Abstract
This review annotates and categorises the glia of adult Drosophila and other model insects and describes the developmental origins of these in the Drosophila optic lobe. The functions of glia in the adult vary depending upon their sub-type and location in the brain. The task of annotating glia is essentially complete only for the glia of the fly's lamina, which comprise: two types of surface glia - the pseudocartridge and fenestrated glia; two types of cortex glia - the distal and proximal satellite glia; and two types of neuropile glia - the epithelial and marginal glia. We advocate that the term subretinal glia, as used to refer to both pseudocartridge and fenestrated glia, be abandoned. Other neuropiles contain similar glial subtypes, but other than the antennal lobes these have not been described in detail. Surface glia form the blood brain barrier, regulating the flow of substances into and out of the nervous system, both for the brain as a whole and the optic neuropiles in particular. Cortex glia provide a second level of barrier, wrapping axon fascicles and isolating neuronal cell bodies both from neighbouring brain regions and from their underlying neuropiles. Neuropile glia can be generated in the adult and a subtype, ensheathing glia, are responsible for cleaning up cellular debris during Wallerian degeneration. Both the neuropile ensheathing and astrocyte-like glia may be involved in clearing neurotransmitters from the extracellular space, thus modifying the levels of histamine, glutamate and possibly dopamine at the synapse to ultimately affect behaviour.
Keywords: Drosophila melanogaster, glia, medulla, lamina, optic lobe, histamine recycling, antennal lobe, olfactory glomeruli, degeneration
1 Introduction
Glia are important but relatively neglected players in nervous system function. They aid neuronal development by providing trophic support, markers for axonal pathfinding, cellular maintenance and neuronal insulation; they also function to regulate the extracellular space of mature neurons by acting in neurotransmitter clearance and recycling as well as in ionic regulation. Changes in glial function can manifest themselves in behaviours as varied as locomotion, sleep cycles, and mate choice. Unlike mammals, in which glia can account for up to 90% of brain cells (Blinkov and Glezer, 1968) insect nervous systems have far fewer glial cells, perhaps only 10% of the 90,000 cells estimated to occur in the adult Central Nervous System (CNS) of the fruit fly Drosophila melanogaster (Ito, pers. comm.).
Various genetic markers, for example expression of the genes reversed polarity (repo) or glial cells missing (gcm) exist for glia of the adult Drosophila nervous system, and as a result glial cells are now easily distinguished from neurons in this species. Amongst glia themselves though, even those with structural similarities, such as the glia of the larval peripheral nervous system (PNS), can have distinct origins and genetic identities (von Hilchen et al., 2008). Just knowing the level of glial diversity within the larval CNS and PNS highlights our general ignorance of the diversity of subtypes in the adult CNS, in which different subpopulations of glial cells are poorly distinguished. While glial subtypes are diverse they can be categorized into five major subclasses, each further distinguished according to the particular neuropile with which the glia is associated. The use of alternative terminologies among members of these five subtypes has been a source of confusion in the field. Apart from the glia of the fly's visual and olfactory system, moreover, glial cell types have not been adequately identified for other regions of the brain. Studies from other systems and insect species, such as the tobacco hornworm moth Manduca sexta or the honeybee Apis mellifera, provide valuable anatomical information, yet the availability of genetic tools and approaches in Drosophila makes this clearly the most propitious insect in which to analyse the functional roles of glia in the adult insect nervous system. These roles include not only their molecular functions but also the effects of glia on nervous system survival, function and ultimately, behaviour.
1.1 Types of glia
Glia can be classified by their location, their ultrastructure (Hoyle, 1986), function or patterns of gene expression (Table 1). In flies such as Drosophila or the housefly Musca domestica, glial types are divisible into four (Freeman and Doherty, 2006) or five (Awasaki et al., 2008) groups, which can then be further subdivided into unique classes for each region of the brain (Freeman and Doherty, 2006). The insect brain is a complex of three fused bilateral ganglia each with its own cell bodies and neuropile: the paired visual protocerebrum, the paired deutocerebrum which processes sensory information from the antennae, and the central tritocerebrum which integrates information from other systems (Mobbs, 1985; Fig. 3B). Each region is composed of many neuropiles. Insect neurons are monopolar and have cell bodies distributed in a rind or cortex that surrounds the neuropile (Strausfeld, 1976). The general classification of nervous system glia assigns the cells to different compartments of the CNS: 1) to the ganglionic surface around the brain, 2) to the cortex, and 3) amongst the synapses in the neuropile. Additional glial cell types such as wrapping glia exist in the peripheral nervous system (Stork et al., 2008).
Table 1.
Specific Driver lines for glia of the Drosophila CNS
| Glial Type | Driver Line | Reference |
|---|---|---|
| LARVA | ||
| eye disc, optic stalk, carpet glia | M1-126 | Choi and Benzer, 1994 |
| larval eye disc surface glia | c527-GAL4 | Hummel et al., 2002 |
| larval eye disc wrapping glia | Mz97-GAL4 | Hummel et al., 2002 |
| larval eye disc wrapping glia | ptc-GAL4 | Murakami et al., 2007 |
| larval eye disc and optic stalk surface glia | NP4702-GAL4 | Murakami et al., 2007 |
| larval eye disc surface glia and wrapping glia | NP2109-GAL4 | Murakami et al., 2007 |
| larval eye disc surface and wrapping glia, optic lobe surface glia |
NP3053-GAL4 | Murakami et al., 2007 |
| PUPA and ADULT | ||
| perineurial | NP6293-GAL4 | Awasaki et al., 2008 |
| subperineural | NP2276-GAL4 | Awasaki et al., 2008 |
| cortex | NP577-GAL4 | Awasaki et al., 2008 |
| cortex | NP2222-GAL4 | Awasaki et al., 2008 |
| astrocyte-like | NP3233-GAL4 | Awasaki et al., 2008 |
| astrocyte-like | NP1243-GAL4 | Awasaki et al., 2008 |
| ensheathing | NP6520-GAL4 | Awasaki et al., 2008 |
| ensheathing and cortex (weakly) | NP1243-GAL4 | Awasaki et al., 2008 |
| cortex (weakly) | NP6520-GAL4 | Awasaki et al., 2008 |
| antenna perineural glia, antennal lobe commissure glia |
Mz317-GAL4 | Sen et al., 2005; Yao et al., 2007 |
| antennal coeloconic independent glia | GH146-GAL4 | Sen et al., 2005 |
| cortex glia, some neuropil glia, chiasm glia | Nrv2-GAL4 | Oland et al., 2008; Górska-Andrzejak et al., 2009; Mayer et al., 2009 |
| ensheathing glia of the antennal lobe | Mz0709-GAL4 | Doherty et al., 2009 |
| astrocyte-like glia of the antennal lobe | dEAAT1-GAL4 | Doherty et al., 2009 |
| astrocyte-like glia of the antennal lobe | alrm-GAL4 | Doherty et al., 2009 |
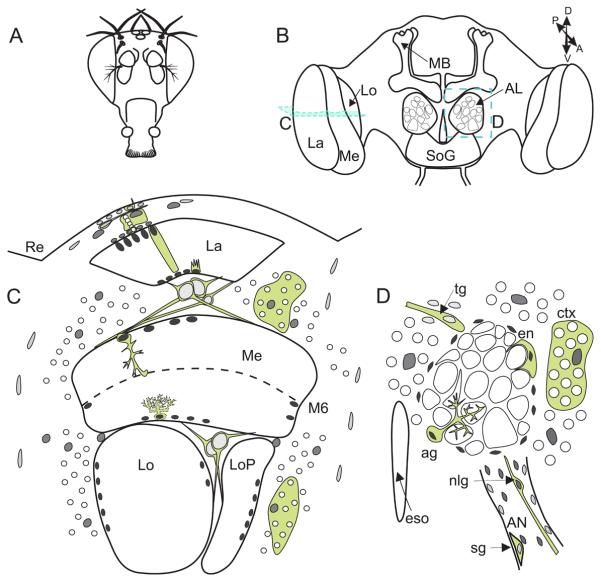
Fig. 3. The glial anatomy of Drosophila.
There are three classes of glia in the insect brain, surface, cortex and neuropile. Figures A and B depict a frontal view of (A) the Drosophila head and (B) underlying brain neuropiles. The lamina (La), medulla (Me), lobula (Lo) and lobula plate (LoP) constitute the visual protocerebrum (B and C) which underlie the retina (Re). The antennal lobes (AL) and mushroom bodies (MB) constitute the deutocerebrum, while all other neuropiles (not illustrated here) including the subesophogeal ganglion (SoG) belong to the tritrocerebrum. A horizontal section through the visual neuropiles (C, section plane C in B) shows the nuclear location of surface (light grey), chiasm (light grey), cortex (medium grey) and neuropile (dark grey) glia relative to their respective neuropiles and their associated neurons (nuclei in white). Subperineurial surface glia and cortex glia (called satellite glia when associated with the visual system) are sparse and only a few satellite glia are required to surround many neuron cell bodies in the cortex. Chiasm glia lie in two locations: between the lamina and medulla neuropile (first optic chiasm) and the medulla and lobula/lobula plate neuropiles (second optic chiasm), in both cases forming an anterior to posterior glial boundary. Within the first optic chiasm two types of glia can be distinguished, small and giant. Neuropile glia lie amongst the axon terminals but for some optic lobe neuropiles no distinction has yet been made between their ensheathing and astrocyte-like glia. Chandelier glia, which have been detected in the neuropile of other Diptera, but have not yet been described in Drosophila, are illustrated in dotted outline at the base of the medulla. On the other hand, within the antennal lobes (D) both types of neuropile glia (dark grey nuclei): ensheathing (en) glia, which wrap the neuropile, and astrocyte-like glia (ag), which extend processes amongst the glomeruli, can be distinguished using different GAL4 driver lines. Outside the neuropile, neuronal cell bodies (white) are ensconced in extensions from cortex glia (ctx; medium grey nuclei) which separate the antennal lobe from neighbouring neuropiles and the oesophagus (eso). Nerve layer glia lie at the base of the antennal nerve (AN) where the nerve enters the antennal lobe, while surface glia (sg) ensheathe the nerve, and tract glia (tg) lie at the edge of the antennal lobe commissures.
1.1.1 Surface glia
Flattened surface glia constitute the externalmost layer of the blood brain barrier (BBB) that isolates the nervous system from the haemolymph of the insect's open circulatory system. They are composed of two types of glia that can be distinguished by their location and cell shape: perineurial (apical) cells, which are covered by a thick extracellular matrix, the neural lamella, and subperineurial (basal) glia.
Perineurial glia lie on the ganglionic surface and have small elongate nuclei. These glia develop postembryonically, in a non-GCM-dependent manner (Awasaki et al., 2008) and thus likely function only in the BBB of the adult. The exact function of the perineurial glia is not known. Subperineurial glia form an inner layer of large, sheet-like glia and are rich in septate junctions, which are proposed to be the principal component of the larval BBB (see Bainton et al., 2005; Schwabe et al., 2005; Stork et al., 2008). Awasaki et al. (2008) propose that these two glial cell layers, with their differential patterns of gene expression, play non-overlapping complementary roles in regulating the permeability of the BBB. There has been some question in the literature whether the immature perineurial cells of embryos are actually glia and not haemocytes (Pereanu et al., 2005). Unlike other glia, which are derived from the epithelium, the perineurial cells derive from the mesoderm (Edwards et al., 1993) and do not express the glial cell marker repo early in their development (Hartenstein et al., 1998). Yet, recent evidence suggests that perineurial cells are, in fact, a specialized subset of repo expressing glia which are developmentally delayed and which do inevitably express glial specific markers in the larva and early pupal stages (Awasaki et al., 2008; Stork et al., 2008). The identification of perineurial cells as glia is further supported by the fact that repo is never expressed in GCM-positive haemocytes (Lee and Jones, 2005).
1.1.2 Cortex glia
Cortex glia are embedded amongst and maintain close contact with the somata of neurons in the cortex of the CNS (Freeman and Doherty, 2006). Anatomically they form a mesh in the cortex and one cortex glial cell can enwrap many neuronal cell bodies (Awasaki et al., 2008). In the visual system all cortex glia are called satellite glia (Eule et al., 1995; Tix et al., 1997), of which there are two distinct types in the lamina, each surrounding the cell bodies of distinct classes of neurons. It is unknown if other neuropiles contain multiple types of cortex glia.
1.1.3 Neuropile glia
Neuropile glia have their nuclei in the synaptic neuropil, are associated with axons and axon fascicles, and extend sheath-like membranes around axon bundles. They help to isolate nerves and may promote neuronal survival through trophic support. Neuropile glia can be either: 1) ensheathing and fibrous; or 2) dendritic and astrocyte-like. Ensheathing glia are lamellar, extending processes along the outer surface of the neuropile to isolate neurons. Astrocyte glia, on the other hand, elaborate extensive processes in the neuropile where spatial considerations dictate that they must be associated with synaptic regions and thus could modulate neural connections. They were depicted from Golgi impregnation in early accounts (e.g., Sánchez y Sánchez, 1935) and have only recently been referred to as astrocyte-like (Awasaki et al., 2008) although they differ genetically from their namesakes, the astrocyte glia of the vertebrate brain.
1.1.4 A previously unclassified subtype: Tract glia?
Another subset of glia wraps axon tracts which project between neuropiles. The tracts in the adult insect brain are too numerous to list comprehensively, but include the outer (between lamina and medulla) and inner (between medulla and lobula neuropiles) optic chiasmata in the visual system; and in the olfactory system, the commissure connecting the two antennal lobes (Yao et al., 2007); multiple antennocerebral tracts that connect the antennal lobes to the mushroom bodies; or the protocerebrocalycal tracts that connect the α- and β-lobes of the mushroom bodies to the calyx through the peduncle (Strausfeld, 1976).
While glia have long been known to surround these tracts they are categorized in only a single study (Tix et al., 1997) as their own subtype within the CNS. Their singularity lies in the fact that they lack characteristics that would allow them to be grouped with any of other known glial subtypes. They are not surface glia because they do not lie on the surface of the brain. Although their cell bodies lie near the cortex they are not cortex glia because they are not restricted to the cortex and do not surround neuronal cell bodies. Nor are they neuropile glia. While they are often classified as neuropile glia, and are similar to the ensheathing subtype, their cell bodies are not within the neuropile nor are they associated with synapses. Some of these glia may be considered equivalent to the interface glia which line axon tracts along the embryonic ventral nerve cord, while the glia of the optic chiasms have drawn comparison to the midline glia of the ventral nerve cord (Tix et al., 1997). The tract glia may, in fact, be two distinct types of glia with the same enwrapping, lamellar morphology, distinguished by those which form chiasms and those which do not. Tract glia within the adult CNS are likely distinct from wrapping glia in the PNS associated with afferent sensory neurons or the GH146-GAL4-expressing nerve layer glia of the antenna (Sen et al., 2005).
1.1.5 Invertebrate glia may have functional similarities to mammalian glia
Glia of the insect brain, while distinct from their mammalian counterparts, do share some common features. The most obvious difference between mammalian and insect glia is simply in their numbers in the CNS, with glia being the most abundant cell type in the mammalian brain, yet comprising as few as 10-25% of the cells in the insect brain (Ito, pers. comm.; Pfrieger and Barres, 1995). Furthermore, gcm is essential for glial specification in Drosophila (Jones et al., 1995), but not in mammals (Kim et al., 1998). While axon bundles in insects are ensheathed by glia, the axons themselves are not associated with any type of Schwann cell or oligodentrocyte-like myelin sheath, although Drosophila glia express Neurexin, a key junctional protein component required for vertebrate myelination (Baumgartner et al., 1996; Bhat et al., 2001). Similarities also exist in glial structure. Astrocyte-shaped glia exist within the synaptic neuropiles of Drosophila (Awasaki et al., 2008). Similarly, neuropile glia could be considered oligodendrocyte-like in that they wrap and guide individual axons during development (Chotard and Salecker, 2004) but astrocyte-like in that they are involved in the regulation of neurotransmitter recycling in the adult (Borycz et al., 2002; Richardt et al., 2002, Richardt et al., 2003). Immune-associated microglia have not been reported in Drosophila. Instead the neuropile ensheathing glia, with cell bodies at the cortex/neuropile border, are responsible for engulfing degenerating axons (Doherty et al., 2009). For a more comprehensive review of the similarites between mammalian and Drosophila glia see Freeman and Doherty (2006).
2 Systems glia
Glia can be further subdivided according to the neuropile with which they are associated and by the locations they occupy within that neuropile.
2.1 Optic lobe glia
The glia of the fly's visual system have several distinct morphological subtypes that have been described in extensive detail for the first optic neuropile, or lamina (Fig. 1), of Musca (Saint Marie and Carlson, 1983a) and the second optic neuropile, or medulla, and the associated chiasmata of the optic lobe in Drosophila (Tix et al., 1997).
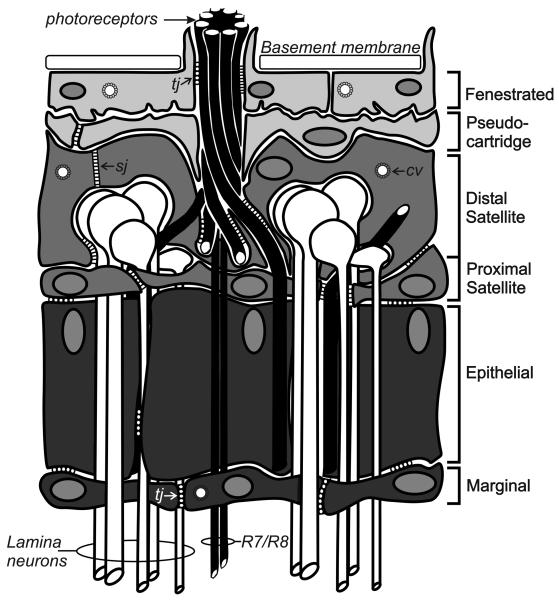
Fig. 1. Glia in the adult fly lamina.
The lamina of the adult optic lobe of the fly is populated by six distinct classs of glia. These include (from distal to proximal) two types of surface glia - the fenestrated and pseudocartridge glia; two types of cortex glia - the distal and proximal satellite glia; and two types of neuropile glia - the epithelial and marginal glia. Septate junctions (sj) connect the distal satellite glia and are an integral part of the blood brain barrier. Tight junctions (tj) are more common in the proximal glial layers. The outermost glia also contain clathrin coated vesicles (cv) and engage in clathrin-mediated endocytosis. Figure modified from Saint-Marie and Carlson (1993a).
2.1.1 Functional anatomy of identified glia in the lamina
The compound eye of Drosophila is composed of approximately 800 unit ommatidia, each containing a fixed complement of cells. These include eight photoreceptor neurons as well as the pigment and cone support cells (Wolff and Ready, 1993). Each photoreceptor axon terminates in one of three different strata of the optic lobe, depending upon the opsin expression of its soma and thus its spectral sensitivity. Photoreceptors R1-R6 have axons that terminate in the lamina, sorting at the distal face of the lamina so as to converge upon a unit column, or cartridge, along with other R1-R6 axons that signal the same point in visual space according to a principle of neuronal superposition (Braitenberg, 1967). The terminals of R1-R6 release the neurotransmitter histamine (Hardie, 1987; Sarthy, 1991) and thereby signal to lamina monopolar neurons L1-L3 and amacrine cells (Burkhardt and Braitenberg, 1976; Nicol and Meinertzhagen, 1982; Meinertzhagen and O'Neil, 1991). To optimize spatial resolution the cartridges are electrically and chemically isolated by the highly organised system of glial barriers which surrounds each cartridge (Shaw, 1984).
Within the lamina six morphologically distinct classes of glial cell form successive populations, arranged from distal to proximal, as follows: the fenestrated glia, pseudocartridge glia, distal and proximal satellite glia, epithelial glia and marginal glia. The functions and expression patterns of some of these subtypes are presented below, followed by an evaluation of their developmental origins. The migration pathways into the lamina and the origins of these cells, as determined by clonal mosaic analysis, suggests that at least some of the subtypes have distinct identities from early in development (Winberg et al., 1992; Perez and Steller, 1996; Dearborn and Kunes, 2004).
2.1.1.1 Fenestrated glia
Fenestrated and pseudocartridge glia, as originally described in Musca (Saint Marie and Carlson, 1983a), are often incorrectly grouped into a single class called the sub-retinal glia (Winberg et al., 1992). However, these two surface glial layers are separate subtypes that are anatomically unique and have distinct functions. In Musca, fenestrated glia are evenly distributed in an array, one per ommatidium (Saint Marie and Carlson, 1983a) so as to form a monolayer with a highly involuted membrane surface. They extend processes which not only ensheathe photoreceptors and trachea but also penetrate the basement membrane and enter the retina (Saint Marie and Carlson, 1983a).
Their location likens them to a type of surface glia, the perineurial glia. Although sometimes proposed to be a part of the BBB their principle function is not as a barrier because they allow the longitudinal passage of colloidal lanthanum (Saint Marie and Carlson, 1983b). While these glia do contain pleated septate junctions, which can provide a barrier to diffusion of solutes through the extracellular space (Juang and Carlson, 1992), those junctions are located principally between the glia and the photoreceptor axons they ensheathe, and not between the glial cells themselves, which are connected only by adhering junctions such as desmosomes or intercellular gap junctions (Chi and Carlson, 1980). Tight junctions have also been reported to exist between glia and photoreceptors in Musca (Saint Marie and Carlson, 1983b), a finding that Shaw (1984) was unable to replicate, however.
Instead of an insulating role, the undulated apical surface, presence of coated vesicles, and ability to take up colloidal lanthanum (Saint Marie and Carlson, 1983a) together suggest that fenestrated glia in Musca have pinocytotic activity and may be involved in clearing neurotransmitter or toxins from the extracellular space and in controlling ion fluctuations (Saint Marie and Carlson, 1983a; Carlson and Saint-Marie, 1990). While the fenestrated glia of Musca contain coated vesicles (Saint Marie and Carlson, 1983a), those in Drosophila express coated-vesicle associated genes such as the clathrin binding AP-3β adaptin gene ruby (Kretzschmar et al., 2000) and are immunoreactive for the vesicular monoamine transporter vMAT (Romero-Calderón et al., 2008). A distinct band of immunolabelling to histamine, presumably of photoreceptor origin, is found in the fenestrated glia in wild-type Drosophila (Borycz et al., 2002; Romero-Calderón et al., 2008). This, along with a lack of the histamine synthesis enzyme histidine decarboxylase (hdc; Burg et al., 1993; Thimgan et al., 2006) suggests that histamine may enter these glia by means of endocytotic uptake. These glia may not have completely identical functions in different Dipteran species, however, since fenestrated glia in Drosophila contain pigment granules (Kretzschmar et al., 2000) while those in Musca do not (Saint Marie and Carlson, 1983a).
2.1.1.2 Pseudocartridge glia
In adult Musca domestica, the pseudocartridge glia can be identified by their position beneath the fenestrated glia and by their abundant horizontal microtubules, long nuclei, and larger cells, which are up to 15μm wide and 2 to 10μm deep, sufficient to enwrap the axons from neighbouring rows of ommatidia. These glia also contain coated vesicles suggesting that they too partake in endocytosis (Saint Marie and Carlson, 1983a), but unlike the fenestrated glia this cell layer contains many more septate junctions, especially between the cell's long interdigitating processes. Both the presence of septate junctions and the increased surface area of these glia that results from interdigitation (Saint Marie and Carlson, 1983b) suggest that these glia are equivalent to the subperineurial glia found elsewhere on the surface of the brain and may form the ‘barrier’ layer of the BBB.
