Abstract
We evaluated intratumor (IT) versus intravenous (IV) administration of the photosensitizer Pc 4 with respect to tumor photosensitizer concentration, specificity, and responses to irradiation. BALB/c mice bearing intradermal EMT6 tumors were given 0.3 mg/kg Pc 4 injected IT or IV through the tail vein. Photosensitizer concentration was evaluated by chloroform extraction and localization assessed by fluorescence imaging and spectroscopy in vivo. Tumors were irradiated at 667 nm, 50 mW/cm2, and 100 J/cm2. Cures were defined as no palpable tumor 90 days after irradiation. Tumor Pc 4 concentrations 1 hour after IT administration were 35,000-fold higher than measured 24 hours after IV administration (0.112 vs 0.317 x 10-5 µg Pc 4/mg tumor). Exquisite tumor selectivity was observed 1 hour after IT injection. Fluorescence imaging of freshly sectioned tumors revealed no regions devoid of sensitizer at this time point, with pixel intensities in a midline section within a factor of 3 of the peak intensity. For identical photosensitizer doses, IT administration significantly improved tumor responses to irradiation, with more than 70% of tumors cured with IT-Pc 4-PDT. In this model, IT-Pc 4 administration provides improved tumor control, greater selectivity, and opportunity for a short drug-light interval.
Introduction
Intravenous (IV) and topical routes of photosensitizer administration are widely used in clinical and preclinical applications of photodynamic therapy (PDT). Topical application of various formulations of the prodrug aminolevulinic acid (ALA) is routinely performed for superficial lesions in the skin [1]. ALA has also been administered orally for PDT of tumors not accessible to topical delivery [2,3] and for fluorescence-guided surgical tumor resection [4].
Direct intratumor (IT) delivery of photosensitizers has been investigated only sporadically, and the previous literature on this subject is relatively sparse and conflicted. Kostron et al. [5] compared intraperitoneal (IP) and IT injection of hematoporphyrin derivative (HPD) in subcutaneous and in intracerebral gliosarcomas in a rat model. They reported three- to four-fold increases in tritiated HPD at both tumor sites after IT versus IP administration. Selectivity was also enhanced significantly. The increased HPD concentrations in tumors after direct IT injection resulted in improved photodynamic inactivation of the tumor cells, as measured using in vivo and in vitro clonogenic assays. In a pair of articles published in 1988, Amano et al. [6] and Lin et al. [7] also reported results of experiments designed to evaluate IT delivery of HPD. Motivated primarily by the persistent skin photosensitivity that results from systemic administration of HPD and a number of other photosensitizers, these authors demonstrated HPD concentrations in a subcutaneous mouse bladder tumor line 3 to 15 times higher with IT relative to IP injection [6]. Increased tumor HPD concentrations did not, however, result in improved tumor cell killing under the conditions of this study [7], which was attributed to a possible lack of efficient tumor blood vessel photosensitization with IT administration. Gibson et al. [8] investigated IT delivery of Photofrin II in a transplanted subcutaneous rat mammary tumor. Photosensitizer levels were not measured directly. Using an in vivo - in vitro protocol, in which Photofrin was injected in vivo through an IT or IP route and tumor and liver mitochondria preparations were subsequently irradiated in vitro, activities of two mitochondrial enzymes were attenuated to a significantly greater degree when irradiation of tumor samples was performed 2 hours after IT injection. Irradiation of tumor and liver mitochondrial preparations at 2 hours after IT Photofrin injection showed tumor selectivity, with activities of enzymes in liver mitochondria 5- to 10-fold less susceptible to PDT-mediated damage. This study also showed increased efficacy of Photofrin PDT as measured by tumor growth delay when irradiation was performed 2 hours after Photofrin injection IT versus IP.
IT delivery of the photosensitizer methylene blue has received slightly more attention in the literature, in part because it is reduced to a colorless, photodynamically inactive form when injected systemically [9]. König et al. [10] showed significant reduction in tumor mass in small subcutaneous Ehrlich carcinomas in mice in response to methylene blue administered through several IT injections and multiple laser irradiations delivered in daily fractions. Two articles by Orth et al. [11,12] described the efficacy of IT-methylene blue-PDT in colorectal tumors in mice. Their latter article reported no evidence of tumor in 79% of mice 5 weeks after an initial PDT treatment, with a second methylene blue injection and irradiation performed at 2 weeks if the tumor had not been eradicated by the first treatment. Encouraging results were reported from a small pilot study in three esophageal cancer patients [13]. Methylene blue was injected directly into inoperable, recurrent carcinomas under endoscopic guidance, and laser irradiation was performed 1 hour later. After two treatments within a 2-week period, there were no adverse side effects and no macroscopic evidence of tumor.
