits the translation of ATF-4 [47], which regulates the promoter of GRP78 [48] but also leads to the transcriptional upregulation of the transcription factor CHOP. Active IRE1α classically cleaves X-box-binding protein (XBP)-1 mRNA, and the resultant spliced protein, sXBP-1, controls the upregulation of a broad spectrum of genes involved in ER-assisted degradation, protein folding and protein quality control [49]. Furthermore, active IRE1α can recruit the adaptor molecule TNF-receptor-associated factor 2, which further recruits the apoptosis-signal-regulating kinase that activates the JNK signaling pathway [50]. While activation of PERK and IRE1α will promote both cytoprotective processes that re-establish homeostasis and proapoptotic processes, ATF6 mainly upregulates the expression of GRP78 and ER degradation enhancing-mannosidase like protein, resulting in increased ER-chaperone activity and degradation of misfolded proteins [51,52].
Previous studies in both genetic and dietary murine models of obesity suggest that ER stress and activation of the UPR in the liver play a determinant role in the development of obesity-induced insulin resistance and Type 2 diabetes [53]. Indeed, it was demonstrated that obesity-induced ER stress led to suppression of insulin receptor signaling through sustained JNK activation which phosphorylates and inactivates insulin receptor substrate-1 [53]. Furthermore, in dietary murine models of hepatic steatosis, ER stress-induced CHOP expression and XBP-1 mRNA splicing are increased in rat liver and correlate with the development of liver injury [16]. Finally, in human patients with NAFLD and NASH, a strong activation of PERK is observed, as reflected by increased phosphorylation of eIF2α [54]; and phosphorylation and activation of JNK, a downstream target of IRE1α, is also observed in liver biopsies from patients with NASH [13,54].
The mechanisms underlying ER stress and the activation of UPR signaling in obesity-induced hepatic steatosis are not fully understood. However, it appears that the composition of fatty acids in the steatotic liver, rather than hepatic steatosis itself, is an important determinant in inducing ER stress-mediated liver damage. Hence, although rats fed a diet enriched in either polyunsaturated fatty acids or saturated fatty acids accumulate hepatic triglycerides to a similar extent, only those rats fed a diet enriched with saturated fatty acids exhibited strong activation of ER stress markers and liver injury [16]. Since intracellular FFAs are trafficked to, and esterified within, the ER, inundation of liver cells with FFAs could disturb ER function and induce an ER-stress response. In accordance with this concept, increased saturated lipid content of ER directly compromises ER morphology and integrity [55]. Recent in vitro studies suggest that saturated FFA-induced liver cell apoptosis relies on ER stress-mediated CHOP induction and JNK activation; and the low toxicity of unsaturated FFAs on liver cells could be due to their incapacity to induce resident ER kinases, PERK- and IRE1α-dependent signaling pathways [15,17,45]. Appropriate ER-calcium levels are necessary for normal protein folding because of the calcium-dependent nature of chaperone proteins (such as GRP78 and calreticulin) [56]. Deletion of ER calcium can result in the misfolding of proteins and can trigger the UPR or ER stress. Indeed, saturated FFAs deplete ER-calcium stores in liver cells and this process partially contributes to ER stress by saturated FFAs [17]. Calcium released by the ER into the cytosol can also be taken up by mitochondria, promoting dysfunction of this organelle and thereby triggering apoptosis [57].
CHOP downstream targets
CCAAT/enhancer-binding homologous protein is an inducible leucine zipper transcription factor that plays an important role in ER stress-induced apoptosis. Although CHOP knockdown confers protection against FFA-mediated ER stress-induced apoptosis in pancreatic β-cells [58] and prevents hepatocyte apoptosis in alcoholic liver injury [59], its role in lipoapoptosis, as well as the mechanisms by which CHOP promotes lipoapoptosis, remains incompletely understood.
Considering that CHOP is a transcription factor, recent studies have revealed that CHOP regulates the transcription of several members of the Bcl-2 family. The Bcl-2 family of proteins regulate the mitochondrial pathway of apoptosis, and this protein family is comprised of both pro- and antiapoptotic members. For example, ER stress-mediated CHOP expression downregulates the antiapoptotic protein Bcl-2 [60], whereas CHOP, plus its heterodimeric partner C/EPBα, directly binds to the promoter of the proapoptotic protein Bim and upregulates Bim transcription [61]. Decrease in Bcl-xL expression and increase in Bim expression have been reported to contribute to saturated FFA-mediated apoptosis in liver cells [11,12,62]. However, whether FFA-induced modulation of these members of the Bcl-2 family depend on CHOP transcriptional activity merits further investigations.
Studies performed in human carcinoma cells demonstrated that CHOP can transcription-ally upregulate TNF-related apoptosis-inducing ligand (TRAIL) receptor 2 (TRAIL-R2 or death receptor 5 [DR5]), a member of the TNF-receptor gene superfamily [63]. Upregulation of death receptors, such as TRAIL receptors and/or Fas can promote apoptosis by the extrinsic cellular pathway [64]. TRAIL has been implicated in steatosis-associated liver injury. For example, enhanced expression of DR5 was observed in liver biopsies from patients with NASH [65]. Similarly, FFAs upregulate DR5 expression in liver cancer cell lines, and silencing DR5 expression protects against FFAs-mediated apoptosis [65]. Although healthy human hepatocytes are resistant to TRAIL, FFA-induced steatosis sensitizes hepatocytes to TRAIL cytotoxicity [66]. Other death receptors may also be implicated in the pathogenesis of steatohepatitis. Indeed, expression of the death receptors Fas (or CD95) and TNF-α-receptor 1 is enhanced in livers of patients with NASH [22,67]. Fas is also increased in experimental models of NASH, and FFA-treated liver cells overexpress Fas [68]. Furthermore, obesity-induced steatosis increases hepatocyte sensitivity to Fas ligand-mediated apoptosis [22,69]. Whether FFA-induced ER stress regulates the expression of these DRs remains unexplored.
JNK-signaling pathway
c-Jun N-terminal kinase, which belongs to the family of mitogen-activated protein kinases, has been implicated as a central mediator of FFA-induced hepatocyte lipoapoptosis in both dietary murine models of nonalcoholic steatohepatitis [70–72] and in human NASH [13,54]; and pharmacological inhibition of JNK prevents FFA-induced hepatocyte apoptosis [11]. Sustained activation of JNK, secondary to saturated FFA-stimulated mitogen-activated protein kinase kinase kinase MLK3 [73] and/or secondary to saturated FFA-induced ER stress [15,50], can cause cell death signals by both transcriptional and post-transcriptional mechanisms [74]. These mechanisms will be discussed in greater detail in this section.
