Summary
High-risk genital human papillomavirus (HPV) infection is aetiologically linked to cervical cancer; however, data on the prevalence and determinants of high-risk HPV infection in Uganda are limited. We conducted a population-based cross-sectional survey among 18–49-year-old women in rural Southwest Uganda. The primary outcome was presence or absence of high-risk HPV DNA (for genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 or 68) in the genital secretions as determined by HPV DNA Hybrid Capture 2 assay (Digene Corp, Beltsville, MD, USA). In 314 women who participated, the prevalence of high-risk HPV was 17.2% (54/314; 95% confidence interval [CI]: 13–21). Older women had a lower proportion of high-risk HPV infection; with a 9% decrease in the odds ratio (OR) of high-risk HPV infection per year increase in age (OR = 0.91; 95% CI: 0.86, 0.96). The odds of detecting high-risk HPV infection was higher among women who were previously tested positive for HIV (OR = 12.1; 95% CI: 2.8, 52.3). In this population of rural Ugandan women, the prevalence of high-risk cervical HPV infection was high. Information on predictors of high-risk HPV infection and intention to receive a vaccine can guide future immunization initiatives for young sexually active women.
Keywords: human papillomavirus (HPV), high-risk HPV, HIV, self-collected vaginal sample, Uganda
INTRODUCTION
Infection with high-risk types of human papillomavirus (HPV) infection is a cause of cervical cancer in women.1 In African countries, rates of cervical cancer have increased concomitantly with AIDS epidemic.2,3 Among African women, estimated HPV prevalence has ranged from 18% to 60% with higher rates observed among HIV-infected women.2–4 Studies performed in East Africa have correlated HIV infection specifically with high-risk HPV genotypes 16 and 18, which are most frequently associated with malignant transformation.5 Although necessary, HPV infection may not be a sufficient cause for cervical neoplasia as other host–environment and virus-related factors are implicated as well.6,7
In Uganda, the rate of cervical cancer has more than doubled since the 1950s to become the most commonly registered tumour among women, and incidence is now the highest recorded on the African continent.8 Compared with the USA, age-adjusted cervical cancer incidence per 100,000 population is 4.7 times higher in Uganda (36.3 vs. 7.7) and age-adjusted rates of death because of cervical cancer is 12.7 times higher (29.3 vs. 2.3).9 The greater disparity in cervical cancer mortality highlights the lack of formal screening programmes for cervical cancer and widely available technologies to establish HPV infection apart from PAP smears, which are not routinely performed.10
Immunization against high-risk HPV may have high value in the developing countries where 80% of the global burden of cervical cancer occurs each year and where Pap screening has been largely ineffective.11 An understanding of the epidemiology of HPV in Uganda is needed to effectively implement an HPV vaccination programme. In this study, we estimate the prevalence of high-risk HPV infection in a population-based sample of sexually active women residing in Bushenyi District, Uganda where a district-wide, home-based HIV testing programme has been in place since 2005. In addition, we examine the extent that established risk factors are associated with HPV infection in this study setting.
MATERIALS AND METHODS
Study design
From July to August 2005, we conducted a cross-sectional study among a population-based sample of the residents of Sheema County in Bushenyi District of rural Southwestern Uganda. Bushenyi District is the site of a district-wide home-based HIV voluntary counselling and testing programme which began in February 2005. Results from testing through December 2006 indicated an HIV prevalence of 4.9%.12
We used a multistage sampling procedure to approach individuals for the study. First, we selected at random one Parish in each of the three sub-counties of Sheema County. Within each Parish, we randomly selected 33% of villages and approached all households that were registered with the local council level 1 (LC1) administration. One person per household was selected at random among those who met the eligibility criteria and was asked to participate in the study. Eligibility criteria included being a woman between 18 and 49 years of age, a resident of the county, having had sexual intercourse in the previous 12 months, no previous hysterectomy, and willingness to provide a self-collected vaginal specimen. Based on the previous studies conducted in Uganda, we expected the prevalence of high-risk HPV to be approximately 15%.13 To estimate this prevalence and its 95% confidence interval with ±4% precision, we calculated a required sample size of 306 women.
