Abstract
Conducting polymers for battery applications have been subject to numerous investigations during the last two decades. However, the functional charging rates and the cycling stabilities have so far been found to be insufficient for practical applications. These shortcomings can, at least partially, be explained by the fact that thick layers of the conducting polymers have been used to obtain sufficient capacities of the batteries. In the present letter, we introduce a novel nanostructured high-surface area electrode material for energy storage applications composed of cellulose fibers of algal origin individually coated with a 50 nm thin layer of polypyrrole. Our results show the hitherto highest reported charge capacities and charging rates for an all polymer paper-based battery. The composite conductive paper material is shown to have a specific surface area of 80 m2 g−1 and batteries based on this material can be charged with currents as high as 600 mA cm−2 with only 6% loss in capacity over 100 subsequent charge and discharge cycles. The aqueous-based batteries, which are entirely based on cellulose and polypyrrole and exhibit charge capacities between 25 and 33 mAh g−1 or 38−50 mAh g−1 per weight of the active material, open up new possibilities for the production of environmentally friendly, cost efficient, up-scalable and lightweight energy storage systems.
There is currently a great interest in the development of thin, flexible, lightweight, and environmentally friendly batteries and supercapacitors.(1) In this process, the preparation of novel redox polymer and electronically conducting polymer-based electrode materials is essential. While it has recently been shown2,3 that it is possible to manufacture redox polymer-based electrodes and batteries with high-capacities and very good cycling performances, the corresponding development within the field of electronically conducting polymers is ongoing. Conducting polymers are particularly interesting materials as devices based on these materials could be used as adaptable energy storage devices due to their inherent fast redox switching, high conductivity, mechanical flexibility, low weight and possibility to be integrated into existing production processes.(4) While conductive polymers are more environmentally friendly and cost-efficient than most metal containing electrode materials, the insufficient cycle stabilities and the high self-discharge rates have so far been limiting their applicability in commercial battery systems.(5)
In the past, several attempts have been made to produce energy storage devices consisting of entirely nonmetal components. Composites with polypyrrole (PPy) have in this respect attracted much interest6−13 but the performance of these materials has up to now not been considered appropriate for this application. One way to improve the performance of nonmetal-based energy storage devices would be to use composite electrode materials of conductive polymers, for example, PPy, deposited as thin layers on a suitable large surface area substrate. Cellulose is in this case an appealing substrate material because of its abundance in nature and its well-established industrial use. Furthermore, the cellulose fibers have been found to be well wetted by polypyrrole,(14) which makes the homogeneous coating of individual cellulose fibers possible. Thus, composites of cellulose with conductive polymers are particularly attractive as these are fully recyclable, lightweight, mechanically robust, and can be manufactured at low costs.15,16 Native cellulose, that is, cellulose I, which is commonly used in paper industry, however, has rather small specific surface area, viz. of the order of 1 m2 g−1.(17) The cellulose extracted from the environmentally polluting Cladophora sp. algae as well as aero gels of microfibrillated cellulose (MFC) produced by mild enzymatic hydrolysis of cellulose from land plants are exceptions as the surface areas of these materials can be up to 100 times larger.15,17 While aerogels of MFC are very sensitive to exposure to moisture and shrink upon removal of absorbed water, we have recently shown that the specific surface area and pore volume of Cladophora cellulose/PPy composites are stable even when exposed to high relative humidities.(18) We have also shown that it is possible to coat highly porous Cladophora cellulose substrates with homogeneous, several nanometer thick layer of PPy to obtain a high-surface area cellulose composite electrode material and that this material exhibits an exceptionally high ion-exchange capacity.18−21 In the present work, we investigate the possibilities of utilizing these materials for energy storage in paper-based batteries.
