Abstract
MicroRNA are small noncoding transcripts involved in many cellular mechanisms, including tumorigenesis. miR-210, in particular, is induced by hypoxia and correlates with adverse outcomes in certain cancers. Because pancreatic adenocarcinomas exhibit extremely hypoxic signatures, we hypothesized that miR-210 may serve as a diagnostic marker for screening or surveillance for pancreatic cancer. Plasma samples were obtained from newly diagnosed pancreatic cancer patients and age-matched noncancer controls. miRNA was extracted directly from plasma and reverse-transcribed to complementary DNA. A known quantity of synthetic Caenorhabditis elegans miR-54 (celmiR-54) was added for normalization. miR-210 and cel-miR-54 were then measured using quantitative reverse transcription polymerase chain reaction. An initial cohort of 11 pancreatic cancer patients and 14 age-matched controls was used as the test set and a second cohort of 11 pancreatic cancer patients and 11 controls was used as the validating set in this study. miR-210 was reliably detected and quantified, with a statistically significant four-fold increase in expression in pancreatic cancer patients compared with normal controls (P < .00004) in the test set. This difference was confirmed in the validation group (P < .018). In summary, circulating miR-210 levels are elevated in pancreatic cancer patients and may potentially serve as a useful biomarker for pancreatic cancer diagnosis.
Introduction
Pancreatic cancer remains one of the most lethal malignancies, with most cases diagnosed after metastatic spread. Median survival in patients with locally advanced/metastatic disease is less than 12 months, with overall 5-year survival less than 5% despite aggressive multimodality treatment [1]. Tumor markers may facilitate earlier diagnosis and have the potential for use in monitoring response to cancer therapies, but there is no current biomarker that reliably serves this purpose. There is, therefore, a tremendous need to identify novel noninvasive biomarkers for early tumor detection.
MicroRNA (miRNA), a class of naturally occurring, noncoding small RNA, regulate the expression of most genes. On a molecular level, miRNA destabilize messenger RNA by repressing translation and shortening the polyA tail. On a cellular level, miRNA play critical roles in differentiation, proliferation, apoptosis, and metabolism and have ultimately been linked to cancer development [2]. Their association with tumorigenesis has given rise to their potential for clinical diagnosis and as therapeutic targets.
Hypoxia, or low oxygen, is an essential feature of the tumor microenvironment, particularly in pancreatic cancer [3]. Cancers with increased hypoxia exhibit poorer prognosis and greater resistance to chemotherapy and radiation. Hypoxia has also been demonstrated to induce differential miRNA expression [4–7]. Several investigators have reported that miR-210, in particular, is increased in response to hypoxia [5,6,8,9] and may in fact be the principal miRNA expressed in a number of different cancer types through a hypoxia-responsive element [4]. Interestingly, miR-210 induction is regulated by hypoxia-inducible factor 1α (HIF-1α), and it serves as an important regulator for inhibiting DNA repair pathways and promoting genomic instability [10]. Furthermore, miR-210 facilitates the mRNA degradation of normoxic genes, providing another mechanism of HIF-regulated gene expression [4].
In the present study, we assessed the potential of circulating miR-210 to function as a diagnostic marker for pancreatic cancer. On the basis of previous work by Mitchell et al. [11] and Chen et al. [12], we have developed a plasma-based assay that reliably quantifies miR-210 from archived patient plasma samples. Here we report that plasma miR-210 expression from patients with newly diagnosed locally advanced pancreatic adenocarcinomas was significantly elevated in comparison to age-matched controls, using a test and a validating set.
Patients and Methods
Cohorts
Plasma samples for pancreatic cancer patients and controls in cohort 1 were collected and archived between June 2006 and January 2008, whereas plasma samples for cohort 2 were collected and archived between March 2005 and October 2006. All samples were collected before any treatment, and miR-210 levels were analyzed retrospectively from these groups. All patients were identically staged with pancreatic protocol computed tomographic scans and evaluated at the Stanford gastrointestinal multidisciplinary tumor board. All patients had locally advanced, unresectable stage T4 disease. Recurrent malignancies were excluded from the study. The protocol described was first tested on a cohort of 11 pancreatic cancer patients and 14 age-matched healthy controls (cohort 1; Table 1). A second validation set of 11 pancreatic cancer patients and 11 age-matched healthy controls (cohort 2; Table 2) was used to confirm our initial results and to achieve greater statistical power. There was no statistically significant difference in plasma miR-210 levels between the two control groups (2-tailed t-test, P = .22).