Very recently, two reports have evaluated IT meso-tetrahydroxyphenyl chlorin (mTHPC, Foscan) administration in rodent models of breast [14] and brain [15] cancer. Both studies were motivated by the prolonged skin photosensitivity associated with this promising photosensitizer. D'Hallewin et al. [14] used the liposomal formulation, Foslip, and showed maximum IT mTHPC fluorescence and irradiation-induced tumor necrosis at a 24-hour drug-light interval. This surprisingly long optimal interval was attributed to concentration quenching within liposomes and a relatively long rate of redistribution, thus drawing attention to the importance of the sensitizer formulation. In an intracranial glioma model in rats, Mannino et al. [15] found comparable tumor uptake and tumor-to-normal tissue ratios after IP and IT Foscan delivery in an ethanol/ethylene glycol (40:60) vehicle but found that favorable results were obtained in the IT case with 20-fold lower administered photosensitizer dose and a shorter (4 hours) drug-light interval.
Despite some promising initial studies, IT photosensitizer administration has not been pursued systematically and it remains an understudied area with significant clinical potential. The purpose of our study was to perform the first investigation of the IT delivery of the second-generation photosensitizer Pc 4, a topical formulation of which has been evaluated in a phase 1 clinical trial for the treatment of cutaneous T-cell lymphoma [16]. Here we describe extremely high sensitizer concentrations, excellent tumor selectivity, and long-term cures using IT-Pc 4-PDT with a short 1-hour drug-light interval.
Materials and Methods
Animal and Tumor Model
Mouse mammary EMT6 tumors were initiated on the backs of female BALB/c mice by the intradermal (ID) injection of 106 cells. Animals were followed daily to track tumor growth and were fed exclusively on a chlorophyll-free diet prepared according to the recipe of Holmes et al. [17] to eliminate chlorophyll-derived fluorescence. Approximately 7 to 10 days after implantation when the tumors reached a volume of approximately 25 to 40 mm3, they were used for control, PDT treatment, or drug extraction studies. For in vivo imaging of host cell infiltration and perfusion, tumors were initiated by the injection of 5 x 105 EMT6 cells into the ID space of the ear pinna [18].
Photosensitizer Administration and Light Treatment
The silicon phthalocyanine photosensitizer, Pc 4 [16], was prepared as described previously [19]. Pc 4 was dissolved in a 50% ethanol- 50% Cremophor solution, and a stock concentration of 2.1 mg/ml was prepared. The stock was diluted in a ratio of 1:9 in 0.9% saline to a final concentration of 0.21 mg/ml. The volume for IT injection was approximately 35 µl, which was injected at a single point near the center of the tumor using a 29-gauge needle. For IV injection, 35 µl of 0.21 mg/ml was further diluted in 65 µl of 90% saline, 5% ethanol, and 5% Cremophor. To facilitate direct comparison, the dose of administered Pc 4 through either IT or IV route was maintained at 0.3 mg/kg. After 1 hour and 24 hours of drug-light intervals for IT and IV administration, respectively, the tumors were subjected to PDT irradiation using 667-nm light from a diode laser (Power Technology, Inc, Little Rock, AR). Light was delivered through a GRIN-lens-terminated multimode fiber (OZ Optics, Ottawa, Ontario, Canada), and the tumors were illuminated with a fluence of 100 J/cm2 at an irradiance of 50 mW/cm2. Controls included untreated ((-) drug, (-) light) animals and mice receiving only 0.3 mg/kg Pc 4 IT (no light).
Drug Extraction
Pc 4 levels in the tumor after IV and IT injection were quantified using chloroform extraction. To accomplish this, 0.3 mg/kg Pc 4 was injected either IT or IV. Either 1 hour (IT) or 24 hours (IV) after injection, the mouse was euthanized and the tumor was excised. For mice that received IT injection, the skin immediately adjacent to the tumor was also harvested. Tumors were weighed and subjected to chloroform extraction (D. Kessel, personal communication). First, tumors were homogenized in a tissue grinder containing 1 ml of Hank's balanced salt solution per 10 mg of tumor. The amount 0.8 ml of MeOH per 10 mg of tumor was added to the solution and vortexed; next, 0.8 ml of chloroform per 10 mg of tumor was added. The solution was then centrifuged at 227g for 10 minutes to allow for the separation of the aqueous and chloroform phases. The bottom layer consisting of the chloroform and the solubilized drug was transferred to a cuvette, and absorbance and fluorescence measurements were performed for IT and IV administration, respectively. The Pc 4 concentration was calculated from these measurements using a calibration curve generated from the absorbance and fluorescence peak amplitudes of known Pc 4 concentrations in the same solvent. Skin sections were frozen in liquid nitrogen for 30 minutes and powdered with a frozen pestle before homogenization. Otherwise, they were treated identically to tumor sections.
