Abstract
Biological radioisotope studies suffer from a lack of sensitive measurement techniques and therefore traditionally require large amounts of labeled material to produce a measurable signal. Such quantities of materials are often significantly higher than naturally-occurring levels preventing these studies from replicating physiological conditions. AMS affords the sensitivity necessary to perform biological radioisotope studies with low levels of labeled material that preserve physiological conditions. The choice of labeled material can substantially affect the ease of interpretation and comprehensiveness of these studies. Here, the benefits and limitations of whole-cell labeling with 14C-glucose and targeted pathway labeling with 14C-nicotinic acid are discussed and compared.
Keywords: AMS, Biology, Cell Labeling, Glucose, HPLC, Metabolism, NAD, Yeast
1. Introduction
The study of biological systems using radioisotope tracers has been limited by the insensitivity of detection instruments such as liquid scintillation counters. Accelerator mass spectrometry (AMS) provides an increase of six orders of magnitude over such conventional decay counting methods. Such an increase in sensitivity allows biological measurements to be made with low levels of radiolabeled material that do not alter the physiological conditions of the system. It has been well established that the pharmacokinetics and biological effects of certain compounds varies with high and low dosages in mammals [1–12]. Similarly, the introduction of a high dosage of radiolabeled material into a cell can alter the cell’s activity, performance, and ultimately the observed effect of the compound on the cell. Physiologically relevant dosages in cell-based studies preserve natural and normal conditions for cell growth and function and provide more relevant data.
To label cells for biological AMS studies, a radiolabeled molecular precursor must be introduced to the cells via cell growth media. Some precursors, such as amino acids, can be taken into the cells and immediately used as building blocks for biosynthesis. Other precursors are transported into the cell and undergo a series of conversions, collectively termed metabolism, that alter the molecular structure of the parent compound into molecules that are suitable for biosynthesis. Because such molecules are broken down within the cell, the position of the 14C label on the precursor is important as a label in one position may result in a loss of signal for a specific downstream product [13].
Equally important to BioAMS studies is the choice of precursor for labeling. Carbon sources such as glucose are metabolized into hundreds of major products within a cell. Assuming that the 14C label is carried into most of these products, the total 14C signal is spread out over a large number of metabolites. The separation of these metabolites into individual 14C-containing metabolite pools using high performance liquid chromatography (HPLC) can be convoluted in this case.
An alternative to whole-cell labeling using carbon sources is specific pathway labeling using precursors with limited, if any, intracellular metabolism. For instance, nicotinamide adenine dinucleotide (NAD) synthesis can occur through a limited number of metabolic processes from an extracellular precursor. NAD and its reduced form, NADH, are small molecules present in all cells that play critical roles in the generation of ATP for protein, DNA, and RNA synthesis and also act as a coenzyme and substrate for enzymatic reactions. Nicotinic acid, an extracellular precursor for NAD and NADH biosynthesis, is broken down into only a handful of components within a cell, all of which are related to the biosynthesis of NAD and NADH [14–16]. By introducing 14C-labeled nicotinic acid into a cellular system, only a handful of specific metabolites will be labeled making HPLC separation simpler. UV detection of these metabolites can be used to quantitate total cellular levels of each metabolite while AMS provides quantitative information about the biosynthetic origin of these metabolites.
We have grown cells in either 14C-glucose or 14C-nicotinic acid for the purpose of comparing the labeling strategies. The amount of label required to produce a reasonable signal for quantitation, AMS trace analysis, and the applicability of each strategy for bioAMS studies is discussed.
2. Methods
14C-nicotinic acid labeling studies
Yeast were cultured and metabolites extracted according to previously published methods [17]. Briefly, saccharomyces cerevisiae (BY4742) cultures were grown to mid-log phase in synthetic complete media supplemented with 1.84 pCi/mL (50 mCi/mmol) 14C-nicotinic acid (Moravek Biochemicals, Brea, CA). Cells were bead beaten in 50-mM ammonium acetate saturated with N2 gas and beads washed with a solution of acetonitrile and 50-mM ammonium acetate (3:1 v/v). Metabolites were extracted using ice-cold chloroform. Samples were lyophilized overnight and resuspended in 50-mM ammonium acetate. Aliquots were then analyzed by reverse phase HPLC using an Agilent 1100 HPLC using a Hydrosphere C18. UV absorbance was monitored at 260 nm and 340 nm and fractions were collected for AMS analysis.
