Abstract
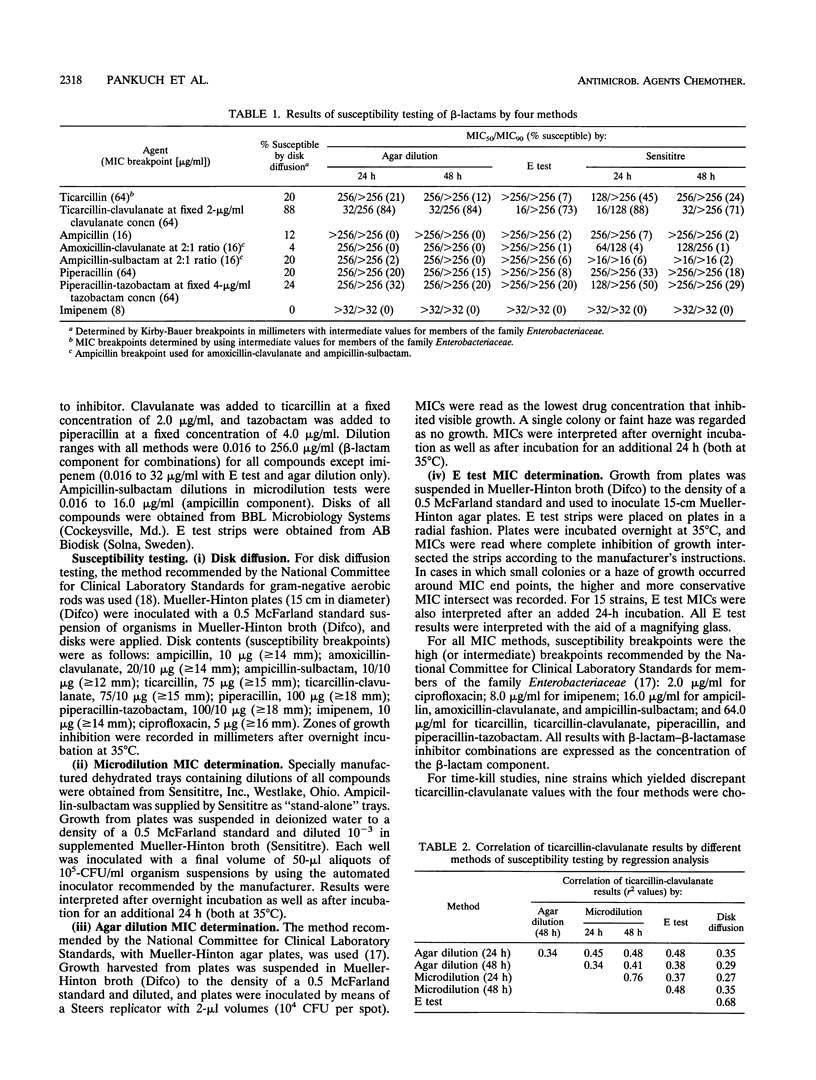
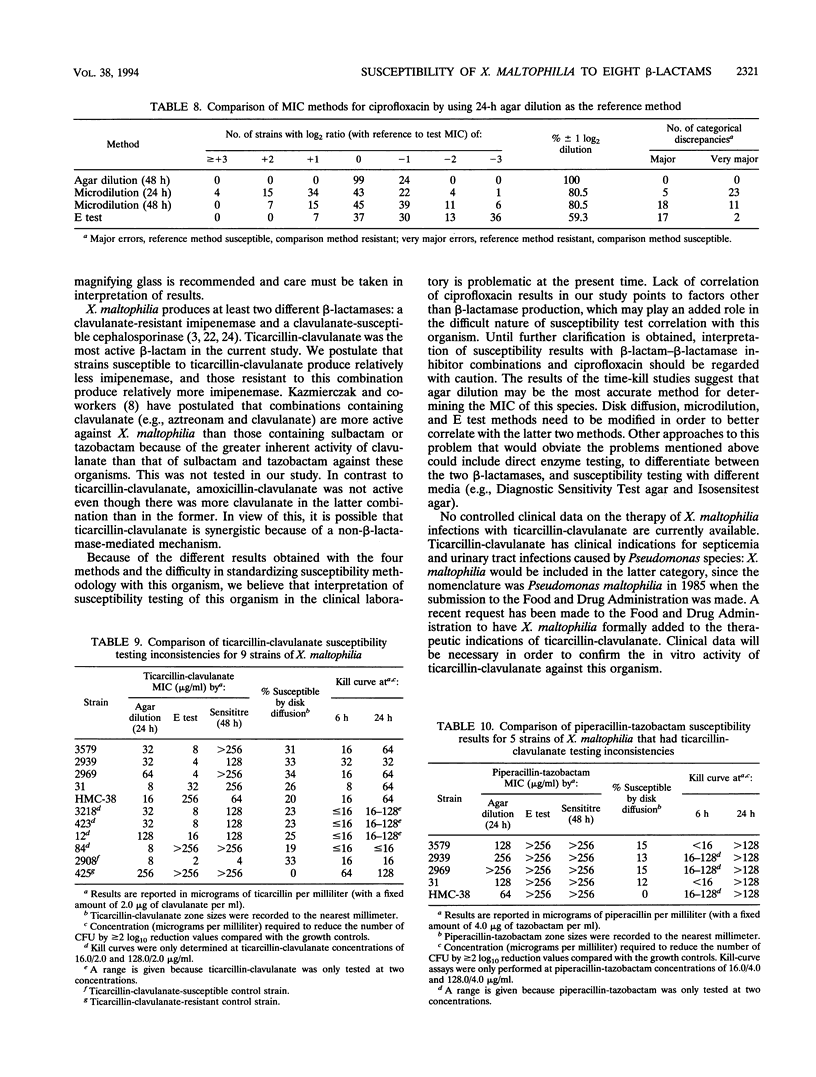
This study evaluated the susceptibility of 123 Xanthomonas maltophilia strains to ticarcillin, ticarcillin-clavulanate, ampicillin, amoxicillin-clavulanate, ampicillin-sulbactam, piperacillin, piperacillin-tazobactam, imipenem, and ciprofloxacin by Kirby-Bauer disk, E test, and Sensititre dehydrated microdilution MIC and conventional agar dilution MIC methodology. Intermediate susceptibility breakpoints for members of the family Enterobacteriaceae were used. When results were analyzed as MICs for 50 and 90% of the strains tested and percentages of strains susceptible at the breakpoint, good correlation between the methods was observed, with ticarcillin-clavulanate clearly the most active beta-lactam by all four methods. However, when the various methods were compared with the agar dilution methodology by regression analysis, poor r2 values (0.3 to 0.7) were obtained for compounds with sufficient on-scale values to permit analysis. When the number of strains with log2 ratios of reference agar dilution MICs to test MICs of +3 to -3 were analyzed, correlation was also poor, with many major and very major discrepancies for all methods tested. Results obtained with time-kill studies of nine strains with discrepant ticarcillin-clavulanate MICs appeared to correlate best when compared at 24 h with agar dilution MICs. The concentration of ticarcillin-clavulanate required to reduce the colony count by > or = 2 log10 reduction values for eight of nine strains compared with that for growth controls was < or = 16.0/2.0 micrograms/ml at 6 h and ranged from 16.0/2.0 micrograms/ml to 128.0/2.0 micrograms/ml at 24 h. The susceptibility method of choice for X. maltophilia has not yet been standardized, but time-kill studies correlated best with agar dilution MICs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Fass R. J., Anhalt J. P., Neu H. C., Thornsberry C., Tilton R. C., Painter B. G., Washington J. A., 2nd Ciprofloxacin disk susceptibility tests: interpretive zone size standards for 5-microgram disks. J Clin Microbiol. 