Table 1.
Cohort 1.
| Cohort 1 | Patient ID | Age (years) | Location | CA 19-9 (U/ml) | miR-210 Expression (Mean ± SD) |
| Pancreas CA | P1 | 85 | Head | 24 | 1.41 ± 0.16 |
| P2 | 60 | Head | 243 | 2.37 ± 0.70 | |
| P3 | 60 | Head | 235 | 4.46 ± 2.04 | |
| P4 | 68 | Head | 4514 | 1.82 ± 0.50 | |
| P5 | 65 | Head | 1270 | 0.51 ± 0.12 | |
| P6 | 57 | Head | <1 | 1.57 ± 0.13 | |
| P7 | 60 | Body | 66 | 3.53 ± 0.08 | |
| P8 | 63 | Head | 1 | 1.43 ± 0.14 | |
| P9 | 51 | Body | 168 | 3.31 ± 0.69 | |
| P10 | 79 | Body | 327 | 1.20 ± 0.31 | |
| P11 | 46 | Head | 40 | 1.77 ± 0.18 | |
| Controls | C1 | 64 | 0.44 ± 0.49 | ||
| C2 | 59 | 0.64 ± 0.48 | |||
| C3 | 58 | 0.48 ± 0.51 | |||
| C4 | 61 | 0.55 ± 0.68 | |||
| C5 | 49 | 0.24 ± 0.48 | |||
| C6 | 58 | 0.45 ± 0.26 | |||
| C7 | 66 | 0.53 ± 0.19 | |||
| C8 | 62 | 0.54 ± 0.12 | |||
| C9 | 55 | 0.17 ± 0.14 | |||
| C10 | 58 | 0.46 ± 0.06 | |||
| C11 | 51 | 0.42 ± 0.03 | |||
| C12 | 61 | 0.68 ± 0.10 | |||
| C13 | 62 | 0.81 ± 0.15 | |||
| C14 | 60 | 0.82 ± 0.04 |
Table 2.
Cohort 2.
| Cohort 2 | Patient ID | Age (years) | Location | CA 19-9 (U/ml) | miR-210 Expression (Mean ± SD) |
| Pancreas CA | P12 | 52 | Head | 8320 | 1.27 ± 0.04 |
| P13 | 77 | Head | 88 | 1.80 ± 0.11 | |
| P14 | 43 | Head | 2950 | 0.69 ± 0.07 | |
| P15 | 58 | Body | <1 | 1.67 ± 0.00 | |
| P16 | 54 | Body | 7.1 | 2.69 ± 0.13 | |
| P17 | 60 | Head | 568 | 2.23 ± 0.11 | |
| P18 | 59 | Body | 1329 | 0.57 ± 0.02 | |
| P19 | 56 | Head | 16.7 | 1.58 ± 0.06 | |
| P20 | 69 | Head | 26 | 0.77 ± 0.00 | |
| P21 | 62 | Head | <3 | 0.68 ± 0.05 | |
| P22 | 64 | Head | 11.6 | 1.65 ± 0.04 | |
| Controls | C15 | 40 | 1.10 ± 0.02 | ||
| C16 | 65 | 0.76 ± 0.10 | |||
| C17 | 55 | 0.35 ± 0.00 | |||
| C18 | 51 | 0.95 ± 0.02 | |||
| C19 | 60 | 0.55 ± 0.06 | |||
| C20 | 44 | 0.97 ± 0.07 | |||
| C21 | 58 | 1.11 ± 0.04 | |||
| C22 | 64 | 1.02 ± 0.02 | |||
| C23 | 46 | 0.62 ± 0.00 | |||
| C24 | 44 | 1.06 ± 0.06 | |||
| C25 | 45 | 0.76 ± 0.09 |
Plasma Extraction
Five to ten milliliters of whole blood was obtained from each patient. Plasma was extracted by centrifuging whole blood at 3000 rpm for 10 minutes at room temperature and then frozen as separate aliquots at -80°C for storage. One hundred microliters of thawed plasma was boiled for 10 minutes at 100°C and then centrifuged at 13,000g for 10 minutes at 4°C. Approximately 1.1 µl of supernatant was collected.