14C-glucose labeling studies
Four yeast cells grown in yeast-peptone-dextrose (YPD) media supplemented with 0.5 mCi/ml (264 mCi/mmol) 14C-glucose (Sigma, St. Louis, MO) were added to approximately 107 unlabeled yeast cells grown to mid-log phase in YPD media. Cells were bead beaten in N2-saturated, ice-cold acetonitrile and 10-mM KH2PO4 (3:1 v/v). Proteins were removed by centrifugation and chloroform extraction. Samples were dried down using centrifugal vacuum evaporation. Metabolites were separated on an LC Packings UltiMate™ Micro-LC System with a C18 column. Absorbance was monitored at 208 nm, 258 nm and 340 nm. Fractions were collected for AMS analysis.
AMS Analysis
For AMS analysis, carbon carrier was added to HPLC fractions and fractions were dried down using centrifugal vacuum evaporation. Fractions were prepared as previously described [18] and the 14C content measured using the 1-MV AMS spectrometer at the Center for Accelerator Mass Spectrometry [19].
3. Results and Discussion
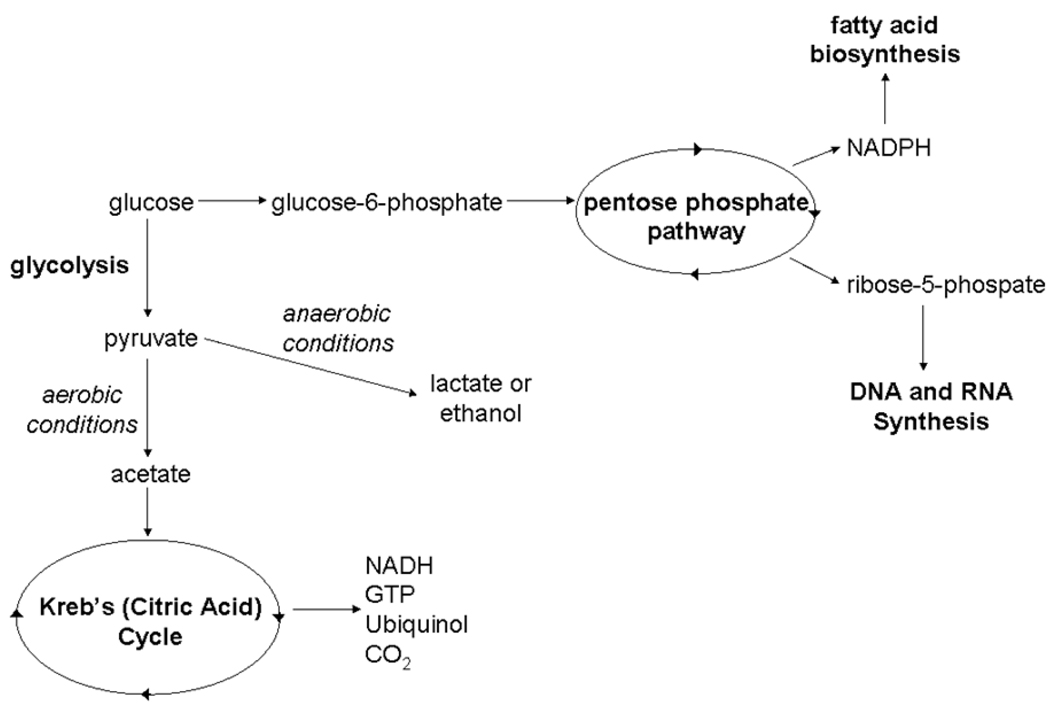
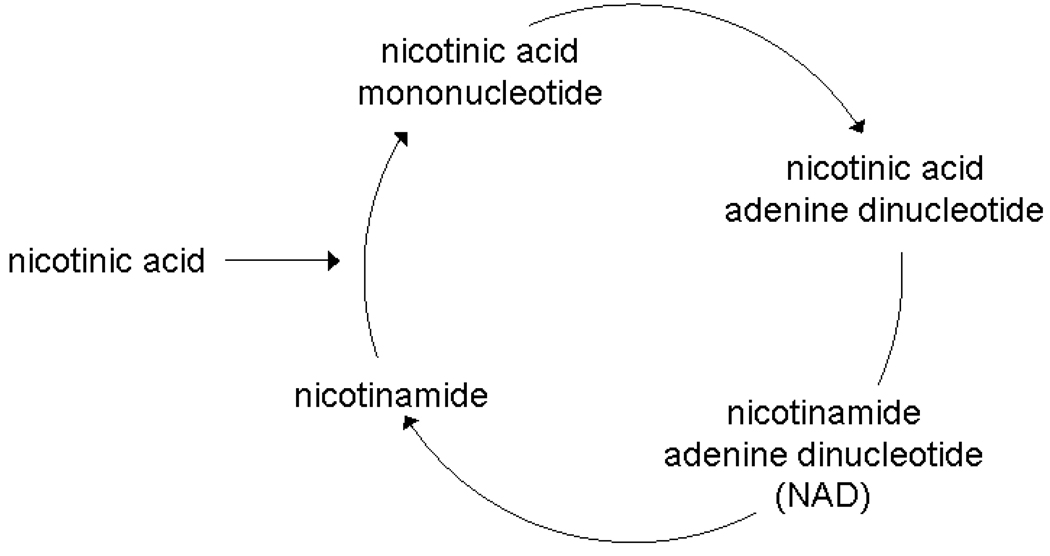
As a major carbon source for yeast, glucose is metabolized through several pathways ultimately resulting in carbohydrates, nucleic acids, and other cell components critical for growth (Figure 1a). Addition of ubiquitously labeled 14C-glucose to cell growth media ensures that all metabolites involved in these pathways contain a 14C label allowing for AMS quantitation. The greatest limitation in the quantitation of these labeled metabolites is the ability to adequately separate each metabolite using HPLC. In contrast to glucose metabolism, nicotinic acid metabolism does not involve a large number of metabolites (Figure 1b). Because these metabolites possess similar structural and chemical characteristics, HPLC separation of each metabolite in this pathway is simplified. Peaks in AMS traces resulting from cells grown in 14C-nicotinic acid are more defined and easier to interpret than peaks from AMS traces for cells grown in 14C-glucose.
Figure 1.
Metabolic fates for glucose and nicotinic acid in yeast. Figure 1a:. A partial, simplified view of glucose metabolism in yeast. Glucose can be broken down into most major components of a cell and even given off as carbon dioxide. Ubiquitously labeled 14C-glucose will label most metabolites in these pathways. Major metabolite pathways are abbreviated and in bold. Figure 1b: Nicotinic acid metabolism in yeast is limited to four metabolites. 14C-nicotinic acid will only label these metabolites creating a very simple AMS trace.
