Abstract
Fast advancements of microfabrication processes in past two decades have reached to a fairly matured stage that we can manufacture a wide range of microfluidic devices. At present, the main challenge is the control of nanoscale properties on the surface of lab-on-a-chip to satisfy the need for biomedical applications. For example, poly(dimethylsiloxane) (PDMS) is a commonly used material for microfluidic circuitry, yet the hydrophobic nature of PDMS surface suffers serious nonspecific protein adsorption. Thus the current major efforts are focused on surface molecular property treatments for satisfying specific needs in handling macro functional molecules. Reviewing surface modifications of all types of materials used in microfluidics will be too broad. This review will only summarize recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology and classify them into two main categories: (1) physical approach including physisorption of charged or amphiphilic polymers and copolymers, as well as (2) chemical approach including self assembled monolayer and thick polymer coating. Pros and cons of a collection of available yet fully exploited surface modification methods are briefly compared among subcategories.
Keywords: Poly(dimethylsiloxane), PDMS, Surface, Microfluidics, Biofouling, Protein adsorption
Introduction
Since the introduction of microelectromechanical system (MEMS) technologies couple decades ago, intensive research efforts have been devoted in the field of microfluidics (Ho and Tai 1998; Stone et al. 2004). At first, silicon and glass based materials were commonly used (Manz et al. 1992; Harrison et al. 1993). Numerous microfluidic components, such as pumps, valves, mixers, and molecules and particles separators and concentrators, have been developed for a broad range of applications including chemistry, biology, physics, and medicine (Auroux et al. 2002; Reyes et al. 2002; Vilkner et al. 2004; Dittrich et al. 2006; West et al. 2008). It is clear that this field has gained enough maturity in the individual component level, as reflected from the number of articles published on this topic in recent years (Abgrall and Gue 2007), and has reached the time to commence an integration of these components into a fully automated system (Whitesides 2006; Haeberle and Zengerle 2007). Technologies already demonstrating multi-components platforms include centrifugal microfluidics (Madou et al. 2006), droplet based microfluidics (Teh et al. 2008) such as electrowetting on dielectric (EWOD, Lee et al. 2002) and two-phase microchannel flow (Song et al. 2006), electrokinetics (Li 2004), and optofluidics (Hunt and Wilkinson 2008).
Yet, silicon and glass based fabrication technologies are fairly time-consuming and require expensive cleanroom usages. On the other hand, poly(dimethylsiloxane) (PDMS) has gained attention for microfluidic applications after two important contributions: soft lithography (McDonald et al. 2000) and microfluidic large scale integration (LSI) (Thorsen et al. 2002). The former was developed for fast fabrication of microchannels while the latter created the possibility for on-chip integration of fluidic elements (valves, mixers, and pumps). The combination of these technologies set a simple pathway to micro total analysis systems (μTAS) technology for a completely integrated and functional system.
Recently, a variety of polymeric materials have been increasingly adopted in producing various microfluidic devices (Quake and Scherer 2000). Commonly used polymers include PDMS (McDonald et al. 2000; Ng et al. 2002; Sia and Whitesides 2003), poly(methyl methacrylate) (PMMA), polycarbonate (PC), poly(ethylene terephthalate) (PET), polyurethane, poly(vinyl chloride) (PVC), and polyester (Shadpour et al. 2006). Techniques commonly used for fabricating polymer microfluidic devices include laser ablation (Roberts et al. 1997), hot embossing (Martynova et al. 1997), injection molding (McCormick et al. 1997), and replica molding (Duffy et al. 1998) and are reviewed by Becker et al. (Becker and Gartner 2000). It is generally agreed that PDMS is among the most popular polymeric materials employed for the fabrication of microfluidic devices owing to a number of advantages: simple fabrication by replica molding, biocompatibility, nontoxicity, excellent optical transparency down to 280 nm, and permeability to gases. In spite of these advantages, the strong hydrophobicity of PDMS surface always impedes PDMS based microfluidic devices from immediate use without any surface processing. The key challenge is the surface fouling problem caused by protein or analyte adsorption on hydrophobic surface enhanced by significant increase in surface to volume ratio in microscale, resulting in low device performance and substantial sample loss. Therefore, it is of key importance to develop efficient surface modification techniques to render PDMS surface protein-resistant.
Extensive amount of works have been performed in developing reliable and reproducible protein-resistant surfaces on various substrate materials. Majority of the works on surface modification of microfluidic device are developed for electrophoresis applications due to the vast exploitation of microchips and demanding stringency on surface properties. Other microfluidic related applications also showed intensive demand on protein resistant surfaces, such as cell culturing and immunoassay (Auroux et al. 2002; Vilkner et al. 2004; Dittrich et al. 2006). Surface modification techniques are basically categorized into physical adsorbed and covalent modifications. Physical adsorbed modification relies on surface bound materials adsorbed via mainly hydrophobic interaction (generally between the hydrophobic PDMS surface and the hydrophobic terminal of, say, amphiphilic molecules or copolymers) or electrostatic interactions while covalent modifications include self assembled monolayer (SAM) and surface grafted polymer chains.
Poly(ethylene glycol) (PEG), also known as poly(ethylene oxide) (PEO), is a well known material for preventing nonspecific adsorption of proteins (Harris 1992; Harris and Zalipsky 1997) as well as its biocompatibility and low toxicity. Besides PEG, many hydrophilic synthetic and natural polymers used for static and dynamic coating in separation science, such as polyacrylamide, poly(vinyl alcohol) (PVA), hydroxylethylcellulose (HEC), poly(N-hydroxyethyl acrylamide) (PHEA), hydroxylpropyl methylcellulse (HPMC), poly(2-hydroxyethyl methacrylate) (pHEMA), poly(vinyl pyrrolidone) (PVP), poly(acrylic acid) (PAA), dextran, hyaluronic acid, and poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) have been derived for physical or covalent surface modifications of the microchannel wall (Doherty et al. 2003; Dolnik 2004, 2006).
Some excellent reviews have documented on surface modification of microchannel made of various silicon-based and polymeric materials for the prevention of biofouling (Doherty et al. 2003; Makamba et al. 2003; Dolnik 2004; Senaratne et al. 2005; Dolnik 2006; Liu and Lee 2006; Pallandre et al. 2006). Yet there are so far limited number of reviews focused on surface modifications of PDMS (Makamba et al. 2003), despite its role as the most extensively exploited polymeric material in the microfluidic community and a bursting number of works published these few years (Muck and Svatos 2007). This review focuses on recent progress on surface modification methods in preparing nonbiofouling coating for PDMS and silica microfluidic devices and is divided into three main categories: surface activation, physical modification, and chemical modification.