Of the three known JNK genes, only JNK1 and JNK2 are expressed in hepatocytes [75], and these two isozymes are alternatively spliced to yield α and β isoforms of both p54 and p46 proteins [76]. Although both JNK1 and JNK2 have been implicated in liver injury, these two isoforms differentially contribute to hepatocyte apoptosis depending on the injurious stimulus. Whereas JNK2 appears to induce apoptosis in a TNF-α-induced liver injury model [77], recent studies have demonstrated that enhanced JNK1-signaling plays a central role in inducing hepatocyte apoptosis in a murine model of steatohepatitis [70,71].
c-Jun N-terminal kinase-1 predominantly contributes to the phosphorylation of the transcription factor c-Jun [13,70,71,78], a critical member of the activator protein-1 (AP-1) transcription factor complex. In experimental models of steatohepatitis, high levels of phosphorylated c-Jun and enhanced AP-1 complex binding activity correlated with increased hepatocyte apoptosis [70,71]. A recent study has determined that JNK1-dependent induction of the proapoptotic member of the Bcl-2 family, p53 upregulated modulator of apoptosis (PUMA), with subsequent activation of Bax, also contributes to hepatocyte lipoapoptosis [13]. In this study, saturated FFA-mediated JNK1/c-Jun phosphorylation resulted in the formation of an active AP-1 complex that directly binds to the promoter of PUMA and upregulates its transcription in liver cells [13]. Genetic deletion of JNK1 prevented FFA-mediated c-Jun activation and PUMA induction [13], and decreased steatohepatitis-mediated hepatocyte apoptosis in mice [70,71]. Similarly, sustained activation of JNK in patients with advanced stages of NAFLD (NASH patients) [54] correlates with a significant increase in DNA-binding activity of hepatic c-Jun containing AP-1 complex [79], a marked increase in hepatic levels of PUMA [13] and the development of hepatocyte injury [22,54].
Alternatively, JNK can also post-transcriptionally phosphorylate and regulate other members of the Bcl-2 family. For example, JNK-mediated phosphorylation of the antiapoptotic proteins Bcl-2 or Bcl-xL suppresses their antiapoptotic activities and promotes apoptosis [80]. By contrast, JNK-dependent phosphorylation of Bad, Bim or Bax enhances the proapoptotic potential of these proteins [81–83]. Whether these post-transcriptional modifications of these pro- and antiapoptotic members of the Bcl-2 family occurs during the lipotoxic insult is not known, nor the exact contribution of each JNK isoform in these processes. Finally, it has been demonstrated that JNK also transcriptionally upregulates DR5 [65] and Fas ligand [84]; two factors that may also contribute to lipoapoptosis, as mentioned previously.
BH3-only proteins & mitochondrial dysfunctions in lipoapoptosis
Proapoptotic BH3-only protein family members, which include Bad, Bid, Bik, Bim, Bmf, Hrk, Noxa and PUMA, are critical regulators of lipoapoptosis. These proteins are the biosensors of cell death and initiate activation of the core proapoptotic machinery. Among these proteins, Bim and PUMA expression were induced in liver cells by the saturated FFA, palmitic acid [11–13]. In this context, induction of Bim was demonstrated to result from transcriptional activation by FoxO3a, which upon dephosphorylation by protein phosphatase 2A, migrates into the nucleus and binds the Bim promoter. Knockdown of Bim using small interfering RNA partially protects liver cells against lipoapoptosis [12]. Prior observations indicate that protein phosphatase 2A can be activated by ER stress [61], and this mechanism could account for its activation during lipoapoptosis in liver cells. As mentioned earlier, saturated FFAs also stimulate PUMA expression by a JNK1/AP-1-dependent mechanism, and genetic deletion of PUMA conferred resistance to murine hepatocyte against palmitic acid-mediated apoptosis [13].
Given the fact that Bim and PUMA have complementary functions, it is likely that these two proapoptotic proteins cooperate in lipoapoptosis, as demonstrated in other cell death processes [85]. FFA-mediated Bim and PUMA induction results in the activation of the multidomain proapoptotic member of the Bcl-2 family, Bax [11,13,86]. Bim and PUMA can directly bind to and activate Bax [87,88]. Furthermore, PUMA may indirectly promote Bax activation by binding to and disabling the function of prosurvival Bcl-2 proteins such as Mcl-1 and/or Bcl-xL [89,90]. Interestingly, palmitic acid-mediated hepatocyte apoptosis can also result in the loss of the antiapoptotic proteins Mcl-1 and Bcl-xL. Inhibition of Mcl-1 degradation or forced overexpression of Bcl-xL attenuates apoptosis by saturated FFAs [14,62].
Therefore, the data discussed previously demonstrate that the toxic saturated FFAs activate the mitochondrial cell death pathway [11]. Indeed, activation and oligomerization of Bax in the outer mitochondrial membrane results in mitochondrial dysfunction, downstream activation of the effector caspases 3, 6 and 7, and ultimately, cell death by apoptosis [91]. FFA-induced Bax activation is regulated by Bim and PUMA [11–13], but lysosomal permeabilization with release of protease, such as cathepsin B, may also contribute to FFA-induced mitochondrial dysfunctions [92]. Bax expression may also be increased during murine experimental steatohepatitis [72], and increased hepatocyte apoptosis, as well as structural and functional mitochondrial abnormalities, are well documented in liver tissue from NASH patients [22,93–95].
Other lipid-signaling molecules
Lipoapoptosis is not only restricted to FFAs. When present in excess, other lipids, such as ceramide and cholesterol, can also trigger cellular apoptotic processes. Although the mechanisms by which these lipids act as signaling molecules are not completely clear, some evidence suggests that they could also contribute to hepatocyte lipoapoptosis.