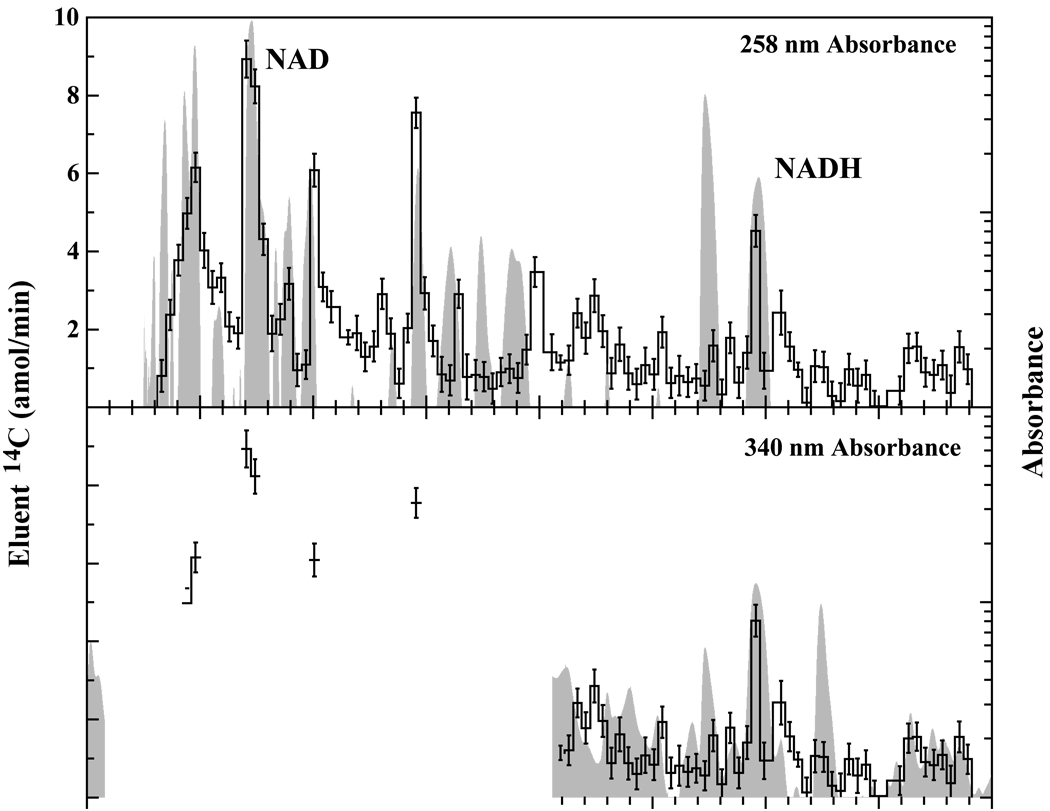
When labeling cells using a 14C-precursor for metabolism, it is crucial that the metabolic fate of the precursor be well-characterized. Metabolites derived from 14C-glucose number in the hundreds, resulting in a high number of 14C-label metabolites dispersed throughout the cell. Extracts from cells grown in 14C-glucose were separated by HPLC and peak identification was accomplished by comparing the retention times of peaks with retention times determined for known standards. Identification of metabolites associated with 14C peaks measured by AMS was accomplished by overlaying the HPLC UV-absorbance trance and the 14C AMS trace. When labeled with 14C-glucose, yeast produce a large number of 14C-labeled metabolites making identification of individual peaks difficult even when HPLC and AMS traces are overlayed (Figure 2). Additionally, because metabolite detection is accomplished using specific absorbances and because some metabolites do not absorb light at these wavelengths, the HPLC trace may not show certain 14C-containing metabolites making identification of these peaks only possible with other qualitative techniques such as mass spectroscopy. Alternatively, some metabolites that appear on the HPLC trace may not contain 14C and will not appear on the AMS trace. Improvements can be made in AMS peak identification for those peaks appearing in HPLC samples by adjusting the buffer concentrations and gradients used for HPLC separation. These modifications will ultimately alter metabolite elution times and greater separation of metabolites can be achieved.
Figure 2.
HPLC-UV trace (gray) overlaid by 14C-AMS trace (black lines) for wild type yeast grown in 14C-glucose. The multitude of peaks containing a 14C label is a result of the large number of metabolites involved in glucose metabolism within yeast. Such traces are difficult to interpret due to insufficient separation and co-elution of peaks.
