Abstract
The performance of solid-contact/coated wire type electrodes with plasticized PVC membranes containing metalloporphyrins as anion selective ionophores is reported. The membranes are deposited on transducers based on graphite pastes and graphite rods. The hydrophobicity of the underlying conductive transducer surface is found to be a key factor that influences the formation of an aqueous layer beneath the polymer film. Elimination of this ill-defined water layer greatly improves the electrochemical properties of the ion-sensors, such as EMF stability and life-time. Only highly lipophilic electrode substrates, namely graphite paste with mineral oil, were shown to prevent the formation of aqueous layer underneath the ion-sensing membrane. The possibility of employing Co(III)-tetraphenylporphyrin both as NO2− selective ionophore and as electron/ion conducting species to ensure ion-to-electron translation was also discussed based on the results of preliminary experiments.
Keywords: solid-state ion-selective electrode, graphite paste, metalloporphyrin, potentiometry
Introduction
Miniaturization has become a growing trend in analytical chemistry, as it can significantly reduce cost of analysis and its environmental impact. It also enables measurements in very small volumes of sample, which is essential in clinical measurements, and for in vivo sensing. Miniaturization has received considerable attention in the field of electrochemical sensors, with many different architectures described over the past 35 years.
In the field of ion-selective electrodes (ISEs), needle-type coated wire electrodes were the first type of miniaturized sensors, intended for intracellular measurements [1,2]. In addition to their small size, such electrodes were exceptionally simple to prepare by dip-coating of platinum wire or a graphite rod into a cocktail containing all the membrane components. Besides above-mentioned solid supports, graphite paste was also used, although rarely, for coated-wire electrodes, first by Ruzicka et al. [3]. In later years, some reports on the use of graphite paste electrodes in potentiometry were published [4], however the vast majority of the reports on this type of electrode material described voltammetric applications [5]. The main drawback of coated-wire electrodes is the instability of the phase boundary potential that develops at the conductor electrode/membrane interface, which changes unpredictably with time [6] and potentially is sensitive to redox gases, including oxygen. The formation of an aqueous layer between the polymeric membrane and solid conductor has been considered as one of the possible reasons for this problem [7]. Such an aqueous layer serves as a pseudo internal electrolyte. However, due to the uncontrollable composition of this solution, the E0 or more appropriately the cell constant (K) of such electrodes tends to change in an unpredictable manner. Another factor that contributes to the poor stability of coated-wire electrodes is an ill-defined charge transfer process at the interface between the ionically conducting membrane and the electronically conducting inner electrode [8].
In recent years other types of solid-state ion-selective electrodes, namely planar sensors, have gained much attention. Again, the problems with response stability have been observed and this has prompted studies toward the design of various intermediate layers which are able to translate the ionic response of an ion-selective membrane into a stable electronic signal by better poising the phase-boundary potential between the conductor and the polymer membrane. Various intermediate layers have been described, including hydrogels [9,10], conducting polymers [10] or redox-active monolayers [11,12,13]. Recently, three-dimensionally ordered macroporous carbon electrodes were also proposed as an intermediate layer for all-solid state ISEs [14]. It was shown that due to the well interconnected pore and wall structure, this material exhibits high ionic and electric conductivity, which resulted in exceptional long-term potential stability, as well as insensitivity to oxygen and light. These promising results prompted us to investigate whether much simpler graphite-paste transducers can be used with similar success, especially in the development of solid-state anion selective membrane electrodes (see below).
As an alternate to employing an intermediate layer for the solid-state ISE construction, attempts have also been undertaken to incorporate certain additives into the polymeric membrane to facilitate the charge transfer between the ion-selective membrane and the metallic transducer. Organic silver ion-ligand complexes were used for this purpose on transducers with metallic silver electrode, and formed a stable Ag/Ag+(ligand) reversible electron redox pair at a membrane/silver electrode interface [15–17]. Conducting polymers, possessing ion/electron conductivity, have also been introduced within the composition of the ion-selective membranes, including poly(3,4-octylthiophene), polyaniline, polyanisidine, and polypyrrole [8,18]. A more stable EMF of solid-contact ISEs can only be obtained with such composite films when the materials have mixed ionic and electronic conductivity, and provide the proper charge transfer at the polymer/conductor interface.