Following the informed consent procedure, women were interviewed in their homes using a pre-tested, 55-item questionnaire about sociodemographic characteristics, reproductive and sexual history, alcohol and tobacco use. Women were also asked to provide a self-collected vaginal sample using the Hybrid Capture (HC) Cervical Sampler (Digene Corporation, Bethesda, MD, USA) according to the manufacturer’s protocol for HPV DNA testing.14 The total encounter with the study participants was 30 minutes on an average.
The specimens were kept at room temperature (18–25°C) and shipped to University Hospitals of Cleveland Biomedical Laboratory (Cleveland, OH, USA) for analysis using the Hybrid Capture II (HC2) microtitre high-risk HPV DNA assay (Digene Corporation). The HC2 assay detects the presence of HPV high-risk DNA types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 and performed according to the manufacturer’s guidelines. Briefly, a cocktail of HPV type-specific RNAs is hybridized to the DNA from the clinical specimen. These RNA:DNA hybrids are captured onto a solid phase and are then detected by alkaline phosphatase-conjugated antibodies. When the alkaline phosphatase is cleaved in a chemiluminescent solution, it produces light that can be detected by a luminometer. Specimens were classified as positive if the relative light units/positive control (RLU/PC) ratio was ≥1 using the 1-pg/mL positive controls supplied in the kit. Special precautions to minimize false-positive results in the HC2 assay were implemented, and selected positive and negative HPV samples were included in the repeat test runs for quality control purposes.
This study was approved by the University Hospitals Health System of Cleveland Institutional Review Board (IRB), Makerere University Institute of Public Health IRB and the Uganda National Council of Science and Technology.
Statistical methods
In a proportion of women high-risk HPV was detected and 95% confidence intervals (CI) were calculated. Exploratory data analysis was conducted to compare women who were and were not infected with high-risk HPV according to categorical variables of interest using Pearson’s chi-square or Fisher’s exact test and continuous variables using the Student’s t-test or Kruskall-Wallis test. Collinearity between variables measuring similar constructs was assessed by calculating the Spearman’s rank correlation coefficient. Logistic regression was performed to determine the factors associated with odds of infection with high-risk HPV. Factors for which unadjusted associations with the presence of high-risk HPV was at least suggestive (P < 0.25) were examined for the possible retention in the adjusted models. In choosing predictors to include in the final models, we desired a parsimonious model containing independent predictors. We evaluated the model fit using the Hosmer-Lemeshow goodness-of-fit test. Statistical analysis was carried out using SAS® software version 9.1 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Epidemiology of high-risk human papilloma virus
Of the 323 women who met the inclusion criteria, 314 (97.2%) agreed to participate in the study and provided a self-collected vaginal swab. Fifty-four women had detectable infection with high-risk HPV (Table 1); thus, the prevalence of high-risk HPV was 17.2% (95% CI: 13.0, 21.4). The median (interquartile range [IQR]) age was 30 (25, 37) years where women with detectable high-risk HPV DNA being significantly younger than women uninfected with high-risk HPV (P < 0.001).
Table 1.