Surface activation
Surface activation step is commonly used for cleaning or oxidization of PDMS surfaces to render surface hydrophilic for promoting aqueous solution filling in microchannel and facilitating PDMS microchip bonding. More importantly, surface activation creates reactive silanol functional groups for subsequent surface functionalizations through various surface chemistries including silanization (Ulman 1996), cerium(IV)-catalyzed polymerization (Slentz et al. 2002), UV mediated polymerization (Hu et al. 2002, 2004), plasma induced polymerization (He et al. 2003), and free radical polymerization (Husseman et al. 1999; Edmondson et al. 2004; Tsujii et al. 2006).
Oxygen plasma (Duffy et al. 1998), UV/ozone (Efimenko et al. 2002; Hillborg et al. 2004), and corona discharges (Hillborg and Gedde 1998; Kim et al. 2000) are commonly used for surface activation purpose. Oxygen plasma contains high energy species including electrons, ions, and radicals which strongly oxidize the organic species on the surface. A milder treatment by UV/ozone is through generation of atomic oxygen in a combination of photochemical processes. The 185 nm line produces ozone from molecular oxygen while 254 nm line converts the ozone to atomic oxygen. This reactive species generated by oxygen plasma and UV/ozone attacks the siloxane backbone of PDMS to form oxygen rich SiOx silica-like layer and Si–OH surface structures. The main drawback of these physical modifications is that the oxidized PDMS surface is known to recover its hydrophobicity in just hours after exposure to air (McDonald et al. 2000). A general agreement to this phenomenon is the migration of low molecular weight (LMW) uncrosslinked polymeric chains from the bulk phase to the surface (Hillborg et al. 2004; Chen and Lindner 2007).
Very recently, Eddington et al. (2006) have shown that by removing these LMW species from the bulk phase through thermal aging, hydrophilicity of the oxidized surface can be retained for a much longer time. Another approach to remove the LMW species is through an extraction/oxidation process introduced by Vickers et al. (2006). Cured PDMS was first extracted in a series of solvents to remove unreacted polymer chains from the bulk phase, followed by a plasma oxidation process to generate a layer of hydrophilic SiO2 surface. No noticeable hydrophobic recovery was observed for at least 7 days of storage in air, as evidenced with X-ray photoelectron spectroscopy (XPS) studies.
Apart from oxygen plasma and UV/ozone treatments, hydrophilicity and surface silanol groups of PDMS surface can also be obtained by using a sol–gel method which created an oxide layer on the PDMS channel wall in situ (Roman et al. 2005; Roman and Culbertson 2006). Roman et al. (2005) formed nanometer-sized silica particles uniformly distributed in a cured PDMS piece. The PDMS piece was first soaked and swollen in a solution of sol–gel precursor tetraethyl orthosilicate (TEOS), followed by incubation in an ethylamine catalyzing solution and heated to form nanoparticles inside the PDMS matrix. Besides forming glasslike layer on PDMS surface, the same group also applied similar sol–gel technique with transition metal sol–gel precursors to in situ deposit TiO2, ZrO2, and vanadia coating inside PDMS microchannel (Roman and Culbertson 2006). Contact angle measurements showed a significant reduction of water contact angles of all the modified surfaces indicating that more hydrophilic surfaces were created with this method. Electroosmotic mobility measurements demonstrated that the surfaces were stable for at least 95 days. Moreover, these created inorganic surfaces have the potential to be functionalized with various silane reagents including amine, perfluoro, mercapto, and oligoethylene glycol (OEG) with contact angles of 45°, 120°, 76°, and 23°, respectively, which were stable over a 30-day period.
Recently, a few improvements of this sol–gel method were presented by other groups. To solve the PDMS swelling problem caused by the precursor solution as well as the contraction and cracking problem during the gelation process, Abate et al. (2008) pre-oligomerized sol–gel precursors of TEOS and methyltriethoxysilane (MTES) into higher molecular weight silane oligomers before introducing into oxygen plasma treated PDMS channel. The device was then heated to 100°C to initiate the gelation reaction. The coating efficiently prevents the diffusion of LMW dye (Rhodamine B), suppresses the swelling of PDMS by toluene, and can be functionalized with various silane reagents for specific interfacial applications. On the other hand, it was pointed out that the glasslike layer formed using Roman’s method (Roman et al. 2005) was not covalently bonded to PDMS and was still in the gel form due to relatively low annealing temperature. To further improve the coating for prevention of diffusion and swelling, Orhan et al. (2008) coated the PDMS channel with a borosilicate glass layer using an active solution of alkali-free precursors, TEOS and trimethoxyboroxine (TMB). This active solution was flushed into tetrabutylammonium fluoride (TBAF) oxidized PDMS channel and cured thermally at 160°C. Resulting surface was characterized as a covalently bonded 800 nm-thick crack-free borosilicate glass. Negligible diffusion of Rhodamine B was observed and the swelling of PDMS was effectively prevented during hours of exposure to toluene.
Physical adsorption
Surface modification via physical adsorption is vastly used in capillary electrophoresis (CE) for suppression of electroosmotic flow (EOF) and prevention of protein nonspecific binding due to its tremendous simplicity and efficiency compared to other covalent surface modification methods. The most conventional examples are blocking proteins such as bovine serum albumin (BSA) or milk powder due to their easy and simple preparations. One example is the use of avidin protein to achieve reconfigurable surface wetting properties taking advantage of the reversible nonspecific adsorption of avidin on hydrophobic surface (Deval et al. 2004). However, the problems of fast denaturation over time and lack of useful functional groups limit the application of this approach (Nelson et al. 2003; Shadpour et al. 2006). Various coating materials, with a majority of polymers, have been developed and could be physically adsorbed onto the microchannel surface via hydrophobic or electrostatic interactions. Examples of such materials include surfactants, amphiphilic copolymers, and charged polymers such as polyelectrolytes, polysaccharides, and polypeptides (Doherty et al. 2003).
Nonionic surfactants
Nonionic surfactants can adsorb strongly on hydrophobic surface rendering it hydrophilic and nonionic, thus preventing the interaction between proteins and the surface (Fig. 1). Glass surface which is hydrophilic can be modified with alkylsilane to render the surface hydrophobic prior to surface coating. Brij-35 (PEO-dodecanol) has long been used to minimize surface adsorption of proteins in CE and microfluidic system. Coating process is simply through direct incubation of surface with the aqueous coating solution (Fig. 1a). Brij 35 is then physisorbed onto hydrophobic surface by its hydrophobic alkyl long chain, extruding the PEO hydrophilic ends to the free surface (Towns and Regnier 1991). The surface prepared by Dou et al. (2004) remarkably reduced the adsorption of large protein molecules and was stable in the range of pH 6–12. The long term stability of this coating, measured by successive determination of EOF, however, depended on the 1-h air drying time, without which the surface was unstable. While high ionic strength buffer tended to cause gradual desorption of coating material, neutral buffer provided a more stable environment for this coating.
Fig. 1.
