Abstract
Background
To date, no study has assessed the effects of modifying carbohydrate intake (specifically decreasing added sugar and increasing fiber) on insulin secretion, nor has any study used an overweight Latino adolescent population. The objective of this study was to examine whether changes in dietary intake, specifically reductions in added sugar and/or increases in fiber, following a 12-week, modified carbohydrate dietary intervention, were associated with changes in insulin secretion and other metabolic risk factors for type 2 diabetes.
Methods
Participants were 16 overweight (≥85th percentile BMI) Latina adolescent females (12–17 years) who completed a 12-week modified carbohydrate intervention. Dietary intake was assessed by 3-day diet records, body composition by dual-energy X-ray absorptiometry, and insulin dynamics by an extended 3-hour oral glucose tolerance test (OGTT) at baseline and post-intervention.
Results
There was a trend for unadjusted change in reported added sugar intake (% of kcals) to be associated with change in insulin secretion, i.e. IAUC (r = 0.47; p = 0.075), and this relationship became significant after controlling for age, baseline insulin secretion, added sugar and adiposity, and change in adiposity (r = 0.85; p < 0.05). No other changes in dietary variables were related to changes in insulin secretion or other metabolic risk factors for type 2 diabetes.
Conclusions
Participants with greater reductions in added sugar intake showed significantly greater improvements in insulin secretion following a modified carbohydrate nutrition intervention. These findings suggest that interventions focused on decreasing added sugar intake have the potential to reduce type 2 diabetes risk in overweight youth.
INTRODUCTION
In the last 2 decades, type 2 diabetes has been reported with increasing frequency in children and adolescents, particularly in the Latino population.1 The increase in diabetes incidence is paralleled by the rising prevalence rates of overweight youth. We have previously shown that Latino children are more likely to be insulin resistant than their Caucasian counterparts, independent of adiposity.2 Furthermore, we found that 32% of overweight Latino children in the Los Angeles area have pre-diabetes (i.e., impaired fasting or 2-hour glucose), 3,4 and 30% have a clustering of diabetes and cardiovascular disease risk factors known as the metabolic syndrome.3
Dietary patterns, specifically high intakes of sugar, may play a prominent role in insulin dynamics and could be associated with type 2 diabetes risk factors. While the intake of all refined sugars has decreased in recent years, intake of dietary fructose, particularly high-fructose corn syrup (HFCS), has dramatically increased.5 National surveys have shown that added sugar intake, defined as sugars eaten separately or as ingredients in processed or prepared foods, constitutes 20% of total energy intake for adolescents in the United States.6 The Nurses' Health Study found that diets high in sugar-sweetened soft drinks and refined grains were significantly associated with increased risk of type 2 diabetes, independent of age, BMI, physical activity, smoking, family history of diabetes, and energy intake.7 Women from this study who consumed one or more sugar-sweetened soft drinks per day had a relative risk of type 2 diabetes of 1.83 (95% CI; p < 0.001), compared with those women who consumed less than one sugar-sweetened soft drinks per month.8
Ludwig et al.9 showed that, with each additional serving of sugar-sweetened beverage consumed per day, the odds of children becoming obese increased by 60%. We have previously reported that higher intakes of total sugar and sugary beverages were the only dietary variables associated with lower acute insulin response and disposition index (an index of β cell function) in 63 overweight Latino children. 10 These results suggest that modest reductions in sugar intake could potentially preserve β cell function and prevent metabolic disorders in these children.
Few intervention studies have assessed whether changes in dietary sugar intake improve adiposity and diabetes risk factors. The majority of these studies were conducted in adults and used a low glycemic index (GI) diet approach. A metaanalysis in adults showed that low-GI diets had small but clinically significant improvements in medium-term glycemic control (as measured with HbA1c and fructosamine levels) in patients with diabetes when compared with conventional (low-fat) or high-GI diets. Similar findings have been observed in a few low-GI intervention studies in children and adolescents.11, 12 No study, however, has assessed the effects of modifying carbohydrate intake (specifically decreasing added sugar and increasing dietary fiber) on insulin secretion, nor has any study used an overweight Latino adolescent population. We believe that a message focused on decreasing added sugar and increasing total fiber is easier to understand and implement than a focus on glycemic index content, especially for a pediatric population. Thus, the purpose of this study was to examine the effects of a 12-week, modified carbohydrate dietary intervention, aimed at reducing added sugar and increasing dietary fiber intake, on adiposity and insulin dynamics in overweight Latina girls.
DESIGN
Subjects
Twenty-three adolescent females were recruited through clinics and word of mouth around the East and Central Los Angeles area for the ALAS (Adolescent Latinas Adjusting Sugars) study. All subjects met the following inclusion criteria: 1) Hispanic origin, (both sets of grandparents required to be of Hispanic heritage); 2) BMI ≥85th percentile for age and sex according to the Centers for Disease Control (CDC) and Prevention Charts;13 3) female; 4) absence of diabetes, determined by an oral glucose tolerance test; 5) not presently taking medication(s) or diagnosed with any syndrome or disease that could influence dietary intake, body composition, fat distribution, or insulin action and secretion; and 6) not currently or in the 6 months prior to participation involved with any dietary or weight loss program. The Institutional Review Board of the University of Southern California approved this study. Parental consent and youth assent were obtained before testing commenced. Other findings from this intervention are in press,14 but no analysis of changes in dietary intake with changes in adiposity or metabolic parameters has been published.
