Abstract
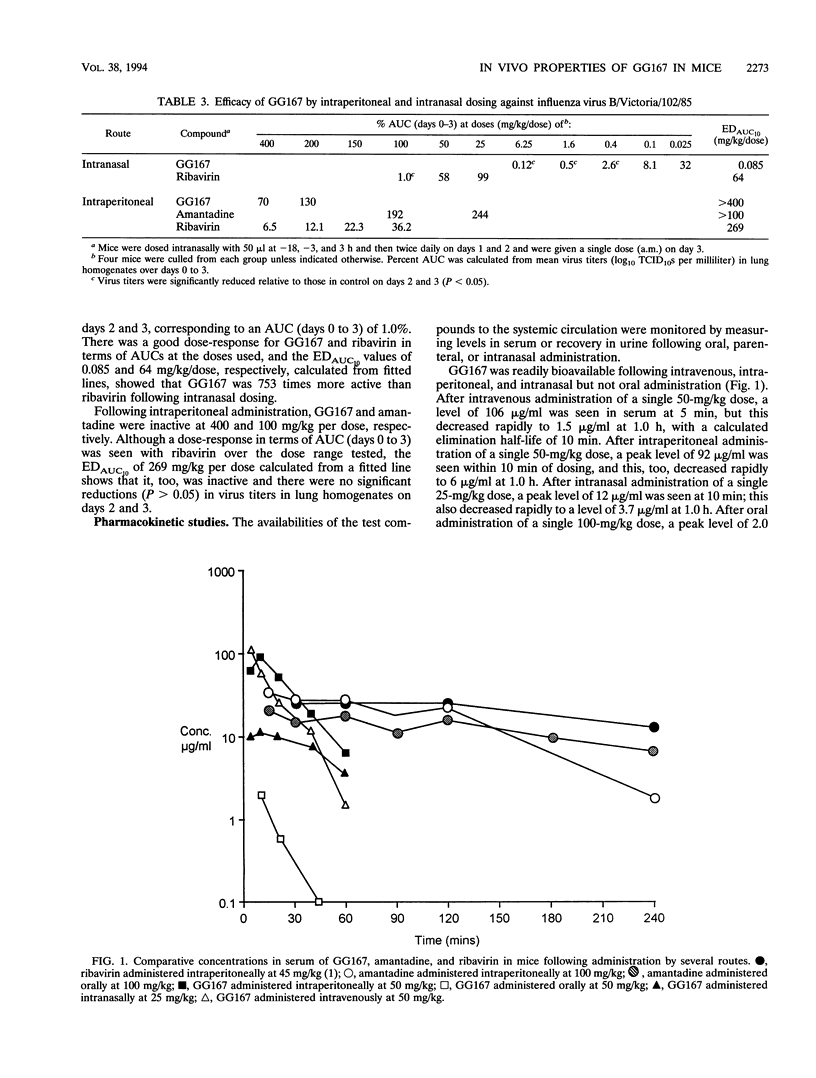
We demonstrate the potent antiviral activity of a novel viral neuraminidase (sialidase) inhibitor, 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid (GG167), administered by the intranasal route in comparison with those of amantadine and ribavirin in experimental respiratory tract infections induced with influenza A and B viruses. In an extended study in which mice were infected (day 0) with influenza A/Singapore/1/57 virus, with treatments given prophylactically plus twice daily over days 0 to 3 and with mice observed to day 10, we show that intranasally administered GG167 at 0.4 and 0.01 mg/kg of body weight per dose reduced mortality, lung consolidation, and virus titers in the lung, with no virus growing back following the cessation of treatment. In other studies with influenza B/Victoria/102/85 virus in which infected mice were culled after the cessation of treatment, the calculated intranasal dose required to reduce virus titers in the lungs of treated animals to 10% of that seen in untreated controls (EDAUC10 [where AUC is area under the virus titer days curve]) was 0.085 mg/kg per dose. GG167 was inactive against influenza viruses A and B when given by the intraperitoneal or oral route (EDAUC10, > 100 mg/kg per dose). GG167 was metabolically stable, with an elimination half-life of 10 min following intravenous administration. While readily bioavailable by systemic routes, it was poorly bioavailable by the oral route. Its potent efficacy by the intranasal route but lack of efficacy by other routes, relative to those of amantadine and ribavirin, was explicable in terms of its in vitro activity, bioavailability, and pharmacokinetic properties and with the extracellular activity of viral sialidase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. K., Trefts P. E., Hintz M., Connor J. D., Kagnoff M. F. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob Agents Chemother. 1983 Nov;24(5):696–701. doi: 10.1128/aac.24.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshe R. B., Smith M. H., Hall C. B., Betts R., Hay A. J. Genetic basis of resistance to rimantadine emerging during treatment of influenza virus infection. J Virol. 1988 May;62(5):1508–1512. doi: 10.1128/jvi.62.5.1508-1512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendt R. F., Walker J. S., Dominik J. W., Stephen E. L. Response of influenza virus-infected mice to selected doses of ribavirin administered intraperitoneally or by aerosol. Antimicrob Agents Chemother. 1977 Jun;11(6):1069–1070. doi: 10.1128/aac.11.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleidner W. E., Harmon J. B., Hewes W. E., Lynes T. E., Hermann E. C. Absorption, distribution and excretion of amantadine hydrochloride. J Pharmacol Exp Ther. 1965 Dec;150(3):484–490. [PubMed] [Google Scholar]
- Chang T. W., Heel R. C. Ribavirin and inosiplex: a review of their present status in viral diseases. Drugs. 1981 Aug;22(2):111–128. doi: 10.2165/00003495-198122020-00002. [DOI] [PubMed] [Google Scholar]
- GRUNERT R. R., MCGAHEN J. W., DAVIES W. L. THE IN VIVO ANTIVIRAL ACTIVITY OF 1-ADAMANTANAMINE (AMANTADINE). I. PROPHYLACTIC AND THERAPEUTIC ACTIVITY AGAINST INFLUENZA VIRUSES. Virology. 1965 Jun;26:262–269. doi: 10.1016/0042-6822(65)90273-4. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Cote K. M., Douglas R. G., Jr Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob Agents Chemother. 1980 May;17(5):865–870. doi: 10.1128/aac.17.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöhle U., Beau J. M., Schauer R. Uptake, metabolism and excretion of orally and intravenously administered, double-labeled N-glycoloylneuraminic acid and single-labeled 2-deoxy-2,3-dehydro-N-acetylneuraminic acid in mouse and rat. Eur J Biochem. 1982 Sep 1;126(3):543–548. doi: 10.1111/j.1432-1033.1982.tb06815.x. [DOI] [PubMed] [Google Scholar]
- Schulman J. L. The use of an animal model to study transmission of influenza virus infection. Am J Public Health Nations Health. 1968 Nov;58(11):2092–2096. doi: 10.2105/ajph.58.11.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H., Sweet C. Lessons for human influenza from pathogenicity studies with ferrets. Rev Infect Dis. 1988 Jan-Feb;10(1):56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- TAKANO K., JENSEN K. E., WARREN J. PASSIVE INTERFERON PROTECTION IN MOUSE INFLUENZA. Proc Soc Exp Biol Med. 1963 Nov;114:472–475. doi: 10.3181/00379727-114-28706. [DOI] [PubMed] [Google Scholar]
- Tisdale M., Bauer D. J. The relative potencies of anti-influenza compounds. Ann N Y Acad Sci. 1977 Mar 4;284:254–263. doi: 10.1111/j.1749-6632.1977.tb21958.x. [DOI] [PubMed] [Google Scholar]
- Woods J. M., Bethell R. C., Coates J. A., Healy N., Hiscox S. A., Pearson B. A., Ryan D. M., Ticehurst J., Tilling J., Walcott S. M. 4-Guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid is a highly effective inhibitor both of the sialidase (neuraminidase) and of growth of a wide range of influenza A and B viruses in vitro. Antimicrob Agents Chemother. 1993 Jul;37(7):1473–1479. doi: 10.1128/aac.37.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Itzstein M., Wu W. Y., Kok G. B., Pegg M. S., Dyason J. C., Jin B., Van Phan T., Smythe M. L., White H. F., Oliver S. W. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993 Jun 3;363(6428):418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]