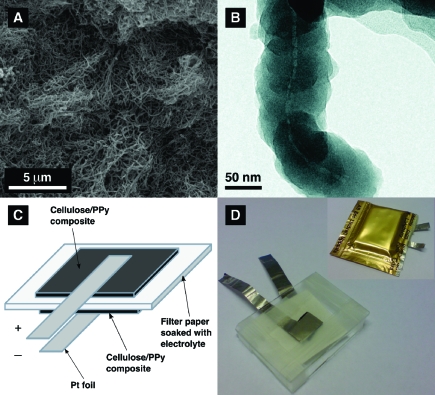
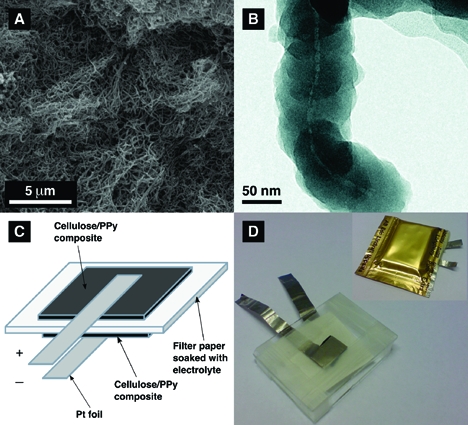
As is seen in Figure 1, the composite material, which was prepared by oxidizing pyrrole on the surface of Cladophora cellulose with the help of Fe(III) chloride (see Supporting Information) and used as the electrode material in the present study, had the appearance of thin black sheets made up from numerous intertwined fibers (see Figure 1a). This structure is in good agreement with that described in our previous reports.18,19 A scanning electron microscopy (SEM) analysis confirmed the presence of an extensive pore structure of the sample, which was further verified by N2 gas adsorption analysis. The measured specific surface area of the composite obtained based on N2 gas adsorption was 80 m2 g−1, while the corresponding total pore volume was ∼0.2 cm3 g−1. The electronic conductivity of the rectangular composite paper sheets was found to be slightly above 1 S cm−1.
Figure 1.
The Cladophora cellulose-PPy conductive paper composite (a) SEM micrograph taken with a magnification factor of 10 000, (b) TEM image of the cellulose composite fiber, (c) schematic image, and (d) photograph of the composite paper battery cell before and after sealing it into an polymer coated aluminum pouch.
From the transmission electron microscopy (TEM) image in Figure 1b, it is clear that the cellulose composite fibers had an average thickness of ∼90−100 nm and that the thickness of the PPy layer was between 40 and 50 nm, which is in good agreement with previous results.(18) The apparent (true) density of Cladophora cellulose has been reported to be about 1.60 g cm−3(22) whereas that of pure PPy has been found to be in the range between 1.55 and 1.65 g cm−3 (ref (23)). It can also be seen that the PPy layer had a characteristic nodular structure which was uninterrupted along the cellulose nanofibers. On the basis of the TEM images, it can thus be concluded that the major component of the composite was PPy, and that the material may be classified as PPy reinforced with cellulose nanofibers.
To determine the amount of polypyrrole in the composite, thermogravimetric analysis (TGA) was used, as is described in more detail in the Supporting Information. This analysis indicated that the composite consists of about two thirds PPy, which is in good agreement with the TEM images and the density values.
To confirm this value, a CHN analysis (Mikro Kemi AB, Uppsala, Sweden) was performed, showing that the nitrogen content of the composite was 13.1 ± 0.5% giving a PPy content in excellent agreement with the TGA results (see Supporting Information).
To evaluate the composite material described above as an electrode material for energy storage, a battery cell (see Figure 1c,d) was assembled in which oxidized (i.e., p-doped) and reduced (i.e., undoped) pieces of the composite were used as the two electrodes (see Supporting Information, Figure S2). The latter were separated by a filter paper soaked in 2.0 M sodium chloride as the electrolyte. Two platinum foils were used as the current collectors while two pieces of microscope glass were utilized to stabilize the cell and to apply pressure on the composites and the Pt foils to improve the contact between these parts. The total thickness of the cell, excluding the glass slides, was ∼2 mm. To avoid evaporation of water from the electrolyte, the cells were packed in polymer-coated aluminum pouches, which previously have been used(24) for the packaging of Li-ion batteries.
The open circuit voltage (OCV) of the battery, composed of oxidized (i.e., p-doped) and reduced (i.e., undoped) pieces of PPy, was found to be approximately one volt when measured with a voltmeter directly after the charging of the battery. This demonstrates the possibility of using the same electrode material as the two electrodes in a battery while still obtaining a sufficiently large cell voltage. It is therefore evident that the present type of composite material significantly facilitates the development of inexpensive paper-based batteries.