Protocol
After an overnight fast, participants underwent testing at the General Clinical Research Center (GCRC) at baseline and postintervention (within one week of the last nutrition class). Height and weight were measured using a beam medical scale and wall-mounted stadiometer, to the nearest 0.1 cm and 0.1 kg, respectively, and the average of two measurements was used for analysis. BMI, BMI percentiles, and BMI z-scores for age and gender were determined using EpiInfo 2005, Version 3.3.2. (CDC, Atlanta, GA). At baseline only, a detailed medical history was obtained, and a physical examination was performed by a licensed healthcare provider (including Tanner staging based on breast stage and pubic hair15). Body composition was assessed with a total body DXA scan using a Hologic QDR 4500W (Hologic, Bedford, MA).
Dietary intake
Dietary intake was assessed from 3-day diet records (2 weekdays, 1 weekend day) at baseline and post-intervention. A nutrition educator clarified diet records at the first nutrition session and the post intervention GCRC visit. All subjects were given measuring cups and 12-inch rulers to assist in proper portion size estimations. Nutrition data were analyzed using the Nutrition Data System for Research (NDS-R version 5.0_35), a software program developed by the University of Minnesota. The NDS-R program calculates key dietary variables targeted during the intervention, such as dietary fiber, added sugar content, refined grains, whole-wheat grains, and sugar-sweetened beverages. Added sugars are defined as those sugars/syrups added to foods during food preparation or commercial food processing, such as high fructose corn syrup, not including naturally occurring sugars like lactose and fructose. Whole grains are defined as foods made from the entire grain seed, usually called the kernel, which consists of the bran, germ, and endosperm. If the kernel has been cracked, crushed, or flaked, it must retain nearly the same relative proportions of bran, germ, and endosperm as the original grain in order to be called whole grain.16 Such whole grains may include barley, buckwheat, bulgur, corn, millet, rice, rye, oats, sorghum, wheat, and wild rice. Refined grains are the resulting product of grain processing, such as white bread, white pasta, white rice, pretzels, and some cereals (this does not include raw sugar).16
Measurement of insulin secretion
Insulin secretion was measured with an extended (3-hour) oral glucose tolerance test (OGTT) and combined insulin and C-peptide modeling. A flexible IV catheter was placed in one arm and fasting blood samples were collected for determination of glucose, insulin, and C-peptide concentrations. At time zero, subjects ingested 1.75 g of oral glucose per kg of body weight (to a maximum of 75g). Blood samples were drawn via the antecubital vein at baseline and every 10 minutes for 3 hours. A total of 18 samples were collected and were assayed for glucose, insulin, and c-peptide.
Prehepatic insulin secretion rates were calculated using the extended combined model approach. 17,18 This model uses kinetic analysis of both plasma insulin and two compartmental C-peptide disappearance rates to estimate the prehepatic insulin secretion. The insulin secretion rates were per-unit C-peptide distribution volume and were corrected to mass per time by assuming a 6.5% body weight C-peptide distribution space.19 Modeling was performed using the MLAB software (Civilized Software, Bethesda, MD). Model-predicted insulin secretion rates are reported in insulin area under the curve (AUC) and incremental insulin area under the curve (IAUC) in nmol/min per L.
Fasting and 2-hour glucose values from the extended OGTT served as the diabetes screen test. Participants with impaired fasting glucose (fasting glucose 100–125 mg/dL) or impaired glucose tolerance (2-hour plasma glucose ≥140 and <200 mg/dL) were eligible to participate in the study. Homeostatic model assessment (HOMA-IR) was used as a measure of insulin resistance; [HOMA-IR = fasting insulin ((U/mL}) × fasting glucose (mmol/L)/22.5].20 Blood samples taken during the OGTT were separated for plasma and immediately transported on ice to the GCRC Core Laboratory. Glucose was assayed using a Yellow Springs Instruments analyzer (Yellow Springs, OH) and insulin and C-peptide were assayed using an automated random access enzyme immunoassay system analyzer (Tosoh Bioscience, Inc., San Francisco, CA).
Nutrition intervention
We developed a nutrition intervention specifically focused on 2 goals: 1) A reduction in added sugar towards a goal of 10% or less of total daily caloric intake from added sugar through a reduction in sugary beverages, candy, syrups, and sweets, which is based on the recommendation of the World Health Organization21; and 2) An increase in dietary fiber to a goal of 14g per 1000 calories, principally through an increase in fruits and vegetables, and a modification of breads, tortillas, and cereals, which is based on the recommendation of the US Institute of Medicine.22 In addition to the target goals stated above, dietary advice worked toward achieving a diet that has 45 to 55% of calories as carbohydrate and 30 to 35% of calories from fat.