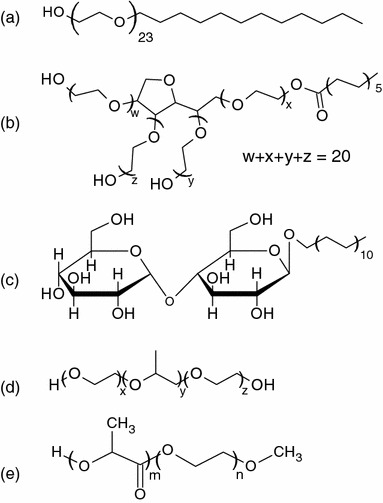
Molecular structures of PEG-copolymers. a Brij 35, b Tween 20, c n-dodecyl-β-d-maltoside (DDM), d pluronic, e poly(lactic acid)-poly(ethylene glycol) (PLA-PEG)
Other commonly used nonionic surfactants such as Tween 20 (Boxshall et al. 2006) (Fig. 1b) and n-dodecyl-β-d-maltoside (DDM) (Huang et al. 2005) (Fig. 1c) have been adopted as well for microchannel coating. Tween 20 is a PEO derivative of sorbitan monolaurate with a hydrophilic PEO head group and an alkyl chain of 12 carbons while DDM is an alkyl polyglucoside. Wall coating with these surfactant molecules demonstrated fairly efficient protein resistance on PDMS substrate though long term stability studies have not been fully documented.
Other amphiphilic PEG-copolymers with various block compositions developed for the modification of biomaterial surface properties in various applications (Tessmar and Gopferich 2007) have also been applied for microchannel coating. Pluronic (Fig. 1d) is a series of powerful and widely used surfactants for dynamic coating in CE. This triblock copolymer of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) can be directly coupled to a variety of hydrophobic polymeric materials through spontaneous surface adsorption of the hydrophobic PPO (Amiji and Park 1992). Stable cell patterning experiments have been demonstrated by Tan et al. (2004) and Liu et al. (2002) using Pluronic F108 on various surfaces, including glass, PDMS, and polystyrene. Hellmich et al. (2005) also investigated the coating of PDMS with Pluronic. PDMS microbioreactor coated with Pluronic to maintain a steady protein level in the culture system was shown to reduce 85% serum protein adsorption compared to native one (Wu et al. 2006b). Poly(lactic acid)–poly(ethylene glycol) (PLA–PEG) (Fig. 1e) is another well-characterized PEG amphiphilic copolymer commonly used in tissue engineering applications (Lucke et al. 2000) and has been shown to be efficient in protein repellency (Salem et al. 2001). Recently, Sinclair and Salem (2006) have successfully demonstrated using microfluidic networks and PLA–PEG–biotin for cell patterning application.
Charged polymer
Positive charged polyelectrolyte such as poly(l-lysine) (PLL) (Blattler et al. 2006), poly(ethylene imine) (PEI) (Brink et al. 1992; Nnebe et al. 2004), and chitosan (Gorochovceva et al. 2005) have been grafted with PEG side chain for preparing nonbiofouling surface via electrostatic adsorption. Poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG) is a polycationic PEG grafted copolymer with a PLL backbone which strongly adsorb onto negatively charged surface in aqueous solution (Fig. 2). Its effective protein repellent properties have been well documented in the literature (Huang et al. 2001; Michel et al. 2002). Recently, Lee and Voros (2005) demonstrated the coating of PLL-g-PEG on oxygen plasma treated PDMS surface and achieved excellent protein resistance and long term stability. The negative charges on the surface generated by oxygen plasma strongly adsorbed polycationic PLL backbone orienting PEG side chain to the aqueous environment creating a very good protein repellent solid/liquid interface. Optical waveguide lightmode spectroscopy (OWLS) and fluorescence microscopy showed excellent protein resistance against 10 successive serum exposure and only trivial amount of serum (98% repelled) was detected after 12 weeks of preservation of the treated surface in HEPES buffer although a clear degradation was observed after exposure to ambient condition. This facile yet reliable aqueous-based coating method is also superior to conventional PEG-silanization process (Papra et al. 2001a, b), as illustrated in the same work. First, the solvent swelling concerns of PDMS when toluene or other organic solvents are used for PEG-silane silanization (Papra et al. 2001b; Lee et al. 2003) can be alleviated with this aqueous-based surface modification. Besides, this method does not suffer from strong surface degradation in neutral HEPES buffer, which was observed in another aqueous-based PEG silanized surface (Papra et al. 2001a).
Fig. 2.
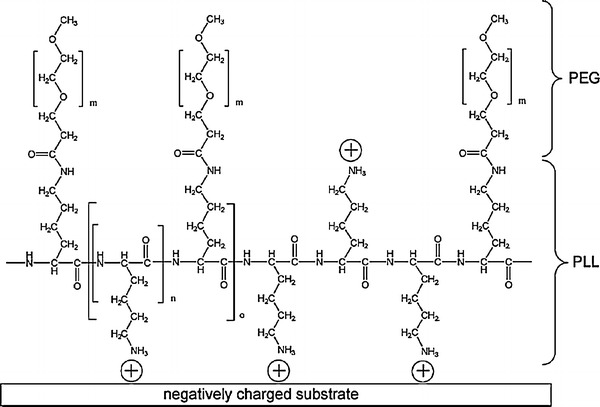
Schematic diagram of the electrostatically adsorbed poly(L-lysine)-g-poly(ethylene glycol) copolymer layer. Reprinted with permission from (Blattler et al. 2006)
Polyelectrolyte multilayer
Despite the simplicity of physical adsorption of molecules for surface modification, long term stability is always difficult to be achieved with this method (Katayama et al. 1998; Dubas and Schlenoff 1999). In another technique utilizing layer-by-layer (LBL) self assembly of polyelectrolyte multilayer (PEM or PEMUs) introduced by Decher (1997), alternating layers of anionic and cationic polymers are electrostatically assembled to create nonbiofouling surface. Figure 3 schematically illustrates the LBL film deposition process. These charged polymers are commonly used for tuning surface charges or control EOF in electrophoresis applications. Polycationic molecules such as Polybrene, polyethyleneimine, poly-(allyamine hydrochloride) (PAH), poly-(diallyldimethylammonium chloride) (PDDA), chitosan, and poly-l-lysine (PLL) can be alternatively adsorbed with polyanionic polymers such as, hyaluronic acid, dextran, and poly(acrylic acid) (PAA) to form the multilayer structure.
Fig. 3.