Complementary DNA Production
To normalize by volume, synthetic Caenorhabditis elegans miR-54 (cel-miR-54) (Integrated DNA Technologies, Coralville, IA) served as a control. cel-miR-54 has previously been shown not to affect human miRNA detection [11]. Complementary DNA was generated by mixing the extracted RNA (1.1 µl) with 5x miR-210 primer (1.0 µl), 5x cel miR-54 primer (0.9 µl), 0.25 nM cel miR-54 oligo (0.1 µl), 5x First Strand buffer (1.0 µl), dithiothreitol (0.5 µl), 25 mM dNTP (0.2 µl), RNAse Out Inhibitor (0.1 µl), and reverse transcriptase (0.1 µl). This mixture was assayed on a standard polymerase chain reaction (PCR) thermal cycler at 16°C for 30 minutes, at 42°C for 30 minutes, at 85°C for 5 minutes, and then at 4°C afterward.
Quantitative Reverse Transcription-PCR
Quantitative reverse transcription-PCR (qRT-PCR) was performed using an ABI 7900 Thermal Cycler, with miR-54 and miR-210 quantified using TaqMan MicroRNA Assays (Applied Biosystems, Foster City, CA). Twenty-five microliters of dH2O was mixed with 5 µl of complementary DNA. Four microliters of this dilution was mixed with 2x Master Mix (5 µl), 20x PCR Primer (0.5 µl), and dH2O (0.5 µl) before being pipetted onto a 394-well plate. qRT-PCR was then performed at 50°C for 2 minutes, at 95°C for 10 minutes, at 95°C for 15 seconds, and at 60°C for 60 seconds, with the last two steps repeated for a total of 40 cycles. Each reaction was performed in triplicate.
Standard curves were generated for miR-54 and miR-210 out to 1:1024 dilutions. Ct values thresholds were set at 0.1.
MicroRNA Stability in Plasma
Samples were subjected to a number of harsh conditions, and microRNA levels were subsequently quantified. Different plasma aliquots of the same sample were used for each condition. Conditions encompassed doubling the boiling time to 20 minutes, incubating at room temperature (at 4-, 12-, and 24-hour intervals), and undergoing multiple freeze/thaw cycles between -80°C storage and room temperature. qRT-PCR was then performed, and raw Ct values of miR-54 and miR-210 were compared with the standard protocol.
Carbohydrate Antigen 19-9 Assay
Two milliliters of blood was drawn from each pancreatic cancer patient before treatment, and the serum component was extracted. Carbohydrate antigen 19-9 (CA 19-9) was quantified by the Stanford Hospital Clinical Laboratory using the ADVIA Centaur 19-9 assay, a two-step sandwich immunoassay using chemiluminescence and 1116-NS-19-9 as a monoclonal antibody (Siemens Diagnostic Healthcare, Deerfield, IL).
Statistics
miR-210 levels were expressed as the mean of the triplicate Ct values on each qRT-PCR run. Normalized values were generated using the ΔΔCt method. Paired Student's t test was used to evaluate the difference between pancreatic cancer patients and normal controls as well between the two control groups. A difference was regarded as statistically significant for a 2-tailed P < .05. Simple regression analysis was used to correlate miR-210 and CA 19-9 levels.
Results
Detection and Quantification of miR-210 in Plasma
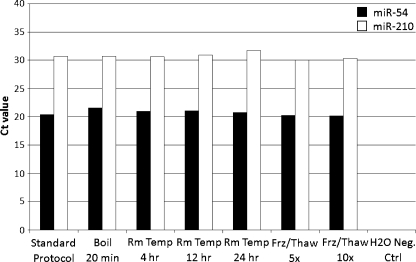
Although plasma is known to contain ribonuclease, we found that miRNA species were present and resistant to RNAse digestion. Moreover, the stability of plasma miRNA persisted even after subjecting samples to different conditions, including boiling, maintaining at room temperature for several hours, and multiple freeze-thaw cycles (Figure 1). The qRT-PCR analysis of miR-54 and miR-210 under these conditions yielded no significant differences compared with untreated plasma, indicating this protocol to be robust and reproducible.