Participants were randomly assigned to either a home-based format (n = 11), where the nutrition educator visited the participant's house for weekly, individualized classes, or the group format (n = 12), which was taught in a classroom on the USC campus. Both groups received identical, 90-minute nutrition classes weekly for 12 weeks and were taught by the same trained nutrition educators. Parents from both intervention groups were required to attend at least 4 of the 12 sessions and were invited to attend all sessions. As previously reported, although this modified carbohydrate dietary intervention led to significant improvements in dietary intake and BMI z-scores, the extremely intensive, individualized, home-based program was no more effective at improving diet, decreasing adiposity, or reducing type 2 diabetes risk factors than the traditional, classroom-based format.14 For this study, we were interested in identifying whether participants who made the most profound changes in their dietary intake had more improvements in metabolic parameters. Therefore, the data was combined from both intervention groups to assess whether changes in dietary intake following the 12-week nutrition intervention were related to changes in metabolic outcomes.
Statistical analysis
Variables that were not normally distributed (insulin secretion, incremental insulin secretion, HOMA-IR, and fasting and 2-hr insulin) were log transformed. Paired t-tests were used to measure differences in physiological and metabolic parameters, and dietary intake between baseline and post-intervention. Simple and partial correlations were employed to assess relationships between dietary variables (at baseline and changes following intervention) and adiposity and metabolic outcomes (at baseline and changes following intervention). Standard covariates used were age, baseline dietary variable of interest, and baseline metabolic variable of interest. Other covariates that were used (either individually or in combination) were baseline adiposity, change in adiposity, Tanner stage, insulin resistance (as measured by HOMA-IR), change in dietary fiber (when assessing change in added sugar), change in energy (when assessing dietary variables expressed in grams per day) and changes in other macronutrients (i.e, protein, carbohydrate, and fat). Hierarchical multiple regression analyses were used to examine the extent to which changes in dietary intake predicted changes in metabolic parameters, independent of covariates. All analyses were performed using SPSS software (version 11.0: SPSS Inc, Chicago, IL), with Type 1 error set at p < 0.05.
RESULTS
Only 16 of the 23 subjects had physiologically reasonable parameter estimates for the extended model, therefore 7 subjects did not have insulin secretion data and were not included in this analysis. This means that either the standard deviations on the estimates were too high, or the residuals, i.e., the difference between the measured data and the model-predicted values, were not randomly distributed around zero. Physiological and metabolic parameters at baseline and post-intervention for sixteen subjects are presented in Table 1. Paired t-tests found no significant differences in physiological or metabolic parameters from baseline to postintervention. Dietary intake at baseline and postintervention are presented in Table 2. Energy (kcals/d), carbohydrate (g/d), fat (g/d), and added sugars (g/d and % kcals) significantly decreased, while dietary fiber (% of kcals) significantly increased from baseline to week 12 of dietary intervention. Among foods targeted to limit, refined grains (servings/d) and sugar sweetened beverages (servings/d) significantly decreased from baseline to week 12 of dietary intervention. Baseline energy (kcals), fat (g/d), protein (g/d), and carbohydrates (g/d) were positively associated with baseline insulin area under the curve (AUC) and incremental insulin area under the curve (IAUC), i.e., measures of insulin secretion. After controlling for baseline body composition and age, however, baseline energy intake was the only variable that remained significantly related to AUC and IAUC (p < 0.05) (data not shown). No other baseline dietary variables were significantly related to any baseline adiposity or metabolic parameter.
TABLE 1.
Physiological and Metabolic Parameters at Baseline and Post-Interventiona
| Physiological & metabolic variablesb | Baseline | Post-Intervention |
|---|---|---|
| Age (y) | 14.5 ± 1.7 | — |
| Tanner | 3.9 ± 0.6 | — |
| BMI z-scores | 2.0 ± 0.5 | 1.9 ± 0.5 |
| Fat mass (kg) | 30.6 ± 8.6 | 30.8 ± 8.9 |
| Lean tissue mass (kg) | 43.2 ± 7.1 | 43.3 ± 7.0 |
| Fasting glucose (mg/dL) | 90.1 ± 8.8 | 88.1 ± 6.0 |
| 20-hr glucose (mg/dL) | 118.9 ± 14.1 | 116.0 ± 15.6 |
| Fasting insulin (µU/mL)c | 17.0 ± 11.1 | 17.3 ± 16.6 |
| 2-hr insulin (µU/mL)c | 93.6 ± 52.7 | 94.4 ± 79.5 |
| HOMA IRIc | 3.8 ± 2.5 | 3.8 ± 3.5 |
| Insulin secretion (nmol/min/L)c | 916.3 ± 677.3 | 856.4 ± 731.2 |
| Incremental insulin secretion (nmol/min/L)c | 650.9 ± 486.1 | 550.2 ± 137.6 |
Mean ± SD
Paired t-test found no significant differences in physiological and metabolic variables from baseline to post-intervention.
Statistical comparisons on non-normally distributed variables are performed using log-transformed data, but data are shown as non-transformed values for ease of interpretation.
TABLE 2.
