Abstract
Mucins are major components in mucus secretions and apical cell membranes on wet-surfaced epithelia. Structurally, they are characterized by the presence of tandem repeat domains containing heavily O-glycosylated serine and threonine residues. O-glycans contribute to maintaining the highly extended and rigid structure of mucins, conferring to them specific physical and biological properties essential for their protective functions. At the ocular surface epithelia, mucin-type O-glycan chains are short and predominantly sialylated, perhaps reflecting specific requirements of the ocular surface. Traditionally, secreted mucins and their O-glycans in the tear film have been involved in the clearance of debris and pathogens from the surface of the eye. New evidence, however, shows that O-glycans on the cell-surface glycocalyx have additional biological roles in the protection of corneal and conjunctival epithelia, such as preventing bacterial adhesion, promoting boundary lubrication, and maintaining the epithelial barrier function through their interaction with galectin-3. Abnormalities in mucin-type O-glycosylation have been identified in many disorders where the stability of the ocular surface is compromised. This review summarizes recent advances in understanding the structure, biosynthesis, and function of mucin-type O-glycans at the ocular surface and their alteration in ocular surface disease.
Keywords: O-glycan, mucin, tear film, ocular surface epithelia, dry eye
I INTRODUCTION
Glycosylation of proteins is the most frequent and diverse form of co- and post-translational modification in living organisms. Indeed, more than half of all proteins carry one or more glycan chains.1 Traditionally, the biochemical analyses of carbohydrates on glycans have been impeded by the limited amounts of sample available. However, the development of new technologies is revealing the vast structural heterogeneity of glycans, as well as their complex biosynthetic pathways. The biological roles of glycans appear to span the spectrum from those that are trivial, to those that are crucial for the development, growth, function or survival of an organism.2
Glycans are primarily defined according to the nature of their linkages. In eukaryotic cells, glycans are covalently attached to a polypeptide backbone, usually via N- or O-linkages. In N-glycans, the carbohydrate N-acetylglucosamine (GlcNAc) is linked to the amide group of an asparagine amino acid in the consensus peptide sequence Asn-X-Ser/Thr where X denotes any amino acid except proline. In O-glycans, the sugar N-acetylgalactosamine (GalNAc) is linked to the hydroxyl groups of serine (Ser) or threonine (Thr) amino acids. A mucin is a large glycoprotein that carries multiple O-glycans (O-GalNAc glycans) clustered in central domains composed of tandem repeats of amino acids rich in Ser and Thr. Several other types of O-glycans also exist, including those O-linked through mannose, fucose, galactose, glucose, N-acetylglucosamine, and xylose.3 In this review, we will focus on O-GalNAc glycans, also known as mucin-type O-glycans.
Mucins are commonly associated to the apical epithelial surfaces of the respiratory, gastrointestinal, and reproductive tracts, as well as those of the ocular surface. Mucin oligosaccharides can provide 50–90% of the molecular mass of the mature glycoprotein, depending on the individual mucin and the pattern of glycosyltransferases expressed in the cell of origin.4,5 The vast majority of oligosaccharides on mucins are O-linked glycans, although N-glycans can also be found on regions flanking the central protein backbone. According to their molecular structure, mucins are classified as secreted or cell-surface associated (also known as membrane associated).4,5 Two types of secreted mucins have been identified, the large gel-forming mucins and the smaller nonpolymeric mucins. At the ocular surface, the major gel-forming mucin expressed by the goblet cells of the conjunctiva is MUC5AC, whereas a small nonpolymeric mucin, MUC7, is expressed by acinar cells of the lacrimal gland.6 The corneal and conjunctival epithelia express three cell-surface-associated mucins, MUC1, MUC4 and MUC16.6 MUC1, MUC4, MUC16, and MUC5AC are the major mucins present in human tear fluid.7 A comprehensive review on the topic of mucins at the human ocular surface epithelia has recently been published.8 In this review, we describe the main discoveries about the structure, biosynthetic pathways, and biological roles of mucin-type O-glycans on the human ocular surface, as well as their alterations in ocular surface disease.
II STRUCTURE OF MUCIN-TYPE O-GLYCANS
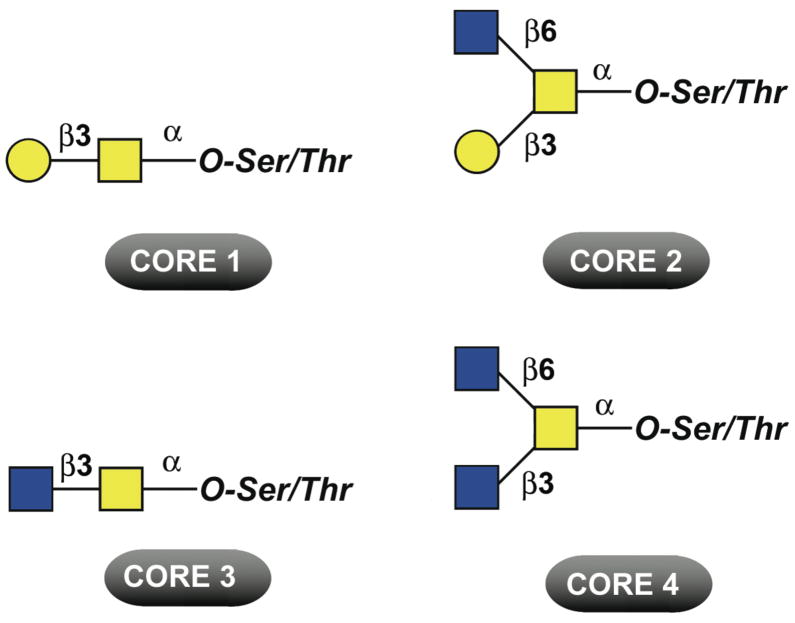
Various types of glycan chains containing between 1 and 20 carbohydrate residues can be found attached to the protein backbone in mucins.9 The simplest is the Tn antigen (GalNAcα1-Ser/Thr), which can be elongated to generate eight different core structures. Figure 1 shows the major core structures found in mammalian mucins: core 1 (Galβ1-3GalNAcα1-Ser/Thr, also known as Thomsen Friedenreich- or T-antigen), core 2 (Galβ1-3(GlcNAcβ1-6)GalNAcα1-Ser/Thr), core 3 (GlcNAcβ1-3GalNAcα1-Ser/Thr) and core 4 (GlcNAcβ1-3(GlcNAcβ1-6)GalNAcα1-Ser/Thr). These core structures may be further elongated by the addition of other carbohydrates (e.g., N-acetyllactosamine) or terminally modified by sialylation, fucosylation, or sulfation. O-glycans can exhibit a remarkable heterogeneity and diversity, as mucins can additionally carry blood group ABH- and Lewis-related carbohydrate antigens. Tissue- and cell-type differences in glycosyltransferase activities contribute to the remarkable variety of complex O-glycans found in nature.
Figure 1. Common O-glycan core structures found on mucins.