Figure 1.
Plasma levels of miR-54 and miR-210 were found to be stable after being subjected to various harsh conditions. Boiling temperatures were at 100°C. Freeze-thaw cycles were at -80°C and room temperature, respectively, reflecting protocol and storage settings.
miR-210 Overexpression in Pancreatic Cancer
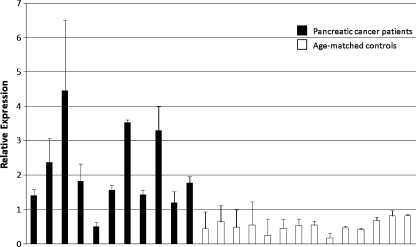
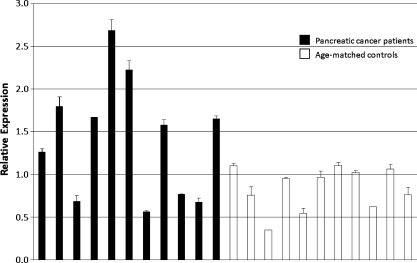
To assess levels of miR-210, plasma samples from human pancreatic cancer patients and age-matched controls were collected (cohorts 1 and 2). Pancreatic cancer was chosen as a model system due to the severe hypoxia present in these tumors [3]. In cohort 1, circulating miR-210, normalized to miR-54, was found to be significantly elevated compared with normal controls, using the ΔΔCt method (4.0x overexpression, P < .00004; Figure 2 and Table 1). A second set of pancreatic cancer patients and healthy controls (cohort 2) was independently analyzed to validate the first set's findings. Once again, significantly higher circulating miR-210 level was noted for cancer patients (1.7x overexpression, P < .018; Figure 3 and Table 2). The qRT-PCR results support the concept that miR-210 may serve as a reliable blood-based biomarker, using a minimum amount of sample (100 µl). The differences between miR-210 levels in the two control groups were not significantly different (P = .22).
Figure 2.
Cohort 1 pancreatic cancer patients exhibited significantly higher circulating miR-210 levels compared with healthy, age-matched controls in plasma (4.0x overexpression, P < .00004). Relative expression was calculated by 2-(Ct value - average Ct value).
Figure 3.
Cohort 2 pancreatic cancer patients demonstrated a similar elevation of miR-210 levels compared with age-matched controls (1.7x overexpression, P < .018). Relative expression was calculated by 2-(Ct value - average Ct value).
No correlation was identified between pretreatment CA 19-9 (Tables 1 and 2) and miR-210 levels in either cohort 1 or cohort 2. The overall correlation coefficient between miR-210 and CA 19-9 levels for all patients was 0.21 (P = .33).
Discussion
Altered miRNA expression has been reported in various cancers, and the dysregulation of tissue miRNA exhibits great potential for identifying malignancy. Hypoxic tumors in particular exhibit poor prognosis and resistance to conventional therapy, and miRNA likely mediate the diverse epigenetic mechanisms resulting in this phenotype [2]. miR-210, in particular, has garnered significant attention in relation to hypoxia and cancer: it is an important mediator of the endothelial cell response to low oxygen tension, and it has emerged as a near-universal responder to hypoxia [5]. Clinically, recent data by Camps et al. [9] show that miR-210 in human breast cancer is not only a marker of tumor hypoxia in vivo but also an indicator of adverse prognosis. We previously found that miR-210 is directly regulated byHIF-1α in a variety of tumor types through a hypoxia-responsive element [4]. Functional studies further suggest that miR-210 suppresses normoxic genes that are no longer necessary for adaptation and survival in a hypoxic environment [4].miR-210 has also been found to promote cell cycle progression by activating c-MYC through inhibition of a c-MYC antagonist [13].