In the pseudocartridge region, septate junctions are found not only between pseudocartridge glia themselves but also between the axons of photoreceptors which enter the lamina (Saint Marie and Carlson, 1983b). The role of pseudocartridge glia as the most external boundary of the BBB is supported by an inability of colloidal lanthanum to penetrate deeply into their septate junctions (Saint Marie and Carlson, 1983b). Despite forming such a barrier, the surfaces of fenestrated and satellite glia that abut the pseudocartridge glia on either face are connected by gap junctions, as are the pseudocartridge glia among themselves (Saint Marie and Carlson, 1983b), suggesting that an intercellular network exists between these three classes of glia, at least in Musca.
2.1.1.3 Satellite glia
Satellite glia are a class of cortex glia divisible into two distinct types, distal (or rind) glia that ensheathe the cell bodies of monopolar neurons in the lamina cortex, and proximal (or interface) glia that invest the photoreceptor axon bundles and the necks of monopolar neurons (Saint Marie and Carlson, 1983a). Both subtypes can also be distinguished in Drosophila (Eule et al., 1995). In Musca, only the distal-most glia contain endocytotic coated vesicles, but both subtypes are part of the glial network by virtue of forming gap junctions with their glial neighbours. They form septate junctions, desmosomes and occluding tight junctions, with more septate junctions found proximally, where the glia wrap axonal bundles of photoreceptors and monopolar neurons (Saint Marie and Carlson, 1983b). As a result of their extensive septate junctions the satellite glia may act as a second layer of the BBB.
2.1.1.4 Epithelial glia
Epithelial glia are neuropile glia which extend throughout the depth of the lamina neuropile. They constitute the sole class of cells with nuclei in the distal neuropile proper, a unique diagnostic characteristic. Groups of lamina neurons are surrounded by a triad of epithelial glia to form a cartridge, and any one glial cell also ensheathes the three neighbouring cartridges, as first identified in Musca (Boschek, 1971) and later confirmed in Drosophila (Meinertzhagen and O'Neil, 1991). Both tight and gap junctions are found at epithelial glia membrane appositions as well as at their base and apex where they contact marginal and satellite glia respectively (Saint Marie and Carlson, 1983b).
Epithelial glia differ from other lamina glia in exhibiting two anatomically distinct organelle junctions: 1) capitate projections, dynamic invaginations from glia with a spherical head (Trujillo-Cenóz, 1965); and 2) bulbous projections called gnarls (Campos-Ortega and Strausfeld, 1973), which are planar in Drosophila (Meinertzhagen and O'Neil, 1991), and extend into the β neurites of T1 neurons. These glia also insinuate themselves at plaque contacts between T1 and photoreceptor terminals and are also occasional postsynaptic elements at histaminergic tetrad synapses (Meinertzhagen and O'Neil, 1991).
Epithelial glia that insert at gnarl junctions between the α processes of amacrine cells and the β neurites of T1 neurons are also technically postsynaptic to those α processes in both Lucilia (Shaw, 1984) and Drosophila (Meinertzhagen and O'Neil, 1991). While the transmitter of amacrine cells is still not known, these cells strongly express immunoreactivity to vesicular glutamate transporter, vGluT (Sinakevitch and Strausfeld, 2004; Kolodziejczyk et al., 2008), while T1 neurons at the same synapse are identifiable with an Excitatory amino acid transporter (EAAT) driver line, dEAAT-GAL4 (Hamanaka and Meinertzhagen, 2010), also compatible with a glutamate phenotype. Thus, it is possible that epithelial glia respond to and regulate clearance of at least two neurotransmitters: histamine at the photoreceptor tetrads, and glutamate at gnarl contacts. Histamine acts as an inhibitory (sign-reversing) neurotransmitter at the former, and glutamate would most probably act as an excitatory neurotransmitter at the latter.
The membranes of the epithelial glia are deeply infolded as well as highly branched amongst the neurons they ensheathe (Saint Marie and Carlson, 1983a) features that contribute to the glia's ability to form individual electrical barriers around each cartridge as well as to produce the high electrical resistance that exists between the retina and lamina (Shaw, 1975). The glia themselves signal their postsynaptic location at the histaminergic tetrad synapses of the photoreceptor (Shaw, 1984; Meinertzhagen and O'Neil, 1991) by expressing the first of two histamine-gated chloride receptor channel proteins, called variously HA-Cl I (Witte et al., 2002), HisCl1 (Zheng et al., 2002) or HclB (Gengs et al., 2002) on their surface (Gao et al., 2008). These “gliapses” and the presumed Cl− influx that results from histamine activation of their receptor appear to play a role in shaping the “on” transient component of the electroretinogram (ERG; Fig. 4C; Shaw, 1984; Meinertzhagen and O'Neil, 1991; Sinakevitch and Strausfeld, 2004; Kolodziejczyk et al., 2008; Pantazis et al., 2008). Finally, epithelial glia are reported to have phagocytic properties, as revealed by their role in engulfing profiles of R1-R6 terminals that undergo degeneration in the Drosophila mutant rdgB (Stark and Carlson, 1982).
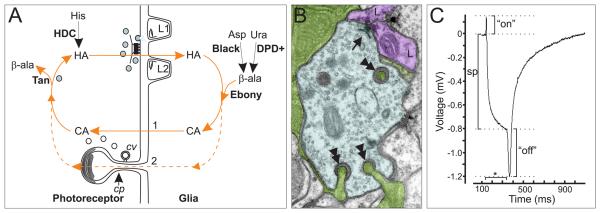
Fig. 4. Visual system function is dependent upon an intimate association of photoreceptors and glia.
A. Histamine (HA) is synthesized from histidine (His) in the photoreceptor by the enzyme histadine decarboxylase (HDC). HA is released from vesicles at the photoreceptor T-bar ribbon. In the synaptic cleft it can act on HA gated chloride channels at the surface of the monopolar cells (L1,L2) or the glia. Excess HA is taken up by the glia where it is then inactivated by conjugation to β-alanine (β-ala) by the protein Ebony. β-ala is produced in the glia from decarboxylation of aspartate (Asp) by Black, or by catabolism of uracil (Ura) along a dihydropyrimidine dehydrogenase (DPD+) dependent pathway (Rawls, 2006). The HA- β-alanyl conjugate, called carcinine (CA), is shuttled from the glia back into the photoreceptor by an unknown mechanism (path 1) or possibly via the capitate projection (cp, path 2), where clathrin-mediated endocytosis of coated vesicles (cv) takes place. In the photoreceptor HA is liberated from CA by Tan. Liberated HA can then be pumped back into recycled vesicles and prepared once again for release at the synapse. B. An EM cross-section of the Drosophila lamina. Photoreceptors (blue) synapse (arrow) onto paired monopolar neurons (L, magenta). Epithelial glia (green) surround the cartridge and invests areas close to synapses. Glia invaginate into photoreceptors at specialised sites called capitate projections (double arrowheads). C. The inverted extracellular response (ERG) recorded from the eye of Drosophila is triggered by a light flash (*) and consists of the combined negative sustained response (sp) of the photoreceptors and the hyperpolarizing (“on”) and depolarizing (“off”) responses of the lamina. The “on” response is modulated by activation of HisCl1 receptors on the epithelial glia.
2.1.1.5 Marginal glia
The lamina is bounded on its proximal surface by a layer of overlapping marginal glia, one per cartridge (Saint Marie and Carlson, 1983a; Eule et al., 1995). At their apices these extend processes into the lamina neuropile, enveloping all axons and trachea as they exit or enter the lamina. Thus, they seal off the lamina extracellular space from the underlying optic lobe. The marginal glia of Musca also contain coated vesicles (Saint Marie and Carlson, 1983a) just like the glia of the distal lamina and may thus have endocytotic properties.
Tight junctions are particularly abundant in marginal glia of Musca and can be found between the glia themselves and between glia and axons. Desmosomes and occasional gap junctions also connect the marginal glia but they lack the septate junctions typical of the BBB in the more distal glia of the lamina (Saint Marie and Carlson, 1983b). Functionally, the marginal glia with their extensive tight junctions form the seat of the resistance barrier that exists in the lamina (Saint Marie and Carlson, 1983b).
2.1.1.6 Glia of the deeper optic lobe regions
The glia of the inner optic neuropiles have been less well characterised using LacZ enhancer trap lines (Eule et al., 1995; Tix et al., 1997) that are no longer available. Nonetheless, they reveal numerous major glial classes (Fig 3C): 1) giant optic chiasm glia which are arranged in rows between successive dorsoventral sheets of intercrossing fibres of the outer and inner chiasmata; these have an early origin (Eule et al., 1995; Tix et al., 1997) and are visible in the larval brain (Meinertzhagen, 1973; Tix et al., 1997); 2) small outer optic chiasm glia associated with the the axon bundles; 3) cortex glia of the medulla (medulla satellite glia), and lobula complex (lobula plate satellite glia); and, 4) other neuropile glia such as the medulla neuropile glia, that form an interface layer with cell bodies in the medulla cortex and have processes that extend deep into the neuropile, and the lobula and lobula plate neuropile glia (Eule et al., 1995; Tix et al., 1997). No distinction has yet been made between the ensheathing and astrocyte-like glia in these neuropiles. Chandelier cells have been consistently described as lying at the limit of the medulla and the inner optic chiasm in some Diptera, Odonata and bees, but have not yet been identified in Drosophila. These are astrocyte-like neuropile glia with a cell body near the chiasma and arbors that extend distally, up into the columns of the medulla (Sánchez y Sánchez, 1935; Cantera and Trujillo-Cenóz, 1996).
2.1.2 The developmental origins of lamina optic lobe glia
The distinction between the three proximalmost layers of lamina glia, the satellite, epithelial and marginal glia, can be observed early in development from the positions these cells occupy in the developing optic lobe (Winberg et al., 1992; Perez and Steller, 1996; Dearborn and Kunes, 2004; Chotard et al., 2005). Epithelial and marginal glia cells originate from the dorsal and ventral glial precursor cell areas, which lie near the bilaterally located lamina furrows of the outer optic anlage (Chotard et al., 2005). This glial precursor zone, alternatively known as subdomain I, can be distinguished in the larva by its expression of wingless, optomotor blind and dachsous (Dearborn and Kunes, 2004). While recent reports do not discuss an origin for the satellite glia from these glial precursor cell areas (Dearborn and Kunes, 2004; Chotard et al., 2005), a group of satellite glia does in fact label with green fluorescent protein (GFP) and bromodeoxyuridine (BrdU) in glial mitotic clones that also label epithelial and marginal glia (Winberg et al., 1992; Perez and Steller, 1996). MARCM analysis of glial origins in other areas of the Drosophila brain suggests that neuropile glia and cortex glia have distinct origins, with cortex glia precursors originating during embryogeneis and neuropile glial precursors developing during larval and pupal stages (Awasaki et al., 2008). If this generalisation were to hold true for the visual system as well, then the distal satellite glia should have a separate origin from the epithelial and marginal glia. The uncertainty regarding the origin of satellite glia, either deriving from subdomain 1 along with the neuropile glia or from a distinct source, may derive from BrdU incorporation into distinct subsets of simultaneously dividing cells. Thus, it is quite likely that satellite glia have distinct origins with all marginal, epithelial, and satellite glia originating concurrently as development of the visual system proceeds from the late second-instar stage onward. For a more extensive review of optic lobe glial development see Chotard and Salecker (2005, 2007) and Perez and Steller (1996) who indicate the developmental origins of these three layers of glia.
For the satellite glia, most workers have not distinguished between the proximal (or interface) and distal (or rind) subtypes previously identified in Musca (Saint Marie and Carlson, 1983a) and Drosophila (Eule et al., 1995). These not only have distinct anatomical features (Saint Marie and Carlson, 1983a; Eule et al., 1995) but also have distinct genetic identities with the proximal satellite glia expressing Ebony (Wagner et al., 2007; interpreted from their figure 4D) and the distal satellite glia expressing the Na+/K+-ATPase Nervana2 (Górska-Andrzejak et al., 2009). They may also have distinct origins in the larva. It might be that only the proximal, or interface, satellite glia originate in the brain, if their proposed ebony expression was to ally them to their possible developmental kin, the epithelial glia, which also express ebony (Richardt et al., 2002). The distal satellite glia, on the other hand, may be derived from a population of glia migrating from the eye disc. It is quite possible that the border between those glia that derive from the eye disc and those that derive from the optic lobe may thus fall between the distal and proximal satellite glia. Resolution of these subtypes and their likely separate developmental origins awaits the isolation of appropriate markers.
Distal to these lamina glia, the nomenclature and developmental origins of the remaining two classes of adult lamina glia, fenestrated and pseudocartridge, are yet more ambiguous. In fact, much of the current literature frequently makes no distinction between these two surface glial layers of the adult lamina, those underlying the basement membrane of the retina. This has resulted in confusing terminology in reference to them, the most common shortcoming of which is referring to them by a single classification - the subretinal glia. Nevertheless, these two layers contain different glial subtypes that are in fact separate entities, possibly, with distinct origins (Winberg et al., 1992; Perez and Steller, 1996). We will start with a review of the terminology in current usage, before proceeding to a suggested resolution.
2.1.2.1 Naming schemes of the larval visual system glia: An historical perspective
Various names have been applied to glial cells that come to underlie the developing retina. Thus, retinal basal glia (RBG) are reported in the larva to arise from the optic stalk and migrate centrifugally into the eye disc (Choi and Benzer, 1994) where they then help guide outgrowing photoreceptor axons towards the optic stalk (Hummel et al., 2002). Another glial cell type, the subretinal glia, is also reported at the distalmost surface of the larval optic lobe (Perez and Steller, 1996). Mitotic clones of glial cells originating in the larval optic stalk give rise to labelled cells in the eye disc, optic stalk and developing distal lamina, suggesting a single origin for these ‘subretinal glia’, ‘optic stalk glia’ and ‘retinal basal glia’ and yet, when the optic stalk and supposed source of glial cells is eliminated, as in disconnected (disco) mutants, some RK2-(Repo) expressing glia persist on the brain's surface, suggesting that there may be two populations of ‘subretinal glia’ and that at least some of these cells have a distinct origin (Perez and Steller, 1996). Reasons that invoke embryonic explanations for how some distal lamina glia may survive despite the loss of the larval optic stalk in disco mutants can be advanced. As the larval photoreceptors in Bolwig's organ develop, their axons navigate a path through the brain along a series guidepost cells and this path is lined by three genetically distinct types of glia (Schmucker et al., 1997). The optic stalk develops around the axons of Bolwig's organ and becomes externalized as the eye disc evaginates from the brain (Younossi-Hartenstein et al., 1992). While the fate of glial cells within the embryonic optic stalk is not known they are likely to be the precursors of the larval optic stalk glia. When the axons of Bolwig's organ mistarget in the disco mutant, the glial cells remain in their appropriate positions along the presumptive embryonic optic stalk (Schmucker et al., 1997). It is possible that in the absence of an optic stalk these glia persist in the brain and are capable of dividing to produce glia that occupy a position at the distalmost surface of the optic lobe. These possible explanations for the action of disco are not mutually exclusive and the validity of each would require separate investigation.
One further distinction needs to be made with respect to the larval subretinal glia. The subretinal glia of the optic lobe (Winberg et al., 1992; Perez and Steller, 1996) are distinct from subretinal cells that contain pigment granules and are described as originating later in development from the pupal eye disc (Cagan and Ready, 1989). Their naming schemes make it easy to confuse these glial terminologies despite the fact that the larval subretinal glia (Winberg et al., 1992; Perez and Steller, 1996) are found in the larval brain while the subretinal cells are found in the eye disc (Cagan and Ready, 1989). Furthermore, there is no evidence in the current literature to support the existence of a true glial cell type in the eye disc with any origin other than the optic stalk (Rangarajan et al., 2001; Hummel et al., 2002; Silies et al., 2007). Despite the terminologies and possibly also their identification being frequently interchanged in the literature (see: Winberg et al., 1992; Xiong et al., 1994), there is no evidence to suggest that larval subretinal glia of the optic lobe that derive, in part, from the optic stalk (Perez and Steller, 1996) and the pupal subretinal cells of the eye disc (Cagan and Ready, 1989) are one and the same. To complicate matters, both the fenestrated and pseudocartridge glia of the adult are grouped as subretinal glia, and yet there is no evidence that either the larval subretinal glia or subretinal cells corresponds to the fenestrated or pseudocartridge glia of the adult. For all these reasons we advocate the abandonment of the term subretinal glia in reference to adult lamina glia as both confusing and inaccurate. To clarify the nomenclature of Drosophila optic lobe glia at various stages of development, we therefore include a table of recommended terminologies (Table 2).
Table 2.
Corresponding glial cell types from the Drosophila literature.
| Preferred glial cell name | Corresponds to | Subtype |
|---|---|---|
| LARVAL EYE DISC/ OPTIC STALK | ||
| wrapping glia Rangarajan et al., 2001; Hummel et al., 2002 |
retinal basal glia Choi and Benzer, 1994 |
peripheral glia? |
| carpet glia Silies et al., 2007 |
retinal basal glia Choi and Benzer, 1994 |
subperineurial glia |
| eye disc surface glia Hummel et al., 2002 |
subretinal glia; retinal basal glia Cagan and Ready, 1989 ;Choi and Benzer, 1994 |
perineurial glia |
| optic stalk surface glia Silies et al., 2007 |
- | perineurial glia |
| edging glia * | marginal glia Silies et al., 2007 |
unknown |
| optic stalk inner glia Silies et al., 2007 |
- | peripheral glia? |
| LARVAL BRAIN | ||
| distal satellite glia | subretinal glia Winberg et al., 1992; Perez and Steller, 1996 |
cortex glia |
| satellite glia Winberg et al., 1992 |
- | cortex glia |
| epithelial glia Winberg et al., 1992 |
- | neuropile glia |
| marginal glia Winberg et al., 1992 |
- | neuropile glia |
| outer chiasm large glia Tix et al., 1987 |
medulla glia | tract glia |
| ADULT FIRST OPTIC NEUROPIL | ||
| fenestrated glia Eule et al., 1995 |
sub-retinal glia Winberg et al., 1992 |
perineurial glia |
| pseudocartridge glia Eule et al., 1995 |
sub-retinal glia Winberg et al., 1992 |
subperineurial glia |
| distal satellite glia Eule et al., 1995 |
satellite glia, rind glia Strausfeld, 1976; Wigglesworth 1959 |
cortex glia |
| proximal satellite glia Eule et al., 1995 |
satellite glia, interface glia Strausfeld, 1976; Wigglesworth 1959 |
cortex glia |
| epithelial glia | - | neuropile glia |
| marginal glia Eule et al., 1995 |
- | neuropile glia |
glial type not definitively classified
suggested new name
2.1.2.2 The glia of the eye disc and optic stalk
The photoreceptors, which develop in a posterior to anterior direction, secrete decepentaplegic (Dpp) and hedgehog (hh) to influence both the proliferative and migratory behaviour of basally located surface glia (Rangarajan et al., 2001). Studies on glial migration in the casein kinase mutant gilgamesh as well as of flies that overexpress tramtrack69, which can repress mitosis in surface glia, support the finding that glia of the eye disc originate in the optic stalk and that they migrate into the disc so as to first occupy positions from the posterior edge of the eye, then more anterior locations (Hummel et al., 2002). Further support comes most recently from the work of Silies et al. (2007) which reveals that glia migrate into the eye disc along large carpet glia, a type of subperineurial glia that expresses moody and otherwise restricts the premature anterior migration of glia. Distinct enhancer trap lines reveal moreover that the eye disc contains at least two types of glial cells, surface and wrapping glia (Hummel et al., 2002), while analysis of glial mitotic clones using repoFLP transgenic flies suggests that throughout the different stages of eye disc development no less than six anatomically distinct glial types can be distinguished (Fig. 2; Silies et al., 2007). These are: 1) fusiform-shaped optic stalk glia; 2) perineurial surface glia which lie along the basal surface of the eye disc and migrate anteriorly; 3) wrapping glia that lie above the outgrowing photoreceptor axons and ensheathe bundles of axons; 4) an undifferentiated population of glia that lies close to the morphogenetic furrow and has filopodia; 5) peripherally located marginal glia cells at the margins of the eye disc, that have an elongated clapboard-like shape; and 6) two large, flat, basally-located, carpet glia with a large nucleus and containing septate junctions (Silies et al., 2007). Other surface glia can be detected between the photoreceptor cell bodies and their axons by the c527-GAL4 reporter line (Hummel et al., 2002). The marginal glia in the larval eye disc (Silies et al., 2007) are not to be confused with the neuropile marginal glia at the base of the lamina in the adult.
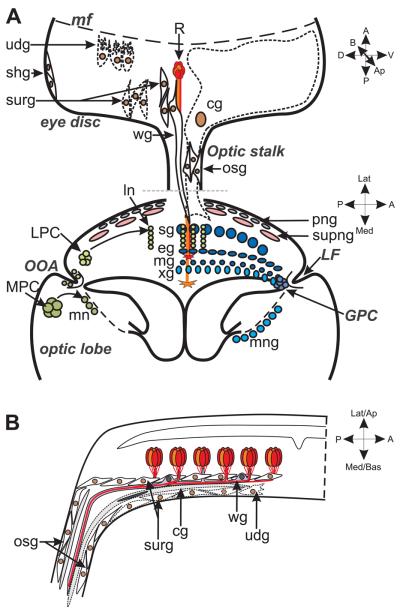
Fig. 2. Glia in the larval visual system.
The larval visual system becomes populated by glia from at least two different sources, the eye disc and in the developing optic lobe, the glial precursor centre (GPC). A. The glia of the eye disc (brown nuclei) all originate in the optic stalk (osg) and migrate into the eye disc. There are four types of differentiated glia in the eye disc: two large basally-located carpet glia (cg, outline in dashed lines), the eye disc marginal glia (edmg; not to be confused with the lamina marginal glia), wrapping glia (wg) and surface glia (surg). Undifferentiated glia (udg, outline in dashed lines) migrate along the basal surface of the eye disc, below the carpet glia, until they come into contact with newly differentiated photoreceptors (R) just posterior to the morphogenetic furrow (mf). Differentiating glia then migrate apically and develop extensions to surround photoreceptor axons, becoming wrapping glia. A grey dashed line indicates a rotation of the brain relative to the optic lobe for illustration purposes, but both wrapping and carpet glia extend into the optic lobes. In the optic lobe, glia are derived from the GPC which lies proximal to the lamina furrow (LF) and the Outer Optic Anlage (OOA), from whence neuronal precursors arise. Three types of lamina glia (dark blue) derive from the GPC; these include at least some of the satellite glia (sg), as well as the epithelial (eg) and marginal glia (mg). The GPC also gives rise to the glia lining the medulla neuropile (light blue) including the outer chiasmal glia (xg) and the medulla neuropile glia (mng). Cells underlying the larval marginal glia are usually labelled medulla glia in the literature, failing to acknowledge that in the adult an additional layer of glia, those of the outer chiasm ‘small’ and ‘giant’ glia (Tix et al., 1997), lies between the marginal glia and medulla glia. Lamina precursor cells (LPC) are displaced to the lamina where, as lamina neurons (ln), their cell bodies come to lie between the satellite and the epithelial glia. Likewise, medulla precusor cells (MPC), possibly ganglion mother cells, ultimately give rise to medulla neurons (mn). Subperineurial glia (supng; pink nuclei) derived from the epithelium and mesodermally derived perineurial glia (png; grey nuclei) surround the entire optic lobe as a sheath to form distinct components of the blood brain barrier. The inner glia of the optic stalk and the medulla cortex glia are not illustrated. B. A cross section of the eye disc shows the relative apical/basal locations of the glia and their locations in relation to the photoreceptors. Figures modified from originals in Chotard et al. (2005) and Silies et al. (2007).
Wrapping glia differ from surface glia, in that they are delayed in migrating to the eye disc, and are not involved in directing photoreceptor axons toward the optic stalk. This delay occurs because wrapping glia derive from a subpopulation of mitotically active surface glia that delaminate and migrate inward. Migrating glia, triggered by Fibroblast Growth Factor (FGF) Receptor activation, differentiate when they reach the anterior edge of the carpet glia and come into contact with the epithelium and newly differentiated photoreceptors (Silies et al., 2007; Franzdóttir et al., 2009). Choi and Benzer's (1994) findings that RBG/wrapping glia labelled by M1-126 do not migrate into the eye in eyeless mutants (eyes absent and sine oculus) may not actually indicate a failure of glia to migrate, but instead a failure of glia to differentiate from perineurial surface glia into M1-126-LacZ labelled wrapping glia, since this would normally occur only when these glia contact photoreceptors expressing the FGF8-like ligand Thisbe (Franzdóttir et al., 2009).
FLP-out clonal analysis of glial cell types (Rangarajan et al., 1999) suggests that the large carpet glia are clonally related to the ‘retinal basal glia’ since both are labelled, although carpet cells are not yet anatomically recognized as a distinct glial subset, in a GFP labelled cell lineage derived from a single clone. Furthermore, Choi and Benzer (1994) report two, and only two, large M1-126-LacZ positive nuclei in the optic stalk of the second-instar larva, which could correspond to the large carpet glia nuclei. Only later in development, at about the third-instar stage, do more M1-126-LacZ positive RBG, with a wrapping glial morphology, appear in the optic stalk. These continue to increase in number over time (Choi and Benzer, 1994). The fact that wrapping glia appear to be clonally related to carpet glia (Rangarajan et al., 1999), and are yet known to form as a result of delamination from surface glia (Silies et al., 2007), suggests that all three glial types (carpet, wrapping and surface) originate from a single precursor type. Again, confirmation of this suggestion must await the availability of suitable markers.
Two further observations on optic stalk glia are pertinent. First, in addition to a population of fusiform perineurial glia (the optic stalk glia) that forms a dense mesh of cells surrounding the photoreceptor axons, the optic stalk also contains an inner glia cell population. While the perineurial glia form a component of the blood brain barrier, the inner glia separate the fascicles of ommatidial axons from those of the unwrapped Bolwig's neuron photoreceptors (Silies et al., 2007). The fate of these inner glia in the adult is unknown since they have not been traced through pupal metamorphosis. Optic stalk glia can also be distinguished by expression of the glial driver line NP7402, even in second-instar larvae, before photoreceptor axons enter the brain (Murakami et al., 2007). As more and more photoreceptor axons grow down into the optic stalk and penetrate the brain, the optic stalk enlarges to accommodate them, but sine oculis mutants demonstrate that the photoreceptors are not required for this enlargement to take place. Glia not only increase in number but the diameter of the optic stalk also increases even before photoreceptor axon ingrowth occurs (Murakami et al., 2007). Glial cell division in the optic stalk depends upon glia-glia intercellular signalling by the FGF8-like ligand Pyramus and activation of its receptor Heartless (Franzdóttir et al., 2009). Expansion of the optic stalk also requires the Focal Adhesion Kinase fak56D and cdGAPr, a GTPase-activating protein domain homologous to that of mammalian CdGAP, for proliferating surface glial cells to migrate in a posterior direction and come to occupy the stalk (Murakami et al., 2007).
2.1.2.3 The eye disc glia likely correspond to adult lamina glia
It is still possible to propose only rather tentative correspondences between these glia identified in the developing visual system and those known in the distal lamina of the adult. The wrapping glia of the larval eye disc are presumed to correspond, in part, to Choi and Benzer's RBG on the following grounds: their smaller numbers, 80 RBG corresponding to 800 ommatidia, and because the RBG actually enwrap photoreceptor axons, about 10 fascicles apiece (Choi and Benzer, 1994). The relatively sparse wrapping glia which extend processes along the surface of developing photoreceptor cell axons (Hummel et al., 2002; Silies et al., 2007) may correspond to the large, sparse, septate junction rich subperineurial pseudocartridge glia. The pseudocartridge glia ensheathe photoreceptor axons extensively as they enter the brain, branching and interdigitating among the axons (Saint Marie and Carlson, 1983a). M1-126 labels both an unidentified glial layer near the lamina of the larval brain and an unidentified layer in the adult lamina (Choi and Benzer, 1994), suggesting perhaps that these unidentified glial cell types in the lamina of larvae and adults are related. The wrapping glia marker Mz97-GAL4 (Hummel et al., 2002) appears, on the other hand, to also label some, as yet unidentified cells in the adult brain (Savarit and Ferveur, 2002) suggesting a possible relationship between glia of the eye disc and those of the adult lamina. The issue of pupal glial reconfiguration is complicated by the fact that carpet glia, not wrapping glia, form the septate-junction-rich subperineurial glia layer in the larva (Silies et al., 2007). However, since wrapping glia far outnumber carpet glia, they seem the most likely candidate to be reconfigured into adult pseudocartridge glia. What becomes of carpet glia in the adult is not known. Alternatively, the outer surface glia (a subset of the perineurial glia) are more numerous than inner wrapping glia, with one glial cell nucleus associated with each ommatidium, and these cells are involved in photoreceptor axon guidance (Hummel et al., 2002). Their exteriormost location, as well as their abundance, makes surface glia a likely candidate for the adult perineurial fenestrated glia.
In summary, we propose that at least some populations of adult lamina glia, fenestrated and pseudocartridge, are derived from glia of the eye disc and that the fenestrated glia may in fact correspond to larval surface glia and pseudocartridge glia to wrapping glia. Again, confirmation of this proposal must await the availability and careful deployment of suitable markers.
2.2 Glia of the olfactory system
Much as the eye conveys spatial information into the brain, the olfactory receptor neurons (ORNs) of the insect antenna project information to distinct glomeruli of the antennal lobe to form a spatial map of odour-specific signalling (Vosshall et al., 2000). The general design of the olfactory network is the same in all insects, with most peripheral sensory neurons synapsing in the antennal lobe and uniglomerular projection neurons from the antennal lobe then conveying this sensory information to higher-order centres in the brain, such as the mushroom bodies and the lateral horn (Galizia and Rössler, 2010).
In Drosophila specifically, the dendrites of the bipolar ORNs expand into sensilla on the third antennal segment and the maxillary palp of the proboscis, with between one and four ORNs per sensillum. Each sensillum is electrically isolated from its neighbour by surrounding support cells that include glia (Venkatesh and Singh, 1984). ORNs are defined by their specific combination of odorant receptors. These are members of either the G-protein coupled receptor gene family (the ORs; Vosshall et al., 1999) or the ionotropic glutamate receptor gene family (the IRs; Benton et al., 2009). In Drosophila there are 1200 ORNs in each antenna (Vosshall and Stocker, 2007) each able to detect a different odour stimulus, and each expressing a combination of between one and four receptor molecules (Couto et al., 2005; Fishilevich and Vosshall, 2005; Goldman et al., 2005) from a group of 45 adult-expressed odorant receptor genes (Clyne et al., 1999; Gao and Chess, 1999; Vosshall et al., 1999). Yet another class of ORN in the coeloconic sensilla expresses a combination of up to five of 15 recently discovered ionotropic receptor genes, some of which are expressed in combination with ORs (Benton et al., 2009). ORNs project to one of the glomeruli in the antennal lobe, with those expressing the same odorant receptor projecting an axon to the same glomerulus to form a chemotopic map (Gao et al., 2000). ORNs expressing 45 unique OR receptor combinations converge on approximately 36 of the 43 distinct glomeruli in the antennal lobe (Laissue et al., 1999) and some of the newly discovered IRs, have been found to project to a subset of glomeruli previously unaccounted for (Benton et al., 2009). Further details are reviewed in Vosshall and Stocker (2007).
While the arrangement and number of sensory neurons may change from species to species, along with a corresponding change in the arrangement of glomeruli in the antennal lobes, the general design of the olfactory system remains the same (Galizia and Rössler, 2010).
2.2.1 Glia of the antenna
In the adult Drosophila antenna there are at least two distinct subsets of glia (Sen et al., 2005). The first is a small population of glia labelled by GH146, which originates in the brain and migrates into the antenna along the ORNs but has no role in patterning the trajectories of ORN axons (Jhaveri et al., 2000; Sen et al., 2005). Once in the antenna these glia, also called the coeloconic independent glia, ensheathe the ORNs, segregating them into groups as their axons enter the brain (Sen et al., 2005). The second group of glia (labelled by MZ319), constitute the majority of glia in the antenna and form an outer sheath around the ORNs and GH146-expressing glia, as well as ensheathing the cell bodies of peripheral sense organs (Sen et al., 2005). These glia are derived from an atonal-dependent coeloconic sensory-order lineage and are involved in sorting ORN axons (Jhaveri et al., 2000; Sen et al., 2005). Despite their role in axon sorting, they do not correspond to the sorting zone glia of the moth Manduca, which arise from the antennal lobe and are located in the brain where the antennal nerve enters the lobe. It is there that axons are sorted into the fascicles which project to distinct glomeruli (Rössler et al., 1999). In fact, because they originate in the brain, at the base of the antennal lobe, the coeloconic independent glia are more likely to be akin to Manduca's sorting zone glia despite the fact that these cells in Drosophila play no role in sorting the ORNs as they enter the antennal lobes (Sen et al., 2005).
2.2.2 Glial organisation in the antennal lobes of Manduca vs Drosophila
The tobacco hornworm Manduca sexta has been a model species for many studies on insect antennal lobe development, structure and function (Tolbert and Hildebrand, 1981). In the adult antennal lobe of Manduca there are five major classes of glia, grouped by the position of their cell bodies: the perineurial and subperineurial glia which ensheathe the antennal nerve and the brain surface, the cortical (or cortex) glia associated with the cell bodies of neurons, the nerve-layer glia (including the sorting zone glia), and the neuropile glia (Oland et al., 1999).
On the way to the first-order olfactory neuropile, the antennal lobe, axons in the antenna are ensheathed by nerve layer glia, of which there is only a single type, with long processes having multiple expansions that enwrap the fascicles of the ORNs. Neuropile glia that surround the olfactory glomeruli fall into two categories. The first are complex glia with large cell bodies and branching astrocyte-like arbors that are associated with axon fascicles as they enter the glomeruli. There are between one and five apically located complex glia per glomerulus. The second are simple glia: small glia with multiple, mostly unbranched processes that form a glomerular envelope with only shallow projections into the neuropile. Each simple glial cell is associated with no more than two or three glomeruli (Oland et al., 1999). Insofar as they lie at the interface between the cell body cortex and the underlying neuropile these are speculated to be modified from interface glia (Edwards and Tolbert, 1998), also known as neuropile cover glia (Cantera, 1993) that have been recognised by various workers (Strausfeld, 1976; Meyer et al., 1987; Cantera 1993; Ito et al., 1995). Glial cells of a single subtype, such as those of the complex neuropile glia can have different shapes depending on their location in the antennal lobe, while the simple neuropile glia can have irregularly shaped nuclei (Oland et al., 1999). Regional subtypes of these glia may also exist, but have not yet been designated.
Drosophila differs from Manduca in that all glial cell bodies remain exclusively in a rind surrounding the glomerulus, and in that the Drosophila glial network is more sparse (Tolbert and Hildebrand, 1981; Oland et al., 1999; Oland et al., 2008). Furthermore, in Drosophila there are no known spatial relationships between the positions of the glial cell bodies and the individual glomeruli (Oland et al., 2008). There are about 80 glial cells in each adult Drosophila antennal lobe. These include the four to six glia that gate and ensheathe tracts on both sides of the antennal commissure (Yao et al., 2007; Oland et al., 2008) and another 2-4 glial cells, possibly nerve layer glia, that encircle the lateral cluster of the antennocerebral tract (Oland et al., 2008) and extend processes around the ORN axons as they enter the lobe (Sen et al., 2005; Oland et al., 2008). Glia have not been indentified at the base of the antennoprotocerebral tract that could ensheathe the efferent projection neurons.
The neuropile glia have been reported to project extensions in three directions. They extend: 1) some processes external to the neuropile to ensheathe incoming olfactory receptor neurons of the antennocerebral tract; 2) out of the antennal lobe to ensheathe projection neuron axons as they extend towards higher-order processing centres in the mushroom bodies; and 3) into the central neuropile to ensheathe large dendrites (Oland et al., 2008). Within the neuropile, these glia insinuate between the glomeruli to form a sparse network around each glomerulus and shallowly invade the synaptic neuropile itself.
Both cortex (Pereanu et al., 2005) and neuropile glia contribute to the sheath surrounding the neuropile. Neuropile glia extend a thin net of velate or branched processes around each glomerulus, which also extend, in a random fashion and at different densities, into the interior of the glomerulus (Oland et al., 2008). Analysis at both the light microscopic and EM levels reveals that glia never completely surround each glomerular neuropile, unlike the situation in the Manduca antennal lobe (Oland et al., 1999); yet individual glomeruli are still able to process distinct odorant information in the absence of any isolating barrier provided by glia (Oland et al., 2008). The glial investment of the neuropile varies in an anterior to posterior direction, with glomerular borders and neuropile investment becoming less visible towards the posterior (Oland et al., 2008).
Using a GAL4/FLP-out based system Awasaki et al. (2008) identified a number of glial cell types in the CNS of Drosophila. Based on cellular location and morphology these constitute the three broad categories: surface, cortex or neuropile, which are further subdivided into distinct groups of surface and neuropile glia (Awasaki et al., 2008). Two types of neuropile glia have been reported: one that preferentially outlines the neuropile compartments and another that fills the interior. Glia specific to the antennal lobes of Drosophila have been defined by similar FLP-out techniques: cell body glia with a nucleus in the cortex, and two types of neuropile glia, ensheathing and astrocyte (Doherty et al., 2009; Fig. 3D). Ensheathing glia are flattened and line the borders of the neuropile to separate it from the cortex. They have processes which surround but do not invade the glomeruli, thus acting to separate these units. Astrocyte-like glia, by contrast, extend membranes into the neuropile to surround synaptic rich regions (Doherty et al., 2009). Thus on morphological grounds these Drosophila glia correspond, respectively, to the simple lamellar glia, and the complex astrocyte-like glia, as described earlier for the antennal lobes of Manduca (Oland et al., 1999).
2.2.2.1 Antennal lobes of bees
Embedded within the somata of the antennal lobe cortex, and restricted to this area, are large multipolar glial cells with numerous cytoplasmic processes that branch amongst the neuronal cell bodies and extend to the surface to form the glomerular envelope. The external and lateral sides of the antennal lobe glomeruli in the honeybee are covered by a cap of glia with flat, spindle-shaped nuclei. These have long lamellar extensions which enwrap the glomeruli with several layers of a glial sheath. As in the Drosophila antennal lobes, glial cell processes fail to invade the neuropile, so that individual glomeruli are not separated by a glial sheath. However, unlike Drosophila a few glial cell bodies can be detected in the neuropilar core of the antennal lobes (Hähnlein et al., 1996). Extensions of these also invade antennoprotocerebral tracts containing the antennal lobe projection neurons (Hähnlein and Bicker, 1996). Thus, much as in the antennal lobes of Drosophila and Manduca, three types of glia can again be identified in bees: a single type of cortex glia and two kinds of neuropile glia, ensheathing and astrocyte-like.
2.2.3 Mushroom body glia
Outputs from the antennal lobes include multiple antennoprotocerebral tracts which project to the lips of the mushroom bodies and the lateral horn (Mobbs, 1985; Galizia and Rössler, 2010). The mushroom body plays a role in higher-order sensory integration, learning, and, in particular, odour-related learning (de Belle and Heisenberg, 1994). The mushroom bodies themselves comprise the calyx input neuropiles, the pedunculus, and the α-, β- and γ-lobes. In social hymenopterans the calyx can be further divided into the lip (olfactory input), collar (visual input) and basal ring (Galizia and Rössler, 2010). Intrinsic neurons, the Kenyon cells, extend dendrites into the calyx. Depending upon the subtype of Kenyon cell the axon may extend through the pedunculus and branch into the α and β lobes (Class I), or just the γ lobe (Class II; Fahrbach, 2006). We will next summarise the glial organisation of these regions for three different insect systems.
In the Drosophila mushroom body, glia can be detected in the cell body clusters of the cortex (Ito et al., 1997). Glia also ensheathe the calyx, pedunculus and lobes. Neuropile glia extend throughout the calyx, not in an organized way that reflects the quadripartite origins of this neuropil, but instead so as to form a loose, unorganised meshwork. Furthermore, they do not enwrap the synaptic microglomeruli (Leiss et al., 2009) and thus are unlikey to have a role in neurotransmitter recycling in this neuropile. Still other glia can be detected between the four fascicles of the pedunculus (Ito et al., 1997).
In the cricket Acheta domestica, at least, the mushroom body cortex is not very rich in glia (Cayre et al., 1996). At the cortex-neuropile interface glia, with large nuclei that are immunoreactive to MAb 5B12 (Glionexin), outline the neuropiles at the bottom of the calyx and extend between the columnar rows of Kenyon cells. The neuropile also contains a distinct subset of microglia labeled by MAb 3G6 (Cayre et al., 1996).
Ethyl gallate staining in the honeybee reveals both cortex and neuropile glia in the mushroom body. Glial cells of varying shapes lie amongst and wrap around the Kenyon cell bodies in the cortex. The size, position and number of glia enwrapping Kenyon cell bodies differ in drones and workers, yet both have small glia which extend processes which wrap around the neuronal somata as well as around the tops of Kenyon cell axons which project towards the calyces. The mushroom body neuropile is completely lined by an envelope of spindle-shaped glia, separating it not only from the cortex but also from the surrounding protocerebral neuropile. Internally, the mushroom body neuropile is divided up into compartments by numerous astrocyte-like glia. These delineate the three columns of the pedunculus that correspond to the lip, collar, and basal ring. There is also a network of mushroom body intrinsic glial cells. They separate the α-lobe from the β-lobe where these branch, as well as separating the lobes from the pedunculus. Although trachea in the calyces are also ensheathed by glia, glia in the calycal neuropile itself are rare, lying only at the border of the collar and basal ring (Hähnlein and Bicker, 1996).
The α- and β lobes have three horizontal strata, with a high density of astrocyte-like glia in the ventral part of the α-lobe. Glia also occur at the boundaries between some of the six layers of the α-lobe and extend processes between the layers where extrinsic neurons arborize into bands. By contrast, only a glial septum-like structure splits the β-lobe into dorsal and ventral halves (Hähnlein and Bicker, 1996).
3. Glial function
Glia fulfill many functions in the insect brain. Of these their role in forming the blood brain barrier and in recycling neurotransmitters have received particular attention.
3.1 Blood brain barrier
In vertebrates, tight junctions between endothelial cells of capillaries in the CNS form the primary barrier to extracellular flow, whereas in insects there is no closed circulatory system and the CNS must instead be isolated from the open circulation of haemolymph. This barrier is necessary to protect the brain from haemolymph fluctuations especially in K+, such as those which occur after feeding in some insects (Treherne, 1985). Glia also surround the tracheoles by which air enters the brain (Cantera and Trujillo-Cenóz, 1996; Pereanu et al., 2007). A role for glia in the BBB can be clearly demonstrated by the diffusion of extracellular tracers, which is blocked at glial sites (Shaw, 1977), an ability believed to be the principal function of occluding junctions such as the pleated septate junctions (Stork et al., 2008). For an extensive review of insect junctions see Carlson et al. (2000).
3.1.1 Impermeability to dyes and tracers
Septate junctions have been implicated in establishing an important diffusion barrier in the insect nervous system. Fly brains that lack glia, or normal glial function, such as in the Drosophila mutant reversed polarity (Repo) are not only permeable to extracellular tracers such as dextran (Stork et al., 2008) but also have abnormal physiological properties such as the reversal in polarity of their ERG, for which they are named (Xiong et al., 1994; Xiong and Montell, 1995). Embryos that are mutant for neurexin IV, a component of the septate junction, allow the diffusion of dextran into the CNS with similar kinetics to that of gcm flies which lack glia altogether, suggesting that the septate junction itself is a vital component of the BBB (Stork et al., 2008).
In the lamina it appears as if the barrier that prevents influx of substances from the haemolymph into the brain is composed of septate junctions formed between pseudocartridge glia and their neighbours. Thus, when introduced to the circulating haemolymph, dyes never enter the optic lobes, and tracer substances such as ionic lanthanum and dyes do not enter the optic lobe from the retina, but instead penetrate only a superficial layer of perineural cells – the fenestrated glia (Shaw, 1984). At the EM level, colloidal lanthanum applied to the retina fails to penetrate deeply into the septate junctions of pseudocartridge glia (Saint Marie and Carlson, 1983b). These findings, along with the fact that tight junctions, the only other occlusive junctional contact, are not readily found in the pseudocartridge glia (Saint Marie and Carlson, 1983b), suggests that the pleated septate junctions of the pseudocartridge zone function principally to restrict movement of substances from the blood into the optic neuropiles.
3.1.2 Molecular components of the septate junction
The septa of the septate junction act like a series of baffles to impede the passage of substances through the extracellular space between glia. In Drosophila embryos smooth and pleated septate junctions have been reported (Tepass and Hartenstein, 1994). These vary morphologically and have different tissue distributions, but are functionally equivalent (Lane et al., 1994).
Pleated septate junctions contain NeurexinIV (Baumgartner et al., 1996) and Coracle (Fehon et al., 1994) which are believed to be components of the septa themselves. A myriad other genes also affects septate junction formation in different Drosophila tissues, including genes for structural proteins such as the polarity determining PDZ protein Scribble (Bilder and Perrimon, 2000); Membrane Associated Guanylate Kinases such as Discs large (Woods and Bryant, 1991; Hough et al., 1997) and Varicose (Wu et al., 2007; Moyer and Jacobs, 2008); the transmembrane claudins Sinuous (Wu et al., 2004) and Megatrachea (Behr et al., 2003); cell adhesion proteins Contactin (Faivre-Sarrailh et al., 2004), Neuroglian (Genova and Fehon, 2003), Fasciclin III (Hortsch and Goodman, 1991; Hough et al., 1997), Gliotactin (Genova and Fehon, 2003; Schulte et al., 2003) and Lachesin (Llimargas et al., 2004; Strigini et al., 2006); the G-protein coupled receptor Moody (Bainton et al., 2005; Schwabe et al., 2005); the transcription factor Grainy head (Narasimha et al., 2008), and the α and β subunits of the Na+/K+ ATPase Nervana 2 (Genova and Fehon, 2003). Gliotactin, Neuroglian and both subunits of Nervana 2 are all required to form a functioning paracellular barrier (Genova and Fehon, 2003) and yet the nervana2 promoter does not drive expression in subperineurial glia (Mayer et al., 2009), the primary cellular component of the BBB in insect nervous systems. It is instead limited to cortex and neuropile glia (Pereanu et al., 2005), such as the cortical distal satellite glia of the Drosophila lamina (Górska-Andrzejak et al., 2009), which lie just beneath the subperineurial pseudocartridge glia.
3.1.3 Other requirements for a properly developed blood brain barrier
In addition to septate junction proteins, the G-protein coupled receptor Moody, which is expressed specifically by surface glia, is essential for proper barrier formation. Loss of moody results in both the reduced formation of septate junctions and a leaky blood–brain barrier because moody mutants purportedly fail to properly regulate the cortical actin cytoskeleton that assembles septate junctions (Bainton et al., 2005; Schwabe et al., 2005). The α and β proteins of Moody immunolocalise in the adult Drosophila brain to a wide band in the lamina that is proposed to include the pseudocartridge glia (Bainton et al., 2005) but may also include the satellite glia which contain abundant septate junctions, at least in Musca (Saint Marie and Carlson, 1983b). Higher resolution studies are needed to clarify this point.