Metalloporphyrins have gained wide attention as ionophores that are useful for preparing anion selective ISEs, since porphyrin complexes with various metal cations induce selectivity patterns that deviate greatly form each other, as well as from the classical Hofmeister series [19]. This occurs owing to selective anion binding as an axial ligand to the central metal cation of these structures. It is especially noteworthy that electrodes with enhanced selectivity towards hydrophilic anions have been realized using metalloporphyrin ionophores, including F−-selective electrodes based on Ga(III)- [20], Zr(III)- [21] and Al(III)-porphyrins [22]. Miniaturized planar electrodes with polymeric membranes containing metalloporphyrins were also described, with a hydrogel as an intermediate layer. Co(III)-tetraphenylporphyrin [23], Zr(IV)- tetraphenylporphyrin and Zr(IV)- octaethylporphyrin [24] were used as ionophores for the preparation of electrodes selective towards nitrite and fluoride anion. It was shown that the working parameters (e.g., selectivity, linear range, slopes of the calibration curves) of the sensors were comparable to classic ISEs with bulk internal electrolyte solutions.
In this work, the performance of miniaturized solid-contact anion selective ISEs prepared with plasticized PVC membranes containing metalloporphyrins as ionophores is reported. The behavior of these anion sensors using different types of conductor/membrane interface configurations is examined in detail. The primary goal of this work is to assess whether the hydrophobicity of the underlying electrode surface and the ability of redox couple formation by the metal ion center of metalloporphyrin can affect the sensor stability. Transducers used in this work include graphite and graphite pastes with various organic solvents.
2. Experimental
2.1. Reagents
The fluoride-selective ionophore chloro(5,10,15,20-tetra-4-tertbutylophenylporphyrinato)aluminum(III) (Al(III)[tBTPP]Cl) was synthesized as follows [25]: 4-tert-butylbenzaldehyde (20 g) was added to propionic acid (500 cm3). The solution was then heated with continuous stirring and when it started boiling, the pyrrole (8.3 g) was slowly added and the heating was continued for 5 h. Then the solution was cooled to room temperature and it was left to stand overnight without stirring. The purple crystalline product was isolated by suction filtration and washed with MeOH until the washing was colorless. The crude product was recrystallized from CH2Cl2/hexane and dried at room temperature to give H2[tBTPP]. The yield of the reaction was 16%.
The aluminum(III) complex of this porphyrin (Al(III)[tBTPP]Cl) was prepared by reaction of H2[tBTPP] (1.0 g) with diethylaluminum chloride (5.0 cm3 of 1 M solution in hexane) in methylene chloride (50 cm3). The reaction was carried out for 6 h at ambient temperature. The mixture was then brought to dryness on a rotary evaporator and extracted with dichloromethane. The organic layer was washed with diluted HCl and then twice with water. The crude product was purified using silica gel column chromatography and crystallized from CH2Cl2/hexane. The yield was 87 %. All the reactants and solvents used in above-described reactions were received from Aldrich (St. Louis, MO).
Acetato(5,10,15,20-tetraphenylporphyrinato)cobalt(III) (Co(III)[TPP]Ac) was prepared according to previous procedures [26,27]. The membrane components poly(vinyl chloride) (PVC), o-nitrophenyl octyl ether (o-NPOE), bis(2-ethylhexyl) sebacate (DOS), potassium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (KTFPB) and tridodecylmethylammonium chloride (TDMACl) were used as received from Fluka. The buffer species glycine (Gly) and 4-morpholinoethanesulfonic acid (MES) was also products of Fluka, while tetrahydrofuran (THF) employed to cast membranes and graphite rod (150 mm long and 3 mm in diameter) were purchased from Aldrich (St. Louis, MO). Polyolefin heat shrink tubing (3.2 mm in diameter) was purchased from Fisher Scientific (Pittsburgh, PA).
All aqueous solutions were prepared with salts of the highest purity available from Fluka. The sample solutions for potentiometric measurements consisted of sodium salts of the given anions in buffer solutions.
2.2. Electrode supports
Graphite rods were prepared by employing a heat shrink tubing as insulator leaving 5 mm at one end for the ion selective membrane coating. The graphite rods were sonicated in acetone then in water and dried in air before use.
Graphite paste electrodes were prepared in PVC tubing, 5 mm i.d., cut to 3 cm length. A copper wire, 3 mm diameter, was inserted into these tubes, forming a 3 mm deep cavity at one end, while second end of copper wire was soldered to the flexible wire with a connection plug. The carbon paste was then placed into the created cavity, polished to form a flat electrode surface. Graphite paste was prepared by grinding the mixture of 75 wt% of graphite powder and 25 wt% of mineral oil (Fluka), DOS or o-NPOE in a mortar. For some of the electrodes, modified carbon paste was prepared by adding the metalloporphyrin and ionic additives in the concentrations equal to those in the ion-selective membrane.