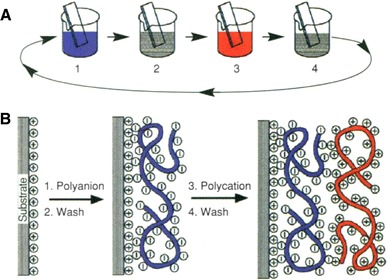
a Scheme of the LBL film deposition process using glass slides and beakers. Steps 1 and 3 represents the adsorption of a polyaninon and polycation, respectively, and steps 2 and 4 are washing steps. The four steps are the basic buildup sequence for the simplest film architecture (A/B)n. The construction of more-complex film architectures requires only additional beakers and a different deposition sequence. b Simplied molecular picture of the first two adsorption steps, depicting film deposition starting with a negatively charged substrate. The polyion conformation and layer interpenetration are an idealization of the surface-charge reversal with each adsorption step. Reprinted with permission from (Decher 1997)
Dubas and Schlenoff (1999) have shown that the adsorption of PEM was not reversible with no spontaneous desorption, yet small extent of desorption by exchange of surface bound polymers with solution took place after a few days, with their PEM model of polystyrene sulfonate (PSS) and poly(diallyldimethyl-ammonium chloride) (PDA) on silicon dioxide surface. Moreover, owing to the fact that PEM surface was strongly dependent on the adsorption conditions and the polyelectrolytes itself and weakly on original substrate surface characteristics, very similar surface properties can be obtained on different substrates treated with the same PEMs (Decher 1997).
The LBL self assembly of polyelectrolytes on a charged surface offers another route of grafting PEG onto substrates. Boulmedais et al. (2004) constructed protein resistant PEM with PEG-derived polypeptides: PLL-g-PEG (Huang et al. 2001; Ruiz-Taylor et al. 2001) and poly(l-glutamic acid)-graft-PEG (PGA-g-PEG). Adhesion of protein was effectively reduced and bacterial adhesion was reduced by 92% with only three bilayers as compared to a glass substrate. PLL-g-PEG was also used as a final topping layer of PEMs on silica surface (Heuberger et al. 2005). Multilayers of poly(allylamine hydrochloride)/poly(styrene sulfonated) (PAH/PSS) were first prepared on oxidized silicon oxide surface. A final layer of PLL-g-PEG is then spontaneously assembled on top. Full serum adsorption on PLL-g-PEG topped PEMs decreased by three orders of magnitude compared to PEMs without the PLL-g-PEG topping layer.
Bovine serum albumin, a well known protein blocking agent, has also been exploited for surface modification with LBL technique. In one example, BSA was applied for PEM preparation with heparin (Tan et al. 2005). Positively charged BSA at pH 3.9 was assembled with heparin on ammonia plasma treated PVC substrate followed with chemical crosslinking with glutaraldehyde for additional stability. The surface showed efficient resistance to platelet adhesion with no thrombus forming. BSA was also applied as a top layer on PEM for PDMS surface modification. Wang et al. (2006a) coated BSA on PEM of chitosan and citrate-stabilized gold nanoparticles to prevent protein adsorption.
Covalent modifications
Modifying the surface via physisorption is experimentally simple and quick, however, these surface always suffer from thermal, mechanical, and solvolytic instabilities due to their weak interactions with the bottom substrate. Covalent modifications with SAM or polymer brushes tethering could improve these inherent difficulties for more surface robustness.
SAM
Self-assembled monolayers (SAMs) are prepared by spontaneous tethering of molecules with active chemical moieties onto reactive solid surfaces. Due to its ease of preparation, low cost, and versatility (Ulman 1996), the field of SAMs has attracted enormous research interests dedicated to many disciplines (Wink et al. 1997; Fendler 2001; Whitesides et al. 2001; Senaratne et al. 2005). Typical examples of SAM systems are organosilane species on oxidized glass, PDMS, metal oxides, and organosulfur (thiol-derivates) on noble metals, grown in either liquid or vapor phase. Many excellent reviews have been published related to chemistries and preparations of SAMs on various surfaces, as well as countless publications dedicated on patterning of SAMs for various bio and nano applications (Ulman 1996; Whitesides et al. 2001; Gooding et al. 2003; Gates et al. 2005; Love et al. 2005). We will focus this section on recent progresses of applying SAMs for surface passivation purposes in microfluidic devices.
In general, trichlorosilanes, triethoxysilanes, and trimethoxysilanes derivates bearing functional groups at the other end of the molecule are regularly used for SAM coating on glass and PDMS surfaces with basically siloxane backbones (Ulman 1996). OEGn alkylsilanes (Lee and Laibinis 1998) was demonstrated in the early works of applying SAMs for biofouling purposes on glass surfaces. Figure 4 shows the schematic illustration of grafting SAM of PEG thin layer on silicon surface. Papra et al. (2001a, b) have coated the PDMS and glass microchannel with SAMs of commercially available PEG-silane to increase the hydrophilicity and protein-resistance for assisting microfluidic networks (μFNs) protein patterning. Besides forming PEG SAMs on oxidized Si/SiO2 surface, Cecchet et al. (2006) prepared PEG SAMs surface with excellent protein repellency through self assembly of poly(ethylene glycol methyl ether) (MPEG) film onto hydrogen-passivated silicon surfaces (Si–H) at elevated temperatures, leading to a formation of Si–O–C bonds between the substrate and the organic layer.
Fig. 4.
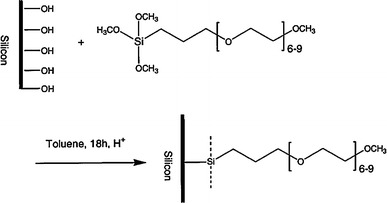
Scheme for grafting a hydrophilic PEG layer onto silicon surface. Reprinted with permission from (Papra et al. 2001b)
In a common practice, an oxidation/activation step on glass and PDMS surface (e.g. oxygen plasma, UV/ozone, or Piranha) is needed to generate surface silanol (Si–OH) groups before the SAM grafting (Makamba et al. 2003). An in situ approach for performing oxidation step inside assembled PDMS microchannel was introduced by Sui et al. (2006). Acidic H2O2 solution was passed through the PDMS microchannel for oxidation purpose, followed with a sequential silanization process by injecting neat PEG-silane solution into the microchannel. This approach alleviated the use of specialized instruments for surface activation and post-assembly process after silanization.
Besides implementation of SAMs as single functional layer on the surface, it also plays a pivotal role as anchoring sites for further attachments of polymer chains or for initiation of surface confined polymerization (Schreiber 2000). Examples include silane reagents with amino, epoxide, mercapto, vinyl, aldehyde, bromo, and phenyl functional groups for further surface conjugation with corresponding functional polymers (Huang et al. 2006; Janssen et al. 2006).
Covalent polymer coatings
The advantage of covalent polymer coatings over other surface modification methods, (e.g. SAM and physisorption) is the superior mechanical and chemical robustness, coupled with a high degree of synthetic flexibility towards the introduction of a variety of functional groups (Pallandre et al. 2006). The pioneering work of grafting polymer on surface to reduce protein adsorption for improving CE was introduced by Hjerten (1985), who used surface vinyl groups introduced by vinyl-silane SAMs on silica to graft linear polyacrylamide chains. The covalent polymer coating techniques are commonly classified into two main categories: “grafting-to” and “grafting-from” (Zhao and Brittain 2000).