Dietary Intake at Baseline and Post-Intervention of the Study Samplea
| Dietary variablesb | Baseline | Post-Intervention |
|---|---|---|
| Energy (kcals)c | 1849.1 ± 734.8 | 1459.5 ± 533.7 |
| CHO (g)c | 259.9 ± 120.4 | 199.1 ± 79.7 |
| CHO (% kcals) | 53.7 ± 6.3 | 54.8 ± 9.3 |
| Protein (g) | 73.6 ± 32.3 | 64.0 ± 19.2 |
| Protein (% kcals) | 15.4 ± 2.6 | 18.2 ± 5.6 |
| Fat (g)c | 69.5 ± 31.3 | 50.0 ± 26.2 |
| Fat (% kcals) | 32.4 ± 5.5 | 29.6 ± 6.9 |
| Dietary Fiber (g) | 15.4 ± 10.0 | 18.4 ± 9.7 |
| Fiber (g/1000 kcal) | 8.1 ± 3.0 | 13.0 ± 6.9 |
| Added sugar (g)d | 90.6 ± 52.3 | 50.4 ± 36.0 |
| Added sugar (% kcals)c | 17.8 ± 6.9 | 12.9 ± 5.7 |
| Refined grains (serv/d)c | 4.2 ± 3.1 | 2.5 ± 1.7 |
| Sugar sweetened beverages (serv/d)c | 1.8 ± 1.8 | 0.7 ± 0.7 |
| Whole-wheat grains (serv/d) | 1.1 ± 1.3 | 1.2 ± 0.8 |
| Total vegetables (serv/d) | 1.1 ± 1.1 | 1.5 ± 1.2 |
| Vegetables excluding those fried (serv/d) | 0.7 ± 0.8 | 1.0 ± 0.8 |
| Fruits (serv/d) | 1.2 ± 1.3 | 2.0 ± 1.6 |
| Fruits excluding juices (serv/d) | 0.6 ± 0.7 | 1.2 ± 1.2 |
All values are mean ± SD
Paired t-test were used to compare differences in dietary variables between baseline and post-intervention.
Denotes significant differences between baseline and post-intervention, p < 0.05.
Denotes significant differences between baseline and post-intervention, p < 0.01.
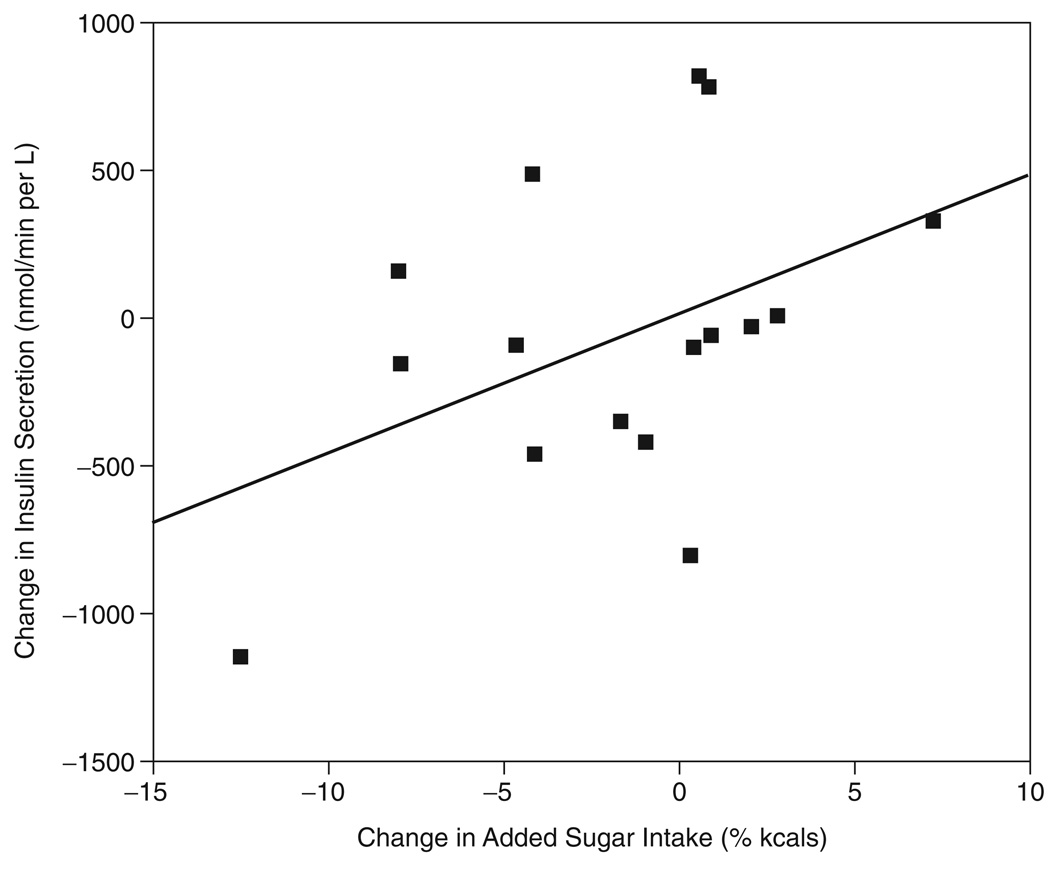
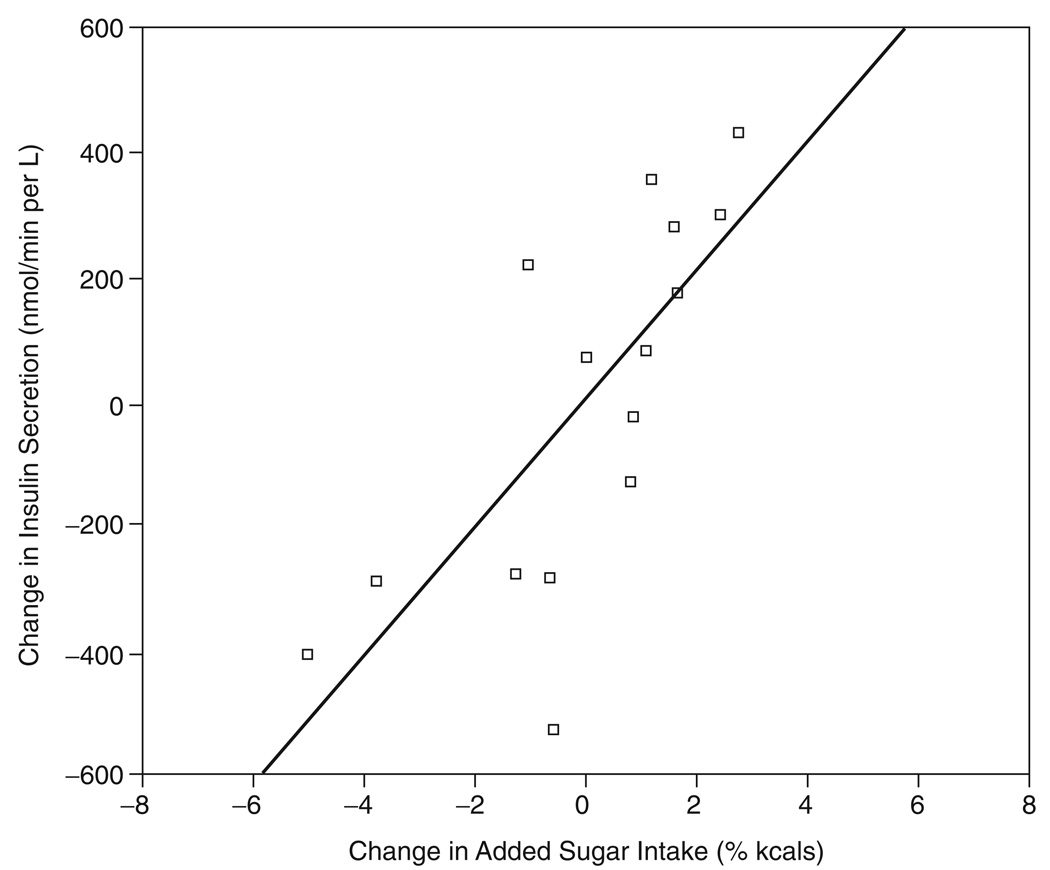
In this current study, we assessed whether changes in dietary intake, specifically added sugar and dietary fiber, were associated with changes in adiposity or metabolic parameters. No change in dietary variables were significantly associated with changes in adiposity parameters, while change