Symbols represent galactose ( ), N-acetylgalactosamine (
), N-acetylgalactosamine ( ), and N-acetylglucosamine (
), and N-acetylglucosamine ( ).
).
A. O-glycans in the Tear Film
The compositional analysis of the carbohydrate content of mucins from human tear secretions was initially carried out in the 1980s.10–12 Using alkaline beta-elimination and sodium borohydride reduction, Chao et al. (1983) demonstrated that at least 22% of all hydroxyamino acid residues in high molecular weight fractions isolated from human ocular mucus secretions were O-glycosidically linked.10 Analysis of the chemical composition of the purified mucins revealed that approximately 55% of the mucin mass was carbohydrate, with galactose, N-acetylgalactosamine, N-acetylglucosamine and sialic acid as major constituents and with minor amounts of fucose, mannose and glucose.12,13 While N-acetylgalactosamine is found on all core O-glycans as the Tn antigen, mannose, and N-acetylglucosamine contribute to forming the N-glycan core (Manα1-6(Manα1-3)Manβ1-4GlcNAcβ1-4GlcNAcβ1-Asn).
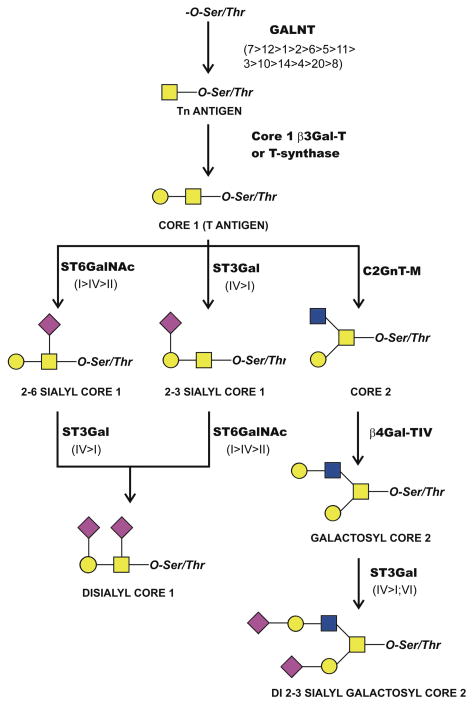
The structure of O-glycan chains in the tear film has been recently characterized.14 In these experiments, mucin-type O-glycans from normal tear fluid were fluorescently labeled and analyzed by normal-phase fluorometric high-performance liquid chromatography (HPLC) coupled with exoglycosidase digestions. It was found that, in normal tear film, the O-glycan profile consists primarily of core 1-based structures and includes core 1, α2-6 sialyl core 1, and α2-3 sialyl core 1 (Figure 2). α2-6 sialyl core 1 was the most abundant (48%), followed by core 1 (16%), and α2-3 sialyl core 1 (8%). These results indicate that O-glycans in the tear film are relatively small as compared to O-glycans found in other mucosal secretions, such as the intestinal and respiratory tracts,15,16 perhaps reflecting the specific requirements of the ocular surface.17,18
Figure 2. Mucin-type O-glycans and their biosynthetic pathways at the ocular surface.
Core 1 and monosialyl core 1 structures are commonly found in human tears and conjunctival epithelium. Disialyl core 1 and modified core 2 structures are present in the conjunctival epithelium. Glycosyltransferases potentially involved in the initial biosynthesis and elongation of mucin-type O-glycans include polypeptide-GalNAc-transferases (GALNT), the core 1 β1,3-galactosyltransferase (T-synthase or c1galt1), the core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT-M), and the β1,4-galactosyltransferase (β4Gal-TIV). In addition to these glycosyltransferases, three α2-6-sialyltransferases (ST6GalNAc-I, II and IV) and three α2-3-sialyltransferases (ST3Gal-I, -IV and VI) are involved in the addition of terminal sialic acid to O-glycan chains. The relative levels of expression of individual glycosyltransferase isoforms are indicated in parentheses. Symbols represent galactose ( ), N-acetylgalactosamine (
), N-acetylgalactosamine ( ), N-acetylglucosamine (
), N-acetylglucosamine ( ), and N-acetylneuraminic acid (
), and N-acetylneuraminic acid ( ).
).
B. O-glycans in Ocular Surface Epithelia
1. Corneal Epithelium
The ability of plant lectins to recognize and bind specific sugar residues has been commonly used to gain information on the carbohydrate composition of epithelial surfaces. Several plant lectins have high affinity for common O-glycan structures.19 Arachis hypogaea agglutinin (peanut lectin, PNA) is very specific for terminal β-D-galactose residues, showing strong reactivity with core 1 O-glycan structures. In neuraminidase-treated paraffin sections of human, cat, and rabbit corneas, PNA stained the entire epithelium, implying the presence of sialylated core 1 O-glycans on the epithelial glycocalyx.20 The presence of core 1 structures has also been detected on the cell-surface of cultured telomerase-immortalized human corneal-limbal epithelial cells, using jacalin, a lectin from Artocarpus integrifolia, specific for the T-antigen.21 Other carbohydrate epitopes, such as the blood group ABH antigens, have also been localized on the human corneal epithelium.22–24 However, it remains unclear whether these structures are present on mucin O-glycans, as the lectins used in these studies can also recognize carbohydrates on other glycoconjugates.
Antibodies to carbohydrate moieties on mucins have been extremely useful to partially determine the O-glycan composition of human ocular surface epithelia. Watanabe et al. (1995) produced a monoclonal antibody designated H185, which bound to apical membranes of apical cells, particularly at the tips of microplicae of human corneal, conjunctival, laryngeal, and vaginal epithelial cells.25 By immunoblot analysis, H185 bound to a high molecular weight protein from both corneal epithelium and cultured corneal epithelium. The H185 binding to blotted proteins was destroyed by sodium periodate treatment and O-glycanase incubation, suggesting that the epitope of H185 is an O-linked carbohydrate. More recently, it has been shown that the H185 epitope is an O-acetylated sialic acid epitope on the cell-surface mucin MUC16.26,27 Interestingly, the H185 antigen is not carried by the membrane-associated mucin MUC1, suggesting that the composition and/or structural conformation of O-glycans on individual mucins is different at the ocular surface.26 Other antibodies to mucin carbohydrate epitopes that bind apical cell membranes of superficial cells of cornea and conjunctiva include the KL-6 and 15H10 monoclonal antibodies, which recognize a sialylated epitope on MUC128 and an uncharacterized carbohydrate epitope on MUC4,29 respectively.
2. Conjunctival Epithelium
Initial attempts to characterize specific O-glycan structures on normal human conjunctival mucins were performed by Berry et al. (1996).17 Using the IE3 and TKH2 antibodies, the authors found that mucins purified from conjunctival tissue were polydisperse and contain mainly short oligosaccharide side chains, including the Tn (GalNAcα1-Ser/Thr) and sialyl-Tn (NeuAcα2-6GalNAcα1-Ser/Thr) antigens. These studies, however, analyzed tissue extracts that contained a mixture of precursor and mature mucins, as well as both cell-surface and secreted mucins, which may explain the presence of mucin glycoforms. Similarly, studies showing that MUC5AC in conjunctival epithelium is present as glycoforms with different charges (containing sialic acid and sulfate residues) were based on a mixture of precursor and mature mucins.30 Larger carbohydrate epitopes, including blood group ABH- and Lewis-related carbohydrate antigens, have also been localized using antibodies within the goblet cell mucin packets and on epithelial cells,31 but it remains unclear whether these larger carbohydrate epitopes are present on glycoconjugates other than mucin O-glycans.