“Grafting-to” polymer coating
In “grafting-to” method, end-functionalized polymers or block copolymers are covalently tethered onto reactive anchoring layer on the surface (e.g. functional groups introduced by SAM). Several approaches for preparing surface anchoring layers were employed, such as silanization (Hjerten 1985) and Grignard chemistry (Cobb et al. 1990). Early demonstrations of nonfouling surface modifications by “grafting-to” techniques are the works by Effenhauser et al. (1997), who grafted PEG and carbohydrates onto silanized fused silica surface to prevent protein adsorption.
Direct grafting of functionalized PEG on silica surface was also demonstrated by Harris et al. (Osterberg and co-workers 1995; Emoto et al. 1998). Epoxide-functionalized PEG molecules were covalently grafted onto aminosilane modified quartz capillary surface (Fig. 5) and showed very efficient protein repellency against fibrinogen. PEG-derived with other functional groups have also been linked onto corresponding reactive silane functionalized glass surface. Recent examples include amine-PEG on aldehyde-silane (Schlapak et al. 2006) and alkyne-PEG on azide via click chemistry (Prakash et al. 2007). Other functionalized hydrophilic polymers have also been grafted onto PDMS microchannel. Wu et al. (2006a) modified hydrophilic polymers (PVA and PVP) with epoxy functional monomer, glycidyl methacrylate, for covalent attachment onto aminosilane treated PDMS surface in aqueous solution. Surface adsorption of lysozyme and BSA was reduced to less than 10% relative to native PDMS surface. This method was further simplified recently by Wu et al. (2007). Epoxy-modified polymers were directly adsorbed onto oxygen plasma treated PDMS surface via hydrogen bond, followed by a thermal treatment at elevated temperature to crosslink the polymer with the surface silanol groups. Effective suppression on protein adsorption was also demonstrated.
Fig. 5.
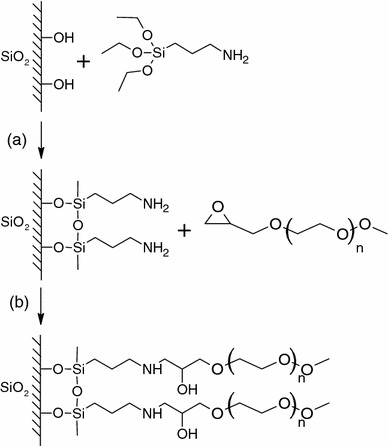
Schematic of grafting PEG onto quartz surface. Step a illustrates the reaction of quartz surface with aminopropyl triethoxysilane (APS). Step b shows the reaction of APS derived surface with PEG. Modified with permission from (Emoto et al. 1998)
Polysaccharides such as dextran (Osterberg et al. 1995) as a potential alternative to PEG as nonfouling materials was grafted onto aminosilane functionalized glass surface (Martwiset et al. 2006). Different ratios of hydroxyl groups in dextran have been converted by sodium periodate (NaIO4) to aldehyde groups which covalently attach surface amine groups. It was found dextran with ~25% conversion of hydroxyl to aldehyde groups provides the best nonfouling surface with negligible protein adsorption whereas molecular weight of dextran does not play an important role in affecting the nonfouling characteristics. The protein resistant dependence on the relative amount of hydroxyl and aldehyde groups on the molecules implied the importance of interactions between surface and water molecules on protein adsorption.
End-functionalized PEG has also been grafted onto PEMs to passivate PDMS channel. Makamba et al. (2005) covalently topped end-functionalized PEG on PEMs of polyethyleneimine (PEI) and poly(acrylic acid) (PAA) crosslinked via carbodiimide coupling between carboxyl and amine groups of the PEM molecules for enhancing the stability of PEM. The treated PDMS surface presented excellent resistance to protein adsorption and high stability against hydrophobic recovery.
Thermal immobilization of polymers onto surface have been presented in the early work of Gilges et al. (1994), who grafted poly(vinyl alcohol) (PVA) onto fused silica capillary surface at elevated temperature without any precoated surface anchoring layer. This water-insoluble permanent coating was stable over a wide range of pH and effective for preventing protein adsorption. Recently, this technique was applied by Wu et al. (2005) to coat PDMS surface with multilayer of partially hydrolyzed (88%) PVA. This method produced a stable, hydrophilic coating on PDMS surface which substantially prevent both acidic and basic proteins, suppress EOF to a negligible value in the range of pH 3–11. Other PVA immobilization methods such as silanization and Grignard chemistry were also demonstrated elsewhere (Moritani et al. 2003).
Despite the simplicity of this “grafting-to” method, it usually suffers from low grafting density due to kinetic hindrance from the grafted polymer film (brushes) at the surface against new coming polymer chains, thus obstructing further attachment (Mansky et al. 1997). Moreover, film thickness is limited by the molecular weight of the functionalized polymer.
“Grafting-from” polymer coating
A powerful alternative for preparing thicker and denser polymer brush is the “grafting-from” technique [also known as surface initiated polymerization (SIP)], in which polymerization of monomers starts from the surface-anchored initiation sites (e.g. created by SAM) compared to the “grafting-to” case, in which prepolymerized polymers with functional groups are attached on the reactive surface. In this way, monomers added to the growing polymer chains from the surface do not endure considerable molecular hindrance, and thereby a thick and dense layer of polymer brushes can be formed.
Free radical polymerization
Early demonstrations of “grafting-from” technique were mostly based on conventional free radical polymerization (Prucker and Ruhe 1998a, b). The free radicals generated mostly photochemically or thermally from the surface immediately attack the double bonds of monomers (such as vinyl groups in methacrylate monomers) and add them to the growing chain. This process at the same time results in the formation of a new radical at the other end of the monomer for successive polymerization until two free radicals meet each other to quench the reactions. Ideally, polymerization is only confined at the surface, not in the solution.