To investigate the electrochemical behavior of the composites, cyclic voltammograms were recorded using scan rates between 3 and 20 mV s−1. These voltammograms (see Supporting Information, Figure S3) showed that the material could be reversibly charged and discharged as oxidation and reduction peaks due to Faradaic processes were seen in the voltammogram during the anodic and cathodic scans. Such oxidation and reduction peaks are generally not seen when thick polymer coatings are used due to the large double layer capacitance associated with such coatings. Rectangularly shaped voltammograms have thus been obtained for ∼100 μm thick PPy films,(25) which is in agreement with the expected behavior for an ideal double-layer capacitor.(26) Our results, on the other hand, indicate that the charging and discharging of the present composite mainly involve surface confined redox reactions. By using a thin electroactive coating on a large surface area substrate such as cellulose it should thus be possible to obtain both a high capacity and rapid oxidation and reduction of the material. This compatibility with high charge and discharge rates stems from the fact that the time needed for the mass transport of the counterions in and out of the coating is short. We have previously shown18−21 that chloride ions will be taken up and expelled from the coating during the oxidation and reduction of the PPy coating. As will be shown below, the results of galvanostatic charge and discharge experiments show that the capacity of these coatings is maintained at high rates and that the capacities are higher than those previously obtained for all-polymer PPy batteries.
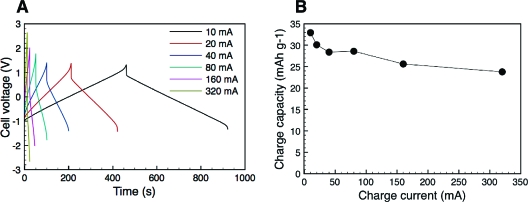
In Figure 2a, the galvanostatic charge−discharge curves are shown for the battery based on the use of different charge and discharge currents. In these experiments, the cell was cycled for 10 cycles at each current (see Supporting Information for details), and the results of the seventh of these cycles are depicted in the figure. To avoid problems due to overoxidation(27) of the PPy coating, the charging of the cell was interrupted at a voltage just below the potential where this was found to take place for the different currents. The results in Figure 2a clearly show that the PPy coatings could be reversibly oxidized and reduced continuously even at very high rates. It can thus be seen that it was possible to decrease the charging time from 8 min at 10 mA to only 11.3 s at a current of 320 mA (corresponding to a current density of 600 mA cm−2). As these currents correspond to rates of 7.5 and 320 C (i.e., charge/discharge within 1/7.5 and 1/320 of an hour), respectively, the results are in excellent agreement with the expected behavior for an electrode material composed of a thin electroactive layer on a large surface area substrate.
Figure 2.
Charge−discharge curves (a) and charge capacities (b) obtained with the conductive paper composite cell for currents ranging from 10 to 320 mA. The capacities were normalized with respect to the total amount of cellulose/PPy composite used in the cell.
Figure 2b shows the charge capacities calculated from the charge curves in Figure 2a, after normalizing with respect to the total weight of the composite material. It is seen that 72% of the electrode capacity obtained with a current of 10 mA was maintained when increasing the current to 320 mA. This demonstrates the outstanding ability of the material to undergo rapid oxidation and reduction. For comparison, most currently employed commercial rechargeable batteries generally require at least an hour to completely recharge because hastened charging increases the demands on the robustness of electrode reactions and shortens the cycling lifetime of the electrode.(28) As is seen in Figure 2b, the charge capacities obtained at 10 and 320 mA were about 33 and 25 mAh g−1. This means that the capacity for this particular cell containing 37.5 mg conductive paper was approximately 1.2 and 0.9 mAh, respectively.
The obtained average charge capacities of between 25 and 33 mAh g−1 are significantly larger than the value of 15 mAh g−1 previously obtained(12) for redox active PPy batteries and also larger than the 22 mAh g−1 reported(11) for an all-polymer PPy battery. In fact, the present capacities are even more competitive since in the latter case the capacity was normalized based only on the weight of the functional PPy layer and not of the entire electrode material as is the case in the present study. On the basis of the results from the TGA measurements and the CHN analysis, showing that about two thirds of the composite consists of PPy, we obtain charge capacities between approximately 38 and 50 mAh g−1 when only considering the electroactive material. These values may be compared with the capacity of a Li+ battery composed of a lithiated graphite (LiC6) anode and a LiCoO2 cathode of approximately 140 mAh g−1 (ref (29)) as well as that of 110 mAh g−1 reported by Pushparaj et al.(30) for a hybrid Li+ ion/carbon nanotube-impregnated paper battery. Since the present system has not yet been fully optimized, it is reasonable to assume that this all polymer-based system may be competitive even when comparing with Li+ ion systems particularly as the present type of batteries can be used in a very wide range of applications for which Li-ion batteries clearly not will be applicable.