The ATP-binding cassette (ABC) transporter gene mdr65 is another essential component of the BBB system with an important role in neuroprotection. Mdr65, which is immunolocalised to the apicalmost surface of the subperineurial glia, near the humoral interface, is able to change the inherent sensitivity of the blood brain barrier to toxic pharmaceuticals (Mayer et al., 2009). Mutations of mdr65 allow abnormal passage and accumulation of ABC transporter substrates, such as the lipophilic dyes Rho123 and RhoB, into the brain from the haemolymph, but do not affect accumulation of 3 kDa FITC-dextran or 10 kD Texas Red dextran, which are not substrates for the ABC transporters. The selective transport of Rho123 and RhoB fluorophores, but not dextrans in mdr65 mutants indicates that the mutation does not disturb the paracellular diffusion barrier. Mutations in mdr65 also increase sensitivity to the anti-microtubule agent vinblastine, allowing mutant brains to accumulate significantly more dextran than wild-type brains or mdr65 brains without co-application of vinblastine. Thus mdr loss of function increases the sensitivity of subperineurial glial cells to vinblastine, suggesting a neuroprotective role for the intact protein (Mayer et al., 2009). The functions of an additional ABC transporter gene, white, and its binding partner genes brown and scarlet, are detailed below.
3.1.4 The blood brain barrier controls the flow of nutrients from haemolymph to neurons
Occluding barriers have the consequence that fluid-borne ions and nutrients have limited access to the underlying avascular neuropile. A possible solution to this conundrum has been found among the lamina glia, where both the proximal (marginal) and distal (fenestrated, pseudocartridge) layers of glia contain clathrin-coated vesicles (Saint Marie and Carlson, 1983a). These indicate the cells' capacity for active endocytosis by which they may acquire nutrients.
3.1.4.1 Transport of material: the role of transporters and gap junctions
For ions, nutrients and metabolites to reach neurons, glia must be able to transport these components from the haemolymph to the brain and in the opposite direction, sometimes with great efficiency. For some components that move over short distances diffusion is sufficient, for which the interglial gap junctions that are reported to exist between all glia of the first optic neuropile (Saint Marie and Carlson, 1983b) to form an intercellular network, may be utilized. This glial intercellular network may be a means by which essential nutrients can be transported in, but also a means by which excess neurotransmitter can be cleared from the brain (Carlson and Saint Marie, 1990).
Not only have gap junctions been detected at the EM level between glia in some species of fly (Saint Marie and Carlson, 1993b), but their molecular components, the innexins, have also been localized to the developing and adult visual system of Drosophila. Thus, innexin 2, innexin 3, and optic gangion reduced (ogre) all express mRNA transcripts in a similar pattern at areas likely to correspond to glial cells (Stebbings et al., 2002).
Altered function of innexins also has implications in the visual system. For example ogrecb8 mutants have not only a reduced number of neurons in the optic lobes (Watanabe and Kankel, 1990) but also an abnormal ERG that lacks ‘on’ and ‘off’ transients (Curtin et al., 2002). The ogre promoter drives expression in photoreceptors and the presence of a functional form of Ogre in the photoreceptors is necessary for a normal ERG. Ogre does not form a homotypic dimer however (Curtin et al., 2002), so some other innexin must be expressed in the photoreceptors to create a functional gap junction. Shaking B (N1 and N2) mRNA is expressed in the lamina monopolar cells (Zhang et al., 1999), where it has an effect on the lamina transients of the ERG (Homyk et al., 1980). This leaves Innexin 1 and 2 as the most likely subunits to constitute the gap junctions of the lamina glia. Their existence and funtion have yet to be confirmed at these sites, however.
3.2 Neurotransmitter transport functions of glia
3.2.1 Optic lobe glial functions
3.2.1.1 Histamine and the role of glia in the ERG response: the HisCl receptor
Drosophila photoreceptors release histamine as a neurotransmitter at their synapses (Hardie, 1987, 1989). Histamine is produced in the retina from histidine, in a single-step reaction regulated by the enzyme HDC (Burg et al., 1993) then pumped into vesicles and released at the photoreceptor synapse. Few of these steps are known in any detail, however. Drosophila photoreceptor synapses are uniformly tetrads, at which release occurs onto a cluster of four postsynaptic elements. These comprise lamina monopolar neurons L1 and L2, which are obligate partners at all tetrads and two other partners from some combination of amacrine cells, L3, or epithelial glia (Fig. 4B; Meinertzhagen and O'Neil, 1991). Released histamine hyperpolarizes the lamina monopolar neurons (Hardie, 1987) when it binds to Ort (HClA, HisCl2), a histamine-gated chloride channel (Gisselmann et al., 2002; Zheng et al., 2002), allowing an influx of Cl− ions into the postsynaptic neurons. The summed electrical response of both photoreceptor depolarization and the lamina response to histamine release together constitute the externally recorded ERG (Fig. 4C). Depolarization of the photoreceptors produces the sustained negative response (Heisenberg, 1971) while hyperpolarization of monopolar cells has been reported to produce the ‘on’ transient and their depolarization the ‘off’ transient (Heisenberg, 1971; Coombe and Heisenberg, 1986). The evidence for this conclusion rests on the concurrent loss of ERG transients and L1 and L2 neurons in the mutant vacuolar medulla, a correlation that does not, however, take into account other possible changes that occur in the lamina with the loss of these cells. In support of the conclusion that the origin of ‘on’ and ‘off’ transients lies in lamina monopolar cells, null mutations of the histamine-gated Cl− channel gene HisCl2 (HClA, ort) that express in these cells (Witte et al., 2002) abolish the synaptic transients of the ERG (Pantazis et al., 2008). The ERG response can, however, be modulated by neighbouring epithelial glia which express the second histamine-gated Cl- channel gene, hisCl1 (Pantazis et al., 2008), or by synaptic feedback from amacrine and monopolar neurons (Zheng et al., 2006; Nikolaev et al., 2009). When compared with wild-type flies, mutants of hisCl1 show a slower rise time in intracellular recordings from monopolar neurons as well as a twofold increase in the amplitude of the “on” transients of their ERG (Pantazis et al., 2008). A number of explanations have been advanced for this difference (Pantazis et al., 2008). First, there is the possible contribution of epithelial glia to the extracellular field potentials that regulate transmitter release from R1-R6 (Shaw, 1984). Second, activation of histamine-gated chloride channels on the epithelial glia may affect glial uptake of histamine, with the histamine remaining in the cleft exerting a prolonged action at the HisCl2 (Ort) channels of L1-L3 that co-occupy the tetrad. Third, HisCl1 channels on the glia compete for histamine with the HisCl2 channels on L1-L3, thus reducing the response of the monopolar cells (Pantazis et al., 2008).
3.2.2 Histamine clearance from the synaptic cleft
After its release from the R1-R6 photoreceptor terminals, liberated histamine must then be cleared from the cleft. While it is not yet known how this clearance occurs there are two possible routes: 1) direct reuptake into the photoreceptor terminal, or 2) uptake into the surrounding epithelial glia. The photoreceptors of barnacle nauplii Balanus amphitrite take up radioactively labelled histamine in a Na+ dependent manner (Stuart et al., 1996; Stuart et al., 2002) but such studies do not clearly demonstrate that the uptake is direct, and not via an unidentified glial or pigment cell pathway. Corresponding evidence that Dipteran photoreceptors can take up histamine directly is lacking. When barnacle eyes take up radioactive histamine, depolarized photoreceptor terminals take up the label more intensely in the light than do hyperpolarized, dark-adapted photoreceptors, and in dark-adapted nauplii it is the glia that label strongly (Stuart et al., 1996). This finding is inconsistent with findings from other Na+ dependent transporters, which take up their transmitter when the cell is relatively hyperpolarized (Tachibana and Kaneko, 1988; Cammack and Schwartz, 1993). Stuart et al. (1996) hypothesise that active uptake of histamine into depolarized photoreceptors may occur either because of the release itself, because the histamine is sequestered more actively into a vesicular pool during transmitter recycling, or because of other presynaptic processes involved with transmitter release, such as ion or second-messenger changes.
The possibility that light-induced release of neurotransmitter might itself also induce or accelerate direct histamine reuptake by the photoreceptors should be seen in the light of two major characteristics of fly photoreceptors, their high gain and rapid rates of tonic neurotransmitter release (Laughlin, 1981; Shaw, 1984; Uusitalo et al., 1995). From likely rates of vesicle shedding, quantum size, and the number of synaptic vesicles in each R1-R6 terminal, it has been calculated that without recycling the terminal's histamine would theoretically deplete within a matter of about 10 seconds (Borycz et al., 2005). It may be possible that given the increased rate of histamine release during light exposure, both direct (into the R-cell) and indirect (via glial) reuptake mechanisms could be utilized, whereas tonic dark release may not utilise direct reuptake.
3.2.3 The histamine/carcinine recycling pathway: The reciprocal roles of Ebony and Tan
Borycz et al. (2002) were the first to propose that a histamine/carcinine (β-alanyl-histamine) recycling pathway exists at fly photoreceptors and this is now known to involve a shuttle between the photoreceptor terminals and their surrounding epithelial glia (Richard et al., 2003; True et al., 2005; Wagner et al., 2007; as reviewed in Stuart et al. 2007). This recycling pathway utilizes the reciprocal actions of two proteins Ebony and Tan (Fig. 4A), encoded by evolutionarily ancient bacterial peptidase (ebony: Hovemann et al., 1998) or fungal isopenicillin-N N-acyltransferase (tan: True et al., 2005) genes. The pathway was first demonstrated by the accumulation of [3H]carcinine in tan, but not ebony flies (Borycz et al., 2002), and can be explained by the reciprocal regulation of β-alanyl conjugation of histamine. Ebony, which is expressed in epithelial glia (Richardt et al., 2002), confines histamine as the inactive conjugate, carcinine. Alternately, Tan protein in the photoreceptors (Wagner et al., 2007) liberates the histamine from its conjugation to β-alanine as carcinine, an action that has been confirmed in vivo (True et al., 2005).
3.2.4 Histamine transport
3.2.4.1 The role of Inebriated and White in carcinine/histamine transport
The proper function of the histamine cycling pathway is dependent on a means by which to transport histamine and carcinine. Histamine in the synaptic cleft may be transported directly back into the photoreceptors or into the glia where it is inactivated, while carcinine must be transported out of the glia and back into the photoreceptors where it can be recycled into histamine. β-alanine must be shuttled back and forth between both photoreceptors and glia. Missing from the equation, however, is the means by which histamine is taken up into the epithelial glia and the means by which carcinine is then returned from the glia to the photoreceptors, fluxes that must be rapid and for which the role of specific transporters would normally be invoked (Stuart et al., 2007).
It has been proposed that inebriated (ine) plays a role in returning carcinine to the photoreceptor (Gavin et al., 2007). Ine is a member of the Na+/Cl− dependent neurotransmitter transporter family which is expressed as multiple transcripts, two of which are characterized: a short form, Ine–RB with 12 transmembrane spanning domains and a long form, Ine–RA with an additional N-terminal domain (Burg et al., 1996; Soehnge et al., 1996). While it is not yet known if these two forms are functionally distinct, flies mutant for either the long form (ine2) or both forms (ine3) have an abnormal ERG characterized by oscillations during the retinal ‘sustained negative response’ and a loss or reduction of both ‘on’ and ‘off’ lamina transients (Burg et al., 1996, Gavin et al., 2007). Two pieces of evidence implicate a relationship between Ine function and carcinine: 1) retinal oscillations are missing in ine2;ebony11 double mutants but not in tan1;ine2 double mutants, suggesting that the product of ebony gene action, carcinine, contributes to these oscillations; and 2) feeding flies carcinine can act partially to mimic the ine2 ERG phenotype, implying that these oscillations result from an excess of carcinine.
Glial specific rescue of the short transcript, ine-RB, in an ine3 mutant background fully restores the ‘on’ and ‘off’ transients to the ERG and eliminates the oscillations, while photoreceptor-specific rescue eliminates the oscillations (Gavin et al., 2007). Gavin et al. (2007) hypothesise that carcinine accumulates in the extracellular cleft of ine mutants because non-functional Ine cannot transport carcinine into photoreceptors. They conclude that an excess of carcinine causes the retinal oscillations, while the loss of transients are said to be due to a consequent reduction in histamine. If flies are able to clear histamine from the cleft via uptake into the glia but are then unable to liberate the inactive carcinine for return to the photoreceptors, as is proposed to occur when ine is defective, histamine recycling would be effectively blocked.
Four major obstacles remain to this hypothesis of ine function. First, ine mRNA has been localized to the retina (Burg et al., 1996) but has yet to be directly localised to the photoreceptors or specifically to the epithelial glia. Second, although Ine functions as a transporter (Huang and Stern, 2002) and despite its ability to modulate the ERG (Gavin et al., 2007) the protein itself has not yet been actually shown to transport carcinine or even histamine. Third, no distinction is made between possible functional differences of the two Ine transcripts, long and short. While mRNA of the long transcript is abundant in the head (Burg et al., 1996) and loss of the long transcript alone (via ine2) can result in an ERG mutant phenotype, this phenotype can be rescued using the short version of the protein (Gavin et al., 2007). Fourth, rescue of ine exclusively in the glia should allow carcinine to be transported out of the glia but not back into the photoreceptor. Thus, there would be excess carcinine in the extracellular space and this, according to the model proposed above, should result in more oscillations in the retinal response, not fewer, as has been demonstrated (Gavin et al., 2007).
The suggested action of Ine in the visual system relies on both the release of carcinine from glia as well as its uptake by photoreceptor neurons (Gavin et al., 2007). Ine (as RosA) has been localised by means of in situ hybridisation to regions throughout the brain of Drosophila, including the retina (Burg et al., 1996). An antibody against the Manduca sexta Ine protein, which has 55% identity with Drosophila protein, immunolocalises to all of the glial cells, neuropiles and axons in the optic lobes of both Manduca and Drosophila (Chiu et al., 2000). In Drosophila, Ine has been reported to localize to the cell bodies of photoreceptors as well as their axons in the lamina and medulla (Chiu et al., 2000) although published data from this study can also be interpreted to suggest expression solely in areas corresponding to the fenestrated, and pseudocartridge glia. There is clearly room for a detailed study of Ine immunoexpression in the visual system, but available data already suggest that expression will be widespread and restricted neither exclusively to neurons, glia, nor to regions of histamine release.
Despite having an unknown substrate (Chiu et al., 2000), the presence of Ine expression throughout the CNS of Manduca sexta and Drosophila (Burg et al., 1996; Chiu et al., 2000), implies a much wider role than just carcinine transport in the photoreceptors and glia of the visual system. Not so far considered is β-alanine, a specific substrate for Ebony action that is presumably widely used as a means to inactivate biogenic amines (Richardt et al., 2003). This also requires a transport mechanism that has so far eluded identification.
Mutants of ABC transporter genes white, brown, and scarlet that control eye pigmentation reveal an additional transport function implicating optic lobe glia. These mutants all have a reduced head content of histamine, as well as reduced levels of other biogenic amines (Borycz et al., 2008) and a redistribution of these amines in head homogenates. In all such mutants less neurotransmitter, be it histamine, dopamine or 5-HT (5-hydroxytryptamine/serotonin), is detected in a vesicle-enriched pellet fraction and more remains in the supernatant. This change is consistent with a redistribution of neurotransmitter that reflects a function for White and its binding partners in pumping amine transmitters into some compartment contained within the pellet fraction of brain homogenates, most likely the synaptic vesicles (Borycz et al., 2008). This explanation is weakened by the fact that histamine is found in photoreceptor terminals while White protein is only weakly expressed in the photoreceptors and is instead found far more obviously in the surrounding epithelial glia (Borycz et al., 2008). A possible explanation can be invoked from this glial expression considering that the mutants also have fewer multiple-headed capitate projections. This is consistent with the possibility that mutants of ABC transporters have altered vesicle endocytosis, which occurs at the capitate projection (Fabian-Fine et al., 2003).
A significant aspect of White function is that the white mutation alters the histaminergic phenotypes of tan and ebony flies. A white,tan double mutant effectively triples the head histamine content relative to single-mutant tan flies; conversely the white;;ebony double mutant has reduced histamine relative to ebony (Borycz et al., 2008). How exactly does a glial-associated ABC transporter such as White affect photoreceptor specific Tan protein function in order to ultimately alter head histamine concentration? The answer may lie in the fly's ability to take up histamine into the glia and convert it to the inactive form carcinine. Thus, both single-mutant white and double-mutant white, tan are unable to convert tritiated histamine into carcinine whereas tan flies can convert tritiated histamine into tritiated carcinine (Borycz et al., 2008), essentially trapping all excess histamine as carcinine (Borycz et al., 2002; True et al., 2005). This difference suggests that white flies lack the ability to take up histamine into their glia for conversion to carcinine under the action of Ebony (Richardt et al., 2002; Richardt et al., 2003; Borycz et al., 2008). However, this does not implicate White itself as an actual neurotransmitter transporter nor does it explain why ABC transporter mutants have an excess of non-vesicular neurotransmitter. More must be done to determine the transporters responsible for shuttling of histamine, carcinine and β-alanine into and out of neurons and the role played by the different optic lobe glia in this process.
3.2.4.2 Capitate projections as specialised recycling organelles
Capitate projection organelles are sites at which epithelial glia make close invaginating appositions into photoreceptors (Fig. 4A, B). Each is characterized by its spherical head, approximately 190 nm in diameter (Stark and Carlson, 1986) that contains a widened extracellular space between the glial and photoreceptor cell membranes. This space is filled with an unknown filamentous electron-dense substance (Saint Marie and Carlson, 1983a). Just beneath the glial cell membrane there also lies an intracellular electron-dense filamentous material (Stark and Carlson, 1986). The capitate heads are also characterized by particles on both glial and photoreceptor cell surfaces that have been visualized in freeze-fractured material (Stark and Carlson, 1986). None of these specializations has been assigned a function. While it is not known what cytoskeletal proteins are associated with the capitate projection on the glial side, on the photoreceptor side the cell surface Extracellular Matrix Metalloprotease Inducer, Basigin, which interacts with Integrin to promote cytoskeletal rearrangement, is required to enable epithelial glia to enter the R1-R6 terminals to form a penetrating capitate projection (Curtin et al., 2005).
Capitate projections, or structurally related organelles, also occur in the terminals of R7 and R8 in the medulla (Takemura et al., 2008) as well as in the terminals of photoreceptors in the ocellus (Stark et al., 1989). These structures are all associated with histamine positive photoreceptors (Nässel et al., 1988; Pollack and Hofbauer, 1991) but the glia that participate at these invaginations obviously differ at each of the three sites. Irrespective of the invaginating glial subtype, the photoreceptor controls the size of the capitate projection head (Edwards and Meinertzhagen, 2009). It is not known what role, if any, glial cytoskeletal proteins play in the formation, penetration, or maintenance of capitate projections but it is clear that photoreceptors and their cytoskeletal proteins play an important role in the shape, extension or maintenance of these specialised organelles, probably via an interaction between photoreceptors and glia that is initiated on the photoreceptor cell surface and requires Basigin (Curtin et al., 2007).
Capitate projections are sites of vesicle endocytosis and the endocytotic proteins Clathrin and Endophilin localize to these organelles (Fabian-Fine et al., 2003). Clathrin coated vesicles occur near capitate projections and endocytotic figures attached to the capitate projection stalk are seen during recovery from endocytotic arrest in the mutant shibire, suggesting a role for the capitate projection in vesicle recovery and a postulated role in direct histamine recovery (Fabian-Fine et al., 2003). The mechanism for the latter lacks evidence, however, and is suggested mostly from the economy of linking vesicle retrieval with histamine recyling in a single organelle and by the intimacy there between epithelial glia, which produce carcinine, and the photoreceptor terminal, which hydrolyses it to liberate trapped histamine. Capitate projections are dynamic and their various stages of development, from shallow to multiheaded, can be identified in a short series of EM sections. Furthermore, the numbers of capitate projections in a photoreceptor terminal can change dependent upon light conditions (Rybak and Meinertzhagen, 1997), pharmacological treatment (Pyza and Górska-Andrzejak, 2004), temperature (Brandstätter and Meinertzhagen, 1995), or genetic mutation - including mutations of tan and ebony (Meinertzhagen and Wang, 1997).
3.2.5 A possible role for other glia
Tan protein localises exclusively to photoreceptors (Wagner et al., 2007) and the fly's rapid histamine recycling pathway in the lamina has been proposed to work via a shuttle pathway between photoreceptor terminals and ebony-containing epithelial glia (Borycz et al., 2002; Richardt et al., 2002; True et al., 2005; Wagner et al., 2007) as reviewed in Stuart et al. (2007). However, histamine immunolabelling itself localises not only to the photoreceptors but also to a narrow band beneath the basement membrane of the eye that corresponds to the fenestrated and/or pseudocartridge glia (Romero-Calderón et al., 2008) as well as to a band beneath the lamina neuropile that corresponds to the location of marginal glia (Borycz et al., 2002; Romero-Calderón et al., 2008). These glia contain neither HDC nor Tan and thus neither produce histamine on their own nor recycle it from carcinine. We propose that marginal glia may act as a sink for excess histamine which cannot be quickly transported back to photoreceptors at sites along the length of their terminals. However, this possibility poses two problems. First, it is not known how marginal glia which are not intimately associated with the R1-R6 terminals at sites of their histamine release, acquire histamine. Two possibilities exist: 1) excess histamine pools at the bottom of the lamina neuropile and is taken up at marginal glia either by transporters or by endocytosis, given that in Musca these glia also contain coated vesicles (Saint Marie and Carlson, 1983b); and 2) in Musca marginal glia are reported to connect to epithelial glia via gap junctions (Saint Marie and Carlson, 1983b), which allow the passage of dyes up to at least 2 kDa in size (Phelan and Starich, 2001), and so could allow excess carcinine to pass from the epithelial glia into the marginal glia for storage. The second problem may thus simply be the reverse of the first, namely how carcinine then finds its way back to the photoreceptor terminal.
Ebony protein localises not only to the epithelial glia but also to medulla neuropile and chiasmal glia (Richardt et al., 2002). In addition, close inspection of previously published confocal microscopy data (Wagner et al., 2007) reveals possible additional sites of Ebony expression in the lamina proximal satellite glia. Ebony does not appear to be expressed in the fenestrated or pseudocartridge glia, however. Table 3 provides a list of cell markers and Drosophila GAL4 lines which drive marker expression in glia and may be used to correctly identify which adult optic lobe glia express Ebony or other histamine-related proteins.
Table 3.