Photosensitizer Fluorescence Imaging and Spectroscopy
Photosensitizer distribution and degree of tumor localization were assessed through in vivo imaging. One hour after IT-Pc 4 injection, anesthetized mice were positioned on the stage of a stereofluorescence microscope (Model SMZ1 500; Nikon Instruments, Melville, NY) equipped with an Xcite illumination source (EXFO, Ontario, Canada) and a computer-controlled x-y translation stage (H101A ProScanII; Prior Scientific, Rockland, MA). Excitation of Pc 4 and fluorescence collection were accomplished using a custom filter cube (HQ560/120x; 635 DCXR; HQ645LPm, Chroma Technology, Rockingham, VT). Individual fields acquired using a 0.5x objective with a 0.75x magnification zoom corresponded to fields of view (FOVs) of 25.2 mm x 19 mm. Images of entire mice were constructed by translating the stage and stitching individual fields to create a montage (MetaVue 6.1; Molecular Devices, Sunnyvale, CA).
To examine the detailed spatial distribution of Pc 4 in the tumors and immediately adjacent normal skin, 1 hour after injection, mice were euthanized and tumors were excised immediately. Tumors were sectioned mediolaterally, and the resulting fresh section was imaged with the stereofluorescence microscope (0.5x objective, 2x zoom). Skin was left on the excised tumor tissue to provide spatial orientation. High-resolution images of 1390 x 1040 pixels with an FOV of 9.5 mm x 7 mm were captured and digitized by a Photometrics 12-bit monochrome CCD camera (CoolSNAPHQ; Roper Scientific, Inc, Trenton, NJ). To assess sensitizer heterogeneity, image analysis was performed on the tumor region using the Surface Plot tool in ImageJ (NIH; URL: http://rsb.info.nih.gov/ij/).
In vivo fluorescence spectroscopy measurements were made using a system and custom multiple-optical-fiber probe described previously [20]. Briefly, Pc 4 fluorescence was excited using a 639-nm laser (Power Technology), and broadband reflectance was acquired using a white light source (75-W xenon arc lamp, model 6263; Oriel Instruments, Stratford, CT). Light from these sources and the fluorescence and reflectance signals were transmitted to/from the tissue surface through dedicated fibers in the probe. Signals were imaged onto a TE-cooled, 16-bit, 512 x 512 pixel CCD camera (Pixis512; Princeton Instruments, Princeton, NJ) through an imaging spectrograph (SpectraPro 275; Acton Research Corp, Acton, MA). A long-pass filter (650AELP; Omega Optical, Brattleboro, VT) in the detection path was positioned to reject excitation light. The signal measured by the CCD was first corrected for instrument response.
To minimize the possibly confounding effects of tissue optical properties on the measured fluorescence, the fluorescence spectrum was divided by the reflectance measured in the same wavelength interval and source-detector geometry. Measurements were obtained at several locations on the tumor as well at both legs. Before measurement, the tumor site and leg areas were shaved, and hair was removed using a commercial depilating agent (Nair; Church & Dwight, Co, Princeton, NJ).
Tumor Response Assay and Statistics
After PDT, tumor dimensions along three orthogonal axes were measured daily using digital calipers. Volumes were computed assuming an approximately ellipsoidal shape with the expression, V = (4/3)πr1r2r3. Mice were removed from the study if the volume of the tumor reached twice the pretreatment volume. Cures were defined as no evidence of palpable tumor 90 days after PDT. Statistical analysis was performed using pairwise comparisons of tumor regrowth curves among control, Pc 4-only, IV-PDT, and IT-PDT treatment groups. A log-rank test was applied to each pair using SAS 9.1 (SAS Institute, Inc, Cary, NC). Bonferroni adjustments were applied to the raw P values of the log-rank tests to guard against type I error.
Host Response and Vascular Perfusion Imaging After PDT
In vivo confocal imaging of host responses or perfusion status in tumors of live mice was performed using a custom laser scanning fluorescence confocal microscope as described previously [21]. Briefly, anesthetized mice were placed in a supine position with the ear tumors facing the top of a coverslip mounted on the stage of the inverted microscope. The fluorophore Alexa Fluor 647 was excited with a 639-nm diode laser and detected using a 647-nm long-pass filter (Semrock, Rochester, NY). Alexa Fluor 488 and fluorescein isothiocyanate (FITC) were excited at 488 nm from an argon ion laser and detected using a combination of 500-nm long-pass and 515/30 nm band-pass filters (Chroma Technology). The combination of a 100-µm-diameter pinhole and a 10x, 0.45 NA objective gave a 6-µm optical section thickness, and the images were acquired at 16 bits with a lateral resolution of 1 µm per pixel.