Since it is generally very difficult to differentiate between currents due to surface confined electrochemical processes and double layer charging and as the influence of double layer charging would be expected to be significant for a high-surface area material, we have tried to estimate the contribution to the charge from the charging of the double layer. In this calculation, a PPy double layer capacitance of 37 μF cm−2 (ref (31)) was assumed together with a specific surface area of 80 m2 g−1 and a sample amount of 37.5 mg. A potential difference of one volt in this case corresponds to a double layer charge of roughly 0.31 mAh. As this corresponds to 29% of the total stored charge (i.e., 1.05 mA) in the cell, this estimation supports the conclusion that the majority of the charge stems from Faradaic reactions in the PPy layer. The contribution from the double layer charging to the overall capacity in the previously published reports11,12 is not known. As our results indicate that the present device performs as a mixed rechargeable battery and capacitor, it should be pointed out that from a pure charge storing perspective, it does not really matter if the charge is stored in the double layer capacitance or as a result of rapid surface confined redox reactions.
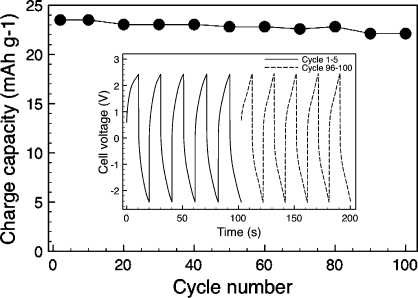
To elucidate the long-term cycling properties of the conductive paper composite electrodes, 100 consecutive galvanostatic charge−discharge cycles were performed using a current of 320 mA (i.e., 600 mA cm−2). In Figure 3, the calculated charge capacities from the charge curves normalized by the total weight of the composite material are plotted versus the cycle number. In the inset of Figure 3 the first and last five cycles of the 100 charge and discharge cycles can also be seen. The latter curves show that the material still cycles well after 100 cycles at a rate of 320 C. As is seen in Figure 3, the decrease in the capacity during the experiment was only 6%. This value can be compared with the 50% decrease found by Song and Palmore(12) for a PPy system after 60 cycles with a current density of 10 μA cm−2. This comparison clearly shows the significant improvement in performance with respect to cycling at high rates that can be obtained with the present composite material.
Figure 3.
Cycling behavior of the cellulose/PPy composite cell for a current of 320 mA (i.e., a current density of 600 mA cm−2). The charge capacities were normalized with respect to total weight of the composite. A 6% decrease in the charge capacity was observed after 100 cycles. The inset shows the first and last five cycles of the experiment.
In conclusion, the presented PPy-cellulose composite material is mechanically robust, lightweight, and flexible. It can be molded into various shapes and its thin sheets can be rolled to make very compact energy storage devices. The widespread availability of cellulose and the straightforward manufacturing of the composite are key factors for producing cost-efficient and fully recyclable paper-based batteries on a large industrial scale. Whereas the system described herein is limited in terms of the delivered cell potential, at least when compared with Li-ion batteries, the present battery holds great promise for applications in areas where Li-ion batteries are difficult to use, for example, in inexpensive large-scale devices or flexible energy storage devices to be integrated into textiles or packaging materials. The present paper-based battery system has also been shown to be compatible with very high charging rates. Together with the good cycling stability this makes the PPy-cellulose composite highly suitable for inclusion in future high-performance energy storage systems.
Acknowledgments
Dr. Alfonso E. Garcia Bennett is gratefully acknowledged for the recording of the TEM images while Henrik Eriksson and Kristina Edström are gratefully acknowledged for help with packaging of the cell into aluminum pouches. The Swedish Foundation for Strategic Research (SSF), the Knut and Alice Wallenberg foundation, as well as the Swedish Science Council (VR) are acknowledged for financial support of this work.
Supporting Information Available
Chemicals and reagents. Preparation of composite. Experimental details on scanning and transmission electron microscopy, surface area and pore volume, thermogravimetric analysis, conductivity measurements, cyclic voltammetry, and galvanostatic charge−discharge measurements. Results from thermogravimetric analysis and cyclic voltammetry. Charge−discharge curves. This material is available free of charge via the Internet at http://pubs.acs.org.