Cell specific markers for glia of the adult optic lobes
| Glial cell type | Gene/Protein/Driver | Function | Reference |
|---|---|---|---|
| fenestrated glia | α dVMAT-B | vesicular monoamine transporter | Romero-Calderón et al., 2008 |
| distal satellite glia; some medulla neuropile glia; outer/inner chiasm glia; most cortex glia |
Nrv2- GAL4 | Na+,K+ -ATPase beta-subunit |
Górska-Andrzejak et al., 2009; Oland et al., 2008 |
| fenestrated glia; central brain glia |
CG33528 | VMAT-like | Thimgan et al., 2006 |
| epithelial glia | black | aspartate/ glutamate decarboxylase, β-alanine production |
Phillips et al., 1993 |
| epithelial, and medulla neuropile glia |
α Ebony | β-alanine-biogenic amine synthetase |
Richardt et al., 2002; Wagner et al., 2007, |
| surface* and satellite glia |
P1478 -GAL4; Basigin |
Extracellular Matrix MetalloPRotease Inducer |
Curtin et al., 2007 |
| fenestrated glia | ruby | clathrin binding AP-3beta adaptin | Kretzschmar et al., 2000 |
| epithelial glia | HisCl1- GAL4 | histamine receptor | Pantazis et al., 2008; Gao et al., 2008 |
| all lamina glia and medulla neuropile glia + |
B380 - LacZ | unknown | Winberg et al., 1992 |
| epithelial glia | α White | ABC transporter subunit | Borycz et al., 2008 |
| surface*, satellite, epithelial, marginal glia + |
3-109 (loco) - LacZ | regulator of G-protein signalling | Winberg et al., 1992; Xiong et al., 1994, Perez and Steller 1996 |
also labels other glial subtypes in the brain
glia labelled as surface are not distinguished as being either fenestrated or pseudocartridge
The wide range of Ebony expression suggests that other glia may be involved in histamine recycling and, in addition, a storage function for lamina glia can be suggested in one further example. Mutant ebony flies, which cannot produce carcinine from histamine and have 50% less total head histamine than wild-type flies, lack the wild-type band of histamine immunolabelling beneath the basement membrane (Borycz et al., 2002). This band corresponds to the location of the fenestrated and pseudocartridge glia, two glial subtypes which do not normally express Ebony protein (Richardt et al., 2002). It is unclear how an ebony mutation affects histamine expression in glia which do not normally express ebony unless we consider these glia a barrier to, or storehouse for, excess histamine; a storehouse not otherwise needed in an ebony fly because of their failure to effectively recycle histamine through the epithelial glia pathway. It is not known how histamine reaches the fenestrated and pseudocartridge glia which in Musca are separated from the lamina neuropile, and thus from the source of histamine, by a band of glia rich in occluding junctions (Saint Marie and Carlson, 1983b).
One way in which histamine could enter the fenestrated and pseudocartridge glia is via the vesicular monoamine transporter DVMAT-B, which has been identified in the fenestrated glia in Drosophila (Thimgan et al., 2006; Romero-Calderón et al., 2008). VMAT transporters normally mediate the transport of monoamine neurotransmitters into secretory vesicles, and Drosophila variants of this protein recognise and possibly transport the biogenic amines, dopamine, 5-HT, octopamine, tyramine and histamine (Greer et al., 2005). DVMAT-A is immunolocalised to dopaminergic and serotinergic neurons, as well as to octopaminergic type II terminals at the neuromuscular junction (Greer et al., 2005), while DVMAT-B immunolocalises to the fenestrated glia (Romero-Calderón et al., 2008). This site is unusual because it is glial and because the fenestrated glia, while they are probable sites of endocytosis, are not known to have vMAT-associated secretory vesicles. vMAT must therefore be associated with the plasma membrane of fenestrated glia, as has been observed in vitro with vMAT-B transcripts in Drosophila S2 cells (Greer et al., 2005). Further implicating a role for dVMAT-B in histamine uptake/recycling in the visual system is the finding that mutants dVMATP1 and dVMATΔ14 have reduced head histamine concentrations as well as reduced histamine immunolabelling in the distal lamina, decreases that are restored by glial specific rescue of vMAT-B (Romero-Calderón et al., 2008). Three main interpretations have been proposed for these VMAT-B containing fenestrated glia: 1) that they store carcinine produced by epithelial glia before transferring it back to the photoreceptor in some way, 2) that they buffer ‘spillover’ between adjacent cartridges, and 3) that they store histamine as a reserve for use under conditions of intense signalling (Romero-Calderón et al., 2008). Direct evidence is available for none of these, however, and must await future analysis.
3.2.6 Other neurotransmitters
Even though Ebony function has been characterised as a β-alanyl-histamine synthetase within the glia of the visual system (Hovemann et al., 1998; Richardt et al., 2002; Richardt et al., 2003), Ebony in fact expresses in glia throughout the CNS (Hovemann et al., 1998). Biochemically it acts to conjugate β-alanine to other biogenic amines in addition to histamine; the requirement for β-alanine is essentially absolute, but many amines can act as a substrate (Richardt et al., 2003). Despite this in vitro evidence, Ebony's role is confirmed only for histamine and visual system functioning (Borycz et al., 2002; Richardt et al., 2002; Richardt et al., 2003) and, apart from its regulation of dopamine in the cuticle and in locomotor behaviour (Suh and Jackson, 2007), there is no other indication for what additional neurotransmitters Ebony might act upon in vivo.
Glia not only take up and inactivate amine neurotransmitters, they also play a vitally important role in clearing other neurotransmitters, such as glutamate, from the extracellular space. As evidence of its importance, loss of the Drosophila glial protein Excitatory Amino Acid Transporter (dEAAT) induced by means of RNA interference (RNAi) shortens lifespan and results in brain neuropile degeneration, apparently as the outcome of glutamate-mediated neurodegeneration resulting from oxidative stress (Rival et al., 2004; Liévens et al., 2005). Finally, in addition to uptake, at least one enzyme for neurotransmitter synthesis is expressed in glia. Thus the gene for dopa decarboxylase, which is required to synthesize dopamine and 5-HT, is expressed in a subset of glia in the larval CNS (Beall and Hirsh, 1987; Mastick and Scholnick, 1992).
In addition to, and possibly associated with, uptake of neurotransmitters, glia also have endocytotic activity. When the temperature-sensitive allele of shibire, which codes for Dynamin and is required for clathrin-mediated endocytosis (e.g., Artalejo et al., 1995), is expressed in most glia of the Drosophila adult by means of a repo-GAL4 driver lethality ensues after only three days at the restrictive temperature (Doherty et al., 2009). Thus, adult glia engage in a number of endocytotic functions which are essential for survival (Doherty et al., 2009). Note, however, that this interpretation rests exclusively on the role played by Dynamin in endocytosis, and does not take into account Dynamin's other functions in cells, especially as a microtubule-associated protein (Shpetner and Vallee, 1989). Evidence for the latter comes from shibire mis-expression, which results in microtubule bundling and signs of degenerative changes in photoreceptor and lamina neurons (Gonzalez-Bellido et al., 2009).
4 Neurotransmitter uptake functions of glia, and glial involvement in behavioural regulation
Glia play an important role in neurotransmitter clearance and this, ultimately, affects vision, locomotion, sexual behaviour, survival and other behaviours as we now detail.
The ability of insect glia to take up neurotransmitters and clear them from the extracellular space has been long established. Cockroach glia take up glutamate at the neuromuscular junction (NMJ; Faeder and Salpeter, 1970) and in the locust glia surrounding the NMJ absorb tritiated GABA (van Marle et al., 1985). In flies, so too, do the satellite, epithelial and marginal glia take up GABA in the lamina (Campos-Ortega, 1974), although in that neuropile endogenous sources of GABA are apparent only for two types of centrifugal neuron (Kolodziejczyk et al., 2008). In addition, as previously discussed, epithelial glia take up histamine released from photoreceptors, clearing it from the cleft (Borycz at al., 2002) and may possibly take up glutamate at sites, known as gnarls, where these glia lie in close association with amacrine cells (Meinertzhagen and O'Neil, 1991).
4.1 The role of glia in glutamate transport and courtship
In Drosophila, glutamate is an essential neurotransmitter in normal adult sexual courtship, and glia play an essential role in the regulation of both glutamate and sexual behaviour. A mutation in the glial cysteine/glutamate amino acid transporter genderblind results in male flies courting other males with the same probability as that with which they would court females (Grosjean et al., 2008).
Genderblind is not only expressed throughout development in a subset of larval CNS glia and in perineurial glia along peripheral nerves (Augustin et al., 2007), but it is also abundant in glia of the adult CNS (Grosjean et al., 2008). While it is difficult to study the exact mechanism of Genderblind action in the adult, its action in the larval PNS provides some clues as to how it may regulate glutamate levels in the adult CNS. At the larval NMJ, perineurial glia take up secreted glutamate and then release it into the haemolymph, thus regulating the amount of glutamate available at the synapse (Augustin et al., 2007). Reduction of Genderblind function in larvae ultimately results in reduced extracellular glutamate (Augustin et al., 2007) and other amino acids (Piyankarage et al., 2008). Furthermore, mutations of the genderblind transporter affect not only the concentration of glutamate but also the number of glutamate receptors at the NMJ. A 50% reduction in extracellular (haemolymph) glutamate coincides with a 2-3 fold increase in postsynaptic GluRIIA and GluRIIB receptors in genderblind larval NMJs. This receptor phenotype consequently affects synaptic transmission in the larvae which can, in turn, be rescued by the addition of glutamate (Augustin et al., 2007).
Reducing Genderblind function exclusively in the adult glia using RNAi alters courtship behaviour (Grosjean et al., 2008) suggesting that this behaviour is dependent on adult regulation of neurotransmitter. Indeed, altered courtship behaviours in male genderblind mutants result because changes in adult ambient extracellular glutamate levels and receptor clustering, in turn, cause improper information processing of a repelling odorant, 7-tricosine, which is emitted by other male flies (Grosjean et al., 2008).
4.2 Glutamate transport via glia dEAATs affects dopaminergic neuron survival and ultimately, motor control
Glial regulation of glutamate concentration is required to prevent neurodegeneration in the brain. In adult Drosophila EAATs that transport both glutamate and aspartate (Donly et al., 1997; Seal and Amara, 1999; Besson et al., 2000) localise to Repo-expressing glial cells in the protocerebral bridge, the optic lobes, and to glial extensions that project close to synaptic areas. Loss or reduction of the glutamate buffering capacity, which requires dEAAT, is neurotoxic, thus revealing the necessity of this glial transporter (Rival et al., 2004).
Flies expressing dEAAT1 RNAi walk normally but have deficits in flying and escape behaviour (Rival et al., 2004). These behavioural deficits are believed to be central in origin since no electrophysiological disruption of synaptic transmission at NMJs is obvious nor does expressing dEAAT1 RNAi in peripheral glia, surrounding the motor neurons, elicit the same behavioural phenotype. Locomotor deficiencies are significantly rescued by expressing human EAAT2 under the control of the Drosophila promoter, suggesting that the behavioural phenotype results in part from a glutamate transport deficiency. Ultrastructural analysis of the CNS in dEAAT flies reveals neurons that appear to be undergoing degeneration. It is proposed that a lack of glial dEAAT results in an accumulation of glutamate in the extracellular space and that this excess glutamate induces oxidative stress in neighbouring neurons, with dopaminergic neurons showing increased vulnerability to degeneration. Flies having reduced dEAAT1 function ultimately exhibit reduced survival, living on average 10-13 days compared with 20-29 days for controls (Rival et al., 2004).
4.3 The role of glia in circadian rhythmicity
In Drosophila, circadian rhythms are controlled by two interconnected molecular loops involving the clock genes timeless, period, cycle, and dclock (Boothroyd and Young, 2008; Helfrich-Förster, 2009). Sites of period expression and function are widespread and include glia as well as neurons (Ewer et al. 1992). Some clock neurons also express the neuropeptide Pigment Dispersing Factor (PDF), which is released to regulate cells downstream in the circadian pathway (Helfrich-Förster and Homberg, 1993; Helfrich-Förster, 1995; Lear et al., 2005; Mertens et al., 2005). The following text summarises the involvement of glia in the fly's circadian clock mechanisms.
4.3.1 Ebony and the clock genes
In the adult, Ebony protein localises exclusively to glia, at areas close to the projections of clock neurons in the optic lobe, protocerebrum and thoracic ganglion (Richardt et al., 2002), with a subset lying close to 5-HT and dopamine-positive neurons (Suh and Jackson, 2007). Some of these Ebony-expressing glia contain clock genes, others do not, and yet ebony RNA, which itself displays robust circadian cycling (Ueda et al., 2002), is not dependent upon PDF, suggesting the internal control of ebony cycling by clock genes in some glia and indirect control by clock neurons in others (Suh and Jackson, 2007).
4.3.1.1 Ebony perturbs Drosophila locomotor activity
The likely involvement of glia in aspects of circadian regulation is immediately obvious in the case of ebony because of this gene's exclusively glial pattern of expression. However, an explanation for the ability of Ebony expressing glia to modulate locomotor behaviour is more elusive and depends upon the proximity of subsets of glia to neurons controlling locomotion. Mutations in the ebony gene perturb Drosophila locomotion, causing the normally ‘day active’ rhythmic pattern of locomotor activity to become arrhythmic (Newby and Jackson, 1991). On the other hand, ebony mutations do not affect the circadian pattern of eclosion, suggesting that these two circadian behaviours are regulated by different mechanisms, only one of which requires ebony function (Suh and Jackson, 2007). The ebony RNA exhibits circadian cycling with peak expression at ZT5 (Claridge-Chang et al., 2001) and cycling both in a normal light:dark cycle and in total darkness (Suh and Jackson, 2007). The spatial distributions of the 5-HT producing enzyme Tyrosine Hydroxylase, as well as of PDF and Timeless proteins, are the same in ebony mutants as in controls. The proximity of Ebony-expressing glia to clock cells, on the one hand, and to 5-HT and dopamine containing neurons on the other, as well as ebony's genetic interactions together indicate that ebony acts downstream of the clock to regulate the fly's circadian behavioural rhythms (Suh and Jackson, 2007). However, Ebony has not yet been shown to modify 5-HT or dopamine in the nervous system in vivo.
Ebony function is required for high levels of daytime activity (Suh and Jackson, 2007). Furthermore, not only is Ebony expression circadian, but the responsiveness of the dopamine receptor-mediated modulation of motor behaviour is, itself, also under circadian control. Ebony protein production increases concurrently with a decrease in postsynaptic dopamine receptor sensitivity, both of which occur during the subjective day (Andretic and Hirsh, 2000; Suh and Jackson, 2007). The gene black, which codes for an aspartate/glutamate decarboxylase responsible for the production of Ebony's substrate, β-alanine (Phillips et al., 1993), also cycles during a normal light–dark cycle. However, somewhat contradicting an increase in Ebony during the day is the finding that black peaks during the night, around ZT16 (Ciriani et al., 2002). While dopamine concentrations have not been shown to cycle throughout the day, a simultaneous increase in the concentration of dopamine-inactivating Ebony protein in the glia and a decrease in dopamine receptor sensitivity suggest a means to offset possible changes in dopamine concentration (Andretic and Hirsh, 2000). Together, this evidence suggests Ebony as a candidate in modulating the dopaminergic control of locomotor behaviour.
4.3.2 Rhythmic changes in epithelial glial size
Not only does ebony mRNA expression display circadian rhythmicity but some ebony-expressing glia also exhibit circadian changes in size (Pyza and Górska-Andrzejak, 2004). Multiple cellular compartments of the fly's lamina cartridge exhibit rhythmic size changes. In Musca the cross-sectional areas of the lamina monopolar L1 and L2 axon profiles normally expand during the day and shrink at night, with the rhythm for L2 being circadian (Pyza and Meinertzhagen, 1996, 1999). The phase of corresponding size changes in L1 and L2 is reversed in Drosophila from that in Musca (Pyza and Meinertzhagen, 1999), although the phenomenon probably shares a similar mechanism in both flies. At least in Musca, the axon size changes are counteracted by opposite changes in the profile area of the epithelial glia, which expand in the night and shrink during the day (Pyza and Górska-Andrzejak, 2004).
Glia, presumably epithelial glia, obviously exert some form of control over the circadian modulation of L1/L2 axon diameter because injecting glial metabolic toxins such as fluorocitrate and iodoacetate increases these day/night size changes, suggesting that the glia normally act to inhibit their extremes (Pyza and Górska-Andrzejak, 2004). However, this interpretation rests on the specificity of these toxins in fly glia and the localisation of their action to epithelial glia. On the other hand octanol, which closes gap junctions, has the opposite effect. It disrupts the circadian changes of L2 in Musca, preventing L2's swelling during the day, while L1 axons are less affected. Octanol also decreases the profile areas of the epithelial glia, preventing their swelling during the subjective night (Pyza and Górska-Andrzejak, 2004). This suggests that inter-glial communication via gap junctions is necessary for epithelial glia to modulate changes in axon profile size, however it fails to rule out any possible contribution from the gap junctions that exist between neurons (Saint Marie and Carlson, 1985). In addition, both octanol and iodoacetate also have an effect on the number of the glial/photoreceptor specific capitate projection organelles. These findings suggest that the glial cells mediate circadian information from the clock neurons to the lamina neurons to modulate processing of visual information during the day and night (Pyza and Górska-Andrzejak, 2004)
5. Cellular and metabolic functions of glia
5.1 Glia and homeostasis: ion buffering and trophic support
A number of studies indicate that functioning glial cells are required for neuronal survival. For example, glia surround photoreceptor axons in the lamina neuropile and cortex, and if these cells are dysfunctional, as in repo, there is increased photoreceptor cell death (Xiong and Montell, 1995). In the embryonic insect's CNS, loss of glial cells, either by targeted glial ablation or by mutantion of gcm, leads to excess neuronal apoptosis among follower neurons (Booth et al., 2000).
Other cases of trophic dependence of neurons have recently been reviewed (Zhu et al., 2008) but in most cases an exact glial source has either not been inferred or closely demonstrated. Recently, a neurotrophin has been discovered which is released from glia and is responsible for the maintenance of dopaminergic neurons, but expression has so far only been demonstrated during development (Palgi et al., 2009). On the other hand, the Drosophila neurotrophin superfamily members DNT1, DNT2 and Spätzle may play a role in neuronal survival of adults (Zhu et al., 2008). DNT1 transcripts are expressed in the CNS throughout development as well as in the optic lobes and central brain of the adult. A loss of function DNT1 induces a significant increase in apoptosis after embryonic stage 17, while over-expressing DNT1 in all neurons is able to suppress naturally occurring apoptosis. However, it is not clear in this particular case whether these neurotrophins have a glial origin. Loss of function of any one of DNT1, DNT2 or Spätzle results in slow, uncoordinated movements in the adult. Yet these motor defects likely stem from abnormalities in development and it is not known if the neurotrophins in question continue to promote cell survival after metamorphosis (Zhu et al., 2008).
It is not exactly known why glial malfunction results in the cell death of its neighbouring neurons. A general proposal is that glia are essential to buffer K+ in the extracellular space and are involved in metabolic signalling to their associated neurons. In repo mutants, adult flies can be identified by a reversal in the polarity of the ERG (Xiong et al., 1994). This reversal has been attributed to defects in the ability of fenestrated, pseudocartridge and satellite glia, which lie between the eye and the lamina neuropile, to buffer K+ correctly. Lamina glia fail to buffer K+ released by the lamina neurons they surround, thus increasing the lamina's response to light to twice that recorded from wild-type flies (Xiong et al., 1994). When the small corneal negative response is summed with an enhanced positive lamina response an overall positive response is recorded. Thus, it is possible that the reversed-polarity ERG observed in repo results from a combined deficiency in K + buffering and impaired resistance to current flow in the lamina. The ultimate result of this loss of lamina glia is the death of both lamina monopolar neurons and photoreceptors (Xiong and Montell, 1995).
5.2 Gliotrophic factors
The above examples document neuronal responses to glial malfunction. In contrast, we also include documentation of the cases in which neurons exert positive influence on the survival, growth or maintenance of glia. Thus, neurons in the developing CNS midline secrete gliotrophic factors, such as Spitz (Kim and Crews, 1993) and Vein (Hidalgo et al., 2001), which activate Epidermal Growth Factor Receptors on the surface of glial cells (reviewed in Hidalgo, 2002). In addition, PVF and PVR promote midline glial survival through ATK and ERK pathways (Learte et al., 2008).
5.3 Glia and neuronal metabolism
The honeybee's retina has proved an excellent model system to study the metabolic relationship between photoreceptor neurons and their glial counterparts in the retina, the pigment cells. These two cell types exhibit a clear separation of metabolic functions, with photoreceptor neurons being aerobically very active and containing a large number of mitochondria, and pigment cells lacking mitochondria but possessing large quantities of glycogen (Tsacopoulos and Poitry, 1982).
In steady-state dark conditions pigment cells transform glucose, stored as glycogen, to phosphorylated glucose-6-phosphate (Tsacopoulos et al., 1988; Tsacopoulos and Magistretti, 1996), which is then converted to pyruvate and ultimately alanine (Tsacopoulos et al., 1994). Light conditions increase glial glycogen turnover and alanine production (Brazitikos and Tsacopoulos, 1991; Evêquoz-Mercier and Tsacopoulos, 1991). Alanine is then released from the pigment cells and taken up by neighbouring photoreceptors where it is converted to pyruvate that enters the Krebs cycle and in the process drives the production of NH3 from the conversion of glutamate to α-ketoglutarate (Tsacopoulos et al., 1994). Exogenous proline also enters the Krebs cycle and is likewise converted to glutamate and α-ketoglutarate with the production of NH3 (Tsacopoulos et al., 1994).
Photoreceptors must first signal in order for pigment cells to increase glycolysis and produce alanine, because these cells themselves fail to respond directly to light stimulation (Tsacopoulos et al., 1987). In the honeybee's retina, metabolically active photoreceptors produce and release glutamate and NH3 which immediately becomes NH4+ at physiological pH (Tsacopoulos et al., 1987). NH4+ is then transported along with glutamate into the neighbouring pigment cells via a member of the K+-Cl− co-transporter family, which is selective for NH4+ over K+ (Marcaggi et al., 1999; Marcaggi and Coles, 2000). Glutamate uptake by the pigment cells regulates the concentration of this potentially toxic amino acid in the extracellular space, in a role that is adopted by glia elsewhere in nervous systems (Seal and Amara, 1999). A rise in the intracellular concentrations of NH4+ and glutamate in the pigment cell triggers glycolysis by acting directly on the enzymes phosphofructokinase, alanine aminotransferase and glutamate dehydrogenase, leading to the production and release of alanine (Tsacopoulos et al., 1987). While some may not consider pigment cells to be glia, this system still implicates an important role for neuronal support cells in assisting the proper function of neurons. The system remains to be validated in Drosophila, but already provides evidence for a nutritive function of glia in maintaining neurons that is both novel and comprehensive.
6. Glial and neuronal interactions during growth and neurodegeneration
Neurons and glia engage in reciprocal signalling so that whenever the integrity of one is threatened, the other may show reactive changes. In addition to these interactions, there are also interactions among the glial cells themselves. For example, after a chemical gliaectomy in the abdominal connective of the cockroach that spares the neighbouring neurons, the surviving glia undergo division (Smith et al., 1990). In adult Drosophila, however, there appears to be only a finite period of time during early adulthood when glia are able to divide in response to degeneration of their neuron neighbours (Kato et al., 2009) and, in this respect, insect glia are unlike their vertebrate counterparts. Thus, although capable of division to replace the loss of their own kind, most glia fail to divide in response to axonal lesions and neuronal death in mature adults. Instead they manifest changes that have received particular attention in Drosophila. We will consider these below, first as the death of neurons when glial function is impaired and then the more intensively studied changes in glia when their neuronal partners are damaged and undergo degeneration.
6.1 Neuron death from glial cell dysfunction
Glia are required for the proper development and survival of neurons. The role of glia in development is well understood and their ability to provide developing axons with localisation signals has been extensively studied in Drosophila (for reviews see Tayler and Garrity, 2003; Chotard and Salecker, 2004). The importance of glia in the adult brain is readily revealed by a number of Drosophila mutants including but not limited to: drop dead (Buchanan and Benzer, 1993), swiss cheese (sws; Kretzschmar et al., 1997), and repo (Xiong and Montell, 1995). It can also be demonstrated by overexpression of mammalian proteins such as the polyglutamine polypeptides of ataxin-3 (Kretzschmar et al., 2005).
Adult drop dead flies survive into adulthood but anatomical abnormalities exist even before the behavioural phenotype manifests itself. Upon eclosion the neuronal morphology appears normal, yet glial cells are stuck in the early stages of development and their stunted processes fail to form a complete glial sheath. As flies age the brain begins to degenerate (Buchanan and Benzer, 1993) and an acceleration of age related markers suggests that theses flies age more rapidly (Rogina et al., 1997). While the early onset mortality phenotype of drop dead may in part result from digestive abnormalities (Blumenthal, 2008), the aberrant shape of glia in the adult suggests rather obviously a role for proper glial development in the maintenance of nervous system function and neuronal survival (Buchanan and Benzer, 1993).