An exquisite analysis of the structures of mucin-type O-glycans isolated from human conjunctiva has been recently performed.32 In this study, mucins were purified from conjunctival tissue using standard protocols, and their O-glycans were released by hydrazinoloysis and subsequently analyzed by a combination of HPLC, exoglycosidase digestions, and tandem mass spectrometry. Over 70% of the oligosaccharides found were core 1-based structures, and included core 1 (4.4%), α2-3 sialyl core 1 (47.4%), α2-6 sialyl core 1 (3.0%) and disialyl core 1 (16.1%) (Figure 2). Interestingly, di 2-3 sialyl galactosyl core 2 (4.1%) and galactosyl core 2 (1.5%), but not sialyl Tn or blood group saccharides, were also identified in human conjunctiva.32 These results are consistent with previous lectin work suggesting that galactose, N-acetylgalactosamine, N-acetylglucosamine, and sialic acid, but not fucose and mannose, were detectable in human conjunctival goblet cells.33 Conjunctival mucins have a species-specific glycan expression pattern, as a high percentage of sialylated structures are found in human conjunctival mucins, whereas fucosylated structures predominate in rabbit and dog conjunctival mucins.32
Of interest is the comparison between O-glycans in human conjunctival epithelium and those found in tears. Overall, short core 1-based structures are prevalent both in human conjunctival and tear mucins.14,32 However, the conjunctival epithelium contains disialyl core 1 O-glycans and core 2-based structures, which are absent in the tear film. Also, α2-3 sialyl core 1 is the main O-glycan in human conjunctival tissue, whereas in tears it is α2-6 sialyl core 1. Possible reasons for these discrepancies include (i) the preparation of the samples before analysis, (ii) degradation of O-glycans by glycosidase activity in tears, (iii) differences in cellular trafficking or, alternatively, (iv) the fact that mucin-type O-glycans in conjunctival tissue are associated to intracellular or cell-surface glycoproteins and not secreted into the tear film. The presence of core 2 O-glycans in conjunctiva, as well as the different amount of α2-3 sialyl core 1 and α2-6 sialyl core 1 in conjunctival epithelial cells and tear fluid suggest unique roles for these mucin-type O-glycans at the ocular surface.
3. Lacrimal Gland and Nasolacrimal Duct
The secreted macromolecules isolated from culture medium of human lacrimal gland explants consist of multiple components, with molecular weights ranging from 104 to 106 daltons, and are composed of approximately 8–26% carbohydrates.34 Both membrane-associated and secreted mucins have been detected in the lacrimal gland.35,36 Although no specific information is available on O-glycan composition or structure in the human lacrimal gland, lectin histochemistry has shown that the gland contains N-acetylglucosamine, N-acetylgalactosamine, D-galactose, D-mannose, sialic acid and L-fucose.37 Similar lectin experiments have been performed to determine the carbohydrate composition of the lacrimal sac and the nasolacrimal duct—a pseudostratified, columnar epithelia rich in goblet cells—that express membrane-associated and secreted mucins.38 N-acetylgalactosamine, N-acetylglucosamine, fucose and α2,6 sialic acid have been detected in goblet cells, whereas α2-3 and α2-6 sialic acids have been detected in epithelial cells.39
III BIOSYNTHESIS OF MUCIN-TYPE O-GLYCANS AT THE OCULAR SURFACE
The biosynthesis of O-glycans is primarily determined by glycosyltransferases that assemble activated monosaccharides (nucleotide sugars) into linear and branched glycan chains. As compared to the biosynthesis of N-glycans, initiated in the endoplasmic reticulum by transfer of a preformed lipid-linked oligosaccharide precursor, mucin-type O-linked glycosylation is initiated in the Golgi complex by a family of polypeptide-GalNAc-transferases (GALNT), which transfer UDP-GalNAc to the hydroxyl groups in serine and threonine residues to form the Tn antigen (Figure 2). There is evidence for 18 distinct mammalian GALNTs, although homology searches reveal the potential for a total of 20 isoforms in humans.40 Glycogene microarray and Taqman real-time PCR analyses have revealed that human conjunctival epithelium expresses 16 GALNTs, including GALNT1-15, and 20.14,41 On ocular surface epithelia, the distribution of GALNT isoforms is cell-layer- and cell-type-specific.42 Immunohistochemistry ha shown that the GALNT4 isoenzyme is found in the apical cell layers of stratified corneal and conjunctival epithelia, whereas GALNT2 is found in the supranuclear region of the basal cell layers. Binding of antibody to GALNT6 is restricted exclusively to conjunctival goblet cells in normal epithelium. This specific distribution of GALNT may ensure proper O-glycosylation of proteins and mucins present in different cell types or, alternatively, a given protein may be differentially glycosylated as the cell migrates from the basal cell layer to the surface of the epithelium to sustain specific functions, such as adhesion in basal cells or hydration in apical cells.42
Analyses of the O-glycan profile in normal tears and conjunctival epithelium have provided the basis for further delineating the potential biosynthetic pathways of mucin-type O-glycosylation at the ocular surface. Glycogene microarray analysis has demonstrated expression of (i) core 1 β1,3-galactosyltransferase (also known as T-synthase or c1galt1), which is responsible for the biosynthesis of core 1 present in tears and conjunctival epithelium, and (ii) the mucin-type core 2 β1,6-N-acetylglucosaminyltransferase (C2GnT-M), which is responsible for the biosynthesis of core 2 present in conjunctival epithelium (Figure 2).14 Core 2 can then be elongated to form galactosyl core 2. By microarray, the galactosyl residue is added by β4Gal-TIV, the most efficient member of the β1,4-galactosyltransferase family in adding galactose to core 2 O-glycans.43
Sialic acids are a group of derivatives of the negatively charged carbohydrate neuraminic acid, the most common of which on human mucins being N-acetyl-neuraminic acid (NeuAc). To date, 20 members of the mammalian sialyltransferase family have been cloned.44 The sialyltransferases ST6GalNAc-I, -II and -IV, identified by microarray in conjunctiva,14 are considered the best candidates to mediate the biosynthesis of α2-6 linkages in mucin-type O-glycans at the ocular surface according to their documented substrate specificity.45 Additionally, ST3Gal-I and -IV, which are known to act preferentially on Galβ1-3GalNAc substrates,46,47 could potentially catalyze the addition of α2-3 sialic acids to core 1, whereas ST3Gal-VI, known to use primarily Galβ1-4GlcNAc as an acceptor substrate,48 could mediate the attachment of α2-3 sialic acid to core 2.
