Abstract
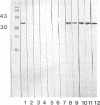
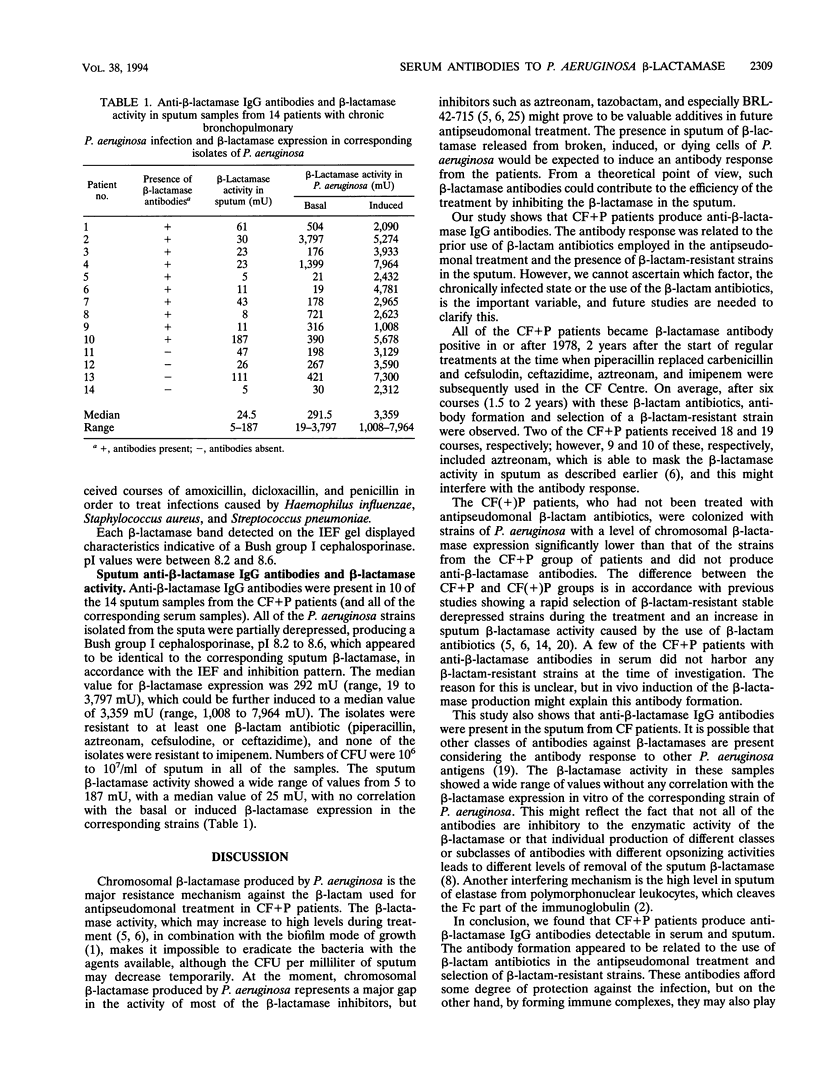
A murine monoclonal anti-chromosomal beta-lactamase antibody was developed and an immunoblotting technique was used to study the presence of serum and sputum antibodies against Pseudomonas aeruginosa chromosomal group 1 beta-lactamase in patients with cystic fibrosis (CF). The serum antibody response was studied with serum samples collected in 1992 from 56 CF patients in a cross-sectional study and with serum samples from 18 CF patients in a longitudinal study. Anti-beta-lactamase immunoglobulin G antibodies were present in all of the serum samples from the patients with chronic bronchopulmonary P. aeruginosa infection (CF + P) but in none of the CF patients with no or intermittent P. aeruginosa infection. Anti-beta-lactamase antibodies were present in serum from CF + P patients after six antipseudomonal courses (median) and correlated with infection with a beta-lactam-resistant strain of P. aeruginosa. The sputum antibody response and the beta-lactamase activity in sputum samples from 14 of the CF + P patients were also studied. beta-lactamase antibodies were present in 10 of these samples. P. aeruginosa strains isolated from these samples were partially derepressed, producing group 1 cephalosporinase. We found a wide range of chromosomal beta-lactamase activity in the sputum samples, with no correlation with basal or induced activity of beta-lactamase expression. The presence of anti-beta-lactamase antibodies in endobronchial sputum could be an important factor in the defense against the infection. On the other hand, immune complexes between the beta-lactamase and corresponding antibodies could play a role in the pathogenesis of bronchopulmonary injury in CF by mediating hyperimmune reactions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Döring G., Goldstein W., Röll A., Schiøtz P. O., Høiby N., Botzenhart K. Role of Pseudomonas aeruginosa exoenzymes in lung infections of patients with cystic fibrosis. Infect Immun. 1985 Sep;49(3):557–562. doi: 10.1128/iai.49.3.557-562.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giwercman B., Lambert P. A., Rosdahl V. T., Shand G. H., Høiby N. Rapid emergence of resistance in Pseudomonas aeruginosa in cystic fibrosis patients due to in-vivo selection of stable partially derepressed beta-lactamase producing strains. J Antimicrob Chemother. 1990 Aug;26(2):247–259. doi: 10.1093/jac/26.2.247. [DOI] [PubMed] [Google Scholar]