Hu et al. (2002, 2004) demonstrated a simple one step UV mediated polymerization technique to coat PDMS surface with various polymers. Figure 6 schematically illustrates the UV graft-polymerization process. PDMS pieces immersed in aqueous solution containing monomer, sodium periodate, and benzyl alcohol were irradiated with UV source which generated surface radicals to initiate polymerization. Sodium periodate served as an oxygen scavenger and benzyl alcohol helped the diffusion of reactive monomers to the surface by decreasing solution viscosity. Hydrophilic polymer such as PEG monomethoxy acrylate (PEGMA) has been successfully coated onto the PDMS surface which substantially prevented the protein adsorption. Hu et al. (2004) further modified this method for in situ surface coating inside assembled PDMS channel. A photoinitiator, benzophenone, in acetone solution was first adsorbed onto the PDMS channel wall prior to filling the channel with monomer solutions. The adsorbed photoinitiator substantially accelerated the polymerization rate on the surface relative to that in solution in order to avoid the gel formation in the solution which may clog the channel. Thinner coating and similar surface quality relative to the former method can be achieved yet under much shorter UV irradiation exposure time. Recently, the same group used similar polymerization process to coat the surface of SU-8 (Wang et al. 2006b). With a proper curing time of SU-8, sufficient photoinitiator remained within cured SU-8 polymer for initiating surface polymerization under UV exposure. Native epoxide functional groups on SU-8 surface can covalently adsorb biomolecules by reacting with the free amino groups and resulted in strong nonspecific protein adsorption. However, surface grafted with PEG methyl ether acrylate efficiently reduced protein adsorption to a negligible extent compared to native SU-8 surface.
Fig. 6.
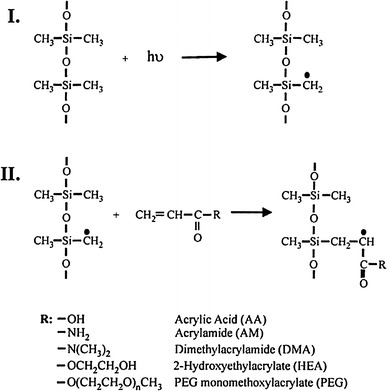
Reaction Scheme of UV graft-polymerization on PDMS surface. Step I illustrates the formation of radicals on PDMS surface by UV light. Step II illustrates the initiation of the polymerization reaction. R is the monomer side groups. Reprinted with permission from (Hu et al. 2002)
Besides PEG, poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC), a phospholipid polymer comprising a methacrylate monomer and a zwitterionic phosphorylcholine head group in the side chain, has shown excellent resistance against nonspecific protein adsorption (Ishihara et al. 1990, 1998) and has been grafted onto various substrates. Goda et al. (2006) grafted PMPC onto PDMS surface with a similar approach as Hu et al. (2004) by immobilizing benzophenone on PDMS surface followed by UV mediated polymerization of monomers from the surface. Protein adsorption on the grafted PDMS surface decreased 50–70% compared to the unmodified PDMS surface. Comparative friction experiments revealed the presence of a highly hydrated thick water layer around the polymer chains is responsible to the reduction of protein adsorption. A recent example of using the UV mediated polymerization approach developed by Hu et al. (2004) was to create reversible bio-fouling/nonfouling surface using poly(N-isopropylacrylamide) (PNIPAAm) (Ebara et al. 2006). PNIPAAm was demonstrated earlier by Huber et al. (2003) to thermally switch between an antifouling hydrophilic state and a protein-adsorbing hydrophobic state. Ebara et al. further extended the use of this reversible surface to control the capture and rapid-release of PNIPAAm-grafted nanobeads. This chromatography system can find various useful applications in immunoassays and enzyme bioprocesses.
Another fast growing surface modification technique worth discussing is plasma-based polymerization. Advantages of this technique include: (1) modification limited to material surface without altering bulk properties, (2) low amount of waste and byproduct compared to wet chemistry, (3) relatively fast deposition rate, and (4) versatility of the method to use different kinds of monomer for a wide range of surface applications (Barbier et al. 2006). Bodas et al. (Bodas and Khan-Malek 2006; 2007) investigated hydrophilic stability of plasma treated polymerization on PDMS surface using hydrophilic monomer 2-hydroxyethyl methacrylate (HEMA). To graft the polymer onto the surface, HEMA monomer solution was spin coated onto plasma treated PDMS surface, followed by oxygen plasma to crosslink the polymer. Hydrophobic recovery test showed an increase in contact angle from 7° to 49° in 2 weeks. Similar oxygen plasma polymerization process was used to graft copolymer of HEMA and acrylic acid (AA) onto PDMS surface (Karkhaneh et al. 2007). O2 plasma treated PDMS surface was immersed in HEMA/AA monomers to allow the monomer to adsorb on the surface before oxygen plasma polymerization. Hydrophobic recovery test revealed that higher HEMA ratio in the mixture yielded a higher contact angle owing to the replacement of hydroxyl groups in AA by methyl groups in HEMA to minimize surface energy.
Besides grafting hydrophilic polymer layer on the surface using plasma, several works have been demonstrated to generate surface functional groups which can be used for further reactions. He et al. (2003) demonstrated a two-step process to generate cyano (CN) functional groups on PDMS surface with long term surface hydrophilic stability. Mildly activated PDMS by microwave plasma in a mixed gas of Ar and H2 was immersed in acrylonitrile solution to generate the hydrophilic functionalities on the surface. The grafted surface exhibited a low water contact angle and was stable at 35° ± 15° for at least 1 month at room temperature. Nitrile groups were also formed on PDMS by Bae et al. (Bae and Urban 2004), who used microwave plasma to graft imidazole and its alkyl-derivatives onto PDMS surface. Pruden et al. (Pruden and Beaudoin 2005), on the other hand, have attempted to modify PDMS surface with primary amine groups using microwave ammonia plasma treatment. A variety of nitrogen containing groups were formed in the reaction with a higher preference of producing amine groups over oxygen groups at higher plasma power, longer reaction time, and higher temperature. Functionalized dextran was also shown to be successfully attached to the primary amine sites.
Despite the versatility and efficiency of free radical polymerization, the main drawback of free radical polymerization is the lack of control of chain length and chain length distribution of the polymer layer, forming branched and highly polydisperse polymer layer (Lou et al. 2006). Furthermore, the polymerization reaction is limited by the initiator efficiency decrement due to the so called cage effect when the primary free radicals recombines forming macroinitiators with increasing molecular weight (Riess 2003). Moreover, no clear experimental evidence has yet reported confirming structure and properties specific to high-density brushes, suggesting that the achieved graft density may still be in a low grafting density regime (Tsujii et al. 2006).
Living radical polymerization
Living radical polymerization (LRP) or controlled radical polymerization (Husseman et al. 1999; Edmondson et al. 2004; Lou et al. 2006; Tsujii et al. 2006), on the other hand, has attracted substantial attention in surface chemistry in recent few years. There are a number of advantages of LRP over conventional free radial polymerization: accurate control on the brush density, composition, and polydispersity, regulated formation of block copolymers on the surface, and allowing polymerizing a wide range of functional monomers. LRP basically relies on a continuous activation/deactivation process of surface-anchored dormant chains immobilized via silane self-assembled on glass surface. Activated polymer chains (capping agents removed), in the presence of monomers, propagates for polymerization until it is randomly deactivated back by the capping agents. Since all chains experience equally frequent activation-deactivation cycles over a long time scale, a slow and nearly simultaneous growing is experienced by all chains, thus producing a low polydispersity polymer brushes. Various capping agents are used for LRP. Examples are halogens with transition metal catalysts for atom transfer radical polymerization (ATRP) (Ejaz et al. 1998; Matyjaszewski et al. 1999), nitroxides for nitroxide mediated radical polymerization (NMP) (Husseman et al. 1999), and dithioester chains for reversible addition-fragmentation chain transfer (RAFT) (Baum and Brittain 2002).