For immunofluorescence imaging in vivo [18,22], antibodies were purchased directly as fluorescent conjugates. We labeled neutrophil infiltration into tumors using ID administration of antimouse GR-1 antibodies (clone RB6-8C5; Biolegend, San Diego, CA). The vessels in the tumor were labeled by ID injection of antimouse CD31 antibodies (clone MEC13.3; Biolegend). The ID injection volumes of antibodies were approximately 30 µl and concentrations were 0.1 mg/ml. Antibodies were administered as a cocktail 3 hours before imaging to allow for clearance of unbound label. To visualize perfusion status in tumors, 200 µl of 5 mg/ml FITC-dextran (FD2000S; Sigma-Aldrich, St Louis, MO) was injected IV through the tail vein. Perfusion in CD31-positive Alexa Fluor 647-labeled tumor vasculature was imaged as early as 5 minutes after injection.
Results and Discussion
Preliminary imaging of Pc 4 fluorescence in vivo indicated that Pc 4 was distributed throughout the tumor within less than 1 hour after IT injection. Published studies of Pc 4-PDT in mice typically used a 24-hour drug-light interval with IV administration of 0.6 mg/kg [23]. Thus, our comparisons were made on the basis of these two intervals. Anticipating favorable Pc 4 concentrations with IT injection, we chose to compare IT versus IV routes of administration using a two-fold lower concentration of 0.3 mg/kg. Pc 4 levels in freshly excised tumors were evaluated using chloroform extraction 1 or 24 hours after IT or IV administration, respectively. As shown in Table 1, the mean Pc 4 concentration recovered from the tumor 1 hour after IT injection was approximately 35,000-fold greater than that extracted 24 hours after IV injection of the same Pc 4 concentration. Because IT administration resulted in some accumulation in the skin immediately adjacent to the ID tumor, we measured Pc 4 concentration there as well. Greater than 95% of the injected Pc 4 was recovered from tumor and immediately adjacent skin, with approximately 65.7% of the injected sensitizer extracted from the tumor and 32.0% from the skin.
Table 1.
Mean (±SD) Pc 4 Concentration in Tumors at 1 and 24 Hours after IT and IV Administration, Respectively, as Quantified by Chloroform Extraction.
| IT - 1 hour (n = 8) | IV - 24 hours (n = 6) |
| 0.112 (±0.025) | 0.317 x 10-5 (±0.707 x 10-6) |
Concentrations are expressed as micrograms of Pc 4 per milligram of tumor.
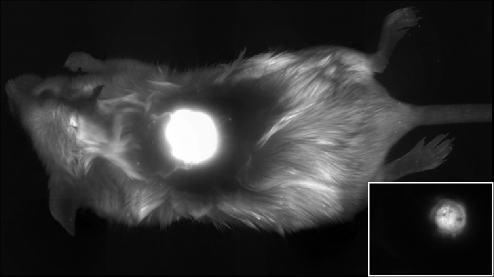
In vivo, whole-mouse fluorescence imaging showed a high degree of localization of Pc 4 in tumors and overlying skin within 1 hour of IT injection at a single point (Figure 1). Image analysis revealed fluorescence counts from the normal skin adjacent to the tumor that were at or very near control levels at this time point. Optical-fiber-based point fluorescence spectroscopy is more sensitive than imaging to low fluorophore levels. Thus, we used this technique to evaluate the systemic distribution of Pc 4 to a site remote from the tumor, the leg of the mouse. At 1 hour after IT injection, singular-value decomposition analysis [20] of spectra like those shown in Figure 2A indicated approximately 50-fold greater Pc 4 fluorescence intensity at the tumor relative to the leg. Spectroscopy measurements performed in mice 24 hours after IV administration showed comparable, low fluorescence emission from the tumor and the remote site, reflecting little if any tumor selectivity (not shown). Pc 4 undergoes modest irradiation-induced photobleaching in human cutaneous T-cell lymphoma lesions [24]. We observed photobleaching in the ID murine tumors as well as shown in Figure 2B. Remarkably, spectroscopy at the tumor site 24 hours after irradiation showed complete recovery of the Pc 4 fluorescence (Figure 2B). Because there is no evidence that the photobleaching of Pc 4 is reversible, we attribute this increase in fluorescence to the monomerization of initially aggregated, concentration-quenched sensitizer, a phenomenon we have observed previously in monolayer cell culture [25]. Photobleaching followed by more modest increases in Pc 4 absorption 4 hours after irradiation of tumors sensitized with 2 mg/kg Pc 4 IV was reported recently by Bai et al. [26].