Ceramide
The role of ceramide in insulin resistance and obesity has been well documented [96,97]. Furthermore, ceramide signaling and, most importantly, de novo synthesis of ceramide, was suggested to play a significant role in lipoapoptosis processes in nonhepatic cells [96,98,99]. Ceramide is the central core lipid in the metabolism of sphingolipids. Ceramide accumulation in the cell can occur from either hydrolysis of sphingomyelin by sphingomyelinases, or by de novo synthesis via serine palmitoyltransferase (SPT) and ceramide synthase. De novo ceramide synthesis occurs in the ER, where a fatty acid moiety, usually palmitoyl CoA, is combined to the amino group of sphingosine. SPT is the rate-limiting enzyme of de novo biosynthesis of ceramide and its activity depends on the availability of the long chain FFAs. Therefore, ceramide concentration increases in liver cells after treatment with saturated FFAs [15]. Palmitic acid and stearic acid-induced apoptosis was demonstrated to be associated with increased de novo ceramide synthesis in murine hematopoietic cell lines [100]; and saturated FFA-induced apoptosis was attenuated with pharmacological inhibitors of de novo ceramide synthesis. Furthermore, increased adipose ceramide levels with associated augmentation of SPT activity are observed in mice fed a high-fat diet [101], and accumulation of ceramide in subcutaneous adipose tissue seems to reflect the development of human fatty liver [102]. A lipidomic analysis revealed a positive correlation between accumulation of triglycerides and ceramide levels in the liver of genetically obese mice when compared with control mice [201]. However, saturated fat diet-induced obesity in mice led to liver injury via extensive ER-stress activation and hepatocyte apoptosis, independently of ceramide generation [16]. Saturated FFA-induced apoptosis in liver cells was also ceramide independent [15,62], as well as the induction of Bim expression by saturated FFAs [12]. The role of ceramide in the pathogenesis of human fatty liver is unclear. Stimulation of the death receptor Fas activates sphingomyelinases, leading to rapid accumulation of ceramide, and this process seems to play an important role in the regulation of Fas-induced apoptosis [103]. As Fas expression is enhanced in livers of patients with NASH [22], it cannot be excluded that ceramide may play a role in lipoapoptosis through the Fas-induced apoptotic signaling cascade. Nevertheless, a recent study has demonstrated that the hepatic ceramide content was unchanged in patients with NAFLD when compared with normal patients [104].
Cholesterol
It is well established that free cholesterol is highly cytotoxic. Analyses of the hepatic lipid composition of subjects with fatty liver disease have demonstrated a progressive increase in free cholesterol in patients with NAFLD and NASH, as compared with controls [41], and despite this increase in free cholesterol, liver cholesterol ester levels are unchanged between the three groups. A recent study has demonstrated that rats fed a high cholesterol diet, with increased free cholesterol loading in hepatocytes, developed a microvesicular steatosis and were sensitized to TNF-α- and Fas-induced hepatocellular death and inflammation [105]. In these rats, mitochondrial free-cholesterol loading accounted for the hepatocellular sensitivity to TNF-α, owing to mitochondrial glutathione depletion. The type rather than the amount of fat was an important determinant in the hepatocellular susceptibility to Fas and TNF-α, as dietary-induced triglyceride accumulation in the liver was insufficient to sensitize rats to DR-mediated toxicity [105]. Dietary supplementation with cholesterol was demonstrated to increase total ceramide levels in plasma in the adipose tissues of rats, which could account for the cytotoxic effect of cholesterol [106]. Free-cholesterol accumulation in atherosclerotic macrophages results in depletion of ER-calcium stores with activation of the UPR and ER stress-induced apoptosis [107,108]. However, free cholesterol loading of the ER in rodent hepatocytes was insufficient to induce an ER-stress response [105]. Therefore, the role of free cholesterol in the pathogenesis of human fatty liver disease is unclear, and the mechanisms by which free cholesterol induce hepatotoxicity requires further investigation.
Therapeutic approaches
To date, there is no proven effective therapy for patients with NASH. Therapy has focused on improvements of associated conditions of the disease, such as obesity, diabetes mellitus and hyperlipidemia. Weight loss in overweight patients with liver disease has demonstrated a sustained improvement in liver function and hepatomegaly [109] and therefore remains the primary nonpharmacological treatment option. Unlike weight loss, pharmacological therapies have not been as consistently positive. Several drugs that decrease insulin resistance and increase hepatic insulin sensitivity are of interest. For example, metformin improved fatty liver disease, reversed hepatomegaly, steatosis and serum alanine aminotransferase abnormalities in obese mice [110]. In human trials, metformin improved insulin sensitivity and decreased serum alanine aminotransferase concentrations in NASH patients but these beneficial effects were transient [111].
Thus, new treatment strategies focus on the molecular causes involved in the development and progression of NASH. Hepatocyte apoptosis is a critical feature of NASH [22], and development of antiapoptotic therapies may be useful in this syndrome. As specific regulation of this apoptosis could be exploited in order to develop new drug therapies, several potential targets of the apoptotic process will be reviewed in this section (Figure 2).
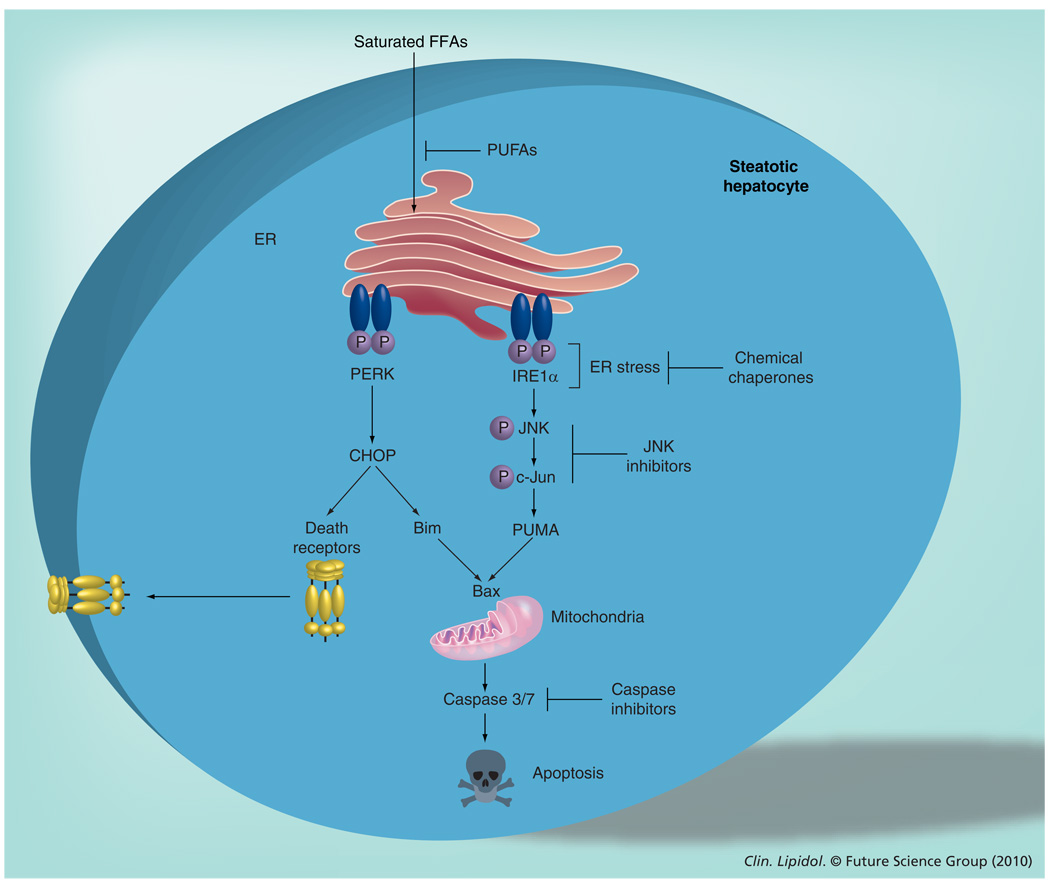
Figure 2. Molecular mechanisms involved in saturated free fatty acid-induced hepatocyte apoptosis.
