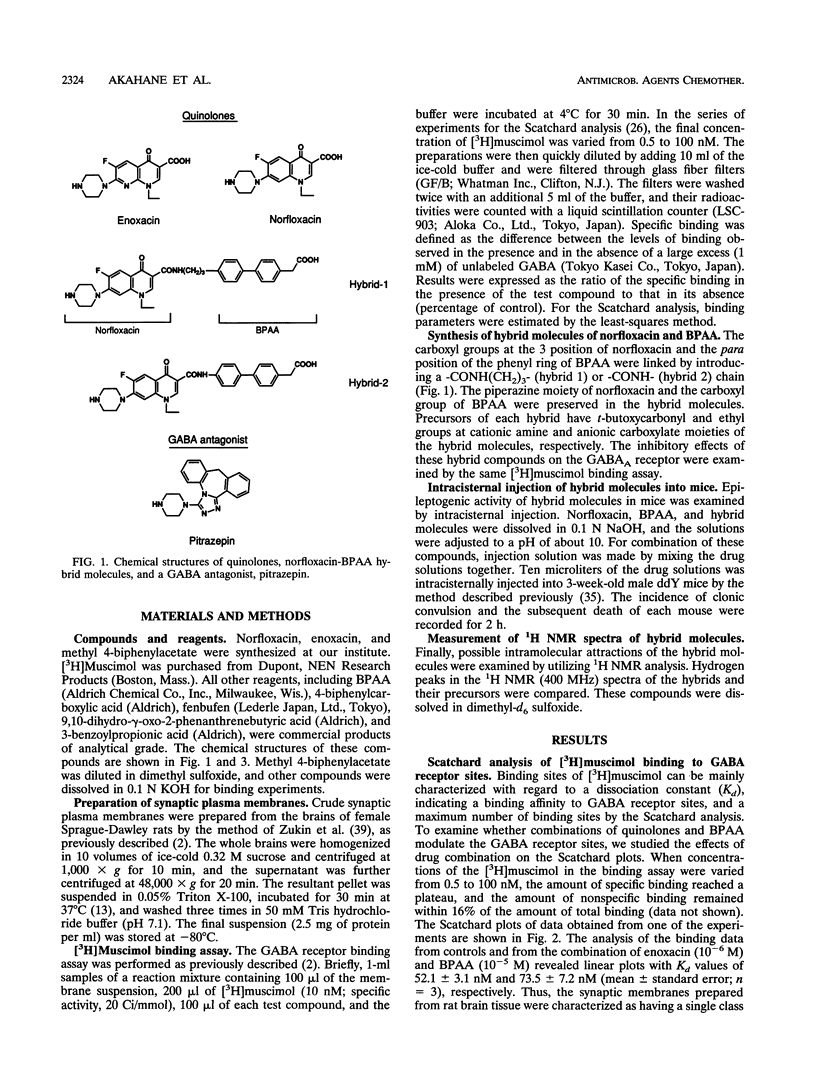
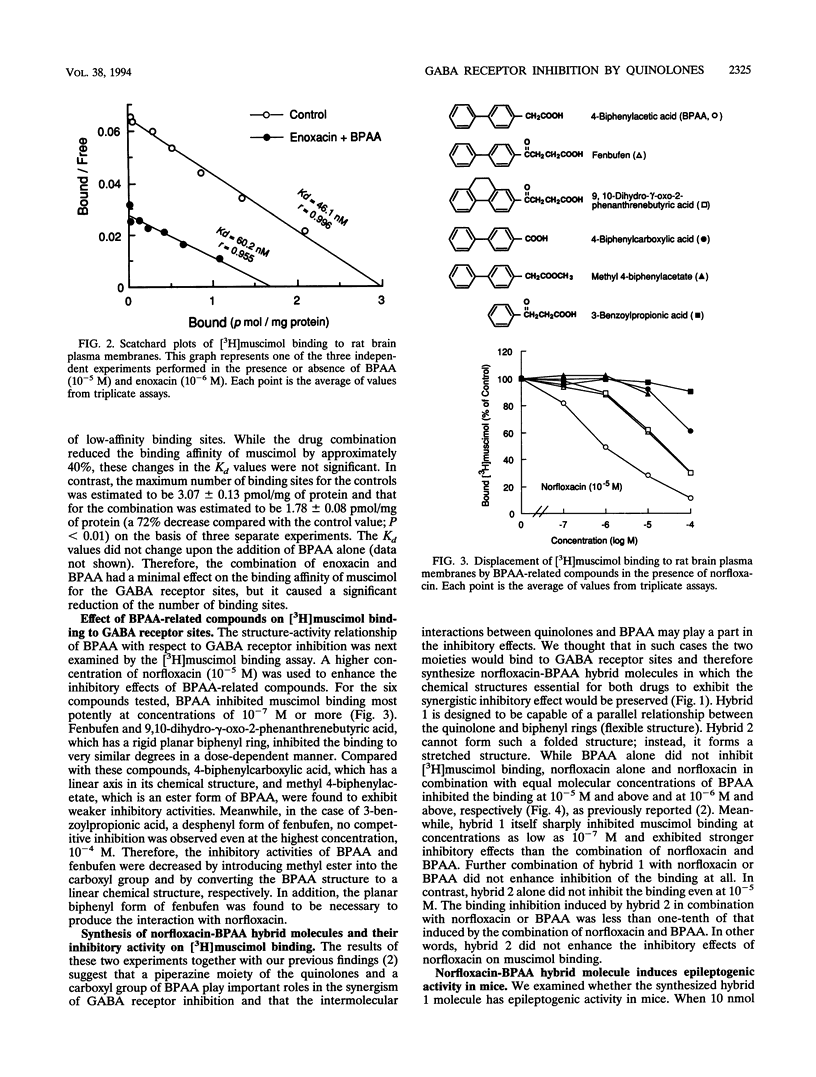
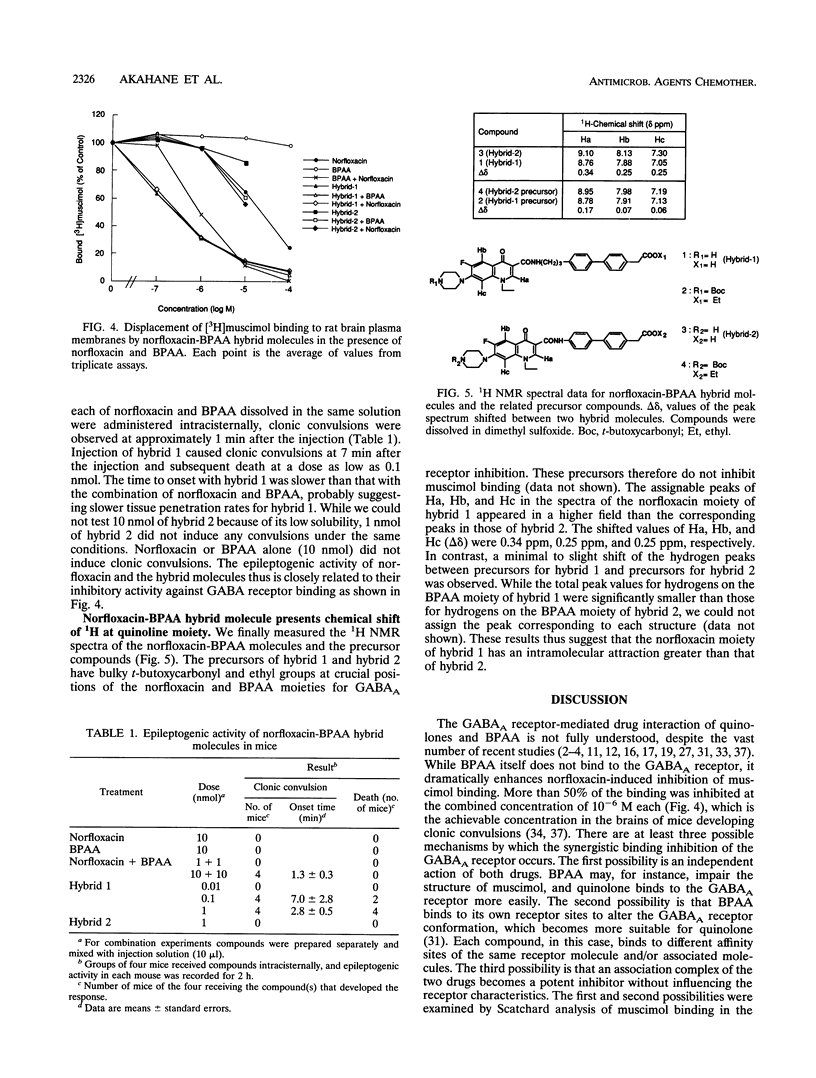
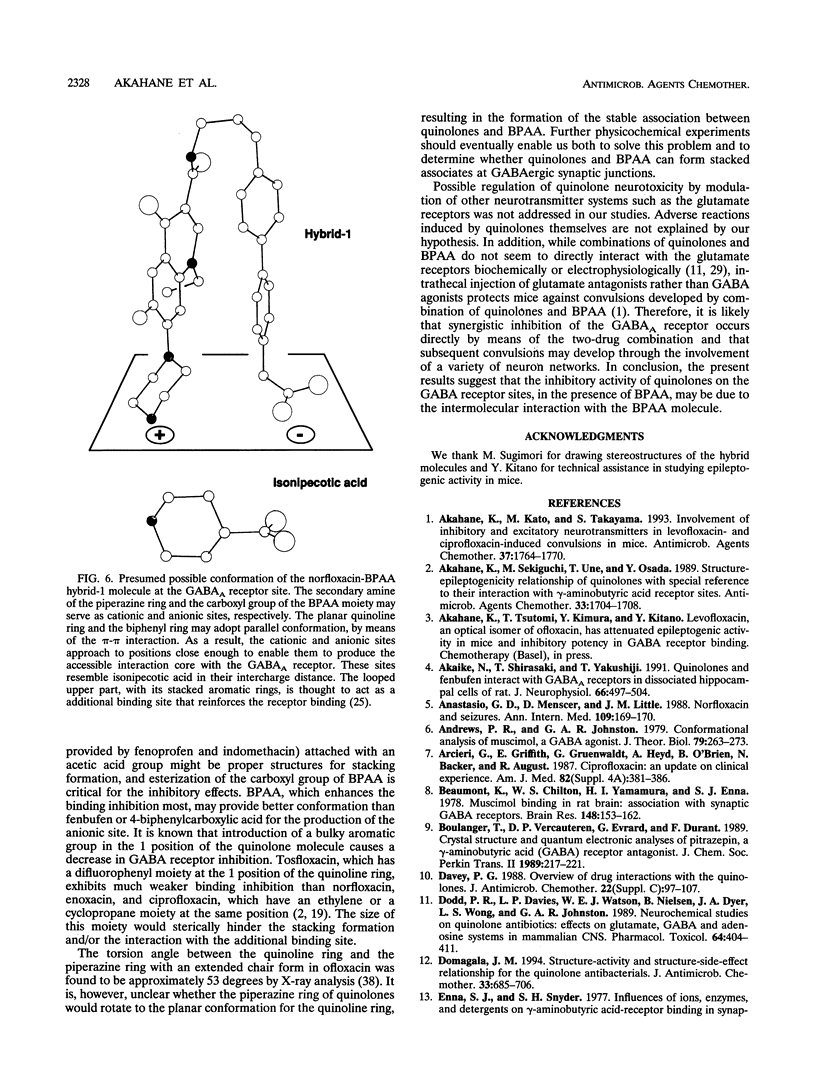
Abstract
The combination of some new quinolone antibacterial agents with 4-biphenylacetic acid (BPAA), a metabolite of fenbufen, is known to specifically induce functional blockade of the gamma-aminobutyric acid (GABA) receptors. The mechanisms of these drug interactions were further examined. Scatchard analysis of [3H]muscimol binding to rat brain plasma membranes in the presence of enoxacin and BPAA revealed that a significant decrease in the number of muscimol binding sites was produced without affecting the affinity of binding to the receptors. In the presence of norfloxacin, BPAA inhibited muscimol binding the most potently of the six BPAA-related compounds tested. Fenbufen and 9,10-dihydro-gamma-oxo-2-phenanthrenebutyric acid also inhibited the binding, and 4-biphenylcarboxylic acid and methyl 4-biphenylacetate inhibited it slightly, but 3-benzoylpropionic acid exhibited no competitive inhibition. Accordingly, hybrid molecules of norfloxacin and BPAA were synthesized for stereochemical analysis of these drug interactions. A hybrid with a -CONH(CH2)3- chain between norfloxacin and BPAA (flexible structure) inhibited muscimol binding, and intracisternal injection of this hybrid caused clonic convulsions in mice more potently than the combination of norfloxacin and BPAA did. In contrast, a hybrid linked by -CONH- (stretched structure) showed almost no such inhibitory effect. 