- Giwercman B., Meyer C., Lambert P. A., Reinert C., Høiby N. High-level beta-lactamase activity in sputum samples from cystic fibrosis patients during antipseudomonal treatment. Antimicrob Agents Chemother. 1992 Jan;36(1):71–76. doi: 10.1128/aac.36.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaconis J. P., Sanders C. C. Purification and characterization of inducible beta-lactamases in Aeromonas spp. Antimicrob Agents Chemother. 1990 Jan;34(1):44–51. doi: 10.1128/aac.34.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen H. K., Nir M., Høiby N., Koch C., Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for delta F508 mutation. Lancet. 1991 Mar 16;337(8742):631–634. doi: 10.1016/0140-6736(91)92449-c. [DOI] [PubMed] [Google Scholar]
- Koch C., Høiby N. Pathogenesis of cystic fibrosis. Lancet. 1993 Apr 24;341(8852):1065–1069. doi: 10.1016/0140-6736(93)92422-p. [DOI] [PubMed] [Google Scholar]
- Kronborg G., Fomsgaard A., Galanos C., Freudenberg M. A., Høiby N. Antibody responses to lipid A, core, and O sugars of the Pseudomonas aeruginosa lipopolysaccharide in chronically infected cystic fibrosis patients. J Clin Microbiol. 1992 Jul;30(7):1848–1855. doi: 10.1128/jcm.30.7.1848-1855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Livermore D. M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. Eur J Clin Microbiol. 1987 Aug;6(4):439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nespolo A., Bianchi G., Salmaggi A., Lazzaroni M., Cerrato D., Malesani Tajoli L. Immunoblotting techniques with picogram sensitivity in cerebrospinal fluid protein detection. Electrophoresis. 1989 Jan;10(1):34–40. doi: 10.1002/elps.1150100109. [DOI] [PubMed] [Google Scholar]
- Pressler T., Pedersen S. S., Espersen F., Høiby N., Koch C. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin Exp Immunol. 1990 Sep;81(3):428–434. doi: 10.1111/j.1365-2249.1990.tb05351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler T., Pedersen S. S., Espersen F., Høiby N., Koch C. IgG subclass antibody responses to alginate from Pseudomonas aeruginosa in patients with cystic fibrosis and chronic P. aeruginosa infection. Pediatr Pulmonol. 1992 Sep;14(1):44–51. doi: 10.1002/ppul.1950140109. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Moland E. S. Characterization of beta-lactamases in situ on polyacrylamide gels. Antimicrob Agents Chemother. 1986 Dec;30(6):951–952. doi: 10.1128/aac.30.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Pedersen S. S., Tilling R., Brown M. R., Høiby N. Use of immunoblot detection of serum antibodies in the diagnosis of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. J Med Microbiol. 1988 Nov;27(3):169–177. doi: 10.1099/00222615-27-3-169. [DOI] [PubMed] [Google Scholar]
- Wood R. E., Boat T. F., Doershuk C. F. Cystic fibrosis. Am Rev Respir Dis. 1976 Jun;113(6):833–878. doi: 10.1164/arrd.1976.113.6.833. [DOI] [PubMed] [Google Scholar]
- Zhou X. Y., Kitzis M. D., Gutmann L. Role of cephalosporinase in carbapenem resistance of clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1993 Jun;37(6):1387–1389. doi: 10.1128/aac.37.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]