Glial hyperwrapping, as it occurs in the Drosophila mutant sws, was also thought to be associated with age-related neuronal apoptosis beginning at approximately 3-4 days of adulthood (Kretzschmar et al., 1997). In these mutants glial hyperwrapping is observed during pupariation and precedes neuronal cell death. Anatomical features of neuronal cell death are followed, except among marginal and epithelial glia of the lamina, by signs of glial cell death (Kretzschmar et al., 1997). Mutations of the sws gene may affect cell death by one of two means. The sws gene is a homologue of the Neuropathy Target Esterase (Zaccheo et al., 2004) protein that codes for a brain-specific phospholipase (Mühlig-Versen et al., 2005). Both SWS and NTE regulate the deacylation of phosphatidylcholine (PtdCho; Zaccheo et al., 2004), a major lipid of cell membranes which is elevated in sws mutants (Mühlig-Versen et al., 2005), and this altered lipid composition can be deleterious (Klein, 2000). SWS is expressed in the endoplasmic reticulum of neurons and some glia, and it is in the endoplasmic reticulum where most PtdCho is processed (Mühlig-Versen et al., 2005). In addition, the SWS protein acts as an inhibitor for the C3 catalytic subunit of cAMP activated protein kinase (PKA-C3). It has been proposed that normal SWS regulates, via inhibition, the localization of kinase activity within the membranes and that an excess of PKA-C3 results in neurodegeneration (Bettencourt da Cruz et al., 2008). Despite the finding that sws acts cell autonomously in both neurons and glia, neuron-specific rescue of the vacuolization phenotype is effective but incomplete, while glial hyperwrapping can be prevented with glial specific rescue and thus the neurodegenerative phenotype may still be affected by dysfunction in the glia (Mühlig-Versen et al., 2005).
In the adult visual system repo is expressed in all lamina and medulla glia, including the fenestrated and pseudocartridge glia that underlie the retina. In the visual system of repo1 mutants, the survival of lamina neurons and photoreceptor cells depends upon repo expression in the associated glia. The photoreceptors degenerate in a retrograde fashion, possibly as a result not only of the loss of their synaptic partners, the lamina monopolar cells, but also the loss of their supporting lamina glia (Xiong and Montell, 1995) but these details need to be elucidated.
In addition, in a Drosophila model of polyglutamate expansion diseases, such as Huntington's disease, overexpression of polyglutamine (polyQ) polypeptides from the C-terminus of human ataxin-3 has been found to reduce life span in a way that correlates with the length and expression level of the polyQ tract. Flies with neuronal overexpression show no signs of degeneration but those with glial expression reveal progressive glial degeneration and apoptosis as well as more severe defects in phototactic behaviour (Kretzschmar et al., 2005). This correlation further demonstrates the necessity of proper glial function for adult neuronal survival.
6.2 The glial response to neurodegeneration
Two kinds of neuronal degeneration are generally acknowledged: developmental degeneration, for example axonal pruning or removal of entire cells by apoptosis, and Wallerian degeneration, damage to a cell that appears in its distal extremities and proceeds in an anterograde direction (Griffin et al., 1995). Neuronal degeneration, either natural or injury induced, elicits reactive gliosis as a response from neighbouring glia that involves changes in gene expression and morphology, so that the glia extend processes to invade the injury site and become actively involved in the clearance of degenerating neurites. In some species, such as ants, experience dependent axonal pruning can occur for up to 60 days post-eclosion (Seid and Wehner, 2009), but most glial phagocytosis of axons in adult insects is in response to neuronal malfunction and axonal injury.
6.2.1 Wallerian degeneration: Physical features of the glial response to neuronal degeneration
The genetic pathways required to induce glial responses exhibit several key differences when these occur either in response to neurite pruning or to injury-induced degeneration. In studies utilizing flies, Wallerian degeneration has been induced by acute transection of antennal lobe neurons (ORNs) or by damage to photoreceptors. Injured ORN axons and their clearance from the CNS have been visualized by their expression of fluorescent fusion gene markers. These reveal changes in glial cell morphology within 24 hours and complete removal of neuronal GFP from the site of injury within 5 days (MacDonald et al., 2006).
6.2.2 Reactive gliosis in the visual system
Degeneration among photoreceptor axons becomes apparent in only 2-5 minutes after mechanical lesions to the Dipteran retina (Griffiths and Boschek, 1976) and this degeneration has been quantified as a series of timed ultrastructural changes in the lamina, from 1 hour to 8 days. Photoreceptor axons that have been injured by photoablation of their cell bodies reveal non-synchronised degeneration of their synaptic terminals that starts within minutes, followed by changes among the surrounding epithelial glia (Brandstätter et al., 1991). Slower changes follow retinal degeneration in mutant Drosophila (Stark and Carlson, 1982, 1985) and in flies exposed to high intensity blue irradiation (Stark and Carlson, 1984). Reactive gliosis and glial hypertrophy accompany these changes in the lamina, becoming obvious within the first week of degeneration as epithelial glia insinuate themselves at postsynaptic sites of former tetrads when these are vacated by the normal L1 and L2 target dendrites (Brandstätter et al., 1992). Gliosis also occurs among the glia of the medulla, after lesion of the central photoreceptor neurons R7 and R8, such that over a six-day period the number of glial profiles increases to occupy a greater portion of the column cross section (Campos-Ortega and Strausfeld, 1972).
Parallel findings come from studies of retinal degeneration mutants. In flies mutant for retinal degeneration A (rdgAPC47) photoreceptors degenerate after one week yet, in the lamina, both the monopolar neuron and glial somata are reported to be ultrastructurally normal (Stark and Carlson, 1985). Gliosis is nevertheless presumed to take place because glia expand into the area occupied by damaged photoreceptors (Stark and Carlson, 1985). In a second retinal degeneration mutant rdgBKS222, there is no obvious change in epithelial glial morphology one week after eclosion even though degenerative changes such as electron dense photoreceptor axons and swollen lamina interneurons are readily evident. Gliosis becomes apparent by 21 days post-eclosion when the lamina contains scattered electron-dense profiles of cells presumed to be R1-R6 axons and other axons having been completely phagocytosed (Stark and Carlson, 1982). While Stark and Carlson (1982) suggest that glial cells multiply and fill the areas left by degenerating axons, they provide no evidence of actual glial cell division and so expansion alone may account for the increased glial volume.
Concurrent with the degenerative changes in the photoreceptor neurons R1-R6, and the expansion of epithelial glial cell profiles, cortex satellite glia which surround the somata of lamina cells also respond quickly by increasing their production of the lysosomal marker, enzyme acid phosphatase (AcPase), at first in the endoplasmic reticulum, then to extracellular sites between the satellite glia and photoreceptor axons, and finally within the synaptic terminals. The glial cells are thought to export hydrolytic enzymes via the golgi-endoplasmic reticulum-lysosome complex to the photoreceptor axon interface (Griffiths, 1979).
6.2.3 Glial factors mediating the response to degeneration of neurons
6.2.3.1 Draper
The glial response to neural degeneration is characterized by dramatic changes in glial cell shape. Early reports (Stark and Carlson, 1982; Shaw, 1984) revealed the phagocytotic activity of lamina epithelial glia after photoreceptor degeneration. In mature adult flies, glia respond by increasing their surface area and extending projections into the site of injury rather than by proliferation or migration (MacDonald et al., 2006). These changes are accompanied by an upregulation of the glial transmembrane protein Draper (Drpr), a Drosophila homologue of the C. elegans cell corpse engulfment gene ced-1 (Freeman et al., 2003). Injured axons fail to be cleared in drpr mutant flies. Furthermore, after ablating antennal segments in drprΔ5 mutants, noticeable changes in glial cell morphology are lacking. Glial processes do not accumulate in antennal lobe glomeruli housing severed axons and the intensity of GFP labelling in glia does not increase. A similar finding has been obtained in drpr RNAi flies. Both mutant and knockdown findings indicate a failure of glia to respond to axonal damage (Freeman et al., 2003).
The Drpr protein itself may act as a glial receptor for molecular cues from severed axons, and it can drive recruitment of glial processes to injured axons for engulfment. Drpr is localised to severed axons 4 h after ablation or axotomy, is concurrent with the appearance of the GFP puncta characteristic of degenerating axons, and is maintained at high levels until all traces of GFP from degenerating axons are eliminated from the CNS (MacDonald et al., 2006).
6.2.3.2 Six microns under
The Drosophila phagocytosis receptor Six Microns Under (SIMU) is another member of the ced-1/drpr family of genes that is expressed in phagocytosing glia and is required to clear apoptotic profiles of degenerating cells from the nervous system (Kurant et al., 2008). Like Drpr, the SIMU protein has a large extracellular EMILIN-like N-terminal domain required for protein recognition and binding but unlike Drpr has only a short intracellular cytoplasmic tail at the C-terminus.
CNS glia appear morphologically normal in SIMU mutants, yet the number of apoptotic cell particles increases twofold and these are less likely to be engulfed by glia. A clue to the role of SIMU is revealed from the behaviour of phagocytes, which have normal search behaviour and mobility, and bind some apoptotic particles, but do not engulf them. Affinity purified HIS-MYC-SIMUΔTM protein readily binds to apoptotic S2 cells, while cells transfected to express SIMU take up more apoptotic cell particles than cells transfected to express only GFP (Kurant et al., 2008).
SIMU and Drpr expression patterns strongly overlap each other, although expression of SIMU appears more patchy. Both SIMU and drpr mutants show a twofold increase in apoptotic particles, which in drpr mutants are found inside phagocytic cells such as glia and macrophages, while SIMU mutants display a three-fold increase in non-engulfed particles relative to wild-type (Kurant et al., 2008). These findings indicate that SIMU mutants fail to recognize and/or engulf apoptotic cells, and because SIMU;drpr double-mutants resemble the SIMU mutant it appears that SIMU acts upstream of Drpr. Despite this conclusion, there is no interaction between SIMU and Drpr in co-immunoprecipitation studies, suggesting that an intermediary factor acts between the two to control engulfment, at least during developmental apotosis (Kurant et al., 2008).
6.2.3.3 Shark, Src42A, and ced-6
The downstream effectors of engulfment in glia are just beginning to emerge. Two examples are the kinases Shark and Src42A (Ziegenfuss et al., 2008). Shark is a non-receptor tyrosine kinase that binds Drpr at its intracellular domain, an immunoreceptor tyrosine-based activation motif. Shark is essential for Drpr-mediated signalling events that result in reactive gliosis. Drpr receptor activation initiates Src family kinase, Src42A, activity, which in turn phosphorylates Drpr. This allows Drpr's association with Shark which ultimately results in phagocytosis. RNAi knockdown of both Shark and Src42A suppresses glial hypertrophy and phagocytosis of damaged axons and interferes with the upregulation of Drpr that is associated with glial phagocytosis (Ziegenfuss et al., 2008). As a member of the CED family of corpse engulfment proteins, Drpr may act in a similar manner to C. elegans CED-1 (see Awasaki et al., 2006), by mediating actin-dependent cytoskeletal reorganisation via Rac1 (Kinchen et al., 2005) and Dynamin (Ziegenfuss et al., 2008).
In C.elegans Ced-6 acts downstream of the Drpr homologue Ced-1 in response to apoptotic cell death (Liu and Hengartner, 1998). Similarly, in Drosophila Ced-6 is upregulated in response to cell death, both by apoptosis and Wallerian degeneration (Awasaki et al., 2006; Doherty et al., 2009). Drosophila dCed-6 and Drpr co-immunolocalize to both neuropile ensheathing glia and cortex glia in the adult, and both proteins are also recruited to the glial surface during degeneration of ORN axons. Furthermore, drpr and dced-6 appear to interact genetically since transheterozygotic animals containing a single copy of both mutations display impaired clearance of degenerating axons, whereas the respective single-mutant heterozygotes are both able to clear degenerating debris. Thus, it is not surprising that repo-driven glial knockdown of dced-6 via RNAi in Drosophila suppresses the recruitment of Drpr to the site of injury and the clearance of degenerating axons (Doherty et al., 2009). What is novel, however, is the finding that in Drosophila the genetic interaction is reversed compared with C. elegans, with drpr acting downstream of dced-6.
6.2.3.4 Ensheathing glia phagocytose degenerating axons
Drpr-mediated engulfment of degenerating neuron profiles, at least in the antennal lobes of Drosophila, is the responsibility of the ensheathing glia which surround the neuropile. Both Drpr and dCed-6 antibodies immunolocalise to the mz0709-GAL4 expressing ensheathing glia, as well as the cortex glia, but only expression of UAS-draperRNAi and UAS-sharkRNAi in ensheathing glia completely blocks clearance of GFP labelled axonal debris. Blocking shibire-mediated endocytosis specifically in ensheathing, but not astrocytic, glia also suppresses glial clearance of degenerating axons (Doherty et al., 2009). Although antennal lobe cortex glia also express Drpr, it appears that these glia do not engage in the phagocytic engulfment of debris following axotomy, since they fail to compensate for the specific role of ensheathing glia in Drpr knockdown experiments.
6.2.4 Neuronal factors mediating the glial response to degeneration of neurons
Expression of the mouse anti-apoptotic factor Wallerian degeneration slow (Wlds) in Drosophila neurons prevents the glial response to Wallerian degeneration, i.e. changes in glial morphology and Drpr accumulation, that accompanies the removal of transected neurons but that plays no role in developmental axon pruning (Hoopfer et al., 2006). Wlds is a fusion protein composed of 70 amino acids from the polyubiquitination protein UDF2/E4 (required for both developmental pruning and injury induced degeneration) and the full-length nicotinamide mononucleotide adenyltransferase (Nmnat) which facilitates nicotinamide adenine dinucleotide (NAD) synthesis. While Wlds is not reported in Drosophila, its component systems the ubiquitin-proteasome system UPS (Zhai et al., 2003) and NAD biosynthetic pathway (Sasaki et al., 2006), are both active in flies (Watts et al., 2003; MacDonald et al., 2006). The ubiquitin activating enzyme Uba1 (E1) and the 19S proteasome regulatory particles Mov34 and Rpn6 have all been implicated in neuronal protein degradation, at least in developmental axon pruning (Watts et al., 2003). Inhibiting the ubiquitin-proteasome system and thereby protein degradation in axons can delay Wallerian degeneration (Zhai et al., 2003). dNmnat1 acts in an opposite sense to the ubiquitin-proteasome system, with overexpression suppressing self destruction of transected ORNs, although at a reduced rate relative to full-length Wlds overexpression. This suggests that the neuroprotective effects of Wlds may proceed via an Nmnat-dependent mechanism (MacDonald et al., 2006). The fact that protective factors elicit molecular signals from transected axons implies that these unknown signals have a role in eliciting the glial response that eventually engulfs the degenerating axons (Hoopfer et al., 2006; MacDonald et al., 2006).
In fact, sensory axons do not need to be transected to undergo Wallerian degeneration. A loss of cell autonomous activity is sufficient to induce degeneration in antennal lobe neurons (Chiang et al., 2009). Antennal sensory neurons must express odorant receptors on their surface in order to transmit signals to the glomeruli of the antennal lobe. When odorant receptors are mistargeted, such as in the or83b mutant, then odour-evoked neuronal activity is lacking and these neurons begin to degenerate concurrent with a glial response. Degeneration also occurs when sensory neurons transduce odorant stimuli but are unable to transmit that information to their postsynaptic partners by release of neurotransmitter, as occurs with light chain tetanus toxin (teTxLC) or shibireK44A overexpression in ORNs (Chiang et al., 2009), or when photoreceptor neurons fail to synapse with lamina monopolar neurons in disco mutants (Campos et al., 1992). This degeneration is not caspase-dependent and cannot be rescued by overexpressing Wlds. but is dependent upon the ubiquitin protease pathway, since expression of UBP2 can rescue the degeneration phenotype. Modulation of Glycogen synthase kinase-3β (GSK-3β) levels affects degeneration, thus suggesting that neuronal activity mediates its effect by inhibiting activity of this kinase in neurons. How GSK-3β modulates these changes is not known, but is likely to occur via a wnt-dependent pathway since wnt overexpression can also reduce degeneration in otherwise inactive neurons (Chiang et al., 2009).
6.2.5 The glial response to degenerating axons is age dependent in Drosophila
While glial cell division in response to the loss of glial cells has been long established (e.g., Smith et al., 1990), whether glia divide in response to axonal injury has been debated. Heat shock inducible mitotic recombination as well as BrdU incorporation labelling reveals low levels of mitotic activity in healthy adult flies, at least up to six days after eclosion. Most of these mitosing adult cells do, in fact, become glia and these glia are concentrated in an area ventrolateral to the antennal lobes, where the antennal nerves enter the brain (von Trotha et al., 2009). Despite a prior lack of evidence for glial mitosis after neuronal lesions, another recent study shows that for the olfactory system and central brain, at least, the ability of glia to divide in response to neuronal degeneration depends on the fly's age. Naturally occurring programmed cell death (PCD), antennal neuron axotomy, and manual damage from stab-injury can all induce glial mitoses in young flies (Kato et al., 2009). This age discrepancy may be the reason why, during axonal degeneration of sensory neurons in the antennal lobes of Drosophila, glia have previously been shown only to expand into the spaces surrounding damaged axons, but not to multiply (MacDonald et al., 2006).
In the Drosophila brain, PCD occurs in a consistent spatiotemporal manner. It is detected at the root of the antennal nerve in a location where BrdU incorporation occurs during the first 10 days post-eclosion but not later (Kato et al., 2009; von Trotha et al., 2009). The glial response is the same following axotomy and BrdU incorporation is not detected if flies are older than 8 days (Kato et al., 2009). This suggests that glial cell division can only occur during specific periods of adult life. BrdU positive glial cells are located within or very close to neuropiles but not deep in the cortex or along the brain surface (Kato et al., 2009), suggesting that they are neuropile glia, of which there are two types: ensheathing and astrocyte-like (Awasaki et al., 2008). Thus, it is likely that only a distinct subset of glia responds to PCD, much as how only ensheathing glia respond to Wallerian degeneration (Doherty et al., 2009). Within the visual system, the epithelial glia, also a type of neuropile glia, fill the space occupied by dying photoreceptors and lamina interneurons and are suspected to undergo division (Stark and Carlson, 1985; Carlson and Saint Marie, 1990).
If PCD in neurons is disrupted by overexpressing the caspase inhibitor gene p35, then glia also fail to incorporate BrdU. This suggests that glia must respond to some signal from dying neurons themselves and not a cell-death signalling cascade that precedes cell death (Kato et al., 2009). It is still not known, however, what the exact signal from dying neurons is that triggers mitosis, or the Shark- (Ziegenfuss et al., 2008) and Drpr- (MacDonald et al., 2006) mediated engulfment responses of glia. Within glia, the tumor necrosis factor Eiger may be the cell autonomous signal promoting cell-death induced mitotic division (Kato et al., 2009). In eiger mutants PCD remains the same as in wild-type, yet glia fail to undergo mitosis. When eiger is rescued in the glia of eiger homozygous mutants, there is a significant increase in the number of BrdU positive glia around PCD or damaged neurons, supporting a cell-autonomous role for Eiger in the glial mitotic response to neuronal degeneration (Kato et al., 2009).
7. Conclusions
In this review we have annotated and categorised the glia of adult Drosophila and their developmental origins in the optic lobe. This task is essentially complete only for the fly's optic lobe lamina. Given the wealth of glial cell types in the simple optic lamina, equal to the numbers of the co-populating neurons, we may only speculate as to how many glial cell types must exist in the rest of the brain. Most of this diversity remains to be discovered, however, and will presumably be matched by a corresponding diversity of glial cell functions.
Glia have widespread functions in the insect brain, analysed most completely in Drosophila. We have enumerated examples in which glia act to modulate and inactivate neurotransmitters, thus modulating behaviour. They also clear neurotransmitter from the extracellular space, so as to affect both neurotransmitter concentration at the receptors of some populations of neurons and reduce oxidative stress in others. In addition they provide nutritive and support functions to maintain their neighbouring neurons, and in the case of Wallerian degeneration are responsible for disposing of the very neurons they once helped maintain. Glia provide a barrier both to, and within, the CNS, isolating it from the haemolymph in the form of the blood brain barrier and selectively transporting substances into and out of the brain. Glia are active and responsive; capable of changing their shape, size and numbers to deal with alterations in their neuronal environment, and to perform these various janitorial, regulatory and policing functions.
These functions may seem disproportionate to the small number of glial cells in insect brains but glial cell function should perhaps be thought of in terms of the numbers of glial cell types, as well as their volume and shape, which in the neuropile is highly elaborated (Strausfeld, 1976). Although progress has been made in the optic lobe, where different glial cell types are clearly distinguishable, we are generally poorly informed on the partition of glial functions in different glial cell types. Thus, while the analysis of glial cell function in insect brains has, as in vertebrate brains, lagged behind the analysis of neuronal function, much progress has been made in recent years in Drosophila because mutants of such function have appeared in genetic screens. In addition to documenting that progress, this review also highlights areas in which future progress can be expected.