1985 Jun;21(6):880–883. doi: 10.1128/jcm.21.6.880-883.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfiglio G., Livermore D. M. Effect of media composition on the susceptibility of Xanthomonas maltophilia to beta-lactam antibiotics. J Antimicrob Chemother. 1991 Dec;28(6):837–842. doi: 10.1093/jac/28.6.837. [DOI] [PubMed] [Google Scholar]
- Bush K. Classification of beta-lactamases: groups 2c, 2d, 2e, 3, and 4. Antimicrob Agents Chemother. 1989 Mar;33(3):271–276. doi: 10.1128/aac.33.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W. Antibiotic susceptibility and outer membrane proteins of clinical Xanthomonas maltophilia isolates. Chemotherapy. 1991;37(4):246–250. doi: 10.1159/000238862. [DOI] [PubMed] [Google Scholar]
- Hawkey P. M., Birkenhead D., Kerr K. G., Newton K. E., Hyde W. A. Effect of divalent cations in bacteriological media on the susceptibility of Xanthomonas maltophilia to imipenem, with special reference to zinc ions. J Antimicrob Chemother. 1993 Jan;31(1):47–55. doi: 10.1093/jac/31.1.47. [DOI] [PubMed] [Google Scholar]
- Hohl P., Frei R., Aubry P. In vitro susceptibility of 33 clinical case isolates of Xanthomonas maltophilia. Inconsistent correlation of agar dilution and of disk diffusion test results. Diagn Microbiol Infect Dis. 1991 Sep-Oct;14(5):447–450. doi: 10.1016/0732-8893(91)90072-n. [DOI] [PubMed] [Google Scholar]
- Khardori N., Elting L., Wong E., Schable B., Bodey G. P. Nosocomial infections due to Xanthomonas maltophilia (Pseudomonas maltophilia) in patients with cancer. Rev Infect Dis. 1990 Nov-Dec;12(6):997–1003. doi: 10.1093/clinids/12.6.997. [DOI] [PubMed] [Google Scholar]