2.3. ISE membrane formulation and EMF measurements
Polymer membranes employed for ISE measurements consisted of 1 wt% ionophore, PVC/o-NPOE or PVC/DOS (1:2) polymeric matrix and 15 mol% of cationic additive (TDMACl) (for Co(III)-porphyrin based membranes only). All membrane components were dissolved in 1.5 mL of THF. Graphite rod electrodes were prepared by dipping the electrodes into the appropriate cocktail solution ten times, leaving 1 min between each dipping to allow solvent evaporation. The electrodes were allowed to stabilize overnight. The thickness of the resulting membrane was 150 μm measured by scanning electron microscope (SEM). Graphite paste electrodes were prepared by drop-casting 24 μl of the membrane cocktail on the surface of graphite paste, placed in a PVC tube. The polymer membrane was left to dry for 1 h. Electrochemical potentials were measured with the following galvanic cell: Ag | AgCl(s) | KCl(sat.) || bridge electrolyte | sample solution | ion-selective membrane | solid support. The bridge electrolyte of the double-junction reference electrode was 1 M potassium chloride. A Gly/H3PO4 solution, pH 3.0, served as conditioning solution for the electrodes based on fluoride selective Al(III)-porphyrin, while MES/NaOH buffer solution was used for electrodes with membranes containing Co(III)-porphyrin. Conditioning solutions contained primary ion at concentration of 10−2 M. Between measurements, electrodes were stored in appropriate buffer solution, containing 10−3 M NaNO2 (for Co(III)-porphyrin-based electrodes) or 10−2 M NaF (for Al(III)-porphyrin-based electrodes). EMF values were measured at ambient temperature (~22 °C) via an IBM computer coupled to a Lawson EMF 16 interface (Lawson Labs, Inc.). Selectivity coefficients were calculated by the separate solution method [24] using EMF values measured in 0.1 M salt solutions and theoretical slope values.
2.4. Studies of aqueous layer formation
Sensors modified with plasticized PVC membranes were initially conditioned in 10−3 M of primary ion solution. After 24 h the solution was changed to 10−3 M NaCl (interfering anion solution). These changes were made in the time intervals sufficient to observe the EMF stability, or to see a definite tendency of the EMF instability and the general picture of its behavior. The observed dynamic EMF response was analyzed in terms of potential drifts upon replacing the primary ions by discriminated interfering ions that indicate the presence of an aqueous film between the membrane and the solid contact [28].
3. Results and Discussion
As already mentioned, various approaches have been proposed in order to improve the stability of miniaturized planar ion-selective electrodes. Quite interesting results were reported, for example, for miniaturized ISEs with polymeric membranes containing Co(III)-porphyrin as an ionophore [23]. A hydrogel (pHEMA) layer, containing aqueous internal electrolyte, was used as a intermediate layer in this earlier work, resulting in pronounced improvement of sensor EMF stability with time, compared to sensors with no hydrogel layer. It must be mentioned, however, that the electrode architecture described in [23], is entirely different from the sensor construction proposed in the present work. Electrodes modified with hydrogel layer containing aqueous solution, deposited on the Ag/AgCl sensing site, are not “all solid-state” sensors in that they mimic conventional ISEs with simply a small volume of gelled internal electrolyte. This is in contrast to the electrodes reported here based on graphite or graphite paste, described in this paper.
Besides intermediate layers, certain species (e.g., conducting polymers and silver complexes) have been incorporated into the polymeric membrane phase to improve electron/ion charge transfer process between the membrane and solid electrode support. The use of a single compound playing the role of ionophore and participating in a redox or ion-exchange equilibrium with the underlying conductive support has been reported only for polyaniline used as pH-sensitive component [29]. Such an approach seems to be quite beneficial given that the addition of any exogenous species to the membrane (e.g., Ag+-complexes, etc.) can affect the analytical properties of the ion-selective membrane, including selectivity and detection limits.
Beyond the incorporation of a suitable species to poise the potential of the polymer membrane/conductor interface, the presence of an aqueous layer between the membrane and electrode solid support may also greatly influence the response parameters of a solid-contract ISE electrode, hindering the examination of unbiased membrane performance [28]. Therefore, in this work, approaches to diminish the formation of such aqueous layer were undertaken by comparing the use of graphite paste electrodes as solid supports for ion-selective membranes to other more traditional solid-contact conductors. The graphite paste materials have the potential to provide an unfavorable environment for the formation of a water layer underneath the membrane, allowing observation of the “true” response of solid-contact electrodes. For direct comparison, electrodes based on bare graphite rods were also prepared. The hydrophobicity of solid supports used as electrode materials was determined by the measurements of contact angle (θaq) for water (Table 1). It was found that the bare graphite possesses significantly greater hydrophilic character (θaq = 75 °), compared to graphite pastes, especially that with mineral oil (θaq = 104°).
Table 1.