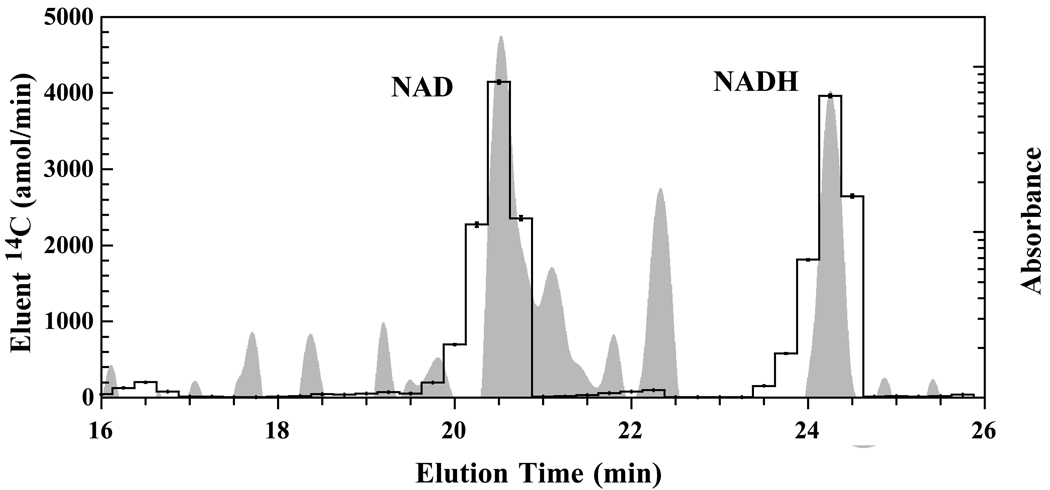
When labeling cells with a precursor for a specific pathway, only the metabolites in that targeted pathway will be labeled resulting in a much simpler 14C-AMS trace with fewer peaks. For 14C-nicotoinic acid-labeled cells, only 2 distinct and 3 minor peaks are visible in the AMS trace (Figure 3). Identification of these peaks is uncomplicated since alignment of the HPLC and AMS traces is straightforward. AMS peaks correspond only to metabolites generated by the NAD salvage pathway for regeneration of degraded NAD metabolites and NAD generation from nicotinic acid. Many non-14C-containing metabolites are present in the HPLC trace indicating that the metabolite extract contains metabolites that are not part of the NAD salvage pathway.
Figure 3.
HPLC-UV trace (gray) overlaid by 14C-AMS trace (black lines) for wild type yeast grown in 14C-nicotinic acid. Only metabolites associated with the NAD salvage pathway are labeled by 14C from nicotinic acid. Specific pathway labeling provides simpler AMS traces with fewer labeled peaks.
There are many advantages and disadvantages to general and targeted labeling of biological samples. General labeling using carbon sources, such as glucose, can result in an overwhelming number of AMS peaks that are difficult to distinguish. However, if adequate separation of peaks can be achieved, then this labeling strategy can be of great use for looking at whole-cell metabolism rates. Targeted pathway labeling using specific precursors, like nicotinic acid, cannot provide as much whole-cell information as general labeling strategies. Conversely, targeted labeling only provides information about specific metabolites present in the pathway of interest. This type of labeling strategy is useful for quantitating the usages of biosynthesis pathways and for measuring the movement of metabolites through these specific pathways.
4. Conclusions
The use of AMS in biology has largely been restricted to pharmacokinetic research although the applicability of AMS extends to basic biological research. The sensitivity of AMS allows biological studies to be conducted with amounts of labeled material that are negligible when compared to total unlabeled concentrations so that physiological conditions may be preserved. Because the application of AMS to basic cell biology is still being developed, several issues have arisen that require careful consideration. Here, we have addressed the choice of 14C-precursors for intracellular metabolite studies in yeast. Two main metabolite labeling strategies, general and targeted, are available for basic biological research studies in cells. While general labeling using 14C-labeled carbon sources provides the most extensive labeling of metabolites, results can be difficult to analyze because of co-eluting peaks. Targeted labeling does not suffer from co-eluting peaks and provides specific biosynthesis pathways but is limited in the number of metabolites available for AMS analysis.