Atom transfer radical polymerization is among the most commonly used LRP technique for SIP due to its compatibility with wide selection of functionalized monomers, easier synthesis of surface-immobilized initiators (i.e. halogen silane) compared to other LRP methods, and mild reaction conditions (Jones et al. 2002). Figure 7 schematically illustrates ATRP graft-polymerization process. The reaction involves reversible transfer of a halogen capping agent from the surface bound initiator to the metal catalyst (activating/deactivating agent) in solution. Upon de-capping the halogen atom from the initiator, chain end radical serves as the initiation site for subsequent polymerization until halogen atom caps back to terminate the propagation.
Fig. 7.
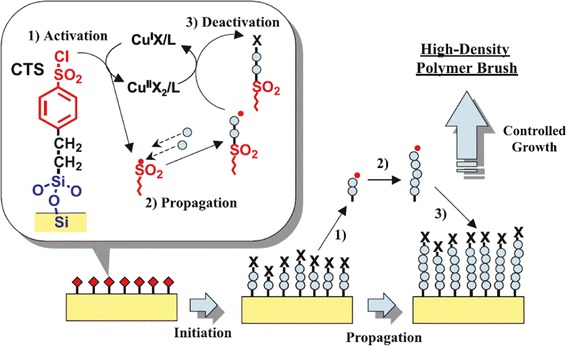
Schematic illustration of surface initiated atom transfer radical polymerization (ATRP). Reprinted with permission from (Tsujii et al. 2006)
In order to achieve a controlled polymerization process via reversible capping (or deactivation) of the growing chains, the very low overall concentration of halogen capping agents released from surface to solution compared to that of the monomer is insufficient. One approach was by adding extra amount of halogen initiator to the monomer solution to increase the capping agent concentration, as introduced in the work of Ejaz et al. (1998). However, increased amount of nontethered polymer chains formed in the solution then has to be removed in a rinsing step. The other approach reported by Matyjaszewski et al. (1999) was by adding appropriate amount of metal deactivating agents prior to polymerization to increase the frequency of deactivation. This approach eliminated the final rinsing step.
Early demonstrations of ATRP suffered from the slow polymerization rate and limited film thickness (<100 nm), owing to the control nature of the polymerization process. Room temperature ATRP (Jones et al. 2002) in aqueous media employed by Huang et al. (2002) accelerated ATRP by incorporating water in the monomer solution. This aqueous reaction produced 700 nm of poly(2-hydroxyethyl methacrylate) (pHEMA) in just 12 h.
Oligo(ethylene glycol) methacrylate (OEGMA) have been recently grafted onto silicon surface by Huck’s group (Brown et al. 2005) with surface initiated ATRP in aqueous solution (Fig. 8). Oxidized silicon wafer was first silylated with ATRP initiator 2-bromo-2-methyl-propionic acid 3-trichloro-silanyl-propyl ester. Oxygen free aqueous solution of OEGMA monomers, metal activator CuICl and deactivator CuIIBr2, and ligand 2,2′-bipyridine (bpy) were then added to the silanized silicon substrates to allow polymerization at 30°C. The grown polymer brushes very effectively inhibited protein adsorption. Feng et al. (2005a) also grafted p(OEGMA) on silicon surface in a similar approach. Due to the importance of grafting density to the performance of inhibiting protein adsorption (Andruzzi et al. 2005), they further characterized the effects of reaction solvents on the graft density of poly(oligo(ethylene glycol) methacrylate) [p(OEGMA)] grown with ATRP. The higher graft density of the polymer brushes prepared in methanol solution than that prepared in water/methanol mixture was correlated to the conformation and hydrodynamic radius of the p(OEGMA) in corresponding solvents. The expanded chain coils in the presence of water limited the diffusion of catalyst and monomers to the surface initiation sites, thus lowering the graft density.
Fig. 8.
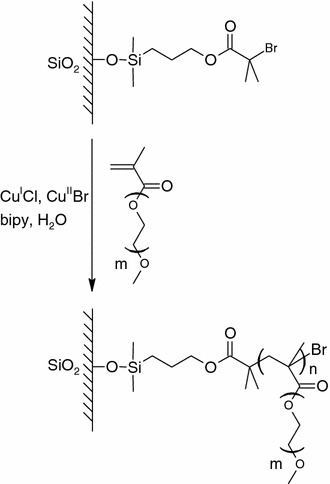
OEGMA brushes grown by atom transfer radical polymerization (ATRP). Modified with permission from (Brown et al. 2005)
Recently, Xiao et al. performed surface initiated ATRP to graft poly(acrylamide) on PDMS surface (Xiao et al. 2002) for fabrication of PDMS CE microchip (Xiao et al. 2004). ATRP initiator was first immobilized by vapor deposition of (1-trichlorosilyl-2-m-p-chloromethylphenyl) ethane onto UV/ozone oxidized PDMS surface. The silanized channel was then filled with oxygen free polymerizing solutions containing acrylamide monomer, Cu(I)Cl, Cu(II)Cl2, and Tris[2-(dimethylamino)ethyl]amine and polymerization was allowed to proceed. The grafted surface exhibited a 20-fold improvement in resisting irreversible adsorption of lysozyme compared to bare PDMS. The grafted surface maintained the hydrophilicity for at least 1 month.
Poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) zwitterionic polymer brush was also grafted on silicon surface with ATRP in room temperature (Feng et al. 2005b). The adsorption of fibrinogen and lysozyme on the modified surface was found to decrease with increasing chain length or layer thickness of the PMPC grafts, which was in turn controlled by the ratio of monomer and free initiator concentration in the solution. With a chain length of 200 U, more than 98% of protein adsorption was reduced compared to unmodified silicon surface. The surface was further characterized to show a strong correlation between fibrinogen adsorption and grafting density (Feng et al. 2006). Another example of growing zwitterionic polymer brushes was demonstrated by Zhang et al. (2006), who produced homopolymer brushes of poly(sulfobetaine methacrylate) (pSBMA) and poly(carboxy-betaine methacrylate) (pCBMA) with surface initiated ATRP to create highly nonfouling surface on glass slides. The reduction of fibrinogen and cell adhesion on these surfaces was shown to be comparable to PEG-like films.