Tissue-based diagnosis remains invasive and time-intensive. Serum and plasma have been extensively researched, yet such minimally invasive tests are still limited for use in tumor detection. Recent studies in prostate cancer by Mitchell et al. [11] and Chen et al. [12] have established that miRNA is stable and quantifiable in plasma. These remarkable properties remain unexplained, although hypotheses include modifications to their structure and associations with protective molecules such as exosomes [11]. In the present study on pancreatic cancer, we have developed a robust protocol that uses minimal plasma sample and is well suited for high-throughput analysis. To normalize sample-to-sample variation in RNA isolation, synthetic C. elegans miR-54 oligonucleotide was spiked into each sample and quantified. cel-miR-54 was chosen as a control based on findings that it does not cross-hybridize with probes for known human miRNA [11].
Using this protocol, we found that miR-210 level was significantly elevated in human pancreatic cancer plasma samples in two independent patient cohorts, 4.0-fold and 1.7-fold, respectively. We chose to evaluate miRNA in pancreatic cancer because the hypoxic tumor microenvironment plays a crucial role in the progression of these malignancies [3,14]. While this article was under review, Wang et al. [15] published similar findings regarding microRNA and pancreatic cancer, demonstrating that combined analyses of four different miRNA (including miR-210) in plasma could distinguish pancreatic cancer patients from healthy individuals. Notably, their extraction of miRNA was different from our methodology, requiring overnight incubation and column purification. Our conclusions corroborate the findings of Wang et al., further establishing miR-210 as a pancreatic cancer biomarker and substantiating the concept of plasma miRNA stability through a novel assay technique.
Our study noted no direct correlation between circulating miR-210 levels and CA 19-9, which was not unexpected because the two factors are presumably regulated by different mechanisms. Because sample size was modest and CA 19-9 was not measured in the control population, it was not feasible to determine the statistical contribution of miR-210 in addition to CA 19-9 for the detection of malignancy in our cohorts.
Drawbacks from our study include its small sample size and its retrospective nature. Nonetheless, the presence of elevated miR-210 in the plasma of pancreatic cancer patients suggests that this may be an important feature of this disease, which may further advance our understanding of the underlying pathogenesis of pancreatic cancer. Moreover, given the exquisite sensitivity of qRT-PCR, miR-210 levels could also facilitate earlier diagnosis and treatment of this malignancy. The abundance and stability of miRNA in plasma further indicate that these blood-based biomarkers have great potential for use to predict the clinical behavior of individual cancers and to monitor therapeutic responses.
Footnotes
This study was supported by PO1 CA67166 (A.C.K., Q.T.L., and X.H.) and R01 CA118582 (Q.T.L. and H.C.). The authors have no potential conflict of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Sassen S, Miska EA, Caldas C. MicroRNA: implications for cancer. Virchows Arch. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koong AC, Mehta VK, Le QT, Fisher GA, Terris DJ, Brown JM, Bastidas AJ, Vierra M. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys. 2000;48:919–922. doi: 10.1016/s0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible miR-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009;35:856–867. doi: 10.1016/j.molcel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivan M, Harris AL, Martelli F, Kulshreshtha R. Hypoxia response and microRNAs: no longer two separate worlds. J Cell Mol Med. 2008;12:1426–1431. doi: 10.1111/j.1582-4934.2008.00398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowland JB, Hother C, Gronbaek K. MicroRNAs and cancer. APMIS. 2007;115:1090–1106. doi: 10.1111/j.1600-0463.2007.apm_775.xml.x. [DOI] [PubMed] [Google Scholar]
- 8.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros D, Weber BL, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7:255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008;14:1340–1348. doi: 10.1158/1078-0432.CCR-07-1755. [DOI] [PubMed] [Google Scholar]
- 10.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant KC, Allen A, et al. Circulating micro-RNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Gou J, Zhang Y, Chen J, Guo X, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 13.Zhang AY, Obagi S. Diagnosis and management of skin resurfacing- related complications. Oral Maxillofac Surg Clin North Am. 2009;21:1–12. doi: 10.1016/j.coms.2008.11.002. v. [DOI] [PubMed] [Google Scholar]
- 14.Yokoi K, Fidler IJ. Hypoxia increases resistance of human pancreatic cancer cells to apoptosis induced by gemcitabine. Clin Cancer Res. 2004;10:2299–2306. doi: 10.1158/1078-0432.ccr-03-0488. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila Pa) 2009;2:807–813. doi: 10.1158/1940-6207.CAPR-09-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]