Contact angles measured for water on various surfaces, used as electrode solid supports in this work.
| graphite/o-NPOE | graphite/DOS | graphite/mineral oil | graphite | |
|---|---|---|---|---|
| Angle, ° | 86 | 90 | 104 | 75 |
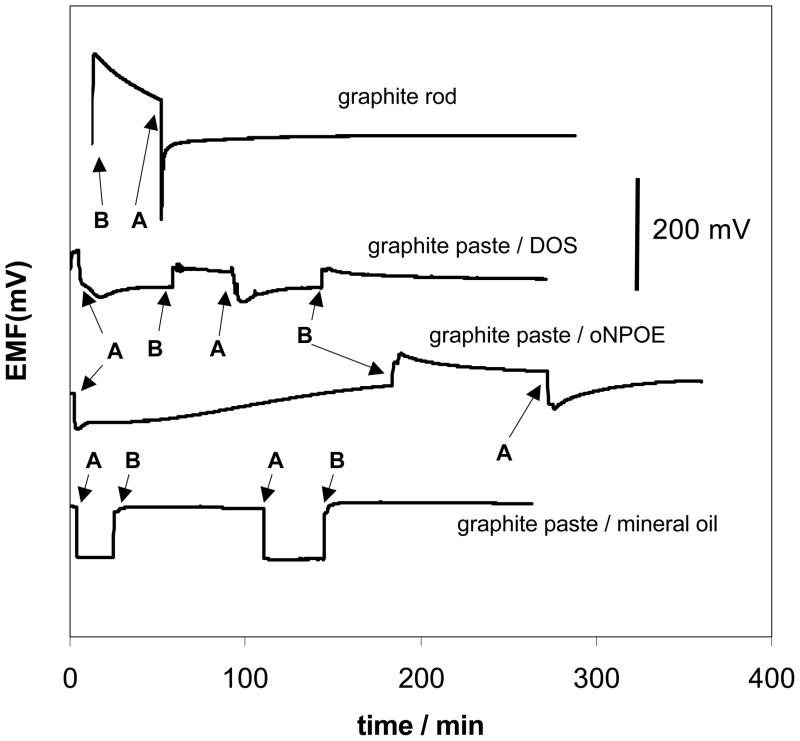
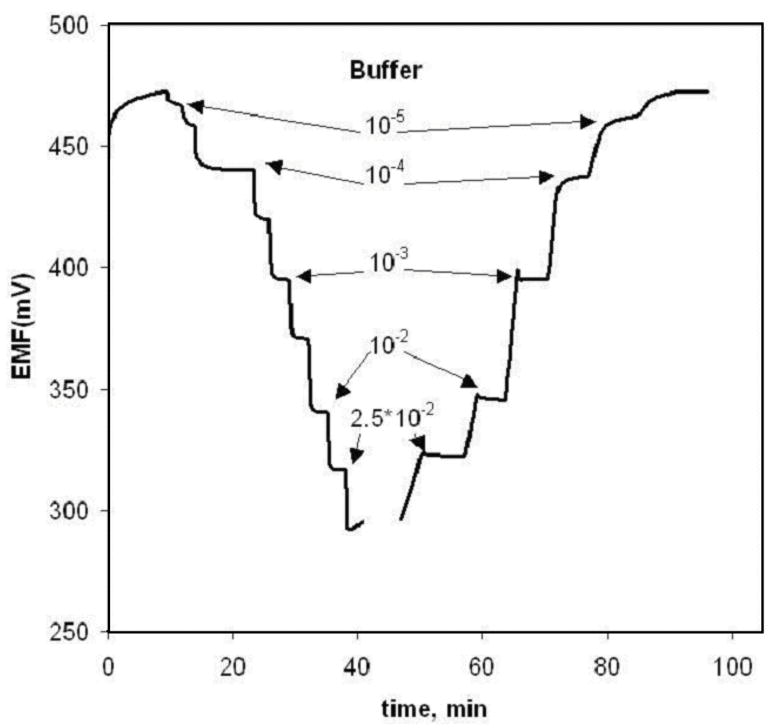
To prove the presence of water layer formation between the underlying electrode and the ion-selective membrane the procedure described by Fibbioli et al. [28] was employed. In this approach, varying the sample solution between primary and interferent ion and observing the degree of drift of the sensor for such changes owing to transmembrane ion fluxes from inner solution to outer and visa-versa were observed. For these fundamental studies, all transducers were modified with plasticized PVC membrane containing Co(III)-TPP and cationic lipophilic additives to create nitrite selective sensors. As it can be seen in Fig. 1, clear evidence for the formation of an aqueous layer was found for electrodes based on a graphite rod and graphite pastes prepared with both DOS and o-NPOE. As it could be foreseen, among the explored electrodes an effect of the water layer on the EMF drift was strongest in a case of graphite rod electrode, where it has reached 152 mV in a primary ion solution. For the o-NPOE graphite paste electrodes the EMF drift was up to 87 mV, and up to 27 mV for the electrodes with DOS. The general form of the time response curse was well reproducible for each type of the electrodes, but the time of EMF stabilization and the value of drift, varied even during one experiment, witch obviously shows unpredictability and instability of the water layer composition. However, an aqueous layer was not observed (little drift after changing solution composition) for electrodes prepared with graphite paste using mineral oil, which can be attributed to higher lipophilicity of this support.