Similar to ATRP in which a halogen atom serves as the capping agent, NMP is based on the use of a nitroxide living group as the reversible capping agents to control the polymerization process. This was first demonstrated by Husseman et al. (1999), who prepared polystyrene brushes on Si surface silylated with alkoxyamine initiator bearing nitroxide functional group (Fig. 9). At elevated temperature the alkoxyamine moiety was cleaved giving off free nitroxide capping agent (known as TEMPO) to the solution while leaving the chain end with an acryl group for subsequent polymerization. Similar to ATRP, extra amount of alkoxyamine initiator was added to the monomer solution to control the polymerization. One advantage of NMP over ATRP is that NMP does not involve metal catalysts which may be difficult to be removed from the polymerization products and therefore may cause undesirable effects in many biological applications (Youngblood et al. 2003). Very recently, Andruzzi et al. (2005) produced highly protein resistant OEG contained styrene-based homopolymer and block copolymer on SiOx surfaces with surface initiated NMP. These polymer brushes presented a superior ability to inhibit cell and protein adsorption compared to SAMs of short OEG, attributed to the greater thickness and surface coverage of polymer brushes compared to SAMs.
Fig. 9.
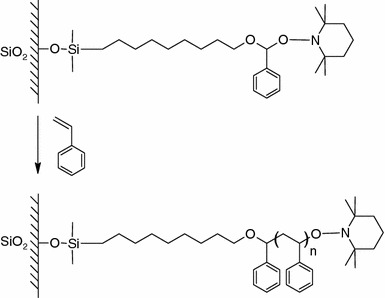
Schematic illustration of polystyrene brushes grown by nitroxide mediated radical polymerization
Conclusion
The increase of using polymeric materials, especially PDMS, becomes the recent trend of fabricating microfluidic devices due to their unique bulk and surface properties and ease of fabrication. With the substantial increase of surface-to-volume ratio in micro scale, careful surface nano-scale treatment is of vital importance to render devices into practical use. One mostly encountered practical issue is the nonspecific protein adsorption on PDMS surface due to its hydrophobic nature.
This review summarizes surface modification methods published recently in constructing nonbiofouling PDMS surfaces under both physical and chemical means. Physical modification, relying basically on hydrophobic or electrostatic surface interactions, is simple to apply and can be employed in applications where long term chemical or mechanical stability is not a concern. When chemical modification is used, SAM can be applied as final functional layer or as an intermediate anchor layer for subsequent polymer grafting. Polymer grafting can be classified into two categories: grafting-to and grafting-from. “Grafting-to” is a relatively simpler method, supported with a large collection of commercially available chemicals. It can be used in general situation to create nonbiofouling layer coupled with various functional groups where defects in surface homogeneity and uniformity do not significantly matter in practice. “Grafting-from” can be applied where thickness, homogeneity, and chemical and mechanical robustness are highly desired. In additional to the aforementioned approaches where only hydrophilic interfaces were created for the anti-biofouling purpose, another promising approach currently under intensive investigation is the nanostructured superhydrophobic surface (Genzer and Efimenko 2006). These surfaces have been demonstrated to be very effective in suppression of protein adsorption. One possible reason is attributed to a decreased contact area between protein molecules and nanostructures which brings less opportunity for protein molecules to adhere to the surface unless they deform (Sun et al. 2005). Another reason may be due to a greater interfacial slip between the superhydrophobic surface and the liquid, which creates stronger shear stress in flow condition to ease the protein removal (Koc et al. 2008). Besides using surface chemistry approach to tune surface properties of polymeric materials, bulk chemistry approach by modifying composition of polymers to acquire specific surface properties is also progressively receiving attention recently (Muck and Svatos 2007) and may in the future become as handy as surface chemistry.
This work reviews recent progresses of surface chemistry applicable for lab-on-a-chip applications and is intended to serve as a reference for choosing an appropriate and technically feasible method for specific applications. It is obvious that these discussed surface modification concepts are not limited to constructing nonbiofouling surfaces on PDMS material. With an understanding of these surface modification concepts, unique surface properties (e.g. hydrophobicity, surface charge) and functionalities can then be achieved on different substrate material by selecting appropriate methods and reagents for the modification. Realizing proper nanoscale surface molecular property modification is essential to achieve desired microfluidic operations.
Acknowledgments
This work is supported by Center for Scalable and Integrated Nano Manufacturing (SINAM) Center (NSF DMI-0327077) and Center for Cell Control (CCC) (NIH 5 PN2EY018228).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Abate AR, Lee D, Do T, et al. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip. 2008;8:516–518. doi: 10.1039/b800001h. [DOI] [PubMed] [Google Scholar]
- Abgrall P, Gue AM. Lab-on-chip technologies: making a microfluidic network and coupling it into a complete microsystem—a review. J Micromech Microeng. 2007;17:R15–R49. [Google Scholar]
- Amiji M, Park K. Prevention of protein adsorption and platelet-adhesion on surfaces by PEO PPO PEO triblock copolymers. Biomaterials. 1992;13:682–692. doi: 10.1016/0142-9612(92)90128-b. [DOI] [PubMed] [Google Scholar]
- Andruzzi L, Senaratne W, Hexemer A, et al. Oligo(ethylene glycol) containing polymer brushes as bioselective surfaces. Langmuir. 2005;21:2495–2504. doi: 10.1021/la047574s. [DOI] [PubMed] [Google Scholar]
- Auroux PA, Iossifidis D, Reyes DR, et al. Micro total analysis systems. 2. Analytical standard operations and applications. Anal Chem. 2002;74:2637–2652. doi: 10.1021/ac020239t. [DOI] [PubMed] [Google Scholar]
- Bae WS, Urban MW. Reactions of antimicrobial species to imidazole-microwave plasma reacted poly(dimethylsiloxane) surfaces. Langmuir. 2004;20:8372–8378. doi: 10.1021/la048916x. [DOI] [PubMed] [Google Scholar]
- Barbier V, Tatoulian M, Li H, et al. Stable modification of PDMS surface properties by plasma polymerization: Application to the formation of double emulsions in microfluidic systems. Langmuir. 2006;22:5230–5232. doi: 10.1021/la053289c. [DOI] [PubMed] [Google Scholar]
- Baum M, Brittain WJ. Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique. Macromolecules. 2002;35:610–615. [Google Scholar]
- Becker H, Gartner C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis. 2000;21:12–26. doi: 10.1002/(SICI)1522-2683(20000101)21:1<12::AID-ELPS12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Blattler TM, Pasche S, Textor M, et al. High salt stability and protein resistance of poly(L-lysine)-g-poly(ethylene glycol) copolymers covalently immobilized via aldehyde plasma polymer interlayers on inorganic and polymeric substrates. Langmuir. 2006;22:5760–5769. doi: 10.1021/la0602766. [DOI] [PubMed] [Google Scholar]
- Bodas D, Khan-Malek C. Formation of more stable hydrophilic surfaces of PDMS by plasma and chemical treatments. Microelectron Eng. 2006;83:1277–1279. [Google Scholar]
- Bodas DS, Khan-Malek C. Fabrