Abstract
Skeletal tissues adapt to their mechanical environments by modulating gene expression, cell metabolism, and extracellular matrix (ECM) architecture; however, the mechanosensory mechanisms for these processes are incompletely understood. Primary cilia have emerged as critical components of the cellular mechanosensory apparatus and have been hypothesized to participate in establishment of cellular and ECM orientation, but their function in skeletal tissues is just beginning to be examined. Here we focused on tendon, a tissue with an oriented matrix that is ideal for analysis of spatial relationships between primary cilia and the ECM. The objective of this study was to characterize the incidence and orientation of tenocyte primary cilia in their native ECM. Primary cilia, nuclei, and collagen were analyzed three-dimensionally in immunofluorescently labeled rat extensor tendon using multiphoton microscopy and semi-automated morphometry. Primary cilia were observed in 64% of tenocytes. The cilia were highly oriented with respect to the ECM: cilia were aligned parallel to the collagen fibers and the long axis of the tendon. This study represents the first quantification of the in situ incidence and orientation of primary cilia in tendon.
Keywords: primary cilia, tendon, tenocyte, collagen, multiphoton microscopy
Introduction
Skeletal tissues are sensitive to their mechanical environments and respond to changes in external forces by modulating gene expression, cell metabolism, and extracellular matrix (ECM) architecture. Physiologic loading promotes homeostasis in a variety of tissues, including tendon, bone, and cartilage; in contrast, supra-physiologic loading is a major cause of pathologic tissue degeneration.1, 2 Such degenerative changes are often associated with altered gene expression patterns, including those that govern synthesis and degradation of ECM molecules.1, 3
Tendon exemplifies a mechanically sensitive tissue that adapts to its loading environment. The tissue consists of a hierarchical matrix of collagen fibers oriented along the primary loading direction, a variety of noncollagenous proteins, and a three-dimensional network of cells with long processes connected by gap junctions. While moderate loading is required for tendon homeostasis, under- and overloading lead to tissue degradation and loss of function.3-6 At the cellular level, both immobilization and high tensile strains increase expression of genes associated with inflammation and tissue resorption, including cyclooxygenase 2, matrix metalloproteinase 1 (MMP1), and MMP13.3, 7 At the tissue level, the absence of mechanical loading is associated with decreased alignment of collagen fibers, resorption of ECM components, and reduction in tissue strength and stiffness.3-6 However, the mechanisms by which tenocytes sense mechanical stimuli and respond with biochemical signals for ECM formation, remodeling, or disassembly remain poorly understood.
Primary cilia are critical components of the cellular mechanosensory apparatus.8, 9 Observed as a solitary, finger-like projection in nearly all vertebrate cells, these immotile, microtubule-based sensory organelles extend from the cell surface into the extracellular environment. Primary cilia have numerous receptors for signaling molecules and are involved in multiple signal transduction complexes, including the platelet-derived growth factor receptor-alpha, hedgehog, and Wnt signaling pathways.8 The mechanosensory function of primary cilia has been characterized most extensively in epithelial cells. Fluid flow through renal tubules bends the primary cilia of the epithelial cells and initiates an intracellular signaling cascade, while removal of the primary cilia eliminates this flow-induced response.10 A major breakthrough in understanding the mechanosensory role of primary cilia occurred with the discovery that defective cilia were linked to impaired flow sensing, disrupted tissue homeostasis, and renal cyst formation.11, 12 Over the past decade, ciliary defects have been linked to a broad array of human diseases and developmental disorders, including polycystic kidney disease, situs inversus, infertility, retinal degeneration, and polydactyly.8, 13
Despite the major progress in understanding the sensory function of primary cilia in epithelial cells, much less is known about primary cilia in the cells of skeletal tissues. Early transmission electron microscopic (TEM) studies of articular chondrocytes and cultured fibroblasts noted ciliary incidence of approximately 100% and 67%, respectively.14-16 Primary cilia have subsequently been observed in a wide range of connective tissue cells, including chondrocytes, fibroblasts, osteocytes, and osteoblasts.17-19 Until recently, however, the function of these organelles remained unclear. The strongest evidence to date that primary cilia participate in mechanotransduction in skeletal tissue cells has emerged from recent studies of cultured bone cells, which showed that primary cilia are essential for key cellular bone formation and resorption responses to dynamic fluid flow in osteoblasts and osteocytes.20 In addition, primary cilia have been hypothesized to play a role in establishing the orientation of cells and their secreted ECM molecules,21 and thus the orientation of the primary cilium may relate to the establishment of anisotropic ECM organization in skeletal tissues.
Characterization of ciliary incidence and orientation in the native ECMs of skeletal tissues will provide quantitative three-dimensional (3D) data as a baseline for further investigation of the primary cilium as a cellular mechanosensor involved in ECM morphogenesis in connective tissues. In the current study we investigated primary cilia in tendon, a tissue with a highly oriented collagenous matrix ideal for analysis of spatial relationships between primary cilia and the ECM. The objective of this study was to characterize the incidence and orientation of tenocyte primary cilia in their native ECM. We hypothesized that tenocytes would have one primary cilium per cell and that primary cilia would be aligned with the collagen fiber direction in the tendon.
Methods
Tissue Collection
Bilateral digital extensor tendons were collected from 3-week-old male and female Sprague-Dawley rats euthanized by pentobarbital overdose under an IACUC-approved protocol. Tissues were collected from 10 rats for analysis of ciliary incidence and 16 rats for analysis of ciliary orientation. Eight of the rats designated for the incidence analysis were administered bromodeoxyuridine (BrdU) (IP, 25 mg/kg) 2 h prior to euthanasia to label S-phase nuclei, and 2 were injected with saline as non-BrdU controls.22
Immunohistochemistry (IHC)
Primary cilia, tenocyte nuclei, and collagen were characterized in immunofluorescently labeled tendon. Paraffin sections were used for the incidence analysis to ensure antibody penetration throughout the tissue, and whole-mount specimens were used for the orientation analysis to preserve the native tendon ECM.
For the incidence analysis, digital extensor tendons were fixed in 10% formalin, embedded in paraffin, and cut into 5-μm-thick sections. One set of sections was immunolabeled for acetylated α-tubulin; a second set was immunolabeled for BrdU; and a third set was double-labeled for acetylated α-tubulin and BrdU. For the single-label specimens, sections were incubated in anti-acetylated α-tubulin or anti-BrdU primary antibodies and then in Alexa 488 or Alexa 568 secondary antibodies, respectively. For the double-labeled sections, following overnight incubation with the acetylated α-tubulin primary antibody, acid denaturation, and neutralization, the sections were incubated in the anti-BrdU primary antibody. The sections were then serially incubated in Alexa 488 and Alexa 568 secondary antibodies. Finally, all sections were coverslipped in a mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI). Details of these IHC protocols are given in the Supplemental Material.
For the orientation analysis, whole fascicles (∼40 μm thick) were fixed in methanol at 4°C for 2 h, permeabilized, and immunolabeled with a mouse anti-acetylated α-tubulin primary antibody and a fluorescent Alexa Fluor 568 secondary antibody to localize primary cilia as described previously.23 The fascicles were stained with Hoechst 33258 for nuclear visualization. Details of the whole-mount IHC protocol are given in the Supplemental Material.
Multiphoton Microscopy (MPM) and Image Analysis
All specimens were imaged with MPM. In this technique, a fast-pulsed laser raster scanned within the tissue excites both multiphoton fluorescence from exogenous fluorophores and endogenous second harmonic generation (SHG) from arrays of collagen molecules.24 The MPM system, described previously, included a Ti:Sapphire laser tuned to 800 nm.25 For the single-label specimens, three signals were collected by detectors equipped with filters corresponding to UV, blue, and visible emissions: SHG from the collagen (400 nm), blue fluorescence from the Hoechst-stained nuclei, and yellow fluorescence from the Alexa 568-labeled primary cilia.23 For the double-label specimens, three signals were collected by detectors equipped with filters for blue, green, and red emissions: SHG, blue-green fluorescence from the Alexa 488-labeled primary cilia, and yellow fluorescence from the Alexa 568-labeled BrdU. In addition, blue fluorescence from the DAPI-stained nuclei was collected in both the blue and green channels.
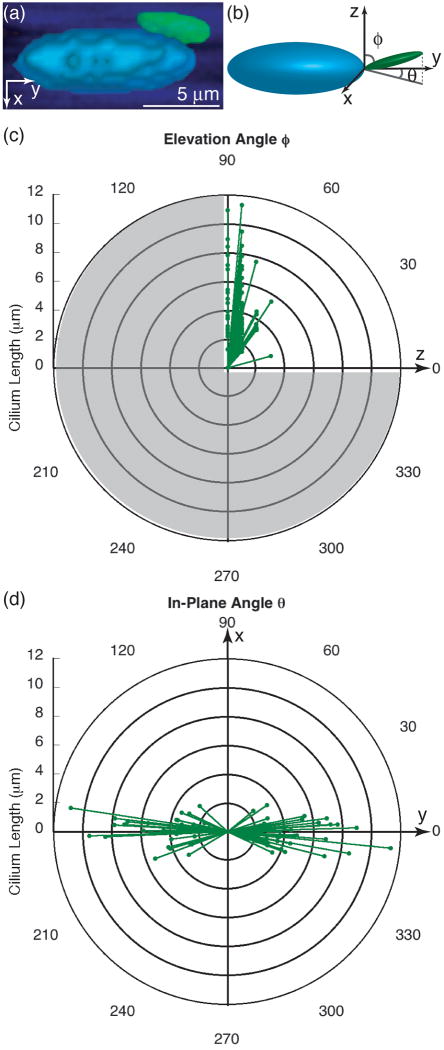
The laser was focused into the tissue in 1-μm steps, generating z-stacks that were deconvolved and reconstructed for 3D visualization (Fig. 1a). Primary cilia and their associated nuclei were tracked throughout the stack. For the incidence analysis, one stack spanning the entire section thickness was collected from each of two sections for each animal. The stacks were analyzed to determine the ratios of a) nuclei with cilia to total nuclei and b) BrdU-labeled nuclei to total nuclei. For the orientation analysis, one image stack spanning the whole fascicle thickness (∼40 μm) was analyzed for each animal using a custom image-processing program in which nuclei and cilia were modeled as ellipsoids and lines, respectively.26 Outcome parameters included the ciliary length in 3D, the in-plane angle θ with respect to the proximal-distal (y) axis, and the elevation angle ϕ with respect to the cranial-caudal (z) axis (Fig. 1b). Details of the imaging are given in the Supplemental Material.
Figure 1.
(a) 3D reconstruction of a multiphoton image stack showing collagen (blue), nucleus (cyan), and primary cilium (green). (b) Schematic of a tenocyte nucleus and primary cilium showing ciliary elevation angle ϕ and in-plane angle θ. Distributions of ciliary (c) elevation angles (0° ≤ ϕ < 90°) and (d) in-plane angles (0° ≤ θ < 360°) showing preferential orientation of primary cilia parallel to the y-axis, which is both the proximal-distal axis of the tendon and the collagen fiber direction.
Results
Primary cilia, tenocyte nuclei, and the collagen ECM were visualized in situ throughout sections and whole fascicles of digital extensor tendon. The SHG signal, as well as the signals from the fluorescently labeled cilia and nuclei, could be detected to a depth of ∼100 μm, often allowing collection of z-stacks encompassing the entire fascicle thickness. In sections and whole fascicles, DAPI-stained ellipsoidal nuclei were observed in rows between the collagen fibers, which appeared bright due to their strong endogenous SHG signal (Fig. 2). The long axes of the nuclei lay parallel to the primary direction of the collagen. The cytoplasmic spaces appeared dark in the multiphoton micrographs due to the absence of the SHG signal from the collagen (Fig. 2, top row).
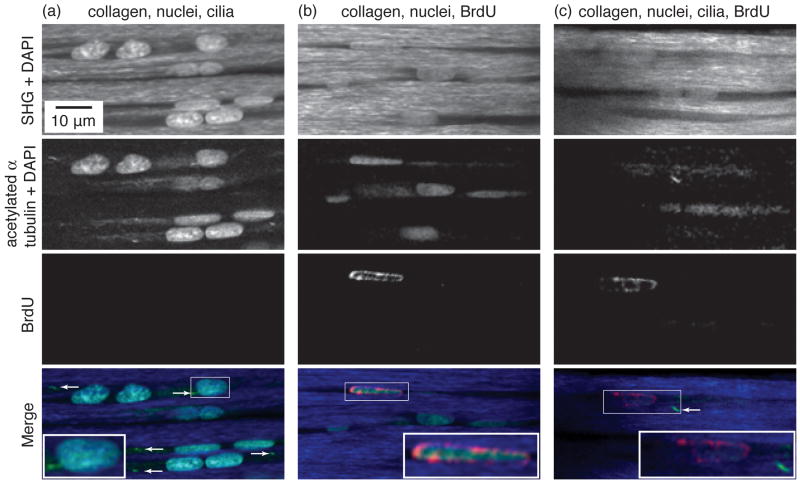
Figure 2.