Figure 1.
Fluorescence image illustrating Pc 4 localization in an ID tumor 1 hour after IT injection at a single location. The whole mouse image was created from a series of adjacent stereofluorescence images, each with 25.2 mm x 19 mm FOVs, which were montaged after acquisition as described in Materials and Methods. Because of the intense, highly localized fluorescence at the tumor site, the display is saturated at the tumor to render the rest of the mouse visible. The inset shows the individual field containing the tumor. Here the display has been rescaled to eliminate saturation. The bright central region corresponds to the tumor.
Figure 2.
(A) Representative fluorescence spectra obtained from tumor and remote leg in vivo 1 hour after IT-Pc 4 administration. (B) Fluorescence spectra from the tumor site before, immediately after, and 24 hours after irradiation (100 J/cm2), as indicated in the legend. In all cases, spectra were acquired with 639 nm excitation, and the fluorescence amplitudes correspond to an acquisition time of 0.1 second.
A concern with IT sensitizer delivery is the possibility of extremely heterogeneous IT distribution. To address this concern directly, we evaluated Pc 4 fluorescence distribution 1 hour after IT injection in freshly excised tumor sections using stereofluorescence microscopy. As illustrated in the representative image of Figure 3A, no gradient relative to the point of injection was observed. An analysis of image pixel intensities shown in Figure 3B revealed an approximately three-fold differential between the maximum and minimum fluorescence counts, with fluorescence in all pixels significantly above control levels. Interestingly, although chloroform extraction revealed a two-fold greater Pc 4 concentration in the tumor relative to the immediately adjacent skin, this is not reflected in the fluorescence recorded in the freshly excised sections. This again is consistent with significant concentration quenching in the tumor, as noted above.
Figure 3.
(A) Stereofluorescence image of Pc 4 distribution in a fresh, excised tumor section. The tumor was excised 1 hour after IT injection of 0.3 mg/kg Pc 4. The white circle bounds the ID tumor, and the bright regions outside of the circle correspond to the skin adjacent to the tumor, where Pc 4 concentrations were half that within the tumor. (B) A surface plot of pixel intensities from the tumor region of A. The Pc 4 fluorescence counts within the tumor ranged from approximately 300 to 1000.
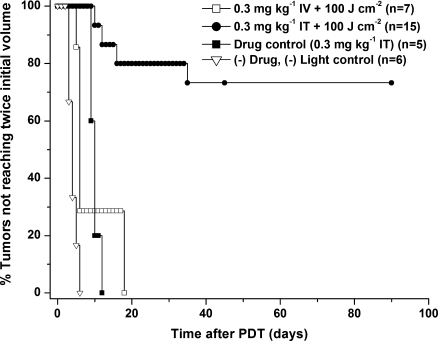
Tumor growth control was used to compare the therapeutic efficacy of IT versus IV-Pc 4 administration with an irradiation regimen consisting of 100 J/cm2 delivered at an irradiance of 50 mW/cm2. The Kaplan-Meier curves of Figure 4 demonstrate that the tumor response to Pc 4-PDTand IT administration was dramatically enhanced relative to that observed with IV injection of the same photosensitizer concentration. Among the 15 mice treated with IT administration, 11 (73%) were cured as defined by no evidence of tumor 90 days after irradiation. No cures were observed in the IV group (n = 8), where the median tumor volume doubling time was 6 days. The difference in tumor control between IV- and IT-PDT was highly significant, with a Bonferroni-adjusted P < .001.Untreated controls ((-) light, (-) drug) displayed a median tumor doubling time of 4 days (n = 6), whereas mice that received Pc 4 injections IT but were not irradiated ((-) light, (+) drug) showed a modest but statistically significant growth delay of 10 days (P = .02, n = 5). Tumor regrowth in response to IV-Pc 4-PDT was statistically indistinguishable from that in response to IT-Pc 4 injection without irradiation (P = 1.0).
Figure 4.
Kaplan-Meier curves of tumor responses to IT-Pc 4-PDT. Laser irradiation at 667 nm was performed at an irradiance of 50 mW/cm2 for a fluence of 100 J/cm2 1 or 24 hours after IT or IV administration of 0.3 mg/kg Pc 4. Improved efficacy of IT- versus IV-Pc 4 was highly statistically significant (P < .001).
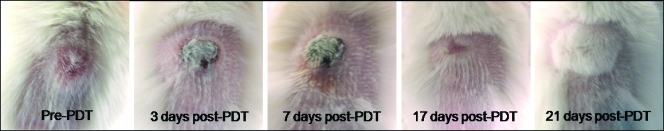
As noted above, Pc 4 was found in the skin immediately adjacent to the ID tumor. PDT resulted in eschar formation directly above the tumor, as shown in Figure 5. The skin healed well with complete regrowth of hair within approximately 3 weeks after irradiation.