Acknowledgements
The authors' work reported in this review was supported by National Institutes of Health grant EY-03592 (to I.A.M.), a Killam Fellowship from the Canada Council, (to I.A.M.), and by a postgraduate scholarship from NSERC, and a stipend from the Lett Fund of the Dalhousie University Biology Department (to T.N.E). We would like to thank S.R. Shaw (Dalhousie University), C. Groh (University of Würzburg), W. Rössler (University of Würzburg) and D.A. Hopkins (emeritus, Dalhousie University) for their careful reading and recommendations on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1873–1878. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J. Neurosci. 2007;27:111–123. doi: 10.1523/JNEUROSCI.4770-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50:855–967. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Lai S-L, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LT-Y, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Beall CJ, Hirsh J. Regulation of the Drosophila dopa decarboxylase gene in neuronal and glial cells. Genes Dev. 1987;1:510–520. doi: 10.1101/gad.1.5.510. [DOI] [PubMed] [Google Scholar]
- Behr M, Riedel D, Schuh R. The Claudin-like Megatrachea Is Essential in Septate Junctions for the Epithelial Barrier Function in Drosophila. Dev. Cell. 2003;5:611–620. doi: 10.1016/s1534-5807(03)00275-2. [DOI] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant iIonotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M-T, Soustelle L, Birman S. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr. Biol. 2000;10:207–210. doi: 10.1016/s0960-9822(00)00339-0. [DOI] [PubMed] [Google Scholar]
- Bettencourt da Cruz A, Wentzell J, Kretzschmar D. Swiss Cheese, a protein involved in progressive neurodegeneration, acts as a noncanonical regulatory subunit for PKA-C3. J. Neurosci. 2008;28:10885–10892. doi: 10.1523/JNEUROSCI.3015-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of mylinated axons requires Neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- Blinkov SM, Glezer II. The Human Brain in Figures and Tables. A Quantitative Handbook. Basic Books; Plenum: New York: 1968. [Google Scholar]
- Blumenthal EM. Cloning of the neurodegeneration gene drop-dead and characterization of additional phenotypes of its mutation. Fly. 2008;2:180–188. doi: 10.4161/fly.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127:237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- Boothroyd CE, Young MW. The In(put)s and Out(put)s of the Drosophila Circadian Clock. Ann. N.Y. Acad. Sci. 2008;1129:350–357. doi: 10.1196/annals.1417.006. [DOI] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Loubani M, Meinertzhagen IA. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J. Neurosci. 2002;22:10549–10557. doi: 10.1523/JNEUROSCI.22-24-10549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borycz J, Borycz JA, Kubów A, Lloyd V, Meinertzhagen IA. Drosophila ABC transporter mutants white, brown and scarlet have altered contents and distribution of biogenic amines in the brain. J. Exp. Biol. 2008;211:3454–3466. doi: 10.1242/jeb.021162. [DOI] [PubMed] [Google Scholar]
- Borycz JA, Borycz J, Kubów A, Kostyleva R, Meinertzhagen IA. Histamine compartments of the Drosophila brain with an estimate of the quantum content at the photoreceptor synapse. J. Neurophysiol. 2005;93:1611–1619. doi: 10.1152/jn.00894.2004. [DOI] [PubMed] [Google Scholar]
- Boschek CB. On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica. Z. Zellforsch. Mikrosk. Anat. 1971;118:369–409. doi: 10.1007/BF00331193. [DOI] [PubMed] [Google Scholar]
- Braitenberg V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp. Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Shaw SR, Meinertzhagen IA. Terminal degeneration and synaptic disassembly following receptor photoablation in the retina of the fly's compound eye. J. Neurosci. 1991;11:1930–1941. doi: 10.1523/JNEUROSCI.11-07-01930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstätter JH, Seyan HS, Meinertzhagen IA. The effects of the loss of target cells upon photoreceptor inputs in the fly's optic lobe. J. Neurocytol. 1992;21:693–705. doi: 10.1007/BF01181585. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Meinertzhagen IA. The rapid assembly of synaptic sites in photoreceptor terminals of the fly's optic lobe recovering from cold shock. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2677–2681. doi: 10.1073/pnas.92.7.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazitikos PD, Tsacopoulos M. Metabolic signaling between photoreceptors and glial cells in the retina of the drone (Apis mellifera) Brain Res. 1991;567:33–41. doi: 10.1016/0006-8993(91)91432-z. [DOI] [PubMed] [Google Scholar]
- Buchanan RL, Benzer S. Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron. 1993;10:839–850. doi: 10.1016/0896-6273(93)90200-b. [DOI] [PubMed] [Google Scholar]
- Burg MG, Sarthy PV, Koliantz G, Pak WL. Genetic and molecular identification of a Drosophila histidine decarboxylase gene required in photoreceptor transmitter synthesis. EMBO J. 1993;12:911–919. doi: 10.1002/j.1460-2075.1993.tb05732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MG, Geng C, Guan Y, Koliantz G, Pak WL. Drosophila rosA gene, which when mutant causes aberrant photoreceptor oscillation, encodes a novel neurotransmitter transporter homologue. J. Neurogenet. 1996;11:59–79. doi: 10.3109/01677069609107063. [DOI] [PubMed] [Google Scholar]
- Burkhardt W, Braitenberg V. Some peculiar synaptic complexes in the first visual ganglion of the fly, Musca domestica. Cell Tissue Res. 1976;173:287–308. doi: 10.1007/BF00220317. [DOI] [PubMed] [Google Scholar]
- Cagan RL, Ready DF. The emergence of order in the Drosophila pupal retina. Dev. Biol. 1989;136:346–362. doi: 10.1016/0012-1606(89)90261-3. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J. Physiol. 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AR, Fischbach K-F, Steller H. Survival of photoreceptor neurons in the compound eye of Drosophila depends on connections with the optic ganglia. Development. 1992;114:355–366. doi: 10.1242/dev.114.2.355. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Autoradiographic localization of 3H-γ-aminobutyric acid uptake in the lamina ganglionaris of Musca and Drosophila. Z. Zellforsch. Mikrosk. Anat. 1974;147:415–431. doi: 10.1007/BF00307474. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Strausfeld NJ. The columnar organization of the second synaptic region of the visual system of Musca domestica. L. I. Receptor terminals in the medulla. Z. Zellforsch. Mikrosk. Anat. 1972;124:561–585. doi: 10.1007/BF00335258. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Strausfeld NJ. Synaptic connections of intrinsic cells and basket arborizations in the external plexiform layer of the fly's eye. Brain Res. 1973;59:119–136. doi: 10.1016/0006-8993(73)90255-2. [DOI] [PubMed] [Google Scholar]
- Cantera R. Glial cells in adult and developing prothoracic ganglion of the hawk moth Manduca sexta. Cell Tissue Res. 1993;272:93–108. [Google Scholar]
- Cantera R, Trujillo-Cenóz O. Glial cells in insect ganglia. Microsc. Res. Tech. 1996;35:285–293. doi: 10.1002/(SICI)1097-0029(19961015)35:3<285::AID-JEMT7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Carlson SD, Saint Marie RL. Structure and function of insect glia. Ann. Rev. Entomol. 1990;35:597–621. [Google Scholar]
- Carlson SD, Juang J-L, Hilgers SL, Garment MB. Blood barriers of the insect. Annu. Rev. Entomol. 2000;45:151–174. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- Cayre M, Strambi C, Charpin P, Augier R, Meyer MR, Edwards JS, Strambi A. Neurogenesis in adult insect mushroom bodies. J. Comp. Neurol. 1996;371:300–310. doi: 10.1002/(SICI)1096-9861(19960722)371:2<300::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chi C, Carlson SD. Membrane specializations in the first optic neuropil of the housefly, Musca domestica L. I. Junctions between neurons. J. Neurocytol. 1980;9:429–449. doi: 10.1007/BF01204835. [DOI] [PubMed] [Google Scholar]
- Chiang A, Priya R, Ramaswami M, VijayRaghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-β to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development. 2009;136:1273–1282. doi: 10.1242/dev.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C-S, Ross LS, Cohen BN, Lester HA, Gill SS. The transporter-like protein Inebriated mediates hyperosmotic stimuli through intracellular signaling. J. Exp. Biol. 2000;203:3531–3546. doi: 10.1242/jeb.203.23.3531. [DOI] [PubMed] [Google Scholar]
- Choi K-W, Benzer S. Migration of glia along photoreceptor axons in the developing Drosophila eye. Neuron. 1994;12:423–431. doi: 10.1016/0896-6273(94)90282-8. [DOI] [PubMed] [Google Scholar]
- Chotard C, Leung W, Salecker I. glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Chotard C, Salecker I. Glial cell development and function in the Drosophila visual system. Neuron Glia Biol. 2007;3:17–25. doi: 10.1017/S1740925X07000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J. Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge-Chang A, Wijnen H, Naef F, Boothroyd C, Rajewsky N, Young MW. Circadian regulation of gene expression systems in the Drosophila head. Neuron. 2001;32:657–671. doi: 10.1016/s0896-6273(01)00515-3. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Coombe PE, Heisenberg M. The structural brain mutant Vacuolar medulla of Drosophila melanogaster with specific behavioral defects and cell degeneration in the adult. J. Neurogenet. 1986;3:135–158. doi: 10.3109/01677068609106845. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr. Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Zhang Z, Wyman RJ. Gap junction proteins are not interchangeable in development of neural function in the Drosophila visual system. J. Cell Sci. 2002;115:3379–3388. doi: 10.1242/jcs.115.17.3379. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J. Cell Sci. 2005;118:2649–2660. doi: 10.1242/jcs.02408. [DOI] [PubMed] [Google Scholar]
- Curtin KD, Wyman RJ, Meinertzhagen IA. Basigin/EMMPRIN/CD147 mediates neuron-glia interactions in the optic lamina of Drosophila. Glia. 2007;55:1542–1553. doi: 10.1002/glia.20568. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Dearborn R, Jr., Kunes S. An axon scaffold induced by retinal axons directs glia to destinations in the Drosophila optic lobe. Development. 2004;131:2291–2303. doi: 10.1242/dev.01111. [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Taşdemir ÖE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donly BC, Richman A, Hawkins E, McLean H, Caveney S. Molecular cloning and functional expression of an insect high-affinity Na+-dependent glutamate transporter. Eur. J. Biochem. 1997;248:535–542. doi: 10.1111/j.1432-1033.1997.t01-1-00535.x. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Swales LS, Bate M. The differentiation between neuroglia and connective tissue sheath in insect ganglia revisited: The neural lamella and perineurial sheath cells are absent in a mesodermless mutant of Drosophila. J. Comp. Neurol. 1993;333:301–308. doi: 10.1002/cne.903330214. [DOI] [PubMed] [Google Scholar]
- Edwards JS, Tolbert LP. Insect neuroglia. In: Locke M, editor. Microscopic Anatomy of the Invertebrates. Wiley-Liss Inc.; 1998. pp. 449–466. [Google Scholar]
- Edwards TN, Meinertzhagen IA. Photoreceptor neurons find new synaptic targets when misdirected by overexpressing runt in Drosophila. J. Neurosci. 2009;29:828–841. doi: 10.1523/JNEUROSCI.1022-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eule E, Tix S, Fischbach KF. Glial cells in the optic lobe of Drosophila melanogaster. 1995 doi: 10.1007/s004410050886. www.flybrain.org, poster accession no. PP00004. [DOI] [PubMed]
- Evêquoz-Mercier V, Tsacopoulos M. The light-induced increase of carbohydrate metabolism in glial cells of the honeybee retina is not mediated by K+ movement nor by cAMP. J. Gen. Physiol. 1991;98:497–515. doi: 10.1085/jgp.98.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J. Neurosci. 1992;12:3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Verstreken P, Hiesinger PR, Horne JA, Kostyleva R, Zhou Y, Bellen HJ, Meinertzhagen IA. Endophilin promotes a late step in endocytosis at glial invaginations in Drosophila photoreceptor terminals. J. Neurosci. 2003;23:10732–10744. doi: 10.1523/JNEUROSCI.23-33-10732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faeder IR, Salpeter MM. Glutamate uptake by a stimulated insect nerve muscle preparation. J. Cell Biol. 1970;46:300–307. doi: 10.1083/jcb.46.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annu. Rev. Entomol. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Franzdóttir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klämbt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–762. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Doherty J. Glial cell biology in Drosophila and vertebrates. Trends Neurosci. 2006;29:82–90. doi: 10.1016/j.tins.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Rössler W. Parallel olfactory systems in insects: anatomy and function. Annu. Rev. Entomol. 2010;55:399–420. doi: 10.1146/annurev-ento-112408-085442. [DOI] [PubMed] [Google Scholar]
- Gao J, Takemura S-y., Ting C-Y, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong S-T, Wang JW, Odenwald WF, White BH, Meinertzhagen IA, Lee C-H. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat. Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- Gavin BA, Arruda SE, Dolph PJ. The role of carcinine in signaling at the Drosophila photoreceptor synapse. PLoS Genet. 2007;3:e206. doi: 10.1371/journal.pgen.0030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengs C, Leung H-T, Skingsley DR, Iovchev MI, Yin Z, Semenov EP, Burg MG, Hardie RC, Pak WL. The target of Drosophila photoreceptor synaptic transmission is a histamine-gated chloride channel encoded by ort (hclA) J. Biol. Chem. 2002;277:42113–42120. doi: 10.1074/jbc.M207133200. [DOI] [PubMed] [Google Scholar]
- Genova JL, Fehon RG. Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila. J. Cell Biol. 2003;161:979–989. doi: 10.1083/jcb.200212054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselmann G, Pusch H, Hovemann BT, Hatt H. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat. Neurosci. 2002;5:11–12. doi: 10.1038/nn787. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Bellido PT, Wardill T, Kostyleva R, Meinertzhagen IA, Juusola M. Overexpressing temperature-sensitive dynamin decelerates phototransduction and bundles microtubules in Drosophila photoreceptors. J. Neurosci. 2009;29:14199–14210. doi: 10.1523/JNEUROSCI.2873-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górska-Andrzejak J, Salvaterra PM, Meinertzhagen IA, Krzeptowski W, Görlich A, Pyza E. Cyclical expression of Na+/K+-ATPase in the visual system of Drosophila melanogaster. J Insect Physiol. 2009;55:459–468. doi: 10.1016/j.jinsphys.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang H-Y, Houshyar R, Bainton RJ, Diantonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. J. Neurobiol. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Griffin JW, George EB, Hsieh ST, Glass JD. Axonal degeneration and disorders of the axonal cytoskeleton. In: Waxman SG, Kocsis JD, Stys PS, editors. The Axon: Structure, Function and Pathophysiology. Oxford University Press; New York: 1995. pp. 375–390. [Google Scholar]
- Griffiths GW. Transport of glial cell acid phosphatase by endoplasmic reticulum into damaged axons. J. Cell Sci. 1979;36:361–389. doi: 10.1242/jcs.36.1.361. [DOI] [PubMed] [Google Scholar]
- Griffiths GW, Boschek CB. Rapid degeneration of visual fibers following retinal lesions in the dipteran compound eye. Neurosci Lett. 1976;3:253–258. doi: 10.1016/0304-3940(76)90051-3. [DOI] [PubMed] [Google Scholar]
- Grosjean Y, Grillet M, Augustin H, Ferveur J-F, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat. Neurosci. 2008;11:54–61. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hähnlein I, Bicker G. Morphology of neuroglia in the antennal lobes and mushroom bodies of the brain of the honeybee. J. Comp. Neurol. 1996;367:235–245. doi: 10.1002/(SICI)1096-9861(19960401)367:2<235::AID-CNE6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hähnlein I, Härtig W, Bicker G. Datura stramonium lectin staining of glial associated extracellular material in insect brains. J. Comp. Neurol. 1996;367:175–187. doi: 10.1002/(SICI)1096-9861(19961209)376:2<175::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hamanaka Y, Meinertzhagen IA. Immunocytochemical localization of synaptic proteins to photoreceptor synapses of Drosophila melanogaster. J. Comp. Neurol. 2010;518:1133–1155. doi: 10.1002/cne.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J. Comp. Physiol. A. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- Hardie RC. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Nassif C, Lekven A. Embryonic development of the Drosophila brain. II. Pattern of glial cells. J. Comp. Neurol. 1998;402:32–47. [PubMed] [Google Scholar]
- Heisenberg M. Separation of receptor and lamina potentials in the electroretinogram of normal and mutant Drosophila. J. Exp. Biol. 1971;55:85–100. doi: 10.1242/jeb.55.1.85. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 1995;92:612–616. doi: 10.1073/pnas.92.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C. Does the morning and evening oscillator model fit better for flies or mice? J Biol Rhythms. 2009;24:259–270. doi: 10.1177/0748730409339614. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C, Homberg U. Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J. Comp. Neurol. 1993;337:177–190. doi: 10.1002/cne.903370202. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Kinrade EFV, Georgiou M. The Drosophila neuregulin Vein maintains glial survival during axon guidance in the CNS. Dev. Cell. 2001;1:1–20. doi: 10.1016/s1534-5807(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Hidalgo A. Interactive nervous system development: control of cell survival in Drosophila. Trends Neurosci. 2002;25:365–370. doi: 10.1016/s0166-2236(02)02186-0. [DOI] [PubMed] [Google Scholar]
- Homyk T, Jr, Szidonya J, Suzuki DT. Behavioral mutants of Drosophila melanogaster. III. Isolation and mapping of mutations by direct visual observations of behavioral phenotypes. Mol. Gen. Genet. 1980;177:553–565. doi: 10.1007/BF00272663. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O'Leary DDM, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Goodman CS. Cell and substrate adhesion molecules in Drosophila. Annu. Rev. Cell Biol. 1991;7:505–557. doi: 10.1146/annurev.cb.07.110191.002445. [DOI] [PubMed] [Google Scholar]
- Hough CD, Woods DF, Park S, Bryant PJ. Organizing a functional junctional complex requires specific domains of the Drosophila MAGUK Discs large. Genes Dev. 1997;11:3242–3253. doi: 10.1101/gad.11.23.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovemann BT, Ryseck R-P, Walldorf U, Störtkuhl KF, Dietzel ID, Dessen E. The Drosophila ebony gene is closely related to microbial peptide synthetases and shows specific cuticle and nervous system expression. Gene. 1998;221:1–9. doi: 10.1016/s0378-1119(98)00440-5. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Glial cells of an insect ganglion. J. Comp. Neurol. 1986;246:85–103. doi: 10.1002/cne.902460106. [DOI] [PubMed] [Google Scholar]
- Huang Y, Stern M. In vivo properties of the Drosophila inebriated-encoded neurotransmitter transporter. J. Neurosci. 2002;22:1698–1708. doi: 10.1523/JNEUROSCI.22-05-01698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Attix S, Gunning D, Zipursky SL. Temporal control of glial cell migration in the Drosophila eye requires gilgamesh, hedgehog, and eye specification genes. Neuron. 2002;33:193–203. doi: 10.1016/s0896-6273(01)00581-5. [DOI] [PubMed] [Google Scholar]
- Ito K, Urban J, Technau GM. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Dev. Genes Evol. 1995;204:284–307. doi: 10.1007/BF02179499. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Jhaveri D, Sen A, Rodrigues V. Mechanisms underlying olfactory neuronal connectivity in Drosophila - the atonal lineage organizes the periphery while sensory neurons and glia pattern the olfactory lobe. Dev. Biol. 2000;226:73–87. doi: 10.1006/dbio.2000.9855. [DOI] [PubMed] [Google Scholar]
- Jones BW, Fetter RD, Tear G, Goodman CS. glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell. 1995;82:1013–1023. doi: 10.1016/0092-8674(95)90280-5. [DOI] [PubMed] [Google Scholar]
- Juang J-L, Carlson SD. A blood brain barrier without tight junctions in the fly central nervous system in the early postembryonic stage. Cell Tissue Res. 1992;270:95–103. [Google Scholar]
- Kato K, Awasaki T, Ito K. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development. 2009;136:51–59. doi: 10.1242/dev.023366. [DOI] [PubMed] [Google Scholar]
- Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, Anderson DJ. Isolation and characterization of mammalian homologues of the Drosophila gene glial cells missing. Proc. Natl. Acad. Sci. USA. 1998;95:12364–12369. doi: 10.1073/pnas.95.21.12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Crews ST. Influence of Drosophila ventral epidermal development by the CNS midline cells and spitz class genes. Development. 1993;118:893–901. doi: 10.1242/dev.118.3.893. [DOI] [PubMed] [Google Scholar]
- Kinchen JM, Cabello J, Klingele D, Wong K, Feichtinger R, Schnabel H, Schnabel R, Hengartner MO. Two pathways converge at CED-10 to mediate actin rearrangement and corpse removal in C. elegans. Nature. 2005;434:93–99. doi: 10.1038/nature03263. [DOI] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J. Neural Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- Kolodziejczyk A, Sun X, Meinertzhagen IA, Nässel DR. Glutamate, GABA and Acetylcholine signaling components in the lamina of the Drosophila visual system. PLOS One. 2008;3:e2110. doi: 10.1371/journal.pone.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 1997;17:7425–7432. doi: 10.1523/JNEUROSCI.17-19-07425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Poeck B, Roth H, Ernst R, Keller A, Porsch M, Strauss R, Pflugfelder GO. Defective pigment granule biogenesis and aberrant behavior caused by mutations in the Drosophila AP-3β adaptin gene ruby. Genetics. 2000;155:213–223. doi: 10.1093/genetics/155.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretzschmar D, Tschäpe J, Bettencourt da Cruz A, Asan E, Poeck B, Strauss R, Pflugfelder GO. Glial and neuronal expression of polyglutamine proteins induce behavioral changes and aggregate formation in Drosophila. Glia. 2005;49:59–72. doi: 10.1002/glia.20098. [DOI] [PubMed] [Google Scholar]
- Kurant E, Axelrod S, Leaman D, Gaul U. Six-microns-under acts upstream of Draper in the glial phagocytosis of apoptotic neurons. Cell. 2008;133:498–509. doi: 10.1016/j.cell.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laissue PP, Reiter C, Hiesinger PR, Halter S, Fischbach KF, Stocker RF. Three-dimensional reconstruction of the antennal lobe in Drosophila melanogaster. J. Comp. Neurol. 1999;405:543–552. [PubMed] [Google Scholar]
- Lane NJ, Martinucci G, Dallai R, Burighel P, editors. Molecular Mechanisms of Epithelial Cell Junctions. R.G. Landes Company; Austin, TX: 1994. Electron microscopic structure and evolution of epithelial junctions. [Google Scholar]
- Laughlin SB. Neural principles in the visual system. In: Autrum H, editor. Comparative Physiology and Evolution of Vision in Invertebrates (Handbook of Sensory Physiology, Vol. VII/6B) Springer-Verlag; Berlin: 1981. pp. 133–280. [Google Scholar]
- Lear BC, Merrill CE, Lin J-M, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Learte AR, Forero MG, Hidalgo A. Gliatrophic and gliatropic roles of PVF/PVR signaling during axon guidance. Glia. 2008;56:164–176. doi: 10.1002/glia.20601. [DOI] [PubMed] [Google Scholar]
- Lee BP, Jones BW. Transcriptional regulation of the Drosophila glial gene repo. Mech. Dev. 2005;122:849–862. doi: 10.1016/j.mod.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Leiss F, Groh C, Butcher NJ, Meinertzhagen IA, Tavosanis G. Synaptic organization in the adult Drosophila mushroom body calyx. J. Comp. Neurol. 2009;517:808–824. doi: 10.1002/cne.22184. [DOI] [PubMed] [Google Scholar]
- Liévens J-C, Rival T, Iché M, Chneiweiss H, Birman S. Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum. Mol. Genet. 2005;14:713–724. doi: 10.1093/hmg/ddi067. [DOI] [PubMed] [Google Scholar]
- Liu QA, Hengartner MO. Candidate adaptor protein CED-6 promotes the engulfment of apoptotic cells in C. elegans. Cell. 1998;93:961–972. doi: 10.1016/s0092-8674(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Llimargas M, Strigini M, Katidou M, Karagogeos D, Casanova J. Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system. Development. 2004;131:181–190. doi: 10.1242/dev.00917. [DOI] [PubMed] [Google Scholar]
- MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Thwaites DT, Deitmer JW, Coles JA. Chloride-dependent transport of NH4+ into bee retinal glial cells. Euro. J. Neurosci. 1999;11:167–177. doi: 10.1046/j.1460-9568.1999.00418.x. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Coles JA. A Cl− cotransporter selective for NH4+ over K+ in glial cells of bee retina. J. Gen. Physiol. 2000;116:125–141. doi: 10.1085/jgp.116.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick GS, Scholnick SB. Repression and activation of the Drosophila dopa decarboxylase gene in glia. Mol Cell Biol. 1992;12:5659–5666. doi: 10.1128/mcb.12.12.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary Conservation of Vertebrate Blood-Brain Barrier Chemoprotective Mechanisms in Drosophila. J. Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinertzhagen IA. Development of the compound eye and optic lobe of insects. In: Young D, editor. Developmental Neurobiology of Arthropods. Cambridge Univ. Press; Cambridge: 1973. pp. 51–104. [Google Scholar]
- Meinertzhagen IA, O'Neil SD. Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J. Comp. Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- Meinertzhagen IA, Wang Y. Drosophila mutants tan and ebony have altered numbers of capitate projections, glial invaginations into photoreceptor terminals. In: Elsner N, Wässle H, editors. Neurobiology From Membrane to Mind. Proceedings of the 25th Göttingen Neurobiology Conference; Georg Thieme Verlag; Göttingen. 1997. p. 457. [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Reddy GR, Edwards JS. Immunological probes reveal spatial and developmental diversity in insect neuroglia. J. Neurosci. 1987;7:512–521. doi: 10.1523/JNEUROSCI.07-02-00512.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs PG. Brain structure. In: Kerkut G, Gilbert LI, editors. Comprehensive Insect Physiology, Pharmacology and Biochemistry of Nervous Systems: Structure and Motor Function. Pergamon Press; Oxford, New York. Toronto, Sydney, Paris and Frankfurt: 1985. pp. 299–370. [Google Scholar]
- Moyer K, Jacobs JR. Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev. Biol. 2008;8:99. doi: 10.1186/1471-213X-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlig-Versen M, Bettencourt da Cruz A, Tschäpe J-A, Moser M, Büttner R, Athenstaedt K, Glynn P, Kretzschmar D. Loss of Swiss cheese/neuropathy target esterase activity causes disruption of phosphatidylcholine homeostasis and neuronal and glial death in adult Drosophila. J. Neurosci. 2005;25:2865–2873. doi: 10.1523/JNEUROSCI.5097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Umetsu D, Maeyama Y, Sato M, Yoshida S, Tabata T. Focal adhesion kinase controls morphogenesis of the Drosophila optic stalk. Development. 2007;134:1539–1548. doi: 10.1242/dev.001529. [DOI] [PubMed] [Google Scholar]
- Narasimha M, Uv A, Krejci A, Brown NH, Bray SJ. Grainy head promotes expression of septate junction proteins and influences epithelial morphogenesis. J. Cell Sci. 2008;121:747–752. doi: 10.1242/jcs.019422. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F. Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res. 1988;253:639–646. doi: 10.1007/BF00219755. [DOI] [PubMed] [Google Scholar]
- Newby LM, Jackson FR. Drosophila ebony mutants have altered circadian activity rhythms but normal eclosion rhythms. J. Neurogenet. 1991;7:85–101. doi: 10.3109/01677069109066213. [DOI] [PubMed] [Google Scholar]
- Nicol D, Meinertzhagen IA. An analysis of the number and composition of the synaptic populations formed by photoreceptors of the fly. J. Comp. Neurol. 1982;207:29–44. doi: 10.1002/cne.902070104. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, Zheng L, Wardill TJ, O'Kane CJ, de Polavieja GG, Juusola M. Network adaptation improves temporal representation of naturalistic stimuli in Drosophila eye: II mechanisms. PLoS ONE. 2009;4:e4306. doi: 10.1371/journal.pone.0004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oland LA, Marrero HG, Burger I. Glial cells in the developing and adult olfactory lobe of the moth Manduca sexta. Cell Tissue Res. 1999;297:527–545. doi: 10.1007/s004410051379. [DOI] [PubMed] [Google Scholar]
- Oland LA, Biebelhausen JP, Tolbert LP. Glial investment of the adult and developing antennal lobe of Drosophila. J. Comp. Neurol. 2008;509:526–550. doi: 10.1002/cne.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palgi M, Lindström R, Peränen J, Piepponen TP, Saarma M, Heino TI. Evidence that DmMANF is an invertebrate neurotrophic factor supporting dopaminergic neurons. PNAS. 2009;106:2429–2434. doi: 10.1073/pnas.0810996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis A, Segaran A, Liu C-H, Nikolaev A, Rister J, Thum AS, Roeder T, Semenov E, Juusola M, Hardie RC. Distinct roles for two histamine receptors (hclA and hclB) at the Drosophila photoreceptor synapse. J. Neurosci. 2008;28:7250–7259. doi: 10.1523/JNEUROSCI.1654-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu W, Shy D, Hartenstein V. Morphogenesis and proliferation of the larval brain glia in Drosophila. Dev. Biol. 2005;283:191–203. doi: 10.1016/j.ydbio.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Pereanu W, Spindler S, Cruz L, Hartenstein V. Tracheal development in the Drosophila brain is constrained by glial cells. Dev. Biol. 2007;302:169–180. doi: 10.1016/j.ydbio.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Steller H. Migration of glial cells into retinal axon target field in Drosophila melanogaster. J. Neurobiol. 1996;30:359–373. doi: 10.1002/(SICI)1097-4695(199607)30:3<359::AID-NEU5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. What the fly's glia tell the brain. Cell. 1995;83:671–674. doi: 10.1016/0092-8674(95)90178-7. [DOI] [PubMed] [Google Scholar]
- Phelan P, Starich TA. Innexins get into the gap. Bioessays. 2001;23:388–396. doi: 10.1002/bies.1057. [DOI] [PubMed] [Google Scholar]
- Piyankarage SC, Augustin H, Grosjean Y, Featherstone DE, Shippy SA. Hemolymph amino acid analysis of individual Drosophila larvae. Anal Chem. 2008;80:1201–1207. doi: 10.1021/ac701785z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Salkoff LB, Kelly LE. A neural gene from Drosophila melanogaster with homology to vertebrate and invertebrate glutamate decarboxylases. J. Neurochem. 1993;61:1291–1301. doi: 10.1111/j.1471-4159.1993.tb13621.x. [DOI] [PubMed] [Google Scholar]
- Pollack I, Hofbauer A. Histamine-like immunoreactivity in the visual system and brain of Drosophila melanogaster. Cell Tissue Res. 1991;266:391–398. doi: 10.1007/BF00318195. [DOI] [PubMed] [Google Scholar]
- Pyza E, Meinertzhagen IA. Neurotransmitters regulate rhythmic size changes amongst cells in the fly's optic lobe. J. Comp. Physiol. A. 1996;178:33–45. doi: 10.1007/BF00189588. [DOI] [PubMed] [Google Scholar]
- Pyza E, Meinertzhagen IA. Daily rhythmic changes of cell size and shape in the first optic neuropil in Drosophila melanogaster. J. Neurobiol. 1999;40:77–88. doi: 10.1002/(sici)1097-4695(199907)40:1<77::aid-neu7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Pyza E, Górska-Andrzejak J. Involvement of glial cells in rhythmic size changes in neurons of the housefly's visual system. J. Neurobiol. 2004;59:205–215. doi: 10.1002/neu.10307. [DOI] [PubMed] [Google Scholar]
- Rangarajan R, Gong Q, Gaul U. Migration and function of glia in the developing Drosophila eye. Development. 1999;126:3285–3292. doi: 10.1242/dev.126.15.3285. [DOI] [PubMed] [Google Scholar]
- Rangarajan R, Courvoisier H, Gaul U. Dpp and Hedgehog mediate neuron-glia interactions in Drosophila eye development by promoting the proliferation and motility of subretinal glia. Mech. Dev. 2001;108:93–103. doi: 10.1016/s0925-4773(01)00501-9. [DOI] [PubMed] [Google Scholar]
- Rawls JM., Jr. Analysis of pyrimidine catabolism in Drosophila melanogaster using epistatic interactions with mutations of pyrimidine biosynthesis and β-alanine metabolism. Genetics. 2006;172:1665–1674. doi: 10.1534/genetics.105.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardt A, Rybak J, Störtkuhl KF, Meinertzhagen IA, Hovemann BT. Ebony protein in the Drosophila nervous system: Optic neuropile expression in glial cells. J. Comp. Neurol. 2002;452:93–102. doi: 10.1002/cne.10360. [DOI] [PubMed] [Google Scholar]
- Richardt A, Kemme T, Wagner S, Schwarzer D, Marahiel MA, Hovemann BT. Ebony, a novel nonribosomal peptide synthetase for β-alanine conjugation with biogenic amines in Drosophila. J. Biol. Chem. 2003;278:41160–41166. doi: 10.1074/jbc.M304303200. [DOI] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson M-T, Iché M, Birman S. Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr. Biol. 2004;14:599–605. doi: 10.1016/j.cub.2004.03.039. [DOI] [PubMed] [Google Scholar]
- Rogina B, Benzer S, Helfand SL. Drosophila drop-dead mutations accelerate the time course of age-related markers. Proc Natl Acad Sci U S A. 1997;94:6303–6306. doi: 10.1073/pnas.94.12.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Calderón R, Uhlenbrock G, Borycz J, Simon AF, Grygoruk A, Yee SK, Shyer A, Ackerson LC, Maidment NT, Meinertzhagen IA, Hovemann BT, Krantz DE. A glial variant of the vesicular monoamine transporter is required to store histamine in the Drosophila visual system. PLoS Genet. 2008;4:e1000245. doi: 10.1371/journal.pgen.1000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler W, Oland LA, Higgins MR, Hildebrand JG, Tolbert LP. Development of a glia-rich axon-sorting zone in the olfactory pathway of the moth Manduca sexta. J. Neurosci. 1999;19:9865–9877. doi: 10.1523/JNEUROSCI.19-22-09865.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak J, Meinertzhagen IA. The effects of light reversals on photoreceptor synaptogenesis in the fly Musca domestica. Eur. J. Neurosci. 1997;9:319–333. doi: 10.1111/j.1460-9568.1997.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. The fine structure of neuroglia in the lamina ganglionaris of the housefly, Musca domestica L. J. Neurocytol. 1983a;12:213–241. doi: 10.1007/BF01148463. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Glial membrane specializations and the compartmentalization of the lamina ganglionaris of the housefly compound eye. J. Neurocytol. 1983b;12:243–275. doi: 10.1007/BF01148464. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Carlson SD. Interneuronal and glial-neuronal gap junctions in the lamina ganglionaris of the compound eye of the housefly, Musca Domestica. Cell Tissue Res. 1985;241:43–52. doi: 10.1007/BF00214624. [DOI] [PubMed] [Google Scholar]
- Sánchez y Sánchez D. Contribucion à l'étude de l'origine et de l'évolution de certains types de neuroglie chez les insectes. Trab. Lab Invest. Biol. (Madrid) 1935;30:299–353. [Google Scholar]
- Sarthy PV. Histamine: a neurotransmitter candidate for Drosophila photoreceptors. J. Neurochem. 1991;57:1757–1768. doi: 10.1111/j.1471-4159.1991.tb06378.x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Araki T, Milbrandt J. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. J. Neurosci. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarit F, Ferveur J-F. Genetic study of the production of sexually dimorphic cuticular hydrocarbons in relation with the sex-determination gene transformer in Drosophila melanogaster. Genet. Res. Camb. 2002;79:23–40. doi: 10.1017/s0016672301005481. [DOI] [PubMed] [Google Scholar]
- Schmucker D, Jäckle H, Gaul U. Genetic analysis of the larval optic nerve projection in Drosophila. Development. 1997;124:937–948. doi: 10.1242/dev.124.5.937. [DOI] [PubMed] [Google Scholar]
- Schulte J, Tepass U, Auld VJ. Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila. J. Cell Biol. 2003;161:991–1000. doi: 10.1083/jcb.200303192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell. 2005;123:133–144. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Seal RP, Amara SG. Excitatory amino acid transporters: A family in flux. Annu. Rev. Pharmacol. Toxicol. 1999;39:431–456. doi: 10.1146/annurev.pharmtox.39.1.431. [DOI] [PubMed] [Google Scholar]
- Seid MA, Wehner R. Delayed axonal pruning in the ant brain: a study of developmental trajectories. Develop. Neurobiol. 2009;69:350–364. doi: 10.1002/dneu.20709. [DOI] [PubMed] [Google Scholar]
- Sen A, Shetty C, Jhaveri D, Rodrigues V. Distinct types of glial cells populate the Drosophila antenna. BMC Dev. Biol. 2005;5:25. doi: 10.1186/1471-213X-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SR. Retinal resistance barriers and electrical lateral inhibition. Nature. 1975;255:480–483. doi: 10.1038/255480a0. [DOI] [PubMed] [Google Scholar]
- Shaw SR. Restricted diffusion and extracellular space in the insect retina. J. Comp. Physiol. A. 1977;113:257–282. [Google Scholar]
- Shaw SR. Early visual processing in insects. J. Exp. Biol. 1984;112:225–251. doi: 10.1242/jeb.112.1.225. [DOI] [PubMed] [Google Scholar]
- Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Silies M, Yuva Y, Engelen D, Aho A, Stork T, Klämbt C. Glial cell migration in the eye disc. J. Neurosci. 2007;27:13130–13139. doi: 10.1523/JNEUROSCI.3583-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinakevitch I, Strausfeld NJ. Chemical neuroanatomy of the fly's movement detection pathway. J. Comp. Neurol. 2004;468:6–23. doi: 10.1002/cne.10929. [DOI] [PubMed] [Google Scholar]
- Smith PJ, Howes EA, Treherne JE. Cell proliferation in the repairing adult insect central nervous system: incorporation of the thymidine analogue 5-bromo-2-deoxyuridine in vivo. J. Cell Sci. 1990;95:599–604. doi: 10.1242/jcs.95.4.599. [DOI] [PubMed] [Google Scholar]
- Soehnge H, Huang X, Becker M, Whitley P, Conover D, Stern M. A neurotransmitter transporter encoded by the Drosophila inebriated gene. Proc. Natl. Acad. Sci. U.S.A. 1996;93:13262–13267. doi: 10.1073/pnas.93.23.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark WS, Carlson SD. Ultrastructural pathology of the compound eye and optic neuropiles of the retinal degeneration mutant (w rdgBKS222) Drosophila melanogaster. Cell Tissue Res. 1982;225:11–22. doi: 10.1007/BF00216214. [DOI] [PubMed] [Google Scholar]
- Stark WS, Carlson SD. Blue and ultraviolet light induced damage to the Drosophila retina: ultrastructure. Curr. Eye Res. 1984;3:1441–1454. doi: 10.3109/02713688409000840. [DOI] [PubMed] [Google Scholar]
- Stark WS, Carlson SD. Retinal degeneration in rdgA mutants of Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int. J. Insect Morphol. Embryol. 1985;14:243–254. [Google Scholar]
- Stark WS, Carlson SD. Ultrastructure of capitate projections in the optic neuropil of Diptera. Cell Tissue Res. 1986;246:481–486. doi: 10.1007/BF00215187. [DOI] [PubMed] [Google Scholar]
- Stark WS, Sapp R, Carlson SD. Ultrastructure of the ocellar visual system in normal and mutant Drosophila melanogaster. J Neurogenet. 1989;5:127–153. doi: 10.3109/01677068909066203. [DOI] [PubMed] [Google Scholar]
- Stebbings LA, Todman MG, Phillips R, Greer CE, Tam J, Phelan P, Jacobs K, Bacon JP, Davies JA. Gap junctions in Drosophila: Developmental expression of the entire innexin gene family. Mech. Dev. 2002;113:197–205. doi: 10.1016/s0925-4773(02)00025-4. [DOI] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klämbt C. Organization and Function of the Blood-Brain Barrier in Drosophila. J. Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld NJ. Atlas of an insect brain. Springer; Berlin-Heidelberg-New York: 1976. [Google Scholar]
- Strigini M, Cantera R, Morin X, Bastiani MJ, Bate M, Karagogeos D. The IgLON protein Lachesin is required for the blood-brain barrier in Drosophila. Mol. Cell Neurosci. 2006;32:91–101. doi: 10.1016/j.mcn.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Stuart AE, Morgan JR, Mekeel HE, Kempter E, Callaway JC. Selective, activity-dependent uptake of histamine into an arthropod photoreceptor. J. Neurosci. 1996;16:3178–3188. doi: 10.1523/JNEUROSCI.16-10-03178.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AE, Mekeel HE, Kempter E. Uptake of the neurotransmitter histamine into the eyes of larvae of the barnacle (Balanus amphitrite) Biol. Bull. 2002;202:53–60. doi: 10.2307/1543222. [DOI] [PubMed] [Google Scholar]
- Stuart AE, Borycz J, Meinertzhagen IA. The dynamics of signaling at the histaminergic photoreceptor synapse of arthropods. Prog. Neurobiol. 2007;82:202–227. doi: 10.1016/j.pneurobio.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Suh J, Jackson FR. Drosophila Ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron. 2007;55:435–447. doi: 10.1016/j.neuron.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Kaneko A. L-Glutamate-induced depolarization in solitary photoreceptors: A process that may contribute to the interaction between photoreceptors in situ. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5315–5319. doi: 10.1073/pnas.85.14.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S-Y, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Garrity PA. Axon targeting in the Drosophila visual system. Curr. Opin. Neurobiol. 2003;13:90–95. doi: 10.1016/s0959-4388(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Berg JS, Stuart AE. Comparative sequence analysis and tissue localization of members of the SLC6 family of transporters in adult Drosophila melanogaster. J. Exp. Biol. 2006;209:3383–3404. doi: 10.1242/jeb.02328. [DOI] [PubMed] [Google Scholar]
- Tix S, Eule E, Fischbach K-F, Benzer S. Glia in the chiasms and medulla of the Drosophila melanogaster optic lobes. Cell Tissue Res. 1997;289:397–409. doi: 10.1007/s004410050886. [DOI] [PubMed] [Google Scholar]
- Tolbert LP, Hildebrand JG. Organization and synaptic ultrastructure of glomeruli in the antennal lobes of the moth Manduca sexta: A study using thin sections and freeze-fracture. Proc. R. Soc. Lond. B. Biol. Sci. 1981;213:279–301. [Google Scholar]
- Treherne JE. Blood-brain barrier. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 5. Oxford; Pergamon: 1985. pp. 115–137. [Google Scholar]
- True JR, Yeh S-D, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, Liou S-R, Han Q, Li J. Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet. 2005;1:e63. doi: 10.1371/journal.pgen.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Cenóz O. Some aspects of the structural organization of the intermediate retina of dipterans. J. Ultrastruct. Res. 1965;13:1–33. doi: 10.1016/s0022-5320(65)80086-7. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Poitry S. Kinetics of oxygen consumption after a single flash of light in photoreceptors of the drone (Apis mellifera) J. Gen. Physiol. 1982;80:19–55. doi: 10.1085/jgp.80.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Coles JA, Van de Werve G. The supply of metabolic substrate from glia to photoreceptors in the retina of the honeybee drone. J. Physiol. (Paris) 1987;82:279–287. [PubMed] [Google Scholar]
- Tsacopoulos M, Evèquoz-Mercier V, Perrottet P, Buchner E. Honeybee retinal glial cells transform glucose and supply the neurons with metabolic substrate. Proc. Natl. Acad. Sci. U.S.A. 1988;85:8727–8731. doi: 10.1073/pnas.85.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Veuthey A-L, Saravelos SG, Perrottet P, Tsoupras G. Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J. Neurosci. 1994;14:1339–1351. doi: 10.1523/JNEUROSCI.14-03-01339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J. Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda HR, Matsumoto A, Kawamura M, Iino M, Tanimura T, Hashimoto S. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J. Biol. Chem. 2002;277:14048–14052. doi: 10.1074/jbc.C100765200. [DOI] [PubMed] [Google Scholar]
- Uusitalo RO, Juusola M, Kouvalainen E, Weckström M. Tonic transmitter release in a graded potential synapse. J. Neurophysiol. 1995;74:470–473. doi: 10.1152/jn.1995.74.1.470. [DOI] [PubMed] [Google Scholar]
- van Marle J, Piek T, Lammertse T, Lind A, Van Weeren-Kramer J. Selectivity of the uptake of glutamate and GABA in two morphologically distinct insect neuromuscular synapses. Brain Research. 1985;348:107–111. doi: 10.1016/0006-8993(85)90365-8. [DOI] [PubMed] [Google Scholar]
- Venkatesh S, Singh RN. Sensilla on the third antennal segment of Drosophila melanogaster Meigen (Diptera: Drosophilidae) Int. J. Insect Morphol. Embryol. 1984:51–63. [Google Scholar]
- von Hilchen CM, Beckervordersandforth RM, Rickert C, Technau GM, Altenhein B. Identity, origin, and migration of peripheral glial cells in the Drosophila embryo. Mech. Develop. 2008;125:337–352. doi: 10.1016/j.mod.2007.10.010. [DOI] [PubMed] [Google Scholar]
- von Trotha J,W, Egger B, Brand AH. Cell proliferation in the Drosophila adult brain revealed by clonal analysis and bromodeoxyuridine labeling. Neural Development. 2009;4:9. doi: 10.1186/1749-8104-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- Wagner S, Heseding C, Szlachta K, True JR, Prinz H, Hovemann BT. Drosophila photoreceptors express cysteine peptidase Tan. J. Comp. Neurol. 2007;500:601–611. doi: 10.1002/cne.21138. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kankel DR. Molecular cloning and analysis of l(1)ogre, a locus of Drosophila melanogaster with prominent effects on the postembryonic development of the central nervous system. Genetics. 1990;126:1033–1044. doi: 10.1093/genetics/126.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: Evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Wigglesworth VB. The histology of the nervous system of an insect, Rhodnius prolixus (Hemiptera). II. The central ganglia. Q. J. Micros. Sci. 1959;100:299–313. [Google Scholar]
- Winberg ML, Perez SE, Steller H. Generation and early differentiation of glial cells in the first optic ganglion of Drosophila melanogaster. Development. 1992;115:903–911. doi: 10.1242/dev.115.4.903. [DOI] [PubMed] [Google Scholar]
- Witte I, Kreienkamp H-J, Gewecke M, Roeder T. Putative histamine-gated chloride channel subunits of the insect visual system and thoracic ganglion. J. Neurochem. 2002;83:504–514. doi: 10.1046/j.1471-4159.2002.01076.x. [DOI] [PubMed] [Google Scholar]
- Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Martinez Arias A, Bate M, editors. The Development of Drosophila. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 1993. pp. 1277–1325. [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu VM, Schulte J, Hirschi A, Tepass U, Beitel GJ. Sinuous is a Drosophila claudin required for septate junction organization and epithelial tube size control. J. Cell Biol. 2004;164:313–323. doi: 10.1083/jcb.200309134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VM, Yu MH, Paik R, Banerjee S, Liang Z, Paul SM, Bhat MA, Beitel GJ. Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development. 2007;134:999–1009. doi: 10.1242/dev.02785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W-C, Okano H, Patel NH, Blendy JA, Montell C. repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev. 1994;8:981–994. doi: 10.1101/gad.8.8.981. [DOI] [PubMed] [Google Scholar]
- Xiong W-C, Montell C. Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron. 1995;14:581–590. doi: 10.1016/0896-6273(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Yao Y, Wu Y, Yin C, Ozawa R, Aigaki T, Wouda RR, Noordermeer JN, Fradkin LG, Hing H. Antagonistic roles of Wnt5 and the Drl receptor in patterning the Drosophila antennal lobe. Nat. Neurosci. 2007;10:1423–1432. doi: 10.1038/nn1993. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Tepass U, Hartenstein V. Embryonic origin of the imaginal discs of the head of Drosophila melanogaster. Develop. Biol. 1992;203:60–73. doi: 10.1007/BF00539891. [DOI] [PubMed] [Google Scholar]
- Zaccheo O, Dinsdale D, Meacock PA, Glynn P. Neuropathy target esterase and its yeast homologue degrade phosphatidylcholine to glycerophosphocholine in living cells. J. Biol. Chem. 2004;27:24024–24033. doi: 10.1074/jbc.M400830200. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of Wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Curtin KD, Sun Y-A, Wyman RJ. Nested transcripts of gap junction gene have distinct expression patterns. J. Neurobiol. 1999;40:288–301. doi: 10.1002/(sici)1097-4695(19990905)40:3<288::aid-neu2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zheng L, de Polavieja GG, Wolfram V, Asyali MH, Hardie RC, Juusola M. Feedback network controls photoreceptor output at the layer of first visual synapses in Drosophila. J. Gen. Physiol. 2006;127:495–510. doi: 10.1085/jgp.200509470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Hirschberg B, Yuan J, Wang AP, Hunt DC, Ludmerer SW, Schmatz DM, Cully DF. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
- Zhu B, Pennack JA, McQuilton P, Forero MG, Mizuguchi K, Sutcliffe B, Gu C-J, Fenton JC, Hidalgo A. Drosophila neurotrophins reveal a common mechanism for nervous system formation. PLoS Biol. 2008;6:e284. doi: 10.1371/journal.pbio.0060284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegenfuss JS, Biswas R, Avery MA, Hong K, Sheehan AE, Yeung YG, Stanley ER, Freeman MR. Draper-dependent glial phagocytic activity is mediated by Src and Syk family kinase signalling. Nature. 2008;453:935–939. doi: 10.1038/nature06901. [DOI] [PMC free article] [PubMed] [Google Scholar]