Multiphoton micrographs of tendon sections immunolabeled for (a) acetylated alpha tubulin (primary cilia), (b) BrdU (S-phase nuclei), or (c) acetylated alpha tubulin and BrdU. The top three rows show the signals from the blue, green, and red detector channels, which arise respectively from endogenous collagen second harmonic generation, Alexa 488-labeled acetylated α-tubulin, and Alexa 568-labeled BrdU. The DAPI signal appears in both the blue and green channels. In the merged images in the bottom row, the collagen, tenocyte nuclei, primary cilia, and S-phase nuclei appear blue, cyan, green, and red, respectively. The insets show enlarged images of regions in the small rectangles.
Primary cilia appeared as bright dots or rods near the tenocyte nuclei in immunofluorescently labeled paraffin sections (Fig. 2a). Diffuse acetylated α-tubulin staining associated with the Golgi was also observed near most nuclei, but the cilia were distinguished by their more intense, focal staining. Cilia often extended from the cytoplasm into the surrounding ECM (Fig. 2a,c). In the analysis of ciliary incidence, the ratio of nuclei associated with a primary cilium to total nuclei was 0.64 ± 0.06 (mean ± SD, n = 687) (Fig. 2a). The ratio of BrdU-labeled nuclei to total nuclei was 0.059 ± 0.24 (n = 241) (Fig. 2b). In the double-immunolabeled sections, BrdU-labeled nuclei were occasionally observed with a primary cilium (Fig. 2c); however, ciliary labeling was poor due to the acid denaturation step required for BrdU labeling, and these sections were excluded from the incidence analysis.
The collagen ECM, tenocyte nuclei, and primary cilia were highly co-oriented (Fig. 3). Three-dimensional reconstruction and analysis of image stacks allowed analysis of ciliary spatial orientation (Figs. 1, 3). The distribution of the ciliary elevation angle ϕ peaked near 90°, indicating that the cilia lay primarily in the x-y (frontal) plane (Fig. 1c). The average elevation angle was 74.5° ± 14.4° (n = 79). The distribution of the in-plane angle θ peaked near 90° and 270°, indicating that the components of the cilia in the x-y plane lay parallel to the y-axis and the primary collagen fiber direction (Fig. 1d). The mean ciliary length was 4.2 ± 2.2 μm.
Figure 3.
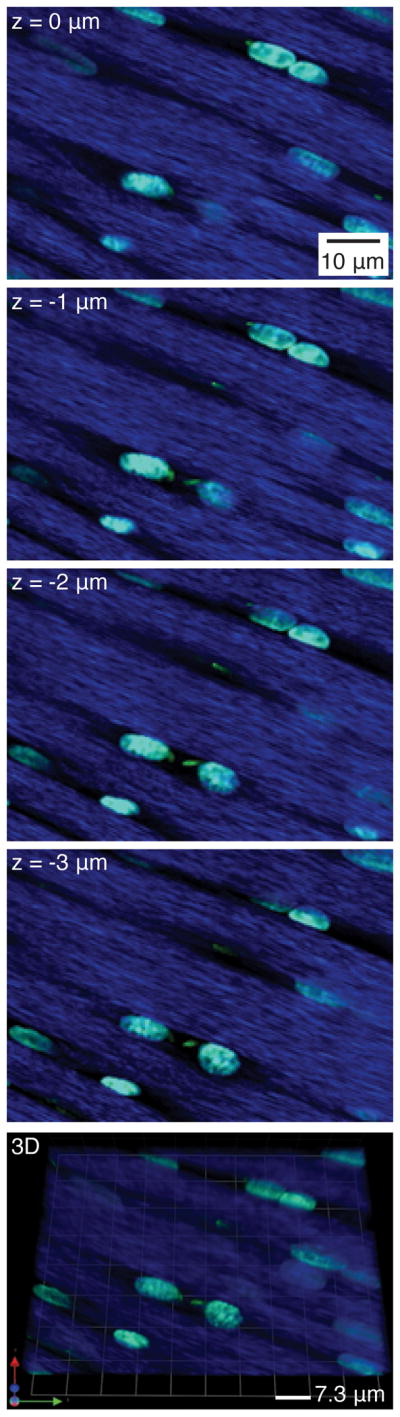
A z-series of a tendon fascicle showing multiple tenocyte nuclei (cyan) and primary cilia (green) in the collagen extracellular matrix (blue). The last panel shows an oblique view of the 3D reconstruction of the image stack.
Discussion
The objective of this study was to characterize the incidence and 3D orientation of primary cilia in native tendon tissue. Consistent with our hypothesis, strong correlations were observed between the in situ alignment of the primary cilium and that of the ECM. Ciliary axonemes of variable length lay in the frontal plane, parallel to the collagen direction and the long axes of the tenocyte nuclei. The observed ciliary incidence of 64% was somewhat lower than hypothesized. This study represents the first in situ quantitative analysis of primary cilia in tendon.
Of the three variables analyzed in the current study – incidence, length, and orientation – only incidence has been analyzed previously in cells of skeletal tissues. A primary ciliary incidence of one per cell was observed in transmission electron microscopic (TEM) analyses of mature murine, equine, and canine articular chondrocytes, and ciliary incidence approaching 100% has been demonstrated for multiple cell types in Go.14, 16, 27, 28 These data motivated our hypothesized incidence of one primary cilium per cell in fully differentiated tissues. The situation is more complex in immature tissues because ciliary incidence depends on the cell cycle, with cilia resorbed prior to mitosis but subsequently reassembled and present throughout most of interphase.29, 30 Our null hypothesis for the current study was that there would be one cilium per tenocyte, and we expected to reject this hypothesis because we analyzed tissues from young animals in which cycling cells were expected to be present. Although it is not possible to calculate cell cycle times or to identify quiescent cells from one pulse of BrdU labeling, our data showed that ∼6% of tenocytes were in the S phase of the cell cycle. The relatively low BrdU labeling index implied either that the cell cycle time was long (>120 hours) or that most tenocytes were no longer cycling. In either case an incidence approaching 100% would be expected, suggesting that cell cycle-related factors cannot fully explain the observed incidence.
In addition to cell-cycle dependence, the ciliary incidence may reflect the local mechanical environment. Recent studies of bovine chondrocytes revealed decreased ciliary incidence in articular regions with greater strain magnitudes31 and in cells cultured in 3D constructs subjected to prolonged compression.32 We observed no systematic spatial variation in ciliated or unciliated cells within the tendon. Although culture conditions are not directly analogous to ours, the incidence of 64% observed here is similar to corresponding values of ∼62% observed in cultured osteoblasts and osteocytes20 and somewhat higher than that of 46% noted in articular cartilage explants.31
Our incidence values may represent a lower bound on the actual incidence. Consistent with studies of other cell types,21, 28, 33 we found that primary cilia were localized close to the nuclei, typically in the same imaging plane. Analysis of MPM image stacks of immunofluorescently labeled whole fascicles showed that primary cilia were generally located <10 μm from the nucleus in the x-y plane and <1 μm from the nucleus in the z direction. To minimize undercounting of ciliated cells in the incidence analysis, we performed the measurements on paraffin sections where antibody penetration was maximized, and we excluded all nuclei that intersected any of the boundaries (top, bottom, or sides) of the imaging volume. Even with these safeguards, some undercounting of ciliated cells was possible in the case of nuclei whose associated cilia were located in a subsequent section. We observed 0-1 “orphan” cilia near the top or bottom of each section, indicating that cilia were rarely located in different sections from their associated nuclei. Because the average number of “orphan” cilia in each section must be equal to the average number of ciliated nuclei that were erroneously counted as unciliated, we can estimate that as many as ∼6% of ciliated cells may have been undercounted due to these factors. Nevertheless, the measured incidence was reproducible and represents a large sample from the digital extensor tendon from similarly aged animals.
A nine-fold range of ciliary lengths (1.3-11.3 μm) was observed in the current study. Few previous studies have analyzed ciliary lengths in normal tissues, and the significance of such variability is unclear. Variability in length may reflect differences in the relative ages of the ciliary axonemes, the shortest being in either the initial stages of formation or the final stages of resorption; and it may also reflect functional differences, e.g., the ability to amplify mechanical inputs. In addition, the ciliary axonemes always appeared to be straight, in contrast to the bent axonemes occasionally seen in primary cilia in epithelial tissues34 and in chondrocytes.17, 26 The extent to which tenocyte ciliary axonemes are capable of bending could be tested in cell culture systems. Additional characterization of tenocyte primary cilia is needed before hypotheses regarding the functional significance of ciliary length and shape can be tested.
A significant contribution of this study is the analysis of the 3D orientation of the ciliary axoneme in tenocytes in their native ECM. The data demonstrate strong correlation between the in situ alignment of the primary cilium with that of the collagen fibers. These results are consistent with a transmission electron microscopic study of embryonic chick flexor tendons, which showed that the ciliary axonemes projected into the ECM and bent so that they lay parallel to the collagen fibers adjacent to the cell surfaces.33 The highly anisotropic ciliary orientation thus mirrored the arrangement of collagen in the ECM, although the cause and effect are unclear. The fluid flow-induced bending response of primary cilia in epithelial cells is well described by a mechanical model of large-angle bending of a cantilevered beam, suggesting that the cilium bends passively in response to external loads.35 Thus, the primary cilia examined here may be aligned with the collagen fibers due to the tensile loading environment. In addition, the ciliary orientation may play a role in determining the orientation of the surrounding ECM. Directionality of collagen secretion arises from an intracellular alignment of collagen fibrils within fibropositiors prior to secretion.36 Tenocytes respond to mechanical stimuli, and their secretion of an oriented ECM is coupled to their mechanosensing ability.37, 38 Primary cilia have been hypothesized to be sensory organelles linking the cellular ability to perceive environmental stimuli to cellular responses through changes in gene expression. This study provides baseline data for future analyses of causal relationships between ciliary alignment and ECM secretion, as well as studies of whether alignment of cilia and surrounding ECM is maintained under conditions known to disrupt the normal tendon organization.
Our approach combining IHC and MPM has several strengths compared to other techniques for 3D characterization of an organelle as small as the primary cilium (0.2 μm diameter). Optical sectioning by MPM allowed tracking of organelles throughout tissue explants and sections with multiple immunofluorescent labels. The efficiency of this approach allowed investigation of a relatively large number of animals (n=16), which would not have been practical with analysis by TEM serial sectioning. In contrast to confocal microscopy, MPM allows collection of signals from the collagen SHG and the immunofluorescently labeled cilia and nuclei. The endogenous SHG signal, which allows visualization of unstained collagen, provides a critical orientation landmark for skeletal tissues, and thus is a key strength of MPM.
One limitation of the current study is that the data are pooled from tissue explants in which the anatomic axes were known during imaging, but the specific anatomic directions were not preserved. For example, the elevation angle is calculated with respect to the known anterior-posterior axis, but it does not discriminate between the anterior vs. posterior directions. Given the tight clustering of the ciliary angles, interpretation of the 3-D orientation of the axonemes is only minimally limited by our inability to map results directly onto the limb. However, this kind of mapping might be significant for tissues that have asymmetric relationships to any limb axis, such as the proximal to distal positioning of articular or growth plate cartilage.
The preferential orientation of the primary cilia observed here coincides with the primary tensile loading direction of the tendon. The current study characterized the normal homeostatic loading environment. Our approach can now be extended to analyze ciliary orientation under altered loading regimes that lead to changes of ECM organization in tendon, testing hypotheses about the possible role of primary cilia as a mechanosensory organelle involved in secreting ECM components in response to mechanical stimuli.
Supplementary Material
Acknowledgments
We thank Dr. Maria Serrat, Barbara Linnehan, and Linell Bigelow for assistance with tissue preparation and Dr. Rebecca Williams and Dr. Karl Kadler for helpful discussions. This work was supported by NIH R21AR053849 to CEF and made use of STC shared experimental facilities supported by the National Science Foundation under Agreement No. ESC-9876771.
References
- 1.Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level: the detection, interpretation, and diversity of responses to mechanical signals. Biochem Cell Biol. 1995;73:349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 2.Buckwalter JA, Martin JA, Brown TD. Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology. 2006;43:603–609. [PubMed] [Google Scholar]
- 3.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166–172. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal S, Long P, Gassner R, et al. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akeson WH, Amiel D, Abel MF, et al. Effects of immobilization on joints. Clin Orthop Relat Res. 1987:28–37. [PubMed] [Google Scholar]
- 6.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 7.Leigh DR, Abreu EL, Derwin KA. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J Orthop Res. 2008;26:1306–1312. doi: 10.1002/jor.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8:97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 9.Marshall WF, Nonaka S. Cilia: tuning in to the cell's antenna. Curr Biol. 2006;16:R604–614. doi: 10.1016/j.cub.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 11.Pazour GJ, Dickert BL, Vucica Y, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 13.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 14.Wilsman NJ. Cilia of adult canine articular chondrocytes. J Ultrastruct Res. 1978;64:270–281. doi: 10.1016/s0022-5320(78)90036-9. [DOI] [PubMed] [Google Scholar]
- 15.Archer FL, Wheatley DN. Cilia in cell-cultured fibroblasts. II. Incidence in mitotic and post-mitotic BHK 21-C13 fibroblasts. J Anat. 1971;109:277–292. [PMC free article] [PubMed] [Google Scholar]
- 16.Wilsman NJ, Farnum CE, Reed-Aksamit DK. Incidence and morphology of equine and murine chondrocytic cilia. Anat Rec. 1980;197:355–361. doi: 10.1002/ar.1091970309. [DOI] [PubMed] [Google Scholar]
- 17.Jensen CG, Poole CA, McGlashan SR, et al. Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int. 2004;28:101–110. doi: 10.1016/j.cellbi.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Z, Zhang S, Mahlios J, et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281:30884–30895. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hellio Le Graverand MP, Ou Y, Schield-Yee T, et al. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525–535. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malone AM, Anderson CT, Tummala P, et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole CA, Jensen CG, Snyder JA, et al. Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol Int. 1997;21:483–494. doi: 10.1006/cbir.1997.0177. [DOI] [PubMed] [Google Scholar]
- 22.Farnum CE, Wilsman NJ. Determination of proliferative characteristics of growth plate chondrocytes by labeling with bromodeoxyuridine. Calcif Tissue Int. 1993;52:110–119. doi: 10.1007/BF00308319. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly E, Williams R, Farnum C. The primary cilium of connective tissue cells: imaging by multiphoton microscopy. Anat Rec (Hoboken) 2008;291:1062–1073. doi: 10.1002/ar.20665. [DOI] [PubMed] [Google Scholar]
- 24.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nature Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 25.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J. 2005;88:1377–1386. doi: 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ascenzi MG, Lenox M, Farnum C. Analysis of the orientation of primary cilia in growth plate cartilage: a mathematical method based on multiphoton microscopical images. J Struct Biol. 2007;158:293–306. doi: 10.1016/j.jsb.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tucker RW, Pardee AB, Fujiwara K. Centriole ciliation is related to quiescence and DNA synthesis in 3T3 cells. Cell. 1979;17:527–535. doi: 10.1016/0092-8674(79)90261-7. [DOI] [PubMed] [Google Scholar]
- 28.Wheatley DN. Cilia in cell-cultured fibroblasts. I. On their occurrence and relative frequencies in primary cultures and established cell lines. J Anat. 1969;105:351–362. [PMC free article] [PubMed] [Google Scholar]
- 29.Rieder CL, Jensen CG, Jensen LC. The resorption of primary cilia during mitosis in a vertebrate (PtK1) cell line. J Ultrastruct Res. 1979;68:173–185. doi: 10.1016/s0022-5320(79)90152-7. [DOI] [PubMed] [Google Scholar]
- 30.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGlashan SR, Cluett EC, Jensen CG, Poole CA. Primary cilia in osteoarthritic chondrocytes: from chondrons to clusters. Dev Dyn. 2008;237:2013–2020. doi: 10.1002/dvdy.21501. [DOI] [PubMed] [Google Scholar]
- 32.McGlashan SR, Chowdhury TT, Joshi P, et al. Chondrocyte primary cilia are mechanosensitive in a duration-dependent manner. Trans Orthop Res Soc. 2009;34 [Google Scholar]
- 33.Poole CA, Flint MH, Beaumont BW. Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil. 1985;5:175–193. doi: 10.1002/cm.970050302. [DOI] [PubMed] [Google Scholar]
- 34.Schermer B, Ghenoiu C, Bartram M, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- 36.Canty EG, Lu Y, Meadows RS, et al. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merrilees MJ, Flint MH. Ultrastructural study of tension and pressure zones in a rabbit flexor tendon. Am J Anat. 1980;157:87–106. doi: 10.1002/aja.1001570109. [DOI] [PubMed] [Google Scholar]
- 38.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.