Acknowledgements
This work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344 and was supported by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program (P41 RR013461).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Turteltaub KW, Vogel JS, Frantz C, Felton JS, McManus M. Princess Takamatsu Symp. 1995;23:93. [PubMed] [Google Scholar]
- 2.Turteltaub KW, Vogel JS, Frantz C, Buonarati MH, Felton JS. Environ. Health Perspect. 1993;99:183. doi: 10.1289/ehp.9399183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turteltaub KW, Mauthe RJ, Dingley KH, Vogel JS, Frantz CE, Garner RC, Shen N. Mutat. Res. 1997;376:243. doi: 10.1016/s0027-5107(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 4.Turteltaub KW, Dingley KH, Curtis KD, Malfatti MA, Turesky RJ, Garner RC, Felton JS, Lang NP. Cancer Lett. 1999;143:149. doi: 10.1016/s0304-3835(99)00116-0. [DOI] [PubMed] [Google Scholar]
- 5.Turteltaub KW, Dingley KH. Toxicol. Lett. 1998;102–103:435. doi: 10.1016/s0378-4274(98)00344-0. [DOI] [PubMed] [Google Scholar]
- 6.Mauthe RJ, Snyderwine EG, Ghoshal A, Freeman SP, Turteltaub KW. Carcinogenesis. 1998;19:919. doi: 10.1093/carcin/19.5.919. [DOI] [PubMed] [Google Scholar]
- 7.Mauthe RJ, Dingley KH, Leveson SH, Freeman SP, Turesky RJ, Garner RC, Turteltaub KW. Int. J. Cancer. 1999;80:539. doi: 10.1002/(sici)1097-0215(19990209)80:4<539::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Lightfoot TJ, Coxhead JM, Cupid BC, Nicholson S, Garner RC. Mutat. Res. 2000;472:119. doi: 10.1016/s1383-5718(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 9.Lang NP, Nowell S, Malfatti MA, Kulp KS, Knize MG, Davis C, Massengill J, Williams S, MacLeod S, Dingley KH, Felton JS, Turteltaub KW. Cancer Lett. 1999;143:135. doi: 10.1016/s0304-3835(99)00142-1. [DOI] [PubMed] [Google Scholar]
- 10.Frantz CE, Bangerter C, Fultz E, Mayer KM, Vogel JS, Turteltaub KW. Carcinogenesis. 1995;16:367. doi: 10.1093/carcin/16.2.367. [DOI] [PubMed] [Google Scholar]
- 11.Dingley KH, Roberts ML, Velsko CA, Turteltaub KW. Chem. Res. Toxicol. 1998;11:1217. doi: 10.1021/tx9801458. [DOI] [PubMed] [Google Scholar]
- 12.Bacon JR, Williamson G, Garner RC, Lappin G, Langouet S, Bao Y. Carcinogenesis. 2003;24:1903. doi: 10.1093/carcin/bgg157. [DOI] [PubMed] [Google Scholar]
- 13.Sauer U. Mol. Syst. Biol. 2006;2:62. doi: 10.1038/msb4100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempel W, Rabeh WM, Bogan KL, Belenky P, Wojcik M, Seidle HF, Nedyalkova L, Yang T, Sauve AA, Park HW, Brenner C. PLoS Biol. 2007;5:e263. doi: 10.1371/journal.pbio.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belenky P, Racette FG, Bogan KL, McClure JM, Smith JS, Brenner C. Cell. 2007;129:473. doi: 10.1016/j.cell.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Katoh A, Hashimoto T. Front. Biosci. 2004;9:1577. doi: 10.2741/1350. [DOI] [PubMed] [Google Scholar]
- 17.Sporty JL, Kabir MM, Turteltaub KW, Ognibene T, Lin SJ, Bench G. J. Sep. Sci. 2008;31:3202. doi: 10.1002/jssc.200800238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ognibene TJ, Bench G, Vogel JS, Peaslee GF, Murov S. Anal. Chem. 2003;75:2192. doi: 10.1021/ac026334j. [DOI] [PubMed] [Google Scholar]
- 19.Ognibene TJ, Bench G, Brown TA, Peaslee GF, Vogel JS. Int. J. Mass. Spectrom. 2002;218:255. [Google Scholar]