Prevalence of high-risk human papilloma virus (HPV) among 314 sexually active women, 18–49 years of age, Bushenyi District, Uganda, 2005
| High-risk HPV |
||||
|---|---|---|---|---|
| Sociodemographic characteristics | Overall (n = 314), No. (%) | Positive (n = 54), No. (%) | Negative (n = 260), No. (%) | P value |
| Median (IQR) age (years) | 30 (25, 37) | 27 (22, 32) | 32 (26, 38) | <0.001 |
| Education attended | ||||
| None | 36 (11.5) | 4 (7.4) | 32 (12.3) | 0.611 |
| Primary school | 221 (70.4) | 39 (72.2) | 182 (70.0) | |
| Secondary school or higher | 57 (18.2) | 11 (20.4) | 46 (17.7) | |
| Place of residence | ||||
| Village | 262 (83.4) | 42 (77.8) | 220 (84.6) | 0.23 |
| Trading centre | 52 (16.6) | 12 (22.2) | 40 (15.4) | |
| Marital status | ||||
| Never married | 1 (0.3) | 1 (1.9) | 0 (0.0) | 0.172 |
| In a monogamous marriage | 250 (79.9) | 41 (75.9) | 209 (80.4) | 0.459 |
| In a polygamous marriage | 35 (11.2) | 8 (14.8) | 27 (10.4) | 0.345 |
| Separated or widowed | 28 (8.9) | 4 (7.4) | 24 (9.2) | 0.798 |
| Religion | ||||
| Anglican | 214 (68.2) | 36 (66.7) | 178 (68.5) | 0.873 |
| Catholic | 57 (18.2) | 8 (14.8) | 49 (18.8) | 0.565 |
| Muslim | 29 (9.2) | 8 (14.8) | 21 (8.1) | 0.125 |
| Other | 14 (4.5) | 2 (3.7) | 12 (4.6) | 1.000 |
| Reproductive health | ||||
| Median (IQR) parity | 4 (2, 6) | 2 (1, 4) | 4 (2, 6) | <0.001 |
| Median (IQR) age at first birth (years) | 19 (18, 21) | 19 (18, 20) | 19 (18, 21) | 0.174 |
| Currently pregnant | 28 (10.8) | 6 (11.1) | 34 (10.8) | 1.000 |
| Ever had a spontaneous abortion or miscarriage | 102 (32.5) | 17 (31.5) | 85 (32.7) | 0.863 |
| Oral contraceptive use in the past year | 47 (14.9) | 8 (14.8) | 39 (15.0) | 0.972 |
| Sexual history | ||||
| Age at first intercourse ≤ 15 years | 63 (20.1) | 17 (31.5) | 46 (17.7) | 0.021 |
| Has sex more than once per week | 193 (61.5) | 39 (72.2) | 154 (59.2) | 0.079 |
| More than one lifetime sexual partner | 109 (34.7) | 22 (40.7) | 87 (33.5) | 0.307 |
| Non-regular sex partner in the past year | 13 (4.1) | 3 (5.6) | 10 (3.8) | 0.474 |
| Partner circumcised | 39 (12.4) | 10 (18.5) | 29 (11.2) | 0.171 |
| Condom use in past year | 25 (7.9) | 5 (9.3) | 20 (7.7) | 0.782 |
| Presently has abnormal vaginal discharge | 102 (32.5) | 16 (29.6) | 86 (33.1) | 0.623 |
| HIV | ||||
| Did not previously test for HIV | 154 (49.0) | 27 (50.0) | 127 (48.8) | 0.877 |
| Previously tested for HIV | 160 (51.0) | 27 (50.0) | 133 (51.2) | |
| HIV status among those previously tested | ||||
| Infected | 9 (5.6) | 6 (23.1) | 3 (2.4) | 0.001* |
| Uninfected | 141 (88.1) | 20 (74.1) | 121 (91.0) | |
| Did not disclose | 10 (6.3) | 1 (3.7) | 9 (6.8) | |
| Substance use | ||||
| Consumed alcohol in the past year | 48 (15.3) | 8 (14.8) | 40 (15.4) | 1.000 |
| Smoked cigarettes in the past year | 18 (5.7) | 5 (9.3) | 13 (5.0) | 0.209 |
Among those with known HIV status
IQR = Interquartile range
Most women who resided in the village (83.4%) had no formal education or attended some primary school (81.9%) were of the Anglican faith (68.2%), and reported being in a monogamous marital relationship (79.9%). Overall, 65% of women had one lifetime sexual partner, few (4.1%) had had a non-regular sexual partner in the previous year, and about one-third of the women reported having an abnormal vaginal discharge. The median (IQR) age at first sexual intercourse was 18 (16, 19) years, and this was significantly correlated with age at first birth (r = 0.622; P < 0.001) among women with children. Women whose age of sexual debut was 15 years or younger were more likely to be infected with high-risk HPV (P = 0.021). Women who reported having sexual intercourse more than once per week were more likely to be infected with high-risk HPV, although this did not attain statistical significance (P = 0.079).
Twenty-eight women (10.8%) were pregnant at the time of the home visit and almost all (96%) women previously had a live-birth with the median (IQR) number of full-term pregnancies being four (2, 6). Parity, which was significantly correlated with age (r = 0.703; P < 0.001), and age at first birth (r = −0.189; P = 0.001) was higher among women not infected with high-risk HPV (P < 0.001). One-third reported ever having a spontaneous abortion or miscarriage. Overall, 14.9% had used oral contraceptives and 7.9% used a condom in the previous year.