Fig. 1.
Test results to assess formation of an aqueous film between membrane and transducer. Membrane composition: 33 wt% PVC, 66 wt% of plasticizer, 1 wt% Co(III) TPPAc, 10 mol% TDMACl, different transducer types. Changes of sample: A – 0.1M NaNO2/B – 0.1M NaCl in MES buffer, pH 5.5.
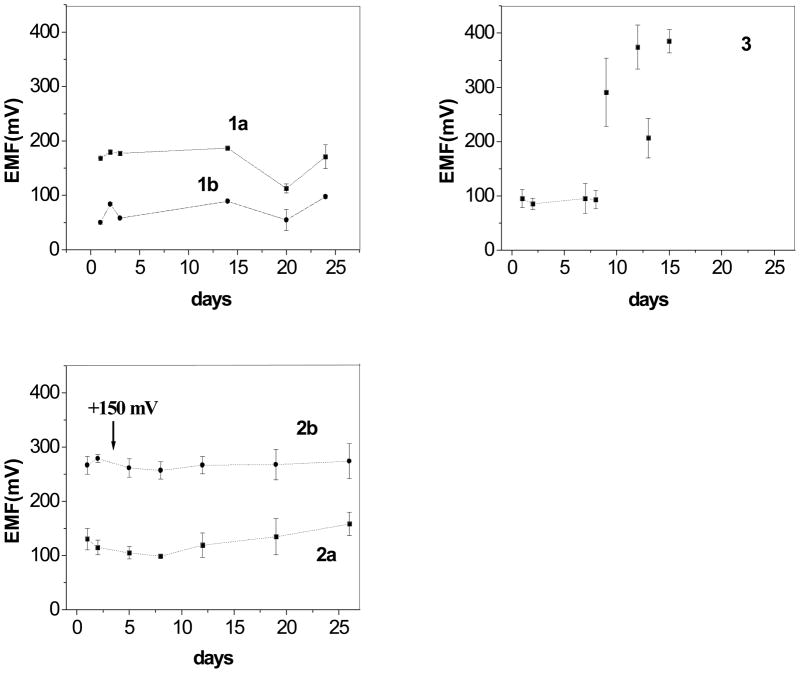
Among the electrode supports examined in this work, the most hydrophobic one was the graphite paste with mineral oil. Since no aqueous layer formation was observed in this case, stable cell EMF values were expected. Indeed, as shown in Fig. 2 (subfig. 3), the potential values, registered at fixed NO2− activity were almost constant during the first week after electrode fabrication. Further, the reproducibility of absolute EMF values for three of the same electrodes was very good (SD ≤ 21.3 mV during first 8 days). Similar trend was observed for other electrodes studied. Surprisingly, after that time, large instabilities were observed. Moreover, the slopes of calibration curves for NO2− decreased in time from the theoretical values for freshly prepared electrodes to approximately −50 mV/decade after two weeks. This may imply that the composition of the membrane changes via diffusion of membrane components into the PVC tubing that holds the graphite paste material.
Fig. 2.
Changes of electrodes EMF with time. Measurements were conducted for 3 electrodes. Standard deviations are shown on the plot. Electrodes prepared with 33 wt% PVC, 66 wt% of plasticizer, 1 wt% ionophore and 10 mol% (relative to the ionophore) TDMACl polymeric membranes, and different transducers. 1). Co(III) TPPAc as ionophore, potentials measured for NaNO2 10−1 M in MES buffer solution, pH 5.5 2). Al(III)t BuTPPCl as ionophore, potentials measured for NaF 10−1 M in Gly/H3PO4 buffer solution, pH 3.0 3). Co(III) TPPAc as ionophore, potentials measured for NaNO2 10−1 M in MES buffer solution, pH 5.5. Transducers used: 1a) graphite paste/oNPOE; 2a). graphite paste/oNPOE; 1b). graphite paste/oNPOE/ionophore and ion exchanger at the same quantities as in a membrane. 2b). graphite paste/oNPOE/ionophore and ion exchanger at the same quantities as in a membrane. 3). graphite paste/mineral oil/ionophore and ion exchanger at the same quantities as in a membrane.