Figure 5.
Digital photographs of the treatment site and surrounding tissue before and at various times after IT-Pc 4-PDT.
When photosensitizers are administered IV, PDT induces a local inflammatory response that is characterized by leukocyte infiltration, with a significant fraction of these infiltrating cells being neutrophils [27–29]. To examine the extent of this response to IT-Pc 4-PDT, we imaged the influx of GR-1+ neutrophils in tumors in vivo 24 hours after irradiation. Figure 6, A and B, illustrates the fluorophore-labeled infiltrating neutrophils imaged in an untreated control and PDT-treated tumor, respectively. Irradiation resulted in a significant three-to five-fold (n = 4) enhanced accumulation of GR-1+ cells at this time point. To the best of our knowledge, these results represent the first demonstration of a PDT-induced inflammatory response visualized directly in vivo through noninvasive optical imaging, and they represent the first characterization of any kind of an acute inflammatory response to Pc 4-PDT.
Figure 6.
(A, B) In vivo confocal images of Alexa Fluor 488-conjugated anti-GR-1+ neutrophils (green) and Alexa Fluor 647-conjugated anti-CD31 vessels (red) in EMT6 tumors grown in the ears of a BALB/c mouse. (A) Control untreated tumor and (B) IT-Pc 4-PDT-treated tumor 24 hours after irradiation. GR-1+ neutrophils and CD31+ vessels were imaged by ID injection of approximately 30 µl of 0.1 mg/ml fluorophore-conjugated antibodies into the ear 3 hours before imaging. (C, D) In vivo confocal fluorescence images of perfusion (green) in CD31-positive vessels (red) in (C) control untreated EMT6 ear tumors and (D) IT-Pc 4-PDT-treated tumor 48 hours after irradiation. Perfusion was imaged by injecting 200 µl of 5 mg/ml FITC-dextran IV through the tail vein, and the perfusion status in CD31+ Alexa Fluor 647-labeled tumor vasculature was imaged as early as 5 minutes after injection. The FOV in the images is 800 µm x 800 µm. Images were acquired at a depth of approximately 100 µm in the tumor.
Perfused tumor blood vessels are required for the survival and regrowth of clusters of PDT-treated tumor cells that escape immediate phototoxicity, but they may also have an important function in facilitating the trafficking of host cells into and out of the treated tumor. This complex scenario serves to illustrate the importance of the perfusion status of vessels and its role in influencing mechanisms of long-term tumor response. With this motivation, we imaged tumor perfusion in live mice before and 24 and 48 hours after IT-Pc 4-PDT using IV-injected FITC-conjugated high-molecular weight dextran as an optical perfusion marker [30]. As illustrated in the representative images of Figure 6, C and D, there was no detectable difference in perfusion status between the control and treated tumors, thus suggesting that IT-Pc 4-PDT does not initiate functional damage to the vasculature, at least up to 48 hours after irradiation.
The recent study published by Bai et al. [26] offers interesting points of comparison with our findings. Using a Pc 4 dose of 2 mg/kg administered IV and assessing tumor and skin sensitizer concentrations with a form of absorption spectroscopy in vivo, those authors demonstrated a relationship between tumor Pc 4 levels at the time of irradiation and tumor response. Their two cures (among 16 PDT-treated mice) after irradiation with 150 J/cm2 occurred in animals with the highest Pc 4 concentrations, and among the 14 mice whose tumors regrew, a correlation between tumor growth delay and IT-Pc 4 concentration was demonstrated. Thus, these data suggest that photosensitizer concentration can be a limiting factor in tumor response to PDT in vivo. Of course, it is not possible to arbitrarily increase the amount of systemically administered drug, which makes IT delivery very attractive in those situations where it is clinically feasible. Indeed, it is unlikely that the extremely high IT-Pc 4 concentrations realized in our experiments could ever be achieved with systemic administration, even if toxicity and skin photosensitivity were of no concern.
In addition to greatly increased sensitizer concentrations in the target tissue, IT administration of Pc 4 offers exceptional selectivity. As observed also by Bai et al. [26], we found no tumor selectivity up to 24 hours after IV Pc 4 delivery. Peak selectivity at 48 hours was a modest 2.1-fold, whereas spectroscopy in our study revealed at least 50-fold tumor-to-normal skin selectivity in as little as 1 hour after IT administration. The ability to achieve high selectivity at very short drug-light intervals would be attractive to patients and clinicians, enabling an entire treatment to be completed within a single, reasonably short visit. Yet another potential advantage of IT administration is the opportunity it presents for using less drug per patient, thereby lowering the cost of PDT substantially. Finally, taken together, reduced persistent skin photosensitivity, good IT distribution, and lower cost would facilitate repeat PDT treatments, which would be more difficult for those photosensitizers that are retained in the skin. Beyond those tumor sites that are obviously accessible to IT injection, the capabilities of modern interventional radiology render many solid tumors throughout the body candidates for this approach.