Nine (5.6%) women reported being HIV-infected and these women were more likely to be infected with high-risk HPV (P = 0.001). Women who had previously tested for HIV but did not disclose their status to study staff (n = 10) were not more likely to be infected with high-risk HPV (P = 1.000, Fisher’s exact test). In addition, these women did not differ from those who disclosed their HIV status (n = 150) or did not previously test for HIV (n = 154) by sociodemographic characteristics, reproductive health, sexual history and substance use. Thus, we classified women as those who did not disclose their HIV status or as those who did not know previously, i.e. status of having unknown HIV status in the subsequent analyses. Overall, 5.7% of women smoked cigarettes and 15.3% had consumed an alcoholic beverage in the previous year.
Risk factors for high-risk human papilloma virus
The association between high-risk HPV detection and age quartile followed a dose–response relationship (Figure 1) that was statistically significant (Cochran-Armitage test for trend; P = 0.0005). The proportion (%; 95% CI) of high-risk HPV among women 19–25, 26–30, 31–37 and 38–49 years of age was 28.6 (20.0, 39.0), 17.3 (10.5, 27.5), 13.9 (7.9, 23.3) and 7.9 (3.7, 16.2), respectively. Thus, relative to women in 19–25 years of age, the odds of detecting high-risk HPV decreased by 48%, 60% and 79% among women in 26–30, 31–37 and 38–49 years of age, respectively. On an average, the odds of detecting high-risk HPV decreased 9% for each one-year increase in age and this remained statistically significant in multivariable analysis (Table 2).
Figure 1.
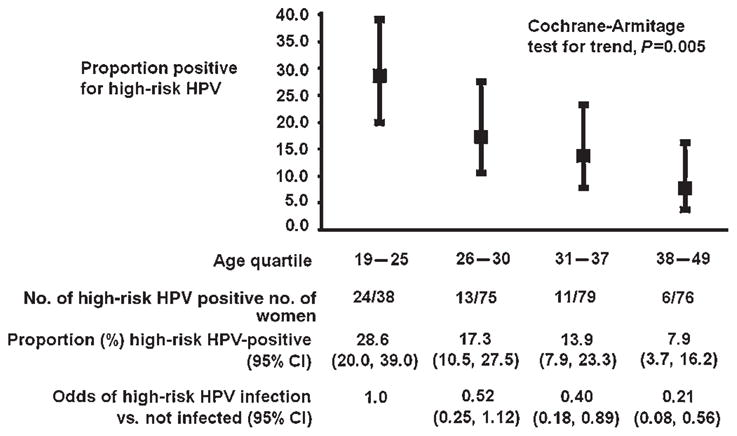
Age-specific prevalence of high-risk human papilloma virus among 314 sexually active women, Bushenyi District, Uganda, 2005
Table 2.
Logistic regression of factors associated with high-risk human papilloma virus infection among 314 sexually active women, 18–49 years of age, Bushenyi District, Uganda, 2005
| Unadjusted |
Adjusted |
|||
|---|---|---|---|---|
| Characteristics | OR | 95% CI | OR | 95% CI |
| Age (per year) | 0.91 | 0.87, 0.96 | 0.90 | 0.85, 0.94 |
| Resides in a trading centre (vs. village) | 1.57 | 0.76, 3.21 | ||
| Muslim (vs. non-Muslim) | 1.98 | 0.83, 4.74 | ||
| Parity (per live-birth) | 0.79 | 0.69, 0.90 | ||
| Age at first birth (per year) | 0.89 | 0.79, 1.01 | ||
| Age at first intercourse ≤ 15 years | 2.14 | 1.11, 4.12 | 2.16 | 1.05, 4.43 |
| Has sex more than once per week (vs. ≤ once per week) | 1.77 | 0.93, 3.38 | ||
| More than one lifetime sexual partner (yes vs. no) | 1.37 | 0.75, 2.45 | ||
| Non-regular sex partner in the past year (yes vs. no) | 1.47 | 0.39, 5.53 | ||
| Partner circumcised (yes vs. no) | 1.81 | 0.82, 3.98 | ||
| Condom use in past year (yes vs. no) | 1.23 | 0.44, 3.42 | ||
| Abnormal vaginal discharge (present vs. not present) | 0.85 | 0.45, 1.61 | ||
| HIV status unknown (vs. known uninfected) | 1.26 | 0.67, 2.32 | 1.38 | 0.71, 2.67 |
| HIV-infected (vs. known uninfected) | 12.10 | 2.80, 52.33 | 16.48 | 3.29, 82.49 |
| Consumed alcohol in the past year (yes vs. no) | 0.96 | 0.42, 2.18 | ||
| Smoked cigarettes in the past year (yes vs. no) | 1.94 | 0.66, 5.69 | 3.74 | 1.15, 12.16 |
On an average, women whose age at sexual debut was 15 years of age or younger had over two times the odds of detection of high-risk HPV. This association was modified by age: for age tertiles, 18–27, 28–35 and 36–49 years, the odds of detecting high-risk HPV among women whose at first sex was ≤ 15 years versus >15 were 3.32 (95% CI: 1.27, 8.72), 1.69 (95% CI: 0.48, 5.90) and 1.19 (95% CI: 0.22, 6.38), respectively. Because of the small sample size of the study, we did not have sufficient power to detect a statistically significant interaction in logistic regression (results not shown).
Smoking cigarettes in the previous year, which was reported by 18 (5.7%) women, was not statistically significant in unadjusted analysis but positively associated with the detection of high-risk HPV (OR = 1.94; 95% CI: 0.66, 5.69). Adjustment for age indicated that the presence of negative confounding as the odds of detecting high-risk HPV was almost four times greater among smokers compared with non-smokers (OR = 3.19; 95% 1.01, 10.04) after controlling for age. The association strengthened after further adjustment for younger age at sexual debut and HIV status (Table 2).
Women who reported being HIV-infected had over 12 times greater odds of detection of high-risk HPV in unadjusted analysis (OR = 12.10; 95% CI: 2.80, 52.33) and had over 16 times greater odds of detection after adjustment (OR = 16.58; 95% CI: 3.30, 83.35) for age, younger age at sexual debut and smoking.
DISCUSSION
In this population-based sample of sexually active women in rural Uganda, the prevalence of high-risk HPV types known to cause cervical cancer was 17.2% (95% CI: 13.0%, 21.4%). Our study demonstrates a large burden of high-risk HPV among the general population of women residing in rural Uganda: none of the women had ever engaged in commercial sex work, all but one woman were currently or previously married, and few (4.1%) had non-regular sexual partners. Our finding is consistent with the two previous studies conducted in Rakai District in rural Uganda that estimated the prevalence of high-risk HPV to be 16.7% and 19% also using the Hybrid Capture 2 test.13 Our study had a high participation rate (97.3%), and women were very willing to provide self-collected vaginal specimens. Other studies have also shown high acceptability rates in rural African communities13,15,16 and further demonstrate that this technique is feasible in screening women for high-risk HPV in such settings.
Infection with high-risk HPV declined with increasing age as observed in other studies.17–20 In the absence of regular Pap screening and technology to test for high-risk HPV infection in Uganda, it is not possible to know when women in our study first became infected with high-risk HPV. Given this and the fact that HPV infection typically lasts 12–18 months before it spontaneously resolves,20 it is likely that infections in younger women represent a recent exposure to high-risk HPV that has not yet resolved, while infections in older women represent a persistent infection.21
Infection in younger women is likely owing to an earlier age of sexual debut as observed in our study. Among university students in the United States, Kahn et al. found that the relationship between younger age of sexual debut and the subsequent HPV infection was mediated by having a higher number of sexual partners, history of sexually transmitted infection, alcohol and drug use, and partner’s number of sexual partners.22 In our study in Bushenyi District, initiation of sexual activity at a younger age was significantly correlated with having a greater number of lifetime sexual partners (data not shown), but we lacked statistical power to determine if lifetime number of partners explained the relationship between earlier age of sexual debut and infection with high-risk HPV. We did not ask women in our study specifically about their partner’s number of sexual partners and lacked power to detect a significant association between polygamous marriage and high-risk HPV infection. However, it is common for men in Uganda to have non-regular sex partners outside monogamous marriage. We found that 15% of women drank alcohol in the previous year, and it was not associated with infection of high-risk HPV. We did not assess drug use, but it was relatively rare in rural Southwestern Uganda. Our finding that smoking cigarettes was associated with high-risk HPV infection, which has been shown to be associated with longer duration of HPV infection,23 should be interpreted with caution given the low prevalence of smoking among women in our study (5.7%).
The variability in the association between infection with high-risk HPV and sexual behaviour could be attributed to the differences in the sexual transmissibility of the different HPV types in this population. Studies conducted in Brazil and Denmark showed weaker associations between sexual behaviour and low-risk HPV types and stronger relationships with infection of high-risk HPV types,24,25 although residual confounding may also be the reason for this observation.
We did not find an association between oral contraceptive use in the past 12 months and high-risk HPV infection. The contraceptive prevalence rate in this community was somewhat low (14.9%). Although in vitro and in vivo evidence shows that hormonal factors may influence the transcription and translation of the HPV genome,26,27 the role of oral contraceptives in the risk of HPV infection is largely unknown.
We observed a non-significant, positive association between condom use and infection with high-risk HPV among women in our study. Relative to other sexually transmitted infections, condoms are less efficacious in preventing transmission of HPV.28 Moreover, using condoms is not the norm among couples in Uganda and women who reported using them in the previous year likely perceived their male partners of being at high-risk. Thus, any condom use among women in our study might actually be a marker of increased exposure to infected sexual partners. In fact, individuals are more likely to use condoms with partners whom they perceive as engaging in high-risk sexual behaviour (e.g. new partners, casual partners and sex workers), but not with partners whom they consider to be safe (e.g. long-term partners, regular partners and spouses).25,29,30 Ascertainment of consistent and correct condom practices was not assessed in our study.
HIV-infection, for which the prevalence was 4.9%, was strongly associated with the infection of high-risk HPV. Women who are immunocompromised because of HIV infection are at increased risk of HPV infection and infection with multiple HPV types.21,31–35 Furthermore, HIV infection is associated with persistence of HPV infection and subsequent development of cervical neoplasia.32 Although we were unable to perform Pap smears or colposcopy in this study, among HIV-infected women attending a sexually transmitted infection clinic in Kampala, Uganda, abnormal Pap smear was associated with the presence of high-risk HPV genotypes.21
A highly efficacious vaccine,36–38 against HPV was approved by the US Food and Drug Administration in 2006 (Gardasil, Merck Inc., Whitehouse Station, NJ, USA). The US Advisory Committee on Immunization Practices recommends routine vaccination of girls 11–12 years of age and administration in girls as young as nine years with ‘catch-up’ vaccination is recommended for females of 13–26 years of age not previously vaccinated.39 For an HPV vaccination programme to be effective in Uganda, it would be important to immunize young girls before their age of sexual debut in light of the high prevalence of high-risk HPV (29%) we observed in women of 19–25 years of age. Although we did not carry out genotyping to determine which specific high-risk HPV types had infected women participating in our study, other studies have demonstrated that the major oncogenic HPV types circulate in Uganda.13,16,21 Furthermore, it has been shown that a vaccine containing the seven most common HPV types would prevent about 87% of cervical cancers worldwide with little variation.40
CONCLUSION
The prevalence of high-risk HPV infection in this population-based sample of women living in this rural setting of Uganda was high. Since the vast majority of women reported having only one lifetime sexual partner, the sexual behaviour of their male partners likely plays an important role to the risk of becoming infected with high-risk HPV. Studies to understand the acceptability of receiving the HPV vaccine among young girls and their parents residing in both urban and rural Uganda are needed.
Acknowledgments
The authors acknowledge the AIDS international training and research program (AITRP) supported by the Forgarty International Center for funding this study (Grant number TW0011). Part of this work was presented as an abstract/poster presentation entitled, ‘Predictors of high-risk genital human papilloma virus infection and HPV vaccine trial acceptance in rural Uganda’, Control Number 61, at the Tenth Annual Conference on Vaccine Research sponsored by the National Foundation for Infectious Diseases. The conference took place from April 30 to May 2, 2007 at the Baltimore Marriott Waterfront Hotel.
References
- 1.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol. 2005;19:269–76. doi: 10.1016/j.bpobgyn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Sun CA, Liu JF, Wu DM, Nieh S, Yu CP, Chu TY. Viral load of high-risk human papillomavirus in cervical squamous intraepithelial lesions. Int J Gynaecol Obstet. 2002;76:41–7. doi: 10.1016/s0020-7292(01)00529-x. [DOI] [PubMed] [Google Scholar]
- 4.Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992;79:328–37. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mayaud P, Gill DK, Weiss HA, et al. The interrelation of HIV, cervical human papillomavirus, and neoplasia among antenatal clinic attenders in Tanzania. Sex Transm Infect. 2001;77:248–54. doi: 10.1136/sti.77.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liaw KL, Hildesheim A, Burk RD, et al. A prospective study of human papillomavirus (HPV) type 16 DNA detection by polymerase chain reaction and its association with acquisition and persistence of other HPV types. J Infect Dis. 2001;183:8–15. doi: 10.1086/317638. [DOI] [PubMed] [Google Scholar]
- 7.Wang SS, Schiffman M, Herrero R, et al. Determinants of human papillomavirus 16 serological conversion and persistence in a population-based cohort of 10,000 women in Costa Rica. Br J Cancer. 2004;91:1269–74. doi: 10.1038/sj.bjc.6602088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkin DM, Wabinga H, Nambooze S, Wabwire-Mangen F. AIDS-related cancers in Africa: maturation of the epidemic in Uganda. AIDS. 1999;13:2563–70. doi: 10.1097/00002030-199912240-00010. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer (IARC) GLOBOCAN 2002 database: summary table by population. 2005. [Google Scholar]
- 10.Chirenje ZM, Rusakaniko S, Kirumbi L, et al. Situation analysis for cervical cancer diagnosis and treatment in east, central and southern African countries. Bull World Health Organ. 2001;79:127–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005;23:2388–94. doi: 10.1016/j.vaccine.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Tumwesigye E, Muganzi E, Tumwebaze N. Bushenyi District Full Access home based HIV counselling and testing program annual report 2006. Feb 15, 2006. [Google Scholar]
- 13.Serwadda D, Wawer MJ, Shah KV, et al. Use of a hybrid capture assay of self-collected vaginal swabs in rural Uganda for detection of human papillomavirus. J Infect Dis. 1999;180:1316–9. doi: 10.1086/315026. [DOI] [PubMed] [Google Scholar]
- 14.Obiso R, Lorincz A. Digene Corporation. Pharmacogenomics. 2004;5:129–32. doi: 10.1517/phgs.5.1.129.25678. [DOI] [PubMed] [Google Scholar]
- 15.Lack N, West B, Jeffries D, et al. Comparison of non-invasive sampling methods for detection of HPV in rural African women. Sex Transm Infect. 2005;81:239–41. doi: 10.1136/sti.2004.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safaeian M, Kiddugavu M, Gravitt PE, et al. Comparability of self-collected vaginal swabs and physician-collected cervical swabs for detection of human papillomavirus infections in Rakai, Uganda. Sex Transm Dis. 2007;34:429–36. doi: 10.1097/01.olq.0000243623.67673.22. [DOI] [PubMed] [Google Scholar]
- 17.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 18.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 19.Wheeler CM, Parmenter CA, Hunt WC, et al. Determinants of genital human papillomavirus infection among cytologically normal women attending the University of New Mexico student health center. Sex Transm Dis. 1993;20:286–9. doi: 10.1097/00007435-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Winer RL, Koutsky LA. Human papillomavirus through the ages. J Infect Dis. 2005;191:1787–9. doi: 10.1086/430275. [DOI] [PubMed] [Google Scholar]
- 21.Blossom DB, Beigi RH, Farrell JJ, et al. Human papillomavirus genotypes associated with cervical cytologic abnormalities and HIV infection in Ugandan women. J Med Virol. 2007;79:758–65. doi: 10.1002/jmv.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn JA, Rosenthal SL, Succop PA, Ho GY, Burk RD. Mediators of the association between age of first sexual intercourse and subsequent human papillomavirus infection. Pediatrics. 2002;109:E5. doi: 10.1542/peds.109.1.e5. [DOI] [PubMed] [Google Scholar]
- 23.Giuliano AR, Sedjo RL, Roe DJ, et al. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States) Cancer Causes Control. 2002;13:839–46. doi: 10.1023/a:1020668232219. [DOI] [PubMed] [Google Scholar]
- 24.Franco EL, Villa LL, Ruiz A, Costa MC. Transmission of cervical human papillomavirus infection by sexual activity: differences between low and high oncogenic risk types. J Infect Dis. 1995;172:756–63. doi: 10.1093/infdis/172.3.756. [DOI] [PubMed] [Google Scholar]
- 25.Kjaer SK, van den Brule AJ, Bock JE, et al. Determinants for genital human papillomavirus (HPV) infection in 1000 randomly chosen young Danish women with normal Pap smear: are there different risk profiles for oncogenic and nononcogenic HPV types? Cancer Epidemiol Biomarkers Prev. 1997;6:799–805. [PubMed] [Google Scholar]
- 26.Howley PM, Schlegel R. The human papillomaviruses. An overview. Am J Med. 1988;85:155–8. [PubMed] [Google Scholar]
- 27.Moodley M, Moodley J, Chetty R, Herrington CS. The role of steroid contraceptive hormones in the pathogenesis of invasive cervical cancer: a review. Int J Gynecol Cancer. 2003;13:103–10. doi: 10.1046/j.1525-1438.2003.13030.x. [DOI] [PubMed] [Google Scholar]
- 28.Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis Sex Transm Dis. 2002;29:725–35. doi: 10.1097/00007435-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Manhart LE, Holmes KK, Koutsky LA, et al. Human papillomavirus infection among sexually active young women in the United States: implications for developing a vaccination strategy. Sex Transm Dis. 2006;33:502–8. doi: 10.1097/01.olq.0000204545.89516.0a. [DOI] [PubMed] [Google Scholar]
- 30.Scheurer ME, Tortolero-Luna G, dler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15:727–46. doi: 10.1111/j.1525-1438.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- 31.Ahdieh L, Munoz A, Vlahov D, Trimble CL, Timpson LA, Shah K. Cervical neoplasia and repeated positivity of human papillomavirus infection in human immunodeficiency virus-seropositive and -seronegative women. Am J Epidemiol. 2000;151:1148–57. doi: 10.1093/oxfordjournals.aje.a010165. [DOI] [PubMed] [Google Scholar]
- 32.Ahdieh L, Klein RS, Burk R, et al. Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682–90. doi: 10.1086/323081. [DOI] [PubMed] [Google Scholar]
- 33.Kirby TO, Allen ME, Alvarez RD, Hoesley CJ, Huh WK. High-risk human papillomavirus and cervical intraepithelial neoplasia at time of atypical squamous cells of undetermined significance cytologic results in a population with human immunodeficiency virus. J Low Genit Tract Dis. 2004;8:298–303. doi: 10.1097/00128360-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Kjaer SK, Svare EI, Worm AM, Walboomers JM, Meijer CJ, van den Brule AJ. Human papillomavirus infection in Danish female sex workers. Decreasing prevalence with age despite continuously high sexual activity. Sex Transm Dis. 2000;27:438–45. doi: 10.1097/00007435-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Palefsky JM, Holly EA. Chapter 6: Immunosuppression and co-infection with HIV. J Natl Cancer Inst Monogr. 2003;31:41–6. doi: 10.1093/oxfordjournals.jncimonographs.a003481. [DOI] [PubMed] [Google Scholar]
- 36.The FUTURE II study group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 37.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–8. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 38.Joura EA, Leodolter S, Hernandez-Avila M, et al. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369:1693–702. doi: 10.1016/S0140-6736(07)60777-6. [DOI] [PubMed] [Google Scholar]
- 39.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 40.Munoz N, Bosch FX, Castellsague X, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–85. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]