Supplementary Material
References
- Nishide H.; Oyaizu K. Science 2008, 319, 737–738. [DOI] [PubMed] [Google Scholar]
- Suga T.; Ohshiro H.; Sugita S.; Oyaizu K.; Nishide H. Adv. Mater. 2009, 21, 1627–1630. [DOI] [PubMed] [Google Scholar]
- Koshika K.; Sano N.; Oyaizu K.; Nishide H. Chem. Commun. 2009, 836–838. [DOI] [PubMed] [Google Scholar]
- Schultze J. W.; Karabulut H. Electrochim. Acta 2005, 50, 1739–1745. [Google Scholar]
- Inzelt G.Conducting Polymers: A New Era in Electrochemistry; Springer: New York, 2008. [Google Scholar]
- Mohammadi A.; Inganäs O.; Lundström I. J. Electrochem. Soc. 1986, 133, 947–949. [Google Scholar]
- Naegele D.; Bittihn R. Solid State Ionics 1988, 28−30, 983–989. [Google Scholar]
- Lee J. Y.; Ong L. H.; Chuah G. K. J. Appl. Electrochem. 1992, 22, 738–742. [Google Scholar]
- Rudge A.; Davey J.; Raistrick I.; Gottesfeld S.; Ferraris J. P. J. Power Sources 1994, 47, 89–107. [Google Scholar]
- Arbizzani C.; Mastragostino M.; Meneghello L. Electrochim. Acta 1996, 41, 21–26. [Google Scholar]
- Killian J. G.; Coffey B. M.; Gao F.; Poehler T. O.; Searson P. C. J. Electrochem. Soc. 1996, 143, 936–942. [Google Scholar]
- Song H.-K.; Palmore G. T. R. Adv. Mater. 2006, 18, 1764–1768. [Google Scholar]
- Grgur B. N.; Gvozdenovic M. M.; Stevanovic J.; Jugovic B. Z.; Marinovic V. M. Electrochim. Acta 2008, 53, 4627–4632. [Google Scholar]
- Johnston J. H.; Kelly F. M.; Moraes J.; Borrmann T.; Flynn D. Curr. Appl. Phys. 2006, 6, 587–590. [Google Scholar]
- Pääkkö M.; Vapaavuori J.; Silvennoinen R.; Kosonen H.; Ankerfors M.; Lindström T.; Berglund L. A.; Ikkala O. Soft Matter 2008, 4, 2492–2499. [Google Scholar]
- Richardson M. J.; Johnston J. H.; Borrmann T. Curr. Appl. Phys. 2006, 6, 462–465. [Google Scholar]
- Mihranyan A.; Llagostera A. P.; Karmhag R.; Strømme M.; Ek R. Int. J. Pharm. 2004, 269, 433–442. [DOI] [PubMed] [Google Scholar]
- Mihranyan A.; Nyholm L.; Garcia Bennett A. E.; Strømme M. J. Phys. Chem. B 2008, 112, 12249–12255. [DOI] [PubMed] [Google Scholar]
- Razaq A.; Mihranyan A.; Welch K.; Nyholm L.; Strømme M. J. Phys. Chem. B 2009, 113, 426–433. [DOI] [PubMed] [Google Scholar]
- Gelin K.; Mihranyan A.; Razaq A.; Nyholm L.; Strømme M. Electrochim. Acta 2009, 54, 3394–3401. [Google Scholar]
- Strømme M.; Frenning G.; Razaq A.; Gelin K.; Nyholm L.; Mihranyan A. J. Phys. Chem. B 2009, 113, 4582–4589. [DOI] [PubMed] [Google Scholar]
- Gustafsson C.; Lennholm H.; Iversen T.; Nyström C. Drug Dev. Ind. Pharm. 2003, 29, 1095–1107. [DOI] [PubMed] [Google Scholar]
- Kerton F. M.; Lawless G. A.; Armes S. P. J. Mater. Chem. 1997, 7, 1965–1966. [Google Scholar]
- Nordlinder S.; Nyholm L.; Gustafsson T.; Edström K. Chem. Mater. 2006, 18, 495–503. [Google Scholar]
- Wang J.; Xu Y.; Chen X.; Du X.; Li X. Acta Phys. -Chim. Sin. 2007, 23, 299–304. [Google Scholar]
- Conway B. E.Electrochemical supercapacitors: Scientific fundametals and technological applications; Kluwer Academic/Plenum Publishers: New York, 1997. [Google Scholar]
- Li Y. F.; Qian R. Y. Electrochim. Acta 2000, 45, 1727–1731. [Google Scholar]
- Winter M.; Brodd R. J. Chem. Rev. 2004, 104, 4245–4270. [DOI] [PubMed] [Google Scholar]
- Arico A. S.; Bruce P.; Scrosati B.; Tarascon J.-M.; Van Schalkwijk W. Nat. Mater. 2005, 4, 366–377. [DOI] [PubMed] [Google Scholar]
- Pushparaj V. L.; Shaijumon M. M.; Kumar A.; Murugesan S.; Ci L.; Vajtai R.; Linhardt R. J.; Nalamasu O.; Ajayan P. M. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 13574–13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L.; Bockris J. O. J. Electrochem. Soc. 1992, 139, 967–976. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.