1H NMR analysis indicated the presence of intramolecular attraction at the quinoline ring of the hybrid exhibiting the antagonistic activity. These results suggest the possibility that quinolones and BPAA interact with the GABA receptor at nearby sites and that the binding affinity of quinolones to the GABA receptors is largely enhanced by the intermolecular interaction with BPAA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akahane K., Kato M., Takayama S. Involvement of inhibitory and excitatory neurotransmitters in levofloxacin- and ciprofloxacin-induced convulsions in mice. Antimicrob Agents Chemother. 1993 Sep;37(9):1764–1770. doi: 10.1128/aac.37.9.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akahane K., Sekiguchi M., Une T., Osada Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with gamma-aminobutyric acid receptor sites. Antimicrob Agents Chemother. 1989 Oct;33(10):1704–1708. doi: 10.1128/aac.33.10.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Shirasaki T., Yakushiji T. Quinolones and fenbufen interact with GABAA receptor in dissociated hippocampal cells of rat. J Neurophysiol. 1991 Aug;66(2):497–504. doi: 10.1152/jn.1991.66.2.497. [DOI] [PubMed] [Google Scholar]
- Anastasio G. D., Menscer D., Little J. M., Jr Norfloxacin and seizures. Ann Intern Med. 1988 Jul 15;109(2):169–170. doi: 10.7326/0003-4819-109-2-169. [DOI] [PubMed] [Google Scholar]
- Andrews P. R., Johnston G. A. Conformational analysis of muscimol, a GABA agonist. J Theor Biol. 1979 Aug 7;79(3):263–273. doi: 10.1016/0022-5193(79)90345-x. [DOI] [PubMed] [Google Scholar]
- Arcieri G., Griffith E., Gruenwaldt G., Heyd A., O'Brien B., Becker N., August R. Ciprofloxacin: an update on clinical experience. Am J Med. 1987 Apr 27;82(4A):381–386. [PubMed] [Google Scholar]