Acknowledgments
The authors thank David Kessel for detailed advice on the sensitizer extraction protocol.
Footnotes
This work was supported by the National Institutes of Health grant CA122093 awarded by the National Cancer Institute.
Disclosure
Malcolm E. Kenney is a founder and member of the scientific advisory board of Fluence, a new start-up company seeking to commercialize Pc 4 for PDT.
References
- 1.Morton CA, McKenna KE, Rhodes LE. British Association of Dermatologists Therapy Guidelines and Audit Subcommittee Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245–1266. doi: 10.1111/j.1365-2133.2008.08882.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackenzie GD, Dunn JM, Selvasekar CR, Mosse CA, Thorpe SM, Novelli MR, Bown SG, Lovat LB. Optimal conditions for successful ablation of high-grade dysplasia in Barrett's oesophagus using aminolaevulinic acid photodynamic therapy. Lasers Med Sci. 2009;24:729–734. doi: 10.1007/s10103-008-0630-7. [DOI] [PubMed] [Google Scholar]
- 3.Beck TJ, Kreth FW, Beyer W, Mehrkens JH, Obermeier A, Stepp H, Stummer W, Baumgartner R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg Med. 2007;39:386–393. doi: 10.1002/lsm.20507. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmeier U, Bink A, Schackert G, Stummer W ALA Glioma Study Group, author. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10:1025–1034. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostron H, Bellnier DA, Lin CW, Swartz MR, Martuza RL. Distribution, retention, and phototoxicity of hematoporphyrin derivative in a rat glioma. Intraneoplastic versus intraperitoneal injection. J Neurosurg. 1986;64:768–774. doi: 10.3171/jns.1986.64.5.0768. [DOI] [PubMed] [Google Scholar]
- 6.Amano T, Prout GR, Lin C-W. Intratumor injection as a more effective means of porphyrin administration for photodynamic therapy. J Urol. 1988;139:392–395. doi: 10.1016/s0022-5347(17)42441-4. [DOI] [PubMed] [Google Scholar]
- 7.Lin C-W, Amano T, Rutledge AR, Shulok JR, Prout GR. Photodynamic effect in an experimental bladder tumor treated with intratumor injection of hematoporphyrin derivative. Cancer Res. 1988;48:6115–6120. [PubMed] [Google Scholar]
- 8.Gibson SL, Van Der Meid KR, Murant RS, Hilf R. Increased efficacy of photodynamic therapy of R3230AC mammary adenocarcinoma by intratumoral injection of Photofrin II. Br J Cancer. 1990;61:553–557. doi: 10.1038/bjc.1990.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. III. Methylene blue-blood levels in the dog and tissue levels in the rat following intravenous administration. J Pharm Sci. 1972;61:1090–1094. doi: 10.1002/jps.2600610711. [DOI] [PubMed] [Google Scholar]
- 10.König K, Bockhorn V, Dietel W, Schubert H. Photochemotherapy of animal tumors with the photosensitizer methylene blue using a krypton laser. J Cancer Res Clin Oncol. 1987;113:301–303. doi: 10.1007/BF00396390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orth K, Russ D, Beck G, Rück A, Beger HG. Photochemotherapy of experimental colonic tumours with intra-tumorally applied methylene blue. Langenbecks Arch Surg. 1998;383:276–281. doi: 10.1007/s004230050132. [DOI] [PubMed] [Google Scholar]
- 12.Orth K, Beck G, Genze F, Rück A. Methylene blue mediated photodynamic therapy in experimental colorectal tumors in mice. J Photochem Photobiol B. 2000;57:186–192. doi: 10.1016/s1011-1344(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 13.Orth K, Rück A, Stanescu A, Berger HG. Intraluminal treatment of inoperable oesophageal tumours by intralesional photodynamic therapy with methylene blue. Lancet. 1995;345:519–520. [PubMed] [Google Scholar]
- 14.D'Hallewin MA, Kochetkov D, Viry-Babel Y, Leroux A, Werkmeister E, Dumas D, Gräfe S, Zorin V, Guillemin F, Bezdetnaya L. Photodynamic therapy with intratumoral administration of lipid-based mTHPC in a model of breast cancer recurrence. Lasers Surg Med. 2008;40:543–549. doi: 10.1002/lsm.20662. [DOI] [PubMed] [Google Scholar]