Despite the lower hydrophobicity of graphite paste with o-NPOE (θaq = 86°), electrodes based on this solid-contact transduction material showed remarkable stability of the cell EMF values (see Fig. 2, subfig. 1). This somewhat surprising observation might be attributed to good compatibility of o-NPOE plasticized PCV membrane with the graphite paste modified with the same o-NPOE plasticizer. Analogous data were obtained for both Co(III)- and Al(III)-porphyrins in this configuration. However, it should be noted that the stability of the Al(III) porphyrin based fluoride electrodes was definitely better compared to the Co(III)-porphyrin based solid-contact nitrite sensors. Indeed, after two weeks, a gradual but pronounced decrease in the slopes of the NO2− calibration curves for Co(III)-porphyrin based electrodes were observed, from initial Nernstian values at first day to ca. −34 mV dec−1. As stated above, this might be explained by the diffusion of electroactive components from the membrane to the graphite paste and/or PVC electrode housing. In contrast, the slopes for fluoride calibration curves for Al(III)-porphyrin based electrodes were near-Nernstian even after 26 days. The difference between absolute EMF values for various pieces of the same electrode configuration increased in time, as shown in Fig. 2 (subfig. 2).
Long-term stability tests were also conducted and they reveal somewhat longer lifetime for electrodes constructed using carbon paste with mineral oil (over 20 d) compared to electrodes based on graphite (14 d). The nitrite selectivity of electrodes based on membranes containing Co(III)[TPP]Ac was quite similar for all types of solid transducers used in this study. Selectivity coefficients calculated for the various sensor configurations tested in this work are also in good agreement with results obtained for classical ISEs with internal solutions (see Table 2). The only exception is electrode 5, showing considerably worse nitrite selectivity. It seems that Co(III)[TPP]Ac is not fully compatible with DOS – plasticizer used in this case, as the slopes of NO2− calibration curves are significantly sub-Nernstian (−29 mV dec−1). Thus, selectivity coefficients for electrode 5 should be treated as approximate values.
Table 2.
Selectivity coefficients, measured for electrodes with plasticized PVC membranes on various supports.
| Transduser type | Membrane composition |
Selectivity coefficients, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ionophore | Plasticizer | Cl− | Br− | NO3− | NO2− | F− | SCN− | ClO4− | |||
| 1 | graphite paste/oNPOE | Al(III)tBuTPP Cl | o-NPOE | −2.0 | −1.4 | −0.7 | - | 0 | 0.1 | 1.2 | |
| 2 | graphite paste/mineral oil | Al(III)tBuTPP Cl | o-NPOE | −4.1 | −5.1 | −4.3 | - | 0 | −0.2 | 0.3 | |
|
Selectivity coefficients,
|
|||||||||||
| 3 | graphite | Co(III)TPP Ac | o-NPOE | −3.9 | −2.4 | −2.4 | 0 | −4.5 | 0.8 | 0.3 | |
| 4 | graphite paste/o-NPOE | Co(III)TPP Ac | o-NPOE | −2.8 | −2.4 | −1.7 | 0 | −4.0 | 1.1 | 1.1 | |
| 5 | graphite paste/DOS | Co(III)TPP Ac | DOS | −1.5 | −1.1 | −0.6 | 0 | −1.6 | 1.0 | 1.7 | |
| 6 | graphite paste/mineral oil | Co(III)TPP Ac | o-NPOE | −3.0 | −2.1 | −1.5 | 0 | −3.8 | 1.2 | 1.2 | |
| 7 | classical ISE | Co(III)TPP Ac | o-NPOE | −3.6 | −3.0 | −2.3 | 0 | −3.6 | 1.1 | 0.4 | |
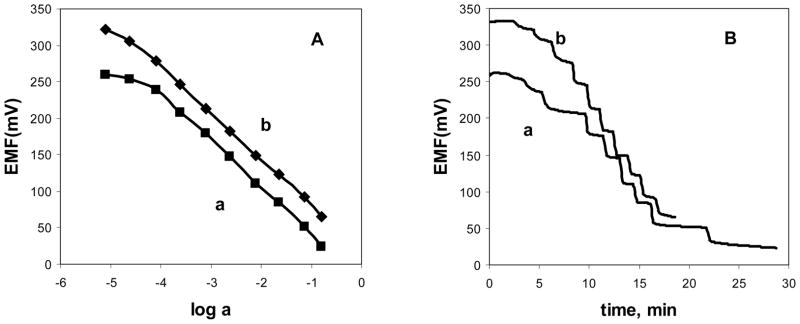
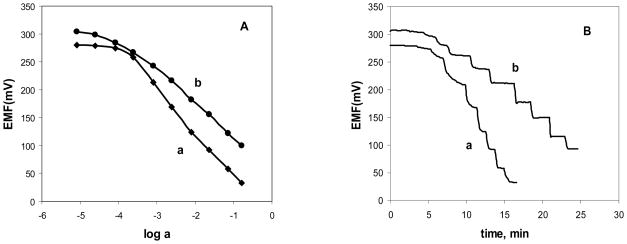
Typical calibration curves for freshly prepared Co(III)-porphyrin based solid-contact nitrite electrodes, fabricated with graphite paste/mineral oil and graphite paste/o-NPOE supports are shown in Fig. 3A. Both types of electrodes show near-Nernstian potentiometric response towards NO2− anion (−63 mV dec−1 for curve a) and −62 mV dec−1 for b) over a broad concentration range (10−4 M to 10−1 M). Response times are rather rapid (t95 < 60 sec) at concentrations > 10−4 M (Fig. 3B), and the EMF values are fully reversible, even for electrodes that are almost a month old (see Fig. 4). For electrodes prepared with the same supports, modified with ion-selective membrane doped with Al(III)-porphyrin, somewhat higher detection limits for the analyte ion (F−) were registered (>10−3 M), as it can be seen in Fig. 5. Additionally, the so-called Hulanicki effect [30] was observed for graphite paste/o-NPOE electrodes, as the slopes of F− calibration curves were super-Nernstian (−80 mV dec−1) at lower analyte activities, with simultaneous sluggish dynamic response. At higher fluoride activities, fast and Nernstian potentiometric response was observed (−63 mV dec−1).