- Beaumont K., Chilton W. S., Yamamura H. I., Enna S. J. Muscimol binding in rat brain: association with synaptic GABA receptors. Brain Res. 1978 Jun 9;148(1):153–162. doi: 10.1016/0006-8993(78)90385-2. [DOI] [PubMed] [Google Scholar]
- Davey P. G. Overview of drug interactions with the quinolones. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):97–107. doi: 10.1093/jac/22.supplement_c.97. [DOI] [PubMed] [Google Scholar]
- Dodd P. R., Davies L. P., Watson W. E., Nielsen B., Dyer J. A., Wong L. S., Johnston G. A. Neurochemical studies on quinolone antibiotics: effects on glutamate, GABA and adenosine systems in mammalian CNS. Pharmacol Toxicol. 1989 May;64(5):404–411. doi: 10.1111/j.1600-0773.1989.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Domagala J. M. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J Antimicrob Chemother. 1994 Apr;33(4):685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- Enna S. J., Snyder S. H. Influences ions, enzymes, and detergents on gamma-aminobutyric acid-receptor binding in synaptic membranes of rat brain. Mol Pharmacol. 1977 May;13(3):442–453. [PubMed] [Google Scholar]
- Gähwiler B. H., Maurer R., Wüthrich H. J. Pitrazepin, a novel GABAA antagonist. Neurosci Lett. 1984 Apr 6;45(3):311–316. doi: 10.1016/0304-3940(84)90244-1. [DOI] [PubMed] [Google Scholar]
- Halkin H. Adverse effects of the fluoroquinolones. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S258–S261. doi: 10.1093/clinids/10.supplement_1.s258. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Davey P. G., Lambert J. J. Antagonism of GABAA receptors by 4-quinolones. J Antimicrob Chemother. 1993 Apr;31(4):457–462. doi: 10.1093/jac/31.4.457. [DOI] [PubMed] [Google Scholar]