in added sugar intake was the only dietary variable significantly associated with any metabolic parameter change. There was a trend for change in added sugar intake (% of kcals) to be associated with change in insulin secretion, i.e., IAUC (r = 0.47; p = 0.075), and this relationship became significant after controlling for age, baseline insulin secretion and baseline added sugar (% kcals) (r = 0.59; p = 0.05). This relationship became stronger when baseline adiposity (i.e., fat mass (FM) and fat free mass (FFM)) and changes in adiposity were entered as covariates (r = 0.85; p < 0.05). The unadjusted and adjusted relationship between change in added sugar and change in insulin secretion is depicted more clearly in Figure 1 and 2.
FIG. 1.
Unadjusted relationship between Δ added sugar vs change in insulin secretion (i.e., IAUC), (r = 0.47; p = 0.075).
FIG. 2.
Adjusted relationship between ( added sugar vs change in insulin secretion (i.e., IAUC). Significant partial correlation (r = 0.85; p < 0.05), covariates used were age, baseline fat mass (FM) & fat-free mass (FFM), change in FM & FFM, baseline added sugar, and baseline insulin secretion.
Using hierarchical multiple regressions, we found that change in added sugar intake (% kcals) explained an additional 32% of the variance in insulin secretion (β = 1.084; p < 0.05) (Table 3), independent of age, BMI z-score, baseline insulin secretion, and added sugar, baseline FM and FFM, and change in FM and FFM. All analyses remained significant when other covariates were entered (either individually or in combination), including insulin resistance (as measured by HOMA-IR), Tanner stage, changes in dietary fiber intake, and changes in macronutrient intake.
TABLE 3.