- 15.Mannino S, Molinari A, Sabatino G, Ciafrè SA, Colone M, Maira G, Anile C, Arancia G, Mangiola A. Intratumor vs. systemic administration of meta-tetrahydroxyphenylchlorin for photodynamic therapy of malignant gliomas: assessment of uptake and spatial distribution in C6 rat glioma model. Int J Immunopathol Pharmacol. 2008;21:227–231. doi: 10.1177/039463200802100126. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD, Baron ED, Scull H, Hsia A, Berlin JC, McCormick T, Colussi V, Kenney ME, Cooper KD, Oleinick NL. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: the case experience with preclinical mechanistic and early clinical-translational studies. Toxicol Appl Pharmacol. 2007;224:290–299. doi: 10.1016/j.taap.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes HJ, Kennedy JC, Pottier R, Rossi R, Weagle G. A recipe for the preparation of a rodent food that eliminates chlorophyll-based tissue fluorescence. J Photochem Photobiol B. 1995;29:199. doi: 10.1016/1011-1344(95)90099-3. [DOI] [PubMed] [Google Scholar]
- 18.Cummings RJ, Mitra S, Lord EM, Foster TH. Antibody-labeled fluorescence imaging of dendritic cell populations in vivo. J Biomed Opt. 2008;13:044041. doi: 10.1117/1.2966122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anula HM, Berlin JC, Wu H, Li Y-S, Peng X, Kenney ME, Rodgers MAJ. Synthesis and photophysical properties of silicon phthalocyanines with axial siloxy ligands bearing alkylamine termini. J Phys Chem. 2006;110:5215–5223. doi: 10.1021/jp056279t. [DOI] [PubMed] [Google Scholar]
- 20.Finlay JC, Conover DL, Hull EL, Foster TH. Porphyrin bleaching and PDT-induced spectral changes are irradiance dependent in ALA-sensitized normal rat skin in vivo. Photochem Photobiol. 2001;73:54–63. doi: 10.1562/0031-8655(2001)073<0054:pbapis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Bigelow CE, Conover DL, Foster TH. Confocal fluorescence spectroscopy and anisotropy imaging system. Opt Lett. 2003;28:695–697. doi: 10.1364/ol.28.000695. [DOI] [PubMed] [Google Scholar]
- 22.Mitra S, Foster TH. In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-l-aspartylchlorin-e6. Neoplasia. 2008;10:429–438. doi: 10.1593/neo.08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson CY, Freye K, Tubesing KA, Li Y-S, Kenney ME, Mukhtar H, Elmets CA. A comparative analysis of silicon phthalocyanine photosensitizers for in vivo photodynamic therapy of RIF-1 tumors in C3H mice. Photochem Photobiol. 1998;67:332–336. [PubMed] [Google Scholar]
- 24.Lee TK, Baron ED, Foster TH. Monitoring Pc 4 photodynamic therapy in clinical trials of cutaneous T-cell lymphoma using noninvasive spectroscopy. J Biomed Opt. 2008;13:030507. doi: 10.1117/1.2939068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang KK-H, Wilson JD, Kenney ME, Mitra S, Foster TH. Irradiation-induced enhancement of Pc 4 fluorescence and changes in light scattering are potential dosimeters for Pc 4-PDT. Photochem Photobiol. 2007;83:1–7. doi: 10.1111/j.1751-1097.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 26.Bai L, Guo J, Bontempo FA, Eiseman JL. The relationship of Phthalocyanine 4 (Pc 4) concentrations measured noninvasively to outcome of Pc 4 photodynamic therapy in mice. Photochem Photobiol. 2009;85:1011–1019. doi: 10.1111/j.1751-1097.2009.00542.x. [DOI] [PubMed] [Google Scholar]
- 27.Krosl G, Korbelik M, Dougherty GJ. Induction of immune cell infiltration into murine SCCVII tumour by Photofrin-based photodynamic therapy. Br J Cancer. 1995;71:549–555. doi: 10.1038/bjc.1995.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J, Cecic I, Parkins CS, Korbelik M. Neutrophils as inflammatory and immune effectors in photodynamic therapy-treated mouse SCCVII tumours. Photochem Photobiol Sci. 2002;1:690–695. doi: 10.1039/b204254a. [DOI] [PubMed] [Google Scholar]
- 29.Gollnick SO, Evans SS, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br J Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown EB, Campbell RB, Tsuzuki Y, Xu L, Carmeliet P, Fukumura D, Jain RK. In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med. 2001;7:864–868. doi: 10.1038/89997. [DOI] [PubMed] [Google Scholar]