- Halliwell R. F., Davey P. G., Lambert J. J. The effects of quinolones and NSAIDs upon GABA-evoked currents recorded from rat dorsal root ganglion neurones. J Antimicrob Chemother. 1991 Feb;27(2):209–218. doi: 10.1093/jac/27.2.209. [DOI] [PubMed] [Google Scholar]
- Hori S., Kurioka S., Matsuda M., Shimada J. Inhibitory effect of cephalosporins on gamma-aminobutyric acid receptor binding in rat synaptic membranes. Antimicrob Agents Chemother. 1985 Apr;27(4):650–651. doi: 10.1128/aac.27.4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Falch E. GABA agonists. Development and interactions with the GABA receptor complex. Mol Cell Biochem. 1981 Aug 11;38(Spec No)(Pt 1):129–146. doi: 10.1007/BF00235692. [DOI] [PubMed] [Google Scholar]
- Lucet J. C., Tilly H., Lerebours G., Gres J. J., Piguet H. Neurological toxicity related to pefloxacin. J Antimicrob Chemother. 1988 Jun;21(6):811–812. doi: 10.1093/jac/21.6.811. [DOI] [PubMed] [Google Scholar]
- Rognan D., Boulanger T., Hoffmann R., Vercauteren D. P., Andre J. M., Durant F., Wermuth C. G. Structure and molecular modeling of GABAA receptor antagonists. J Med Chem. 1992 May 29;35(11):1969–1977. doi: 10.1021/jm00089a005. [DOI] [PubMed] [Google Scholar]
- Segev S., Rehavi M., Rubinstein E. Quinolones, theophylline, and diclofenac interactions with the gamma-aminobutyric acid receptor. Antimicrob Agents Chemother. 1988 Nov;32(11):1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T., Harata N., Nakaye T., Akaike N. Interaction of various non-steroidal anti-inflammatories and quinolone antimicrobials on GABA response in rat dissociated hippocampal pyramidal neurons. Brain Res. 1991 Oct 25;562(2):329–331. doi: 10.1016/0006-8993(91)90641-8. [DOI] [PubMed] [Google Scholar]