IV BIOLOGICAL ROLES OF MUCIN-TYPE O-GLYCANS
Mucin-type O-glycans are critical not only to protection and maintenance of mucosal epithelial integrity, but also for viability of the entire organism. Insights into the biological roles of mucin-type O-glycans in mammalians have been recently obtained with knockout mice, targeting glycosyltransferases critical for the biosynthesis of core O-glycans. Genetic ablation of core 1 β1,3-galactosyltransferase (T-synthase) in mice causes defective angiogenesis and fatal brain hemorrhages by embryonic day 14.49 Mice deficient in mucin-type core 2 β1,6-N-acetylglucosaminyltransferase (C2GnTM) exhibit impaired mucosal barrier function and increased susceptibility to colitis.50 Similarly, mice lacking core 3 O-glycan in the colon through a targeted deletion of the core 3 β1,3-N-acetylglucosaminyltransferase (C3GnT) gene have reduced expression of Muc2 and are highly susceptible to experimental colitis and colorectal adenocarcinoma.51 These results highlight the critical involvement of mucin-type O-glycans in maintaining a wet-surfaced phenotype. This section describes the functional roles of mucin-type O-glycans at the ocular surface epithelia.
A. Tear Film O-glycans
1. Clearance of Molecules and Microorganisms
Mucus lining epithelial surfaces provides an important innate immune function by clearing noxious molecules, and by trapping and removing pathogens and particulates from mucosal surfaces.52 Fleiszig et al. (1994, 2003) have shown that human tear fluid can protect corneal epithelial cells against P. aeruginosa virulence through a mechanism that does not involve bacteriostatic activity, but instead binding of the pathogen to the ocular mucin and, therefore, modulating accessibility to the epithelial glycocalyx.53,54 P. aeruginosa appears to bind preferentially to a high molecular weight glycoprotein in human tears that contains α2-6 sialic acid, as shown by staining with Sambucus nigra agglutinin.55 As described above, secreted mucin-type O-glycans in tear fluid contain extensive amounts of α2-6 sialic acids as compared to conjunctival epithelial tissue (which contains primarily α2-3 sialic acids), constituting potential ligands for P. aeruginosa binding.
2. Hydration and Lubrication
Mucin-type O-glycans contribute to preventing the desiccation of the ocular surface by providing a hydrophilic character to mucins.56 As compared to the nonglycosylated domains of mucins, which are rich in hydrophobic amino acids, the glycosylated regions of mucins have the capacity to bind water.57 The differing affinities for water of these two domains can lead to physical gelation by a mechanism similar to that seen in synthetic multiblock copolymers.57 Mucus secretions are, therefore, thought to have important protective and lubricating properties, primarily owing to their ability to form a gel.
B. Cell-surface O-glycans
1. Barrier Function
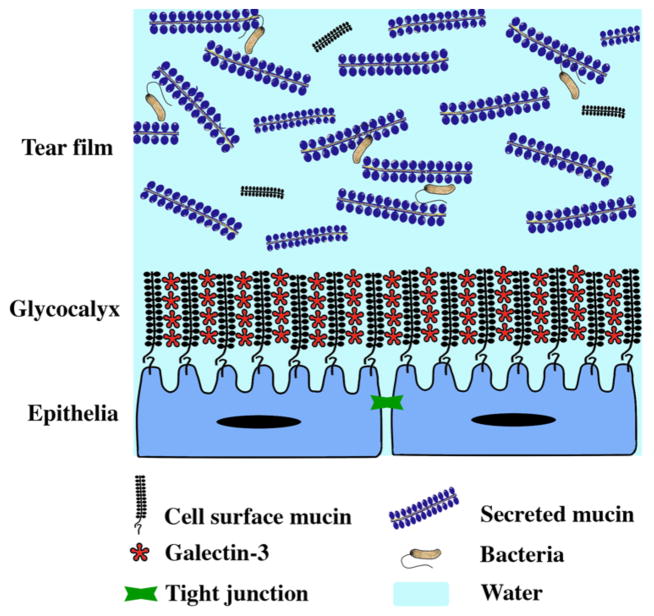
O-glycans are important to maintaining the highly extended and rigid structure of mucins, as clustered O-glycans induce the peptide core to adopt a stiff and extended conformation that prevents folding into a globular structure.9,58,59 As a result of this extended conformation, mucins and their O-glycans can extend up to 300 nm above the apical membrane of apical cells at the ocular surface,60 therefore, constituting a candidate to maintain the mucosal epithelial barrier on the apical glycocalyx (Figure 3). Evidence has shown that protection of ocular surface epithelial cells against penetrance of diagnostic dyes seems to be dependent on the biosynthesis of O-glycans. In vitro, appearance of islands of corneal epithelial cells that exclude the rose bengal diagnostic dye correlates with the biosynthesis of core 1 O-glycans on the apical surface.21 More recently, a mechanism for epithelial barrier formation at the ocular surface has been proposed and involves interaction of cell-surface-associated mucins and their O-glycans with the carbohydrate-binding protein galectin-3.61 Galectin-3 is a 35-kDa β-galactoside-binding lectin that can interact in a multivalent fashion and crosslink glycan ligands on cell-surface receptors to generate molecular lattices. In corneal and conjunctival epithelia, experiments have shown that galectin-3 colocalizes with MUC1 and MUC16 on the apical cell surface.61 Moreover, abrogation of the mucin-galectin interaction using competitive carbohydrate inhibitors of galectin binding has resulted in decreased levels of galectin-3 on the corneal epithelial cell surface, with concomitant loss of barrier function, as indicated by increased permeability to rose bengal. Likewise, downregulation of mucin O-glycosylation, using a stable tetracycline-inducible system to knockdown T-synthase, also resulted in impaired barrier function, with a concomitant reduction of galectin-3 on the apical cell surface of human corneal epithelial cells. These results provide a new model of barrier formation at the ocular surface that involves association of mucin O-glycans with galectin-3 (Figure 3). This model includes components of the cell-surface glycocalyx in addition to the previously characterized tight junctions. Other components (e.g., galectin-8 and -9, trefoil peptides, divalent cations) may also contribute to stabilizing the glycocalyx barrier at the ocular surface, but their specific contributions remain unclear.
Figure 3. Functions of mucin-type O-glycans at the ocular surface.
Cell-surface O-glycans at the apical membrane of epithelial cells bind multiple galectin-3 oligomers to form a highly organized cell-surface barrier. This barrier, in conjunction with tight junctions which seal the space between adjacent cells, contributes to protection of the epithelia against noxious molecules and pathogens. Cell-surface O-glycans also confer a disadhesive character to the ocular epithelia that limits pathogen colonization and prevents the tarsal conjunctival epithelial surface of the eye from adhering to the cornea during blinking or sleep. Secreted O-glycans in the tear film contribute to retention of water and lubrication of the surface of the eye, and facilitates the clearing and discharge of pathogens from the ocular surface.
2. Prevention of Apical Cell-surface Adhesion
Cell-surface-associated mucins and their O-glycans are constitutively expressed on apical membranes of epithelia that require a lumen and are, thus, anti-adhesive. At the ocular surface, the prolonged contact between the corneal and conjunctival epithelial surfaces requires a specialized apical cell surface with a disadhesive character. It is generally recognized that the extended mucin protein backbone on cell-surface-associated mucins confers an anti-adhesive character to cell surfaces. Overexpression of membrane-associated mucins induces morphological changes, cell detachment, and cell-cell dissociation of tumor cells in culture.62 The anti-adhesive character of mucins from human conjunctival tissue has been observed using atomic force microscopy, where the number of mucin-mucin interactions is minimal as compared to mucin-mica interactions.63 More recently, Sumiyoshi et al. (2008) used static and dynamic flow adhesion assays in corneal epithelial cells to demonstrate that O-glycans have an anti-adhesive character at the apical cell surface.64 In these experiments, disruption of mucin O-glycosylation using the chemical primer benzyl-α-GalNAc resulted in an increased adhesive character of the apical surface of differentiated corneal epithelial cells, supporting the involvement of mucin-type O-glycans in boundary lubrication and prevention of adhesion of opposite surfaces during blinking or sleeping.
3. Protection Against Pathogen Colonization
The ability of microorganisms to adhere to the epithelial cell glycocalyx is thought to be one of the first steps in the colonization and infection of mucosal surfaces. Both viruses and bacteria use receptors in the glycocalyx to gain access to the underlying epithelium. It has been proposed that cell-surface mucins contribute to host defense against Campylobacter jejuni infection in the gastrointestinal tract by expressing diverse and complex oligosaccharides that act as releasable decoy ligands for bacterial adhesins, thereby limiting attachment of pathogens to other cell-surface molecules and subsequent invasion.65 On the other hand, cell-surface mucins and their O-glycans are also known to restrict adenoviral vector access to apical receptors expressed in respiratory epithelium,66 suggesting a dual role for exposed mucin O-glycans by masking specific cell-surface receptors or acting as decoys. Abrogation of mucin O-glycosylation in corneal epithelial cells using the chemical primer benzyl-α-GalNAc resulted in increased adherence of Staphylococcus aureus to apical human corneal epithelial cells and to biotinylated cell-surface proteins in static and liquid phase adhesion assays, consistent with the role of mucin O-glycans in preventing bacterial adhesion by limiting access to the epithelial cell surface.67 Similarly, surface mucins and their sialic acids seem to act as a protective barrier against Streptococcus pneumoniae adhesion in conjunctival epithelial cells.68 In addition, cell-surface mucins at the ocular surface contain sialic acid O-acetyl groups, known to confer resistance against degradation by some bacterial and viral sialidases, therefore, constituting an alternative mechanism that prevents mucin degradation and access to specific receptors at the ocular surface.27
V O-GLYCOSYLATION IN OCULAR SURFACE DISEASE
Abnormalities in mucin-type O-glycosylation have been observed in many diseases such as cancer, inflammatory bowel disease, and cystic fibrosis. Mucins produced in cancer cells have an altered expression of glycosyltranferases that commonly results in short and highly sialylated O-glycan chains, such as the Tn and sialyl-Tn antigens.69 Alterations in O-glycan structures in cancer have many biological and pathological consequences for the function of epithelial cells—altering their antigenic and adhesive properties, as well as their potential to invade and metastasize.70 Inflammatory bowel diseases and cystic fibrosis are characterized by alterations in sulfation and sialylation of terminal O-glycan chains, which may result in a detrimental effect on the physico-chemical properties of the mucus, its protective barrier function, which would in turn impact bacterial colonization.15,71 Other reported diseases with defective mucin-type O-glycosylation include the Tn syndrome, a rare autoimmune disease in which subpopulations of blood cells carry an incompletely glycosylated membrane glycoprotein due to mutations in Cosmc, a molecular chaperone required for the proper folding and activity of T-synthase;72 IgA nephropathy, an immune disorder characterized by reduced galactosylation of the Tn-antigen in IgA1;73 and familial tumoral calcinosis, an autosomal recessive disorder characterized by a mutation in GALNT3.74 This section provides an overview of the alterations in mucin-type O-glycosylation identified in ocular surface disorders.
A. Dry Eye
Dry eye is a multifactorial disease of the ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface.75 Several authors have reported changes in the distribution of mucin-type O-glycans in the epithelial glycocalyx of patients with dry eye. These include an alteration in the distribution of O-acetyl sialic acids on MUC16, as determined by H185 antibody binding, in the apical conjunctival epithelium of patients with dry eye and superior limbic keratoconjunctivitis.27,76,77 In these patients, H185 binding to the apical cell surface was lost, whereas it was increased in goblet cells. To date, it is unknown whether the H185 antigen is present on the goblet cell-specific MUC5AC. In addition, a decrease in core 1 has been detected by transmission electron microscopy in secretory granules of goblet cells in dry eye,78 perhaps reflecting a decrease in T-synthase activity in the goblet cells. Using the KL-6 antibody, Hayashi et al. (2004) have also shown that sialylation of MUC1 in conjunctival epithelium is altered in opposite ways in dry eye as the disease progresses, increasing in mild and moderate dry eye, but diminishing in severe dry eye, suggesting that upregulation of cell-surface O-glycosylation may in part alleviate the consequences caused by goblet cell mucin dysfunction in the disease’s early stages.28
As compared to the epithelial glycocalyx, a recent study showed no differences in the total amount and structures of mucin O-glycans in the tear film between patients with dry eye and control subjects, by either HPLC or lectin blot.14 These results contrast with those reported by Garcher (1998) and Nakamura (2004), which show a decrease in sialic acid and sialyl-Lewis a in tears of dry eye patients, using HPLC and the CA 19-9 ELISA test.79,80 The decrease reported by these authors, however, could be attributed to changes in glycolipids and N-linked glycans present in the tear film, as no sialyl-Lewis a has been reported in tear and conjunctival mucin O-glycans.14,32
The expression of glycosyltransferase genes involved in mucin-type O-glycosylation in the conjunctival epithelium of patients with dry eye has been analyzed by real time RT-PCR and microarray.14,41 No differences in mRNA expression of mucin-type glycosyltransferases have been detected between normal subjects and patients with dry eye. In these studies, however, samples were harvested by both impression and brush cytology, therefore, mixing the RNA from both goblet and non-goblet cells in the stratified epithelium. On the other hand, immunofluorescence studies have revealed alterations in the cell-type and cell-layer distribution of GALNT glycosyltranferases in patients with ocular cicatricial pemphigoid (OCP).42 In this study, GALNT1, -2, -3, -4, and -6 (GALNT6 is goblet cell-specific in normal epithelium) increased in the nonkeratinized conjunctival epithelium of patients with OCP, suggesting an initial attempt by the epithelium to maintain a wet-surface phenotype by upregulating glycosyltranferase expression at early stages in this pathology. As the disease progresses and the epithelia become keratinized, the expression of GALNT enzymes is lost. Although no localization studies have been performed with other mucin-type glycosyltransferases, it has been hypothesized that alteration in the local distribution of these enzymes, not in their overall expression, might correlate with altered cell-surface O-glycosylation and epithelial damage in dry eye.14
B. Contact Lens Wear and Infection
As discussed in section IVB3, the ability of pathogens to adhere to the epithelial cell glycocalyx is thought to be one of the first steps in the colonization and infection of mucosal surfaces. Extended contact lens wear is commonly associated with an increased incidence of microbial keratitis.81 It has been hypothesized that microabrasions on the apical epithelial cell surface caused by contact lens shear stress, dryness, or hypoxia alter the mucin O-glycan composition of the epithelial glycocalyx, resulting in a more adhesive surface, and contributing to bacterial adhesion and invasion.67
In humans, the apical ocular surface glycocalyx of patients with superior limbic keratoconjunctivitis (a disease commonly associated with contact lens wear) contains reduced levels of O-acetyl sialic acid on MUC16,27,77 which suggests that alteration of terminal O-glycans may compromise the mucin barrier and increase the risk of bacterial keratitis. Furthermore, the presence of terminal carbohydrates also appears to be altered by histochemistry in non-goblet conjunctival epithelial cells of contact lens wearers.82 Core 1 structures, on the other hand, seem to be altered in goblet cells of asymptomatic contact lens wearers, but not in their stratified epithelia.83 Experimental work in the 1990s with lectin binding in rabbits has also revealed that contact lens wear modifies the corneal epithelial glycocalyx, which has been shown by electron microscopy to be thinner and have altered glycan chains.84,85 Unfortunately, no data are available on the effect of newer generations of contact lenses with improved polymer chemistry and oxygen permeability in the structural integrity of ocular surface epithelial glycocalyx. Overall, these data suggest that alterations in the amount and composition of cell-surface O-glycans associated with contact lens wear could contribute to a higher risk of infection.
C. Pterygium
Pterygium is a disorder of the ocular surface associated with chronic UV exposure and characterized by proliferation, inflammatory infiltrates, fibrosis, angiogenesis and extracellular matrix breakdown.86 Several authors have reported abnormalities in the biosynthesis of O-glycans in pterygium. By lectin histochemistry, apical conjunctival epithelial cells in pterygium have been shown to have increased expression of core 1 as compared to normal tissue.87 Similarly, increased sialylation on MUC1 has also been detected in pterygium using the KL-6 antibody.88 On the other hand, decreased sialyl-Lewis a immunoreactivity and lower ST3Gal-III expression have been reported in conjunctival epithelium of patients with pterygium.89 In the latter study, however, it remains unclear whether this decrease relates to mucin-type O-glycosylation, as no sialyl-Lewis a is found on mucin O-glycans in human conjunctival epithelium32 and the acceptor substrate specificity of ST3Gal-III is preferential to non-mucin substrates.14
D. Ocular Rosacea
Rosacea is a chronic skin disorder frequently associated with inflammation of the ocular surface and eyelids. Analyses by mass spectrometry have shown that the O-glycan content in human tear fluid of patients with ocular rosacea contains high levels of anionic oligosaccharides.90 Some of these oligosaccharides seem to be distinctive markers of the disease and have, therefore, been proposed as biomarkers for these patients, particularly in those lacking the typical facial skin manifestations.
VI SUMMARY AND CONCLUSIONS
During the last decade, many aspects of the structure and biological roles of mucin-type O-glycans have been revealed, due in part to the development of new analytical tools. At the ocular surface, O-glycans are relatively small as compared to those found in other mucosal secretions, perhaps reflecting specific requirements of exposed ocular surface epithelia. Their functions are diverse, ranging from clearance of pathogens in the tear film to the formation of an organized cell-surface barrier through their interaction with galectin-3. Some of these functional properties of mucin-type O-glycans could be used to design new methods to prevent infection of the ocular surface or enhance the delivery of topical drugs into the epithelia. Alterations in mucin-type O-glycosylation have been observed in different pathologies, but many others remain to be studied. Further efforts to determine additional functions of O-glycans and their alteration in disease could lead to the development of new approaches to prevent ocular surface disease and to restore a healthy phenotype after the ocular surface has been compromised.
Acknowledgments
Supported by the National Eye Institute of the National Institutes of Health, EY014847 (PA), and a postdoctoral fellowship from the Alfonso Martin Escudero Foundation in Spain (AG).
Abbreviations
- C2GnT-M
core 2 β1,6-N- acetylglucosaminyltransferase
- C3GnT
core 3 β1,3-N- acetylglucosaminyltransferase
- GalNAc
N-acetylgalactosamine
- GALNT
polypeptide-GalNAc-transferase
- GlcNAc
N-acetylglucosamine
- HPLC
high-performance liquid chromatography
- NeuAc
N-acetyl-neuraminic acid
- OCP
ocular cicatricial pemphigoid
- PNA
Arachis hypogaea agglutinin (peanut lectin)
- Ser
Serine
- ST3Gal
Galactoside α2,3-sialyltransferase
- ST6GalNAc
N-acetylgalactosaminide α2,6- sialyltransferase
- Thr
Threonine
- Tn antigen
GalNAcα1-Ser/Thr
- T-synthase or c1galt1
core 1 β1,3-galactosyltransferase
Footnotes
The authors have no proprietary or commercial interests in any concept or product discussed in this article.
References
- 1.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 2.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A. Essentials of Glycobiology. 2. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2009. p. xxix.p. 784. [PubMed] [Google Scholar]
- 4.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–57. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 5.Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–86. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- 6.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–48. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 7.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–50. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–83. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Steen P, Rudd PM, Dwek RA, et al. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 10.Chao CC, Vergnes JP, Brown SI. O-glycosidic linkage in glycoprotein isolates from human ocular mucus. Exp Eye Res. 1983;37:533–41. doi: 10.1016/0014-4835(83)90129-x. [DOI] [PubMed] [Google Scholar]
- 11.Moore JC, Tiffany JM. Human ocular mucus. Chemical studies Exp Eye Res. 1981;33:203–12. doi: 10.1016/s0014-4835(81)80069-3. [DOI] [PubMed] [Google Scholar]
- 12.Chao CC, Butala SM, Herp A. Studies on the isolation and composition of human ocular mucin. Exp Eye Res. 1988;47:185–96. doi: 10.1016/0014-4835(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 13.Argueso P, Herreras JM, Calonge M, et al. Analysis of human ocular mucus: effects of neuraminidase and chitinase enzymes. Cornea. 1998;17:200–7. doi: 10.1097/00003226-199803000-00015. [DOI] [PubMed] [Google Scholar]
- 14.Guzman-Aranguez A, Mantelli F, Argueso P. Mucin-type O-glycans in tears of normal subjects and patients with non-Sjogrens dry eye. Invest Ophthalmol Vis Sci. 2009;50:4581–7. doi: 10.1167/iovs.09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia B, Royall JA, Damera G, et al. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15:747–75. doi: 10.1093/glycob/cwi061. [DOI] [PubMed] [Google Scholar]
- 16.Podolsky DK. Oligosaccharide structures of human colonic mucin. J Biol Chem. 1985;260:8262–71. [PubMed] [Google Scholar]
- 17.Berry M, Ellingham RB, Corfield AP. Polydispersity of normal human conjunctival mucins. Invest Ophthalmol Vis Sci. 1996;37:2559–71. [PubMed] [Google Scholar]
- 18.McMaster TJ, Berry M, Corfield AP, et al. Atomic force microscopy of the submolecular architecture of hydrated ocular mucins. Biophys J. 1999;77:533–41. doi: 10.1016/S0006-3495(99)76910-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peumans WJ, Van Damme EJ. Plant lectins: specific tools for the identification, isolation, and characterization of O-linked glycans. Crit Rev Biochem Mol Biol. 1998;33:209–58. [PubMed] [Google Scholar]
- 20.Panjwani N, Moulton P, Alroy J, et al. Localization of lectin binding sites in human, cat, and rabbit corneas. Invest Ophthalmol Vis Sci. 1986;27:1280–4. [PubMed] [Google Scholar]
- 21.Argueso P, Tisdale A, Spurr-Michaud S, et al. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–9. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe H, Gipson IK. Detection of blood group differences in human corneal epithelium using a monoclonal antibody and lectins. Arch Ophthalmol. 1994;112:667–73. doi: 10.1001/archopht.1994.01090170111032. [DOI] [PubMed] [Google Scholar]
- 23.Holmes MJ, Mannis MJ, Lund J, et al. Lectin receptors in the human cornea. Cornea. 1985;4:30–4. [PubMed] [Google Scholar]
- 24.Brandon DM, Nayak SK, Binder PS. Lectin binding patterns of the human cornea. Comparison of frozen and paraffin sections. Cornea. 1988;7:257–66. [PubMed] [Google Scholar]
- 25.Watanabe H, Fabricant M, Tisdale AS, et al. Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci. 1995;36:337–44. [PubMed] [Google Scholar]
- 26.Argueso P, Spurr-Michaud S, Russo CL, et al. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–95. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 27.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16:1219–28. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi Y, Kao WW, Kohno N, et al. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Vis Sci. 2004;45:2212–7. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]
- 29.Pflugfelder SC, Liu Z, Monroy D, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–26. [PubMed] [Google Scholar]
- 30.Ellingham RB, Berry M, Stevenson D, et al. Secreted human conjunctival mucus contains MUC5AC glycoforms. Glycobiology. 1999;9:1181–9. doi: 10.1093/glycob/9.11.1181. [DOI] [PubMed] [Google Scholar]
- 31.Garcher C, Bara J, Bron A, et al. Expression of mucin peptide and blood group ABH- and Lewis-related carbohydrate antigens in normal human conjunctiva. Invest Ophthalmol Vis Sci. 1994;35:1184–91. [PubMed] [Google Scholar]
- 32.Royle L, Matthews E, Corfield A, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–73. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 33.Kawano K, Uehara F, Sameshima M, et al. Application of lectins for detection of goblet cell carbohydrates of the human conjunctiva. Exp Eye Res. 1984;38:439–47. doi: 10.1016/0014-4835(84)90122-2. [DOI] [PubMed] [Google Scholar]
- 34.Chao CC, Vergnes JP, Freeman IL, et al. Biosynthesis and partial characterization of tear film glycoproteins. Incorporation of radioactive precursors by human lacrimal gland explants in vitro. Exp Eye Res. 1980;30:411–25. doi: 10.1016/0014-4835(80)90056-1. [DOI] [PubMed] [Google Scholar]
- 35.Jager K, Wu G, Sel S, et al. MUC16 in the lacrimal apparatus. Histochem Cell Biol. 2007;127:433–8. doi: 10.1007/s00418-006-0246-6. [DOI] [PubMed] [Google Scholar]
- 36.Paulsen F, Langer G, Hoffmann W, et al. Human lacrimal gland mucins. Cell Tissue Res. 2004;316:167–77. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed A, Grierson I. Cellular carbohydrate components in human, rabbit and rat lacrimal gland. Studies using fluorescein and peroxidase labelled lectins. Graefes Arch Clin Exp Ophthalmol. 1989;227:78–87. doi: 10.1007/BF02169831. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen FP, Corfield AP, Hinz M, et al. Characterization of mucins in human lacrimal sac and nasolacrimal duct. Invest Ophthalmol Vis Sci. 2003;44:1807–13. doi: 10.1167/iovs.02-0744. [DOI] [PubMed] [Google Scholar]
- 39.Paulsen F, Thale A, Kohla G, et al. Functional anatomy of human lacrimal duct epithelium. Anat Embryol (Berl) 1998;198:1–12. doi: 10.1007/s004290050160. [DOI] [PubMed] [Google Scholar]
- 40.Tian E, Ten Hagen KG. Recent insights into the biological roles of mucin-type O-glycosylation. Glycoconj J. 2009;26:325–34. doi: 10.1007/s10719-008-9162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imbert Y, Jumblatt MM, Foulks GN, et al. Expression in human ocular surface tissues of the GalNAc-transferases that initiate mucin-type O-glycosylation. Cornea. 2006;25:1193–9. doi: 10.1097/01.ico.0000240099.16420.17. [DOI] [PubMed] [Google Scholar]
- 42.Argueso P, Tisdale A, Mandel U, et al. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 43.Ujita M, McAuliffe J, Schwientek T, et al. Synthesis of poly-N-acetyllactosamine in core 2 branched O-glycans. The requirement of novel beta-1,4-galactosyltransferase IV and beta-1,3-n-acetylglucosaminyltransferase. J Biol Chem. 1998;273:34843–49. doi: 10.1074/jbc.273.52.34843. [DOI] [PubMed] [Google Scholar]
- 44.Takashima S. Characterization of mouse sialyltransferase genes: their evolution and diversity. Biosci Biotechnol Biochem. 2008;72:1155–67. doi: 10.1271/bbb.80025. [DOI] [PubMed] [Google Scholar]
- 45.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, et al. The human sialyltransferase family. Biochimie. 2001;83:727–37. doi: 10.1016/s0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa H, Paulson JC. Cloning of a novel alpha 2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 1994;269:1394–401. [PubMed] [Google Scholar]