Regression of Change in Added Sugar (% kcals) vs. Change in Insulin Secretiona
| R2 | R2 Change | Standardized β | p-value for β |
|
|---|---|---|---|---|
| Model 1 | 0.552b | 0.552 | ||
| Age | 0.621 | 0.150 | ||
| Total fat | 0.154 | 0.767 | ||
| Total LTM | 0.066 | 0.915 | ||
| Δ Total fat | 0.277 | 0.609 | ||
| Δ Total LTM | −0.769 | 0.158 | ||
| Baseline % added sugar | 0.312 | 0.413 | ||
| Baseline insulin secretion | 0.185 | 0.613 | ||
| Model 2 | 0.935b | 0.322 | ||
| Age | 0.978 | 0.010c | ||
| Total fat | −0.261 | 0.439 | ||
| Total LTM | 0.230 | 0.539 | ||
| Δ Total fat | −0.115 | 0.733 | ||
| Δ Total LTM | −0.875 | 0.026 | ||
| Baseline % added sugar | −0.330 | 0.281 | ||
| Baseline insulin secretion | −0.142 | 0.550 | ||
| Δ % Added sugar | 1.084 | 0.016c |
Linear Regression: dependent variable insulin secretion (i.e., IAUC), statistical analyses were performed using log-transformed insulin secretion value.
indicates significance of model; p < 0.05.
indicates significance of β; p < 0.05.
DISCUSSION
The primary purpose of this study was to assess whether changes in dietary intake, specifically added sugar and dietary fiber, following a 12-week, modified carbohydrate dietary intervention, were associated with changes in adiposity and metabolic outcomes, specifically insulin secretion, in Latina adolescents. Our results are the first to demonstrate that a reduction in added sugar intake (% kcals) was associated with a reduction in insulin secretion. These results suggest that dietary interventions focusing on decreasing added sugar intake could have an impact on reducing type 2 diabetes in these high-risk girls.
Although the dietary goal of reducing added sugar by 10% or less of total daily calorie intake was not achieved, the mean added sugar intake postintervention was 12.9% of caloric intake. For those participants with a reduction in insulin secretion (n = 10), added sugar intake decreased by about 40 grams per day or 5% of calories to a mean of 50 g/d and 13% of daily caloric intake. These changes suggest that a modest reduction of 40 g/d and as little as 5% of kcals from added sugar could reduce type 2 diabetes risk factors. This reduction could be achieved by simply omitting 1–2 servings per day of sugar-sweetened beverages or refined grains, such as candy or baked goods.
In this study we did not observe a significant effect of increased dietary fiber intake on changes in adiposity or metabolic outcomes. This is probably due, in part, to the relatively modest increase in dietary fiber throughout the intervention and the fairly small contribution of this nutrient to energy intake. Although the dietary goal to increase fiber intake to 14g/1000 kcals was not met, mean dietary fiber did increase from 8.1 to 13.0 g/1000 kcals. Even so, change in dietary fiber intake was entered as a covariate in all analyses and did not significantly effect the change in added sugar findings.
Very few studies have employed reliable and effective methods for the estimation of insulin secretion. The combined model approach used in the current study takes into account the kinetics of endogenous plasma insulin and C-peptide, and provides an accurate estimation of prehepatic secretion rate,17, 18, 23 and we are the only group thus far that has used this approach to estimate insulin secretion rates in children 2. To our knowledge, this is the first intervention study to show that decreases in added sugar intake are associated with decreases in insulin secretion using the combined model approach. In addition, changes in dietary intake were not associated with changes in rudimentary measures of insulin dynamics, i.e., fasting or 2-hr glucose and insulin levels or HOMA-IR, and significant results were found only when examining changes in insulin secretion using the combined model approach. This suggests that more sensitive measurements of insulin dynamics may be required to capture the relationship between dietary intake patterns and diabetes risk factors.
It is difficult to determine the mechanism by which a reduction in added sugar intake has on decreases in insulin secretion, because decreases in insulin secretion could be an adaptive response to changes in insulin resistance. For this study, we used the HOMA index as a measure of insulin resistance, which is not a direct measure of whole body insulin resistance and may, in fact, reflect hepatic insulin resistance to a greater extent than peripheral insulin resistance.24 When HOMA was entered into the model as a covariate, it did not affect the significant association between reductions in added sugar intake and insulin secretion. We have previously shown in a similar population that 2-hour insulin was significantly related to whole body SI as measured by frequently sampled intravenous glucose tolerance test (FSIVGTT), while fasting insulin values were not related to SI, (insulin sensitivity) after adjusting for body fat. These results suggest that peripheral resistance, reflected in the post-oral glucose challenge insulin values, may affect whole body SI, as measured by the FSIVGTT, proportionately more than hepatic resistance. In the present study, however, change in added sugar intake was not significantly associated with change in 2-hr insulin. Even so, we cannot exclude the likelihood that the decrease in insulin secretion may be an adaptive response to improvements in insulin sensitivity, and recognize the lack of a direct measure of whole body insulin resistance as a limitation of this study. Nevertheless, we are currently conducting a much larger intervention in Latino children (n = 60), where we are measuring both insulin secretion using the 3-hour OGTT model and insulin sensitivity using the frequently sampled intravenous glucose tolerance test (FSIVGTT). In the near future, we intend to assess the relationship of changes in dietary intake, specifically added sugar, to the change in both insulin sensitivity and secretion using the more precise measures.
In addition, we are assuming that a decrease in insulin secretion essentially means an improvement in insulin secretion, i.e., less strain on the β-cells to secrete insulin. But one might argue that a decrease in insulin secretion suggests that the β-cells are already exhausted. Had this been the case, however, we might have expected an increase in circulating glucose levels following the intervention, as children with inadequate insulin secretion for the degree of their insulin resistance (i.e., β-cell dysfunction) tend to have higher levels of glycemia.4, 25 We thus conclude that the reduction in insulin secretion following a reduction in dietary sugars is likely due to improvements in underlying whole body insulin sensitivity as discussed above, reflecting a reduction in β-cell demand and a potential decrease in diabetes risk.
Subjects who decreased their added sugar intake (% of kcals; n = 8) had approximately 20% and 34% reductions in insulin secretion and incremental insulin secretion, respectively. These reductions could potentially lead to decreased subsequent development of type 2 diabetes. In the Troglitazone in Prevention of Diabetes (TRIPOD) study, a 3 month treatment with troglitazone resulted in a modest, albeit significant, 13% reduction in insulin secretion compared to the control (placebo) group in 266 Hispanic women.26 This modest reduction in insulin secretion was significantly associated with a 6.3% reduction in the onset of annual type 2 diabetes in this population. Therefore the decrease in insulin secretion (20%) and incremental insulin secretion (30%) seen in subjects who decreased their added sugar is novel and could have important implications on the subsequent development of type 2 diabetes.
Several limitations of this study should be noted. This study employed a fairly small sample size (n = 23), and only 16 of these subjects had accurate insulin secretion rates. The small sample size, however, is somewhat offset by the use of precise measurements for adiposity and insulin dynamics and the focus on an understudied high-risk population. Another limitation is that we did not employ a dietary control group; the initial study design was to assess the difference between an individualized, home-based format versus a group, classroom-based format using an identical nutrition curriculum. We intended the group-classroom format to serve as the control, but both intervention groups had similar improvements in dietary intake and health outcomes, and were therefore combined for this study. The specifics of the between group comparison have been previously reported.14 Nevertheless, this was a pilot feasibility study and we intend to test this modified carbohydrate nutrition education in larger and longer intervention studies, using both genders and with the appropriate control group. Another limitation is that we did not employ a dietary control group; the initial study design was to assess the difference between an individualized, home-based format versus a group, classroom-based format using an identical nutrition curriculum. We intended the group-classroom format to serve as the control, but both intervention groups had similar improvements in dietary intake and health outcomes, and were therefore combined for this study. The specifics of the between group comparison have been previously reported.14 Nevertheless, this was a pilot feasibility study and we intend to test this modified carbohydrate nutrition education in larger and longer intervention studies, using both genders and with the appropriate control group.
Another limitation is that we would expect that such large decreases in energy (21%) and fat intake (28%) would have resulted in significant reductions in weight or adiposity, which was not the case. Obviously, the current study is limited by the use of self-reported 3-day diet records, which rely solely on the subjects’ self-reporting and are often prone to errors. It is highly possible that participants under-reported perceived unhealthy foods and over-reported perceived healthy foods, especially after a dietary intervention. However, we would expect to see larger under-reporting of added sugars and over-reporting of dietary fibers which were the targeted dietary variables in this intervention. In this study we saw rather modest increases in dietary fiber (approximately 3 g/day, which equates to only 1 piece of whole wheat bread or an extra serving of vegetables) and modest decreases in added sugar (about 40 grams a day, which equates to only 1–2 servings of sugar sweetened beverages or refined grains). Although the decreases in added sugars were modest, they were still the only change in a dietary variable related to improvements in insulin secretion, which suggests that modest improvements in added sugar could reduce risk of type 2 diabetes. Regardless, we would expect the degree of underreporting of energy and fat to be similar across the whole sample, given that they were not targeted dietary variables in the intervention, and therefore not affect the relationship found with added sugar and insulin secretion.
Another limitation of this study is the inability of the NDS-R dietary analysis program to discriminate between high fructose corn syrup (HFCS) and other types of added sugar. However, we feel that the message of decreasing added sugar in the diet is much easier to understand than separating out aspects of added sugar, especially in a pediatric population. Our results suggest that future dissemination and testing of this modified carbohydrate nutrition curriculum with bigger samples and true controls is definitely warranted.
In summary, this is the first study to examine the association between changes in dietary intake following a nutrition intervention on changes in insulin secretion and other metabolic risk factors for type 2 diabetes in overweight Latina adolescents. We found a significant association between the degree of reduction in added sugar intake and decreases in insulin secretion, independent of age, puberty, adiposity, insulin resistance, and other macronutrients. These results suggest that interventions focusing on modest reductions in added sugar intake, as little as 40 grams a day or 5% of kcals, could reduce the strain placed on β-cells and potentially reduce the progression and/or development of type 2 diabetes in youth.
ACKNOWLEDGMENTS
Supported by the Dr. Robert C. Atkins Foundation, the General Clinic Research Center (National Center for Research Resources Grant MO1 RR 00043), and the American Diabetes Association, Mentor-Based Postdoctoral Fellowship 2005 (awarded to M.I.G.)