Fig. 3.
Calibration curves (A) and dynamic response (B) toward nitrite in MES buffer solution, pH 5.5. Electrodes with 33 wt% PVC, 66 wt% of o-NPOE as plasticizer, 1 wt% Co(III)-TPPAc and 10 mol% (relative to the ionophore) TDMACl membranes, prepared with different transducers: a) graphite paste/oNPOE b) graphite paste/mineral oil
Fig. 4.
Reversibility of EMF response toward nitrite in MES buffer, pH 5.5. Electrode with 33 wt% PVC, 66 wt% of oNPOE as plasticizer, 1 wt% Co(III) TPPAc and 10 mol% (relative to the ionophore) TDMACl membranes, prepared with graphite paste/mineral oil transducer.
Fig. 5.
Calibration curves (A) and dynamic response (B) for fluoride in Gly/H3PO4 buffer solution, pH 3.0. Electrodes with 33 wt% PVC, 66 wt% of oNPOE as plasticizer, 1 wt% Al(III)-tBuTPPCl and 10 mol% (relative to the ionophore) TDMACl membranes, prepared with different transducers: a) graphite paste/oNPOE b) graphite paste/mineral oil
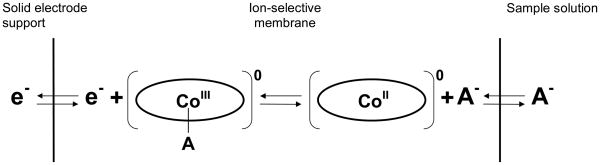
The possibility of the metalloporphyrins (e.g., Co(III)-tetraphenylporphyrin) also participating in a reversible redox reaction at the underlying solid conductor was also considered as a possible explanation of good EMF stability for sensors based on carbon paste supports. The schematic representation of such a possible ion/electron equilibrium for the electrode based on solid conductor, modified with membranes containing a Co(III)-porphyrin ionophore, is shown in Fig. 6. Such direct redox reaction for ionophore can only be considered if there is no aqueous layer beneath the polymeric membrane, as the presence of such layer would not allow the lipophilic ionophore within the polymer film to exhibit a reversible electron transfer equilibrium with the solid-contact conductor. Based on the stable NO2− response of graphite paste electrodes, it might be supposed that the membrane/electrode support interface is well defined. However, similar data were also recorded for analogous electrodes based on Al(III)-porphyrin, which is not redox-active. Thus, further fundamental experiments related to this issue must be carried out before clear-cut conclusions can be drawn.
Fig. 6.
The schematic representation of the possible ion (A−)/electron (e−) equilibrium for electrode based on solid conductor, modified with membranes containing Co-porphyrin.
Conclusions
Based on the results presented above, it can be concluded that the formation of an aqueous layer underneath a polymeric ion-selective membrane depends strongly on the lipophilicity of solid-contact conducting support employed to prepare the solid-contact ion-selective electrodes. Formation of such layer was observed for electrodes prepared with graphite and graphite paste with o-NPOE and DOS as the organic binding material. Some unfavorable response parameters related to the presence of aqueous layer were observed for these electrodes, including instability of EMF values. However, the compatibility of PVC plasticized membrane and electrode support is also an important factor in improving electrode EMF stability, as it was shown for graphite paste modified with o-NPOE. The possibility of facilitating ion-to-electron charge transfer by the Co(III)-tetraphenylporphyrin was also tested, however, further experiments are necessary to fully explain the results observed.