- 47.Lee YC, Kojima N, Wada E, et al. Cloning and expression of cDNA for a new type of Gal beta 1,3GalNAc alpha 2,3-sialyltransferase. J Biol Chem. 1994;269:10028–33. [PubMed] [Google Scholar]
- 48.Okajima T, Fukumoto S, Miyazaki H, et al. Molecular cloning of a novel alpha2,3-sialyltransferase (ST3Gal VI) that sialylates type II lactosamine structures on glycoproteins and glycolipids. J Biol Chem. 1999;274:11479–86. doi: 10.1074/jbc.274.17.11479. [DOI] [PubMed] [Google Scholar]
- 49.Xia L, Ju T, Westmuckett A, et al. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol. 2004;164:451–9. doi: 10.1083/jcb.200311112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone EL, Ismail MN, Lee SH, et al. Glycosyltransferase function in core 2-type protein O-glycosylation. Mol Cell Biol. 2009;29:3770–82. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.An G, Wei B, Xia B, et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–29. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–12. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- 53.Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71:3866–74. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fleiszig SM, Zaidi TS, Ramphal R, et al. Modulation of Pseudomonas aeruginosa adherence to the corneal surface by mucus. Infect Immun. 1994;62:1799–804. doi: 10.1128/iai.62.5.1799-1804.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aristoteli LP, Willcox MD. The adhesion of Pseudomonas aeruginosa to high molecular weight human tear film species corresponds to glycoproteins reactive with Sambucus nigra lectin. Exp Eye Res. 2006;83:1146–53. doi: 10.1016/j.exer.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res. 2001;73:281–89. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- 57.Bansil R, Stanley E, LaMont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–57. doi: 10.1146/annurev.ph.57.030195.003223. [DOI] [PubMed] [Google Scholar]
- 58.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–4. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 59.Hanisch FG. O-glycosylation of the mucin type. Biol Chem. 2001;382:143–9. doi: 10.1515/BC.2001.022. [DOI] [PubMed] [Google Scholar]
- 60.Nichols B, Dawson CR, Togni B. Surface features of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 1983;24:570–6. [PubMed] [Google Scholar]
- 61.Argueso P, Guzman-Aranguez A, Mantelli F, et al. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem. 2009;284:23037–45. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Komatsu M, Carraway CA, Fregien NL, et al. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–54. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 63.Berry M, McMaster TJ, Corfield AP, et al. Exploring the molecular adhesion of ocular mucins. Biomacromolecules. 2001;2:498–503. doi: 10.1021/bm000145y. [DOI] [PubMed] [Google Scholar]
- 64.Sumiyoshi M, Ricciuto J, Tisdale A, et al. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203. doi: 10.1167/iovs.07-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McAuley JL, Linden SK, Png CW, et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J Clin Invest. 2007;117:2313–24. doi: 10.1172/JCI26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stonebraker JR, Wagner D, Lefensty RW, et al. Glycocalyx restricts adenoviral vector access to apical receptors expressed on respiratory epithelium in vitro and in vivo: role for tethered mucins as barriers to lumenal infection. J Virol. 2004;78:13755–68. doi: 10.1128/JVI.78.24.13755-13768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ricciuto J, Heimer SR, Gilmore MS, et al. Cell surface O-glycans limit Staphylococcus aureus adherence to corneal epithelial cells. Infect Immun. 2008;76:5215–20. doi: 10.1128/IAI.00708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williamson YM, Gowrisankar R, Longo DL, et al. Adherence of nontypeable Streptococcus pneumoniae to human conjunctival epithelial cells. Microb Pathog. 2008;44:175–85. doi: 10.1016/j.micpath.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochim Biophys Acta. 1999;1473:67–95. doi: 10.1016/s0304-4165(99)00170-1. [DOI] [PubMed] [Google Scholar]
- 70.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corfield AP, Carroll D, Myerscough N, et al. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–57. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 72.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 73.Barratt J, Smith AC, Feehally J. The pathogenic role of IgA1 O-linked glycosylation in the pathogenesis of IgA nephropathy. Nephrology (Carlton) 2007;12:275–84. doi: 10.1111/j.1440-1797.2007.00797.x. [DOI] [PubMed] [Google Scholar]
- 74.Topaz O, Shurman DL, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36:579–81. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 75.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 76.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–9. [PubMed] [Google Scholar]
- 77.Watanabe H, Maeda N, Kiritoshi A, et al. Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol. 1997;124:751–7. doi: 10.1016/s0002-9394(14)71691-5. [DOI] [PubMed] [Google Scholar]
- 78.Versura P, Maltarello MC, Cellini M, et al. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. II. A quantitative study in dry-eye patients. Acta Ophthalmol (Copenh) 1986;64:451–5. doi: 10.1111/j.1755-3768.1986.tb06952.x. [DOI] [PubMed] [Google Scholar]
- 79.Garcher C, Bron A, Baudouin C, et al. CA 19-9 ELISA test: a new method for studying mucus changes in tears. Br J Ophthalmol. 1998;82:88–90. doi: 10.1136/bjo.82.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura Y, Yokoi N, Tokushige H, et al. Sialic Acid in human tear fluid decreases in dry eye. Jpn J Ophthalmol. 2004;48:519–23. doi: 10.1007/s10384-004-0111-x. [DOI] [PubMed] [Google Scholar]
- 81.Bourcier T, Thomas F, Borderie V, et al. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greiner JV, Weidman TA, Korb DR, et al. Histochemical analysis of secretory vesicles in nongoblet conjunctival epithelial cells. Acta Ophthalmol (Copenh) 1985;63:89–92. doi: 10.1111/j.1755-3768.1985.tb05222.x. [DOI] [PubMed] [Google Scholar]
- 83.Versura P, Maltarello MC, Cellini M, et al. Detection of mucus glycoconjugates in human conjunctiva by using the lectin-colloidal gold technique in TEM. III. A quantitative study in asymptomatic contact lens wearers. Acta Ophthalmol (Copenh) 1987;65:661–67. doi: 10.1111/j.1755-3768.1987.tb07060.x. [DOI] [PubMed] [Google Scholar]
- 84.Klotz SA, Misra RP, Butrus SI. Contact lens wear enhances adherence of Pseudomonas aeruginosa and binding of lectins to the cornea. Cornea. 1990;9:266–70. [PubMed] [Google Scholar]
- 85.Latkovic S, Nilsson SE. The effect of high and low Dk/L soft contact lenses on the glycocalyx layer of the corneal epithelium and on the membrane associated receptors for lectins. Clao J. 1997;23:185–91. [PubMed] [Google Scholar]
- 86.Chui J, Di Girolamo N, Wakefield D, et al. The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf. 2008;6:24–43. doi: 10.1016/s1542-0124(12)70103-9. [DOI] [PubMed] [Google Scholar]
- 87.Kawano K, Uehara F, Ohba N. Lectin-cytochemical study on epithelial mucus glycoprotein of conjunctiva and pterygium. Exp Eye Res. 1988;47:43–51. doi: 10.1016/0014-4835(88)90022-x. [DOI] [PubMed] [Google Scholar]
- 88.Kase S, Kitaichi N, Furudate N, et al. Increased expression of mucinous glycoprotein KL-6 in human pterygium. Br J Ophthalmol. 2006;90:1208–9. doi: 10.1136/bjo.2006.094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Creuzot-Garcher C, Guerzider V, Assem M, et al. Alteration of sialyl Lewis epitope expression in pterygium. Invest Ophthalmol Vis Sci. 1999;40:1631–6. [PubMed] [Google Scholar]
- 90.An HJ, Ninonuevo M, Aguilan J, et al. Glycomics analyses of tear fluid for the diagnostic detection of ocular rosacea. J Proteome Res. 2005;4:1981–7. doi: 10.1021/pr0501620. [DOI] [PubMed] [Google Scholar]