- Shirasaki T., Harata N., Nakaye T., Akaike N. Quinolones do not interact with NMDA receptor in dissociated rat hippocampal neurons. Brain Res. 1991 Oct 25;562(2):344–346. doi: 10.1016/0006-8993(91)90645-c. [DOI] [PubMed] [Google Scholar]
- Simpson K. J., Brodie M. J. Convulsions related to enoxacin. Lancet. 1985 Jul 20;2(8447):161–161. doi: 10.1016/s0140-6736(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Squires R. F., Saederup E. Indomethacin/ibuprofen-like anti-inflammatory agents selectively potentiate the gamma-aminobutyric acid-antagonistic effects of several norfloxacin-like quinolone antibacterial agents on [35S]t-butylbicyclophosphorothionate binding. Mol Pharmacol. 1993 May;43(5):795–800. [PubMed] [Google Scholar]
- Squires R. F., Saederup E. Mono N-aryl ethylenediamine and piperazine derivatives are GABAA receptor blockers: implications for psychiatry. Neurochem Res. 1993 Jul;18(7):787–793. doi: 10.1007/BF00966774. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Sato H., Kume Y., Tamai I., Okezaki E., Nagata O., Kato H. Inhibitory effects of quinolone antibacterial agents on gamma-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob Agents Chemother. 1988 Feb;32(2):190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Amano H., Shiomi H., Takagi H. Comparison of the analgesic effects of various opioid peptides by a newly devised intracisternal injection technique in conscious mice. Eur J Pharmacol. 1979 Jun 15;56(3):265–268. doi: 10.1016/0014-2999(79)90181-x. [DOI] [PubMed] [Google Scholar]
- Zukin S. R., Young A. B., Snyder S. H. Gamma-aminobutyric acid binding to receptor sites in the rat central nervous system. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4802–4807. doi: 10.1073/pnas.71.12.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]