Acknowledgments
The authors gratefully acknowledge the Ministry of Science and Higher Education (N N204 0294 33) and Warsaw University of Technology for financial support of this work. We also thank the National Institutes of Health for partial support of this work (grant # EB-000784)
References
- 1.Cattrall RW, Freiser H. Anal Chem. 1971;43:1905. doi: 10.1021/ac60350a026. [DOI] [PubMed] [Google Scholar]
- 2.James H, Carmack G, Freiser H. Anal Chem. 1972;44:856. doi: 10.1021/ac60312a046. [DOI] [PubMed] [Google Scholar]
- 3.Ruzicka J, Lamm CG, Tjell JC. Anal Chim Acta. 1972;62:15. doi: 10.1016/s0003-2670(01)95648-x. [DOI] [PubMed] [Google Scholar]
- 4.Kalcher K, Kauffmann J-M, Wang J, Svancara I, Vytras K, Neuhold C, Yang Z. Electroanal. 1995;7:5. [Google Scholar]
- 5.Wang J. Analytical Electrochemistry. Wiley-VCH; 2000. p. 115. [Google Scholar]
- 6.Buck RP, Freiser H. Ion Selective Electrodes in Analytical Chemistry. Vol. 1. Plenum; New York: 1978. p. 58. [Google Scholar]
- 7.Hauser PC, Chiang, David WL, Graham AW. Anal Chim Acta. 1995;302:241. [Google Scholar]
- 8.Bobacka J, Lindfors T, McCarrick M, Ivaska A, Lewenstam A. Anal Chem. 1995;67:3819. [Google Scholar]
- 9.Dybko A, Zachara J, Golimowski J, Wróblewski W. Anal Chim Acta. 2003;485:103. [Google Scholar]
- 10.Gyurcsányi RE, Rangisetty N, Clifton S, Pendley BD, Lindner E. Talanta. 2004;63:89. doi: 10.1016/j.talanta.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Fibbioli M, Bandyopadhyay K, Liu SG, Echegoyen L, Enger O, Diederich F, Gingery D, Bühlmann P, Persson H, Suter UW, Pretsch E. Chem Mater. 2002;14:1721. [Google Scholar]
- 12.Grygołowicz-Pawlak E, Wyglądacz K, Sęk S, Bilewicz R, Brzózka Z, Malinowska E. Sens Acuators B. 2005;111:310. [Google Scholar]
- 13.Grygolowicz-Pawlak E, Plachecka K, Brzozka Z, Malinowska E. Sens Actuators B. 2007;123:480. [Google Scholar]
- 14.Lai C-Z, Fierke MA, Stein A, Bühlmann P. Anal Chem. 2007;79:4621. doi: 10.1021/ac070132b. [DOI] [PubMed] [Google Scholar]
- 15.Liu D, Meruva RK, Brown RB, Meyerhoff ME. Anal Chim Acta. 1996;321:173. [Google Scholar]
- 16.Lutze O, Ravi KM, Ramamurthy AFN, Brown RB, Hower R, Meyerhoff ME. Fresenius J Anal Chem. 1999;364:41. [Google Scholar]
- 17.Decker M, Cammann K. Sens Actuators B. 1994;19:359. [Google Scholar]
- 18.Zachara JE, Toczyłowska R, Pokrop R, Zagórska M, Dybko A, Wróblewski W. Sens Actuators B. 2004;101:207. [Google Scholar]
- 19.Górski Ł, Malinowska E, Parzuchowski P, Zhang W, Meyerhoff ME. Electroanal. 2003;15:1229. [Google Scholar]
- 20.Steinle ED, Schaller U, Meyerhoff ME. Anal Sci. 1998;14:79. [Google Scholar]
- 21.Malinowska E, Górski Ł, Meyerhoff ME. Anal Chim Acta. 2002;468:133. [Google Scholar]
- 22.Mitchell-Koch JT, Pietrzak M, Malinowska E, Meyerhoff ME. Electroanal. 2006;18:551. [Google Scholar]
- 23.Wygladacz K, Malinowska E, Jazwinski J, Brzózka Z, Sens Actustors B. 2002;83:109. [Google Scholar]
- 24.Górski Ł, Malinowska E. Anal Chim Acta. 2005;540:159. doi: 10.1016/j.aca.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 25.Pietrzak M, Meyerhoff ME, Malinowska E. Anal Chim Acta. 2007;596:201. doi: 10.1016/j.aca.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alder AD, Longo FR, Kampas F, Kim J. J Inorg Nucl Chem. 1970;32:2443. [Google Scholar]
- 27.Sugimoto H, Ueda N, Mori M. Bull Chem Soc Jpn. 1981;54:3425. [Google Scholar]
- 28.Fibbioli M, Morf WE, Badertscher M, de Rooij NF, Pretsch E. Electroanal. 2000;12:1286. [Google Scholar]
- 29.Lindfors T, Ervelä S, Ivaska A. J Electroanal Chem. 2003;560:69. [Google Scholar]
- 30.Hulanicki A, Lewandowski R. Chem Anal (Warsaw) 1974;19:53. [Google Scholar]