MIG conceived the study. With the assistance of MIG, JND designed the study, collected and analyzed the data, and drafted the manuscript. EEV and JND wrote the curriculum and EEV assisted with the design of the study and collection of data. DSM assisted with the design of the study and trained/supervised the behavioral component of the intervention. RMW assisted with the insulin secretion modeling. GQS and MJW assisted with the interpretation of the data. All co-authors assisted with the revision of the manuscript. We are grateful to the nurses and nutrition staff at the USC-GCRC. Finally, we express our gratitude to the children and their families for making this study possible.
REFERENCES
- 1.Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- 2.Goran M, Bergman R, Cruz M, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2001;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 3.Cruz ML, Weigensberg MJ, Huang T, Ball GDC, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. JCEM. 2004;89:108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 4.Weigensberg MJ, Ball GD, Shaibi GQ, Cruz ML, Goran MI. Decreased beta–cell function in overweight Latino children with impaired fasting glucose. Diabetes Care. 2005;28:2519–2524. doi: 10.2337/diacare.28.10.2519. [DOI] [PubMed] [Google Scholar]
- 5.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 6.Guthrie JF, Morton JF. Food sources of added sweeteners in the diets of Americans. J Am Diet Assoc. 2000;100:43–51. doi: 10.1016/S0002-8223(00)00018-3. quiz 49–50. [DOI] [PubMed] [Google Scholar]
- 7.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005 Sep;82(3):675–684. doi: 10.1093/ajcn.82.3.675. quiz 714-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 10.Davis J, Ventura E, Weigensberg M, et al. The relation of sugar intake to beta-cell function in overweight Latino children. Am J Clin Nutr. 2005;82:1004–1010. doi: 10.1093/ajcn/82.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spieth LE, Harnish JD, Lenders CM, Raezer LB, Pereira MA, Hangen SJ, Ludwig DS. A low-glycemic index diet in the treatment of pediatric obesity. Arch Pediatr Adolesc Med. 2000;154:947–951. doi: 10.1001/archpedi.154.9.947. [DOI] [PubMed] [Google Scholar]
- 12.Ebbeling C, Leidig M, Sinclair K, Hangen J, Ludwig D. A reduced-glycemic load diet in the treatment of adolescent obesity. Archs Pediatr Adolesct Med. 2003;157:773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention: CDC growth Charts. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Atlanta, GA: National Center for Health Statistics; 2000. (U.S. Publ. no. 314) [Google Scholar]
- 14.Davis JN, Ventura EE, Alexander KA, Salguero LE, Weigensberg MJ, Crespo NC, Spruijt-Metz D, Goran MI. Development and testing of a culturally tailored nutrition education program for reducing sugar and increasing fiber intake in overweight Latina adolescents. Int J Ped Obes. 2007;2:22–30. [Google Scholar]
- 15.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans. [Accessed on January 2, 2007];2005 www.healthierus.gov/dietaryguide-lines.
- 17.Watanabe R, Steil G, Bergman R. Critical evaluation of the combined model approach for estimation of pre-hepatic insulin secretion. Am J Phys. 1998;274:E172–E183. doi: 10.1152/ajpendo.1998.274.1.E172. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe R, Bergman R. Accurate measurement of endogenous insulin secretion does not require separate assessment of c-peptide kinetics. Diabetes. 2000;49:373–382. doi: 10.2337/diabetes.49.3.373. [DOI] [PubMed] [Google Scholar]
- 19.Van Cauter E, Mestrez F, Sturis J, Polonsky K. Estimation of insulin secretion rates from c-peptide levels: Comparison of individual and standard kinetic parameters for c-peptide clearance. Diabetes. 1992;41:363–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 20.Mathews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Geneva: World Health Organization; 2003. Diet, nutrition and the prevention of chronic diseases. Technical report Series, Number 916. [PubMed] [Google Scholar]
- 22.Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington, DC: Institute of Medicine of the National Academies; 2002. [DOI] [PubMed] [Google Scholar]
- 23.Kjems LL, Christiansen E, Volund A, Bergman RN, Madsbad S. Validation of methods for measurement of insulin secretion in humans in vivo. Diabetes. 2000;49:580–588. doi: 10.2337/diabetes.49.4.580. [DOI] [PubMed] [Google Scholar]
- 24.Weigensberg MJ, Cruz ML, Goran MI. Association between insulin sensitivity and post-glucose challenge plasma insulin values in overweight Latino youth. Diabetes Care. 2003;26:2094–2099. doi: 10.2337/diacare.26.7.2094. [DOI] [PubMed] [Google Scholar]
- 25.Goran M, Bergman R, Avilla Q, Watkins M, Ball GDC, Shaibi GQ, Weigensberg MJ, Cruz ML. Impaired glucose tolerance and reduced beta-cell function in overweight Latino children with a positive family history of type 2 diabetes. JCEM. 2004;89:207–212. doi: 10.1210/jc.2003-031402. [DOI] [PubMed] [Google Scholar]
- 26.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]