- Khardori N., Reuben A., Rosenbaum B., Rolston K., Bodey G. P. In vitro susceptibility of Xanthomonas (Pseudomonas) maltophilia to newer antimicrobial agents. Antimicrob Agents Chemother. 1990 Aug;34(8):1609–1610. doi: 10.1128/aac.34.8.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecso-Bornet M., Pierre J., Sarkis-Karam D., Lubera S., Bergogne-Berezin E. Susceptibility of Xanthomonas maltophilia to six quinolones and study of outer membrane proteins in resistant mutants selected in vitro. Antimicrob Agents Chemother. 1992 Mar;36(3):669–671. doi: 10.1128/aac.36.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A. J., Jr, Hoffmann K. K., Wenzel R. P. Associated mortality and clinical characteristics of nosocomial Pseudomonas maltophilia in a university hospital. J Clin Microbiol. 1986 Jul;24(1):52–55. doi: 10.1128/jcm.24.1.52-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muder R. R., Yu V. L., Dummer J. S., Vinson C., Lumish R. M. Infections caused by Pseudomonas maltophilia. Expanding clinical spectrum. Arch Intern Med. 1987 Sep;147(9):1672–1674. [PubMed] [Google Scholar]
- Nagai T. Association of Pseudomonas maltophilia with malignant lesions. J Clin Microbiol. 1984 Nov;20(5):1003–1005. doi: 10.1128/jcm.20.5.1003-1005.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Inoue M., Mitsuhashi S. Purification and properties of an inducible cephalosporinase from Pseudomonas maltophilia GN12873. Antimicrob Agents Chemother. 1984 Mar;25(3):362–365. doi: 10.1128/aac.25.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino Y., Kobayashi F., Inoue M., Mitsuhashi S. Purification and properties of inducible penicillin beta-lactamase isolated from Pseudomonas maltophilia. Antimicrob Agents Chemother. 1982 Oct;22(4):564–570. doi: 10.1128/aac.22.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuravleff J. J., Yu V. L. Infections caused by Pseudomonas maltophilia with emphasis on bacteremia: case reports and a review of the literature. Rev Infect Dis. 1982 Nov-Dec;4(6):1236–1246. doi: 10.1093/clinids/4.6.1236. [DOI] [PubMed] [Google Scholar]