Saturated FFAs accumulate in the ER, which leads to disturbance of ER function and induction of an ERstress response, mediated by the phosphorylation and activation of two ER-resident kinases, IRE1α and PERK. Phosphorylated IRE1α activates an apoptotic pathway involving JNK activation. Active JNK phosphorylates the transcription factor c-Jun, which leads to the transcriptional upregulation of the proapoptotic BH3-only protein, PUMA. Activation of PERK results in the induction of the transcription factor CHOP, which upregulates the expression of the proapoptotic BH3-only protein, Bim. Bim, in concert with PUMA, activates the proapoptotic executioner protein, Bax, resulting in mitochondrial dysfunction and activation of effector caspases and apoptosis. CHOP can further upregulate the expression of death receptors, such as death receptor 5, which sensitize fatty hepatocytes to circulating death ligands (e.g., TNF-related apoptosis-inducing ligand).
CHOP: CCAAT/enhancer-binding homologous protein; ER: Endoplasmic reticulum; IRE: Inositol-requiring enzyme; FFA: Free fatty acid; JNK: c-Jun N-terminal kinase; P: Phosphate; PERK: PKR-like endoplasmic reticulum kinase; PUFA: Polyunsaturated fatty acid; PUMA: p53 upregulated modulator of apoptosis.
Caspase inhibitors
As mentioned previously, Feldstein et al. have demonstrated that caspase 3 activation and hepatocyte apoptosis are prominent pathologic features of human NAFLD and correlate with disease severity [22]. Saturated FFA-induced apoptosis in liver cells depends on mitochondrial dysfunction, cytochrome c release and caspase 3/7 activation. Inhibition of caspase activity using pan-caspase inhibitors, Z-VAD-fmk or IDN-6556, markedly reduced cell death in liver cells treated with palmitic acid or stearic acid [11]. Similar effects were observed in vivo in experimental murine models of steatohepatitis. Indeed, prolonged treatment with the pan-caspase inhibitor, VX-166, decreased caspase-3 activation and reduced the number of tunnel-positive cells in mice fed a murine model of NASH [112]. Despite amelioration in hepatic steatosis and an improvement in liver fibrosis, the inhibitor was not sufficient to completely abrogate liver cell death and did not decrease serum alanine aminotransferase concentrations in this mouse model of NASH [112]. Nonetheless, a novel caspase inhibitor, GS9450, was developed by Gilead Sciences, Inc. (CA, USA) and is now being evaluated in a Phase IIA clinical trial in NASH patients.
Cathepsin B inhibitors
Some evidence suggests that structural and functional mitochondrial abnormalities, which are observed in liver cells from NASH patients [94,95], are involved in the progression of NAFLD [113]. Saturated FFAs induce mitochondrial dysfunction in both primary and transformed hepatocytes [11,62,92]. Recent data suggest that impaired mitochondrial functions in this context results from FFA-induced Bax translocation to lysosomes, with subsequent lysosomal permeabilization and cathepsin B release [62,92]. Thus, both genetic and pharmacological inhibition of cathepsin B in liver cells reduced saturated FFA-induced mitochondrial membrane permeabilization and cytochrome c release [62,92]; genetic deletion of cathepsin B attenuated steatohepatitis-mediated mitochondrial dysfunction, hepatocyte apoptosis and liver damage in mice [92,114]. Lysosomal permeabilization and release of cathepsin B into the cytosol was also observed in human liver tissues from patients with NAFLD, and correlated with the degree of inflammatory activity [114]. Therefore, inhibition of cathepsin B enzyme activity could represent a potential strategy to prevent mitochondrial dysfunctions associated with NASH.
Dietary supplementation with unsaturated free fatty acids
Saturated FFAs are more toxic to liver cells than unsaturated FFAs [11–17], and unsaturated FFAs can rescue liver cells from saturated FFA-induced caspase activation and apoptosis [15,17,18,44,45]. The protective effects of unsaturated FFAs include the inhibition of biomarkers of ER stress, namely CHOP induction and JNK activation, the prevention of ER stress-dependent upregulation of the proapoptotic BH3-only proteins, PUMA and Bim, and the reduction in Bax activation and subsequent mitochondrial dysfunction [45]. Thus, supplementation of n-3 polyunsaturated fatty acid (PUFA) in vivo to high-fat diet-fed rats ameliorated fatty liver and the degree of liver injury [115].
Furthermore, depletion of long-chain n-3 PUFAs has been observed in the hepatic tissue of patients with NAFLD [116] and likely promotes the pathogenesis of the disease [117]. Dietary supplementation with n-3 PUFAs reduced hepatic triglyceride content and serum alanine aminotransferase levels, and improved insulin resistance in patients with NAFLD [118,119]. Nevertheless, clinical trials have yet to confirm the therapeutic benefit of a dietary supplementation with n-3 PUFAs in patients with NAFLD.
JNK inhibitors
Chronic or excessive JNK activation results in systemic insulin resistance, promoting diabetes and metabolic syndrome. JNK, which is activated exclusively in liver biopsies from NASH patients [13,54], contributes to the pathogenesis of NAFLD [11,13,70,71]. In cellular models of saturated FFA-induced toxicity, sustained JNK activation mediates PUMA and Bim-dependent Bax activation and apoptosis in liver cancer cells and isolated murine and human hepatocytes [13], both of which are prevented when using JNK inhibitors [11,13]. While the JNK1 isoform mediates the proapoptotic processes in experimental steatohepatitis, the JNK2 isoform appears to have an antiapoptotic effect on lipoapoptosis [71]. Indeed, genetic deletion of JNK2 exacerbates hepatocellular injuries induced by a high-fat diet in mice; the cytoprotective function of JNK2 is mediated by its ability to enhance Bim degradation and cellular elimination [71]. Thus, while indiscriminate JNK inhibition could interfere with the beneficial antiapoptotic effect of JNK2 isoform, selective inhibition of the JNK1 isoform could represent a potentially effective treatment for NASH.
Chemical chaperones
Perturbations in the ER may be associated with lipotoxicity, and ER stress was detected in both rodent and human steatohepatitis [16,54]. Saturated FFAs activate the PERK-and IRE1α-dependent arm of ER stress, which results in CHOP induction and JNK activation in various liver cancer cells [15,17,45]. Interestingly, saturated FFAs seem to regulate only selective components of the UPR. Indeed, saturated FFAs did not promote the beneficial ER responses involved in protein folding, such as upregulation of molecular chaperones (GRP75 and calreticulin), as well as enhancing-mannosidase like protein [15]. Similarly, the level of the major ER chaperone, GRP78, is not, or is only modestly, altered after saturated FFAs treatment [Akazawa Y et al., Unpublished Data, 15]. Given that ATF6 activation controls the expression of GRP78 [51,52], ATF6 may remain silent during lipoapoptosis processes and no study has ever reported the involvement of ATF6 activation during saturated FFA-mediated apoptosis.
Since the failure to activate the protective responses of the UPR may contribute to lipotoxicity, re-establishment of ER homeo-stasis may represent a novel strategy to limit lipoapoptosis. A recent study has reported that oral administration of chemical chaperones, (e.g., 4-phenyl butyric acid) or bile acid derivatives (e.g., tauroursodeoxycholic acid) to genetically obese mice decreased biochemical markers of ER stress in the liver (e.g., PERK, IRE1α and JNK activation), improved insulin sensitivity, reduced hepatic steatosis and normalized the liver functional enzyme, alanine aminotransferase [120]. Thus, molecular agents that modulate ER and increase folding capacity may have therapeutic potential in reducing hepatic impairments associated with obesity and NAFLD. However, it should be noted that ursodeoxycholic acid failed to improve NAFLD in a human trial [121].
Conclusion
Nonalcoholic fatty liver disease, the most common liver disorder in the developed world, is associated with insulin resistance, a component of metabolic syndrome, which leads to the accumulation of fat, mainly triglycerides, in the liver. In NAFLD, the liver fails to cope with an excess of lipids. Although hepatic steatosis is not toxic, the high serum concentration of FFAs observed in advanced stages of NAFLD (NASH) induce hepatocyte apoptosis, and lipoapoptosis may represent a key mechanism resulting in the progression of simple steatosis to steatohepatitis. The lipotoxic effects of FFAs are multiple and complex, and involve impairment of the proper function of liver cell organelles, such as the ER. FFA-mediated ER stress probably plays a pivotal role in the activation of the numerous intracellular processes leading to hepatocyte apoptosis. These include JNK- and CHOP-dependent upregulation of proapoptotic BH3-only proteins and death receptors, Bax activation, mitochondrial permeabilization and subsequent activation of effector caspases. At present, there are no proven effective therapies for NASH. Thus, a better understanding of the molecular mechanisms involved in lipoapoptosis should help identify novel therapeutic approaches for this disease process.
Future perspective.
The phenomenon of lipotoxicity is among a multitude of events associated with NASH. Although an important amount of information has been accumulated in the past 10 years concerning the molecular factors involved in lipoapoptosis, the mechanisms by which saturated FFAs induce an ER-stress response remain obscure. A plausible explanation is the impairment of ER morphology and function, as the result of an overload of saturated lipid content of the ER [55]. However, the nontoxic FFA, oleate, which failed to induce ER stress in liver cells [15,17,45], has also been reported to cause ER distension in pancreatic cells [122]. Therefore, future studies will need to focus on uncovering the specific upstream mechanisms induced by saturated FFAs which lead to the activation of ER stress.
Furthermore, saturated FFAs seem to regulate only selective components of the UPR. Indeed, saturated FFAs did not induce, or only modestly induced, the protective ER responses, such as upregulation of ER chaperones [Akazawa Y et al., Unpublished Data, 15] in order to increase protein folding and re-establish ER homeostasis. This observation suggests that FFAs may not induce a full ER-stress response, as selective ER responses remain silent during the lipoapoptosis processes. A further examination of the ER stress-mediated mechanisms during lipoapoptosis is necessary.
Executive summary.
Nonalcoholic steatohepatitis & lipoapoptosis
Nonalcoholic steatohepatitis (NASH), an inflammatory stage of nonalcoholic fatty liver disease (NAFLD), is characterized by high serum concentration of circulating free fatty acids (FFAs) and hepatocyte apoptosis.
Hepatic lipids
Circulating FFAs, mainly derived from the lipolysis of adipose tissue triglycerides, are transported into hepatocytes by the fatty acid transporter protein 5 and FAT/CD36. Within the hepatocyte, these FFAs can be esterified to form neutral triglycerides resulting in hepatic steatosis, as observed in NAFLD patients.
Esterification of FFAs appears to be a detoxification process, as nonesterified FFAs are inherently toxic to hepatocytes and induce apoptosis.
Saturated and unsaturated FFAs differ with regard to their potential for lipoapoptosis; saturated long-chain FFAs are significantly more toxic than unsaturated FFAs and unsaturated FFAs can rescue liver cells from saturated FFA-induced apoptosis.
Impaired cellular capacity to incorporate toxic FFAs into neutral triglycerides may account for the development of liver injury in NASH.
Hepatocyte cell death in steatosis & steatohepatitis
Hepatocyte lipoapoptosis could mainly arise from the disturbance of endoplasmic reticulum (ER) function and the induction of an ER-stress response mediated by the phosphorylation and activation of two ER-resident kinases, inositol-requiring enzyme) (IRE) 1α and PKR-like endoplasmic reticulum kinase (PERK).
A prolonged and persistent ER stress triggers proapoptotic signals mediated by PERK-dependent activation of the transcription factor CCAAT/enhancer-binding homologous protein (CHOP) and IRE1α-dependent induction of c-Jun N-terminal kinase (JNK) signaling pathways. JNK and CHOP upregulate the expression of the proapoptotic BH3-only proteins, p53 upregulated modulator of apoptosis (PUMA) and Bim, respectively.
PUMA, in concert with Bim, activates the proapoptotic executioner protein, Bax, resulting in mitochondrial dysfunction and activation of effector caspases. Both contribute to FFA-induced apoptosis.
In NASH patients, upregulation of death receptors, such as TNF-related apoptosis-inducing ligand receptors and/or Fas, can also sensitize fatty hepatocytes to the extrinsic cellular pathway of apoptosis.
Other lipid-signaling molecules
Lipids others than FFAs, such as ceramide and free cholesterol, could also contribute to hepatocyte lipoapoptosis. Although both ceramide and free cholesterol are proapoptotic molecules, the mechanisms by which these lipids trigger apoptosis are not completely clear and the contribution of these two lipids in human nonalcoholic steatohepatitis merits further investigation.
Therapeutic approaches
To date, there are no proven effective therapies for NASH and the development of an antiapoptotic therapy may be useful in this disease.
Caspases inhibitors, cathepsin B inhibitors, dietary supplementation with polyunsaturated FFAs, JNK inhibitors and chemical chaperones could represent efficient novel therapeutic approaches in the treatment of lipoapoptosis associated with NASH.
Acknowledgment
The authors thank Erin Nystuen-Bungum for her excellent secretarial assistance.
Footnotes
Financial & competing interests disclosure
This work was supported by grant DK 41876 from the NIH and the Mayo Foundation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Angulo P, Lindor KD. Non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2002;17 Suppl.:S186–S190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 2.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129(1):113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 4.Ratziu V, Poynard T. Assessing the outcome of nonalcoholic steatohepatitis? It’s time to get serious. Hepatology. 2006;44(4):802–805. doi: 10.1002/hep.21391. [DOI] [PubMed] [Google Scholar]
- 5.Chavez-Tapia NC, Mendez-Sanchez N, Uribe M. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann. Intern. Med. 2006;144(5):379. doi: 10.7326/0003-4819-144-5-200603070-00021. author reply 380. [DOI] [PubMed] [Google Scholar]
- 6.Parekh S, Anania FA. Abnormal lipid and glucose metabolism in obesity: implications for nonalcoholic fatty liver disease. Gastroenterology. 2007;132(6):2191–2207. doi: 10.1053/j.gastro.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 7.Lee RG. Nonalcoholic steatohepatitis: tightening the morphological screws on a hepatic rambler. Hepatology. 1995;21(6):1742–1743. doi: 10.1002/hep.1840210636. [DOI] [PubMed] [Google Scholar]
- 8.Roden M. Mechanisms of disease: hepatic steatosis in Type 2 diabetes – pathogenesis and clinical relevance. Nat. Clin. Pract. Endocrinol. Metab. 2006;2(6):335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 9.Unger RH. Lipotoxic diseases. Annu. Rev. Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 10.Kusminski CM, Shetty S, Orci L, et al. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14(12):1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 11. Malhi H, Bronk SF, Werneburg NW, et al. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006;281(17):12093–12101. doi: 10.1074/jbc.M510660200. ▪ Reports for the first time the involvement of the c-Jun N-terminal kinase (JNK) signaling pathway in lipoapoptosis processes.
- 12.Barreyro FJ, Kobayashi S, Bronk SF, et al. Transcriptional regulation of Bim by FoxO3a mediates hepatocyte lipoapoptosis. J. Biol. Chem. 2007;282(37):27141–27154. doi: 10.1074/jbc.M704391200. [DOI] [PubMed] [Google Scholar]
- 13.Cazanave SC, Mott JL, Elmi NA, et al. JNK1-dependent PUMA expression contributes to hepatocyte lipoapoptosis. J. Biol. Chem. 2009;284(39):26591–26602. doi: 10.1074/jbc.M109.022491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuoka HC, Mott J, Bronk SF, et al. Mcl-1 degradation during hepatocyte lipoapoptosis. J. Biol. Chem. 2009;284(44):30039–30048. doi: 10.1074/jbc.M109.039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Wang D, Topczewski F, et al. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006;291(2):E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 16. Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147(2):943–951. doi: 10.1210/en.2005-0570. ▪ Demonstrates that the induction of an endoplasmic reticulum (ER) stress in diet-induced models of steatohepatitis depends on the free fatty acid content of the diet and not on the development of a hepatic steatosis.
- 17.Wei Y, Wang D, Gentile CL, et al. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Mol. Cell. Biochem. 2009;331(1–2):31–40. doi: 10.1007/s11010-009-0142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Listenberger LL, Han X, Lewis SE, et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl Acad. Sci. USA. 2003;100(6):3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi K, Yang L, McCall S, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45(6):1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 20.Larter CZ, Yeh MM, Haigh WG, et al. Hepatic free fatty acids accumulate in experimental steatohepatitis: role of adaptive pathways. J. Hepatol. 2008;48(4):638–647. doi: 10.1016/j.jhep.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Larter CZ, Yeh MM, Williams J, et al. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J. Hepatol. 2008;49(3):407–416. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 22. Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. ▪▪ Observes that the severity of nonalcoholic fatty liver disease (NAFLD) correlates with an increase in hepatocyte apoptosis, which constitutes a hallmark of nonalcoholic steatohepatitis (NASH).
- 23.Nehra V, Angulo P, Buchman AL, et al. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig. Dis. Sci. 2001;46(11):2347–2352. doi: 10.1023/a:1012338828418. [DOI] [PubMed] [Google Scholar]
- 24.Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis GF, Carpentier A, Adeli K, et al. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and Type 2 diabetes. Endocr. Rev. 2002;23(2):201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 26.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115(5):1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohl J, Ring A, Hermann T, et al. Role of FATP in parenchymal cell fatty acid uptake. Biochim. Biophys. Acta. 2004;1686(1–2):1–6. doi: 10.1016/j.bbalip.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Febbraio M, Wada T, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPAR-γ in promoting steatosis. Gastroenterology. 2008;134(2):556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 29.Doege H, Baillie RA, Ortegon AM, et al. Targeted deletion of FATP5 reveals multiple functions in liver metabolism: alterations in hepatic lipid homeostasis. Gastroenterology. 2006;130(4):1245–1258. doi: 10.1053/j.gastro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Doege H, Grimm D, Falcon A, et al. Silencing of hepatic fatty acid transporter protein 5 in vivo reverses diet-induced non-alcoholic fatty liver disease and improves hyperglycemia. J. Biol. Chem. 2008;283(32):22186–22192. doi: 10.1074/jbc.M803510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonen DP, Jacobs RL, Febbraio M, et al. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56(12):2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 32.Baumgardner JN, Shankar K, Hennings L, et al. A new model for nonalcoholic steatohepatitis in the rat utilizing total enteral nutrition to overfeed a high-polyunsaturated fat diet. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(1):G27–G38. doi: 10.1152/ajpgi.00296.2007. [DOI] [PubMed] [Google Scholar]
- 33.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294(5):G1281–G1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 34.Westerbacka J, Kolak M, Kiviluoto T, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56(11):2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 35.Mitsuyoshi H, Yasui K, Harano Y, et al. Analysis of hepatic genes involved in the metabolism of fatty acids and iron in nonalcoholic fatty liver disease. Hepatol. Res. 2009;39(4):366–373. doi: 10.1111/j.1872-034X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 36.Newberry EP, Xie Y, Kennedy S, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J. Biol. Chem. 2003;278(51):51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 37.Newberry EP, Kennedy SM, Xie Y, et al. Diet-induced alterations in intestinal and extrahepatic lipid metabolism in liver fatty acid binding protein knockout mice. Mol. Cell. Biochem. 2009;326(1–2):79–86. doi: 10.1007/s11010-008-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlton M, Viker K, Krishnan A, et al. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49(4):1375–1384. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Almeida IT, Cortez-Pinto H, Fidalgo G, et al. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin. Nutr. 2002;21(3):219–223. doi: 10.1054/clnu.2001.0529. [DOI] [PubMed] [Google Scholar]
- 40.Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and re-esterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes Metab. 2003;29(5):478–485. doi: 10.1016/s1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- 41.Puri P, Baillie RA, Wiest MM, et al. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 42.Allard JP, Aghdassi E, Mohammed S, et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver (NAFLD):a cross-sectional study. J. Hepatol. 2008;48(2):300–307. doi: 10.1016/j.jhep.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 43.Mu YM, Yanase T, Nishi Y, et al. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology. 2001;142(8):3590–3597. doi: 10.1210/endo.142.8.8293. [DOI] [PubMed] [Google Scholar]
- 44.Li ZZ, Berk M, McIntyre TM, et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J. Biol. Chem. 2009;284(9):5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akazawa Y, Cazanave S, Mott JL, et al. Palmitoleate attenuates palmitate-induced Bim and PUMA upregulation and hepatocyte lipoapoptosis. J. Hepatol. 2009 doi: 10.1016/j.jhep.2010.01.003. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 47.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004;167(1):27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo S, Baumeister P, Yang S, et al. Induction of Grp78/BiP by translational block: activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 2003;278(39):37375–37385. doi: 10.1074/jbc.M303619200. [DOI] [PubMed] [Google Scholar]
- 49.Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21(1):81–93. doi: 10.1016/j.immuni.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Urano F, Wang X, Bertolotti A, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287(5453):664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Rutkowski DT, Dubois M, et al. ATF6a optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13(3):351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Ozcan U, Cao Q, Yilmaz E, et al. Endoplasmic reticulum stress links obesity, insulin action, and Type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 54. Puri P, Mirshahi F, Cheung O, et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):568–576. doi: 10.1053/j.gastro.2007.10.039. ▪▪ Observes the activation of ER-stress responses in liver biopsies from NASH patients.
- 55.Borradaile NM, Han X, Harp JD, et al. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006;47(12):2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J. Chem. Neuroanat. 2004;28(1–2):51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Scorrano L, Oakes SA, Opferman JT, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 58.Cunha DA, Hekerman P, Ladriere L, et al. Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J. Cell Sci. 2008;121(Pt 14):2308–2318. doi: 10.1242/jcs.026062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ji C, Mehrian-Shai R, Chan C, et al. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol. Clin. Exp. Res. 2005;29(8):1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCullough KD, Martindale JL, Klotz LO, et al. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001;21(4):1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puthalakath H, O’Reilly LA, Gunn P, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 62.Feldstein AE, Werneburg NW, Li Z, et al. Bax inhibition protects against free fatty acid-induced lysosomal permeabilization. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(6):G1339–G1346. doi: 10.1152/ajpgi.00509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279(44):45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 64.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23(6):1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malhi H, Barreyro FJ, Isomoto H, et al. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56(8):1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volkmann X, Fischer U, Bahr MJ, et al. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46(5):1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro PS, Cortez-Pinto H, Sola S, et al. Hepatocyte apoptosis, expression of death receptors, and activation of NF-κB in the liver of nonalcoholic and alcoholic steatohepatitis patients. Am. J. Gastroenterol. 2004;99(9):1708–1717. doi: 10.1111/j.1572-0241.2004.40009.x. [DOI] [PubMed] [Google Scholar]
- 68.Feldstein AE, Canbay A, Guicciardi ME, et al. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J. Hepatol. 2003;39(6):978–983. doi: 10.1016/s0168-8278(03)00460-4. [DOI] [PubMed] [Google Scholar]
- 69.Siebler J, Schuchmann M, Strand S, et al. Enhanced sensitivity to CD95-induced apoptosis in ob/ob mice. Dig. Dis. Sci. 2007;52(9):2396–2402. doi: 10.1007/s10620-006-9148-7. [DOI] [PubMed] [Google Scholar]
- 70.Schattenberg JM, Singh R, Wang Y, et al. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43(1):163–172. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 71. Singh R, Wang Y, Xiang Y, et al. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49(1):87–96. doi: 10.1002/hep.22578. ▪▪Characterizes the proapoptotic effects of JNK1 isoform and the antiapoptotic functions of JNK2 isoform in murine model of steatohepatitis.
- 72.Wang Y, Ausman LM, Russell RM, et al. Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J. Nutr. 2008;138(10):1866–1871. doi: 10.1093/jn/138.10.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaeschke A, Davis RJ. Metabolic stress signaling mediated by mixed-lineage kinases. Mol. Cell. 2007;27(3):498–508. doi: 10.1016/j.molcel.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Czaja MJ. The future of GI and liver research: editorial perspectives. III. JNK/AP-1 regulation of hepatocyte death. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284(6):G875–G8759. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 75.Czaja MJ. Cell signaling in oxidative stress-induced liver injury. Semin. Liver Dis. 2007;27(4):378–389. doi: 10.1055/s-2007-991514. [DOI] [PubMed] [Google Scholar]
- 76.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103(2):239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 77.Kodama Y, Taura K, Miura K, et al. Antiapoptotic effect of c-Jun N-terminal kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136(4):1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 78.Sabapathy K, Hochedlinger K, Nam SY, et al. Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation. Mol. Cell. 2004;15(5):713–725. doi: 10.1016/j.molcel.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 79.Videla LA, Tapia G, Rodrigo R, et al. Liver NF-κB and AP-1 DNA binding in obese patients. Obesity (Silver Spring) 2009;17(5):973–979. doi: 10.1038/oby.2008.601. [DOI] [PubMed] [Google Scholar]
- 80.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol. Cell. Biol. 1999;19(12):8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donovan N, Becker EB, Konishi Y, et al. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J. Biol. Chem. 2002;277(43):40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- 82.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl Acad. Sci. USA. 2003;100(5):2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J. Biol. Chem. 2006;281(30):21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 84.Eichhorst ST, Muller M, Li-Weber M, et al. A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol. Cell. Biol. 2000;20(20):7826–7837. doi: 10.1128/mcb.20.20.7826-7837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Erlacher M, Labi V, Manzl C, et al. Puma cooperates with Bim, the rate-limiting BH3-only protein in cell death during lymphocyte development, in apoptosis induction. J. Exp. Med. 2006;203(13):2939–2951. doi: 10.1084/jem.20061552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamaguchi H, Wang HG. Bcl-xL protects BimEL-induced Bax conformational change and cytochrome C release independent of interacting with Bax or BimEL. J. Biol. Chem. 2002;277(44):41604–41612. doi: 10.1074/jbc.M207516200. [DOI] [PubMed] [Google Scholar]
- 87.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26(9):1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gallenne T, Gautier F, Oliver L, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J. Cell Biol. 2009;185(2):279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chipuk JE, Fisher JC, Dillon CP, et al. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc. Natl Acad. Sci. USA. 2008;105(51):20327–20332. doi: 10.1073/pnas.0808036105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jabbour AM, Heraud JE, Daunt CP, et al. Puma indirectly activates Bax to cause apoptosis in the absence of Bid or Bim. Cell Death Differ. 2009;16(4):555–563. doi: 10.1038/cdd.2008.179. [DOI] [PubMed] [Google Scholar]
- 91.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9(3):231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 92.Li Z, Berk M, McIntyre TM, et al. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47(5):1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120(5):1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 94.Caldwell SH, Swerdlow RH, Khan EM, et al. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 1999;31(3):430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 95.Caldwell SH, Chang CY, Nakamoto RK, et al. Mitochondria in nonalcoholic fatty liver disease. Clin. Liver Dis. 2004;8(3):595–617. doi: 10.1016/j.cld.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 96.Summers SA. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006;45(1):42–72. doi: 10.1016/j.plipres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 97.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Shimabukuro M, Zhou YT, Levi M, et al. Fatty acid-induced β cell apoptosis: a link between obesity and diabetes. Proc. Natl Acad. Sci. USA. 1998;95(5):2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim. Biophys. Acta. 2002;1585(2–3):202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 100.Paumen MB, Ishida Y, Muramatsu M, et al. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J. Biol. Chem. 1997;272(6):3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 101.Shah C, Yang G, Lee I, et al. Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. J. Biol. Chem. 2008;283(20):13538–13548. doi: 10.1074/jbc.M709950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56(8):1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 103.Paris F, Grassme H, Cremesti A, et al. Natural ceramide reverses Fas resistance of acid sphingomyelinase−/− hepatocytes. J. Biol. Chem. 2001;276(11):8297–8305. doi: 10.1074/jbc.M008732200. [DOI] [PubMed] [Google Scholar]
- 104.Kotronen A, Seppanen-Laakso T, Westerbacka J, et al. Hepatic stearoyl-CoA desaturase (SCD)-1 activity and diacylglycerol but not ceramide concentrations are increased in the nonalcoholic human fatty liver. Diabetes. 2009;58(1):203–208. doi: 10.2337/db08-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mari M, Caballero F, Colell A, et al. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 2006;4(3):185–198. doi: 10.1016/j.cmet.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 106.Ichi I, Nakahara K, Kiso K, et al. Effect of dietary cholesterol and high fat on ceramide concentration in rat tissues. Nutrition. 2007;23(7–8):570–574. doi: 10.1016/j.nut.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 107.Feng B, Yao PM, Li Y, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003;5(9):781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 108.Devries-Seimon T, Li Y, Yao PM, et al. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the Type A scavenger receptor. J. Cell Biol. 2005;171(1):61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Calamita G, Portincasa P. Present and future therapeutic strategies in non-alcoholic fatty liver disease. Expert Opin. Ther. Targets. 2007;11(9):1231–1249. doi: 10.1517/14728222.11.9.1231. [DOI] [PubMed] [Google Scholar]
- 110.Lin HZ, Yang SQ, Chuckaree C, et al. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat. Med. 2000;6(9):998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 111.Nair S, Diehl AM, Wiseman M, et al. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol. Ther. 2004;20(1):23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 112.Witek RP, Stone WC, Karaca FG, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed] [Google Scholar]
- 113.Fromenty B, Robin MA, Igoudjil A, et al. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30(2):121–138. doi: 10.1016/s1262-3636(07)70098-8. [DOI] [PubMed] [Google Scholar]
- 114.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-α expression via a lysosomal pathway. Hepatology. 2004;40(1):185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 115.Svegliati-Baroni G, Candelaresi C, Saccomanno S, et al. A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-α and n-3 polyunsaturated fatty acid treatment on liver injury. Am. J. Pathol. 2006;169(3):846–860. doi: 10.2353/ajpath.2006.050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Araya J, Rodrigo R, Videla LA, et al. Increase in long-chain polyunsaturated fatty acid n-6/n-3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin. Sci. (Lond) 2004;106(6):635–643. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- 117.Videla LA, Rodrigo R, Araya J, et al. Oxidative stress and depletion of hepatic long-chain polyunsaturated fatty acids may contribute to nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2004;37(9):1499–1507. doi: 10.1016/j.freeradbiomed.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 118.Capanni M, Calella F, Biagini MR, et al. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol. Ther. 2006;23(8):1143–1151. doi: 10.1111/j.1365-2036.2006.02885.x. [DOI] [PubMed] [Google Scholar]
- 119.Spadaro L, Magliocco O, Spampinato D, et al. Effects of n-3 polyunsaturated fatty acids in subjects with nonalcoholic fatty liver disease. Dig. Liver Dis. 2008;40(3):194–199. doi: 10.1016/j.dld.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 120. Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of Type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. ▪▪ Reports that chemical chaperones, which enhance the adaptative capacity of the ER, may have therapeutic potential in the treatment of obesity and NAFLD.
- 121.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39(3):770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 122.Cnop M, Igoillo-Esteve M, Cunha DA, et al. An update on lipotoxic endoplasmic reticulum stress in pancreatic β-cells. Biochem. Soc. Trans. 2008;36(Pt 5):909–915. doi: 10.1042/BST0360909. [DOI] [PubMed] [Google Scholar]
Website
- 201.Yetukuri L, Katajamaa M, Medina-Gomez G, et al. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007 doi: 10.1186/1752-0509-1-12. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1839890. [DOI] [PMC free article] [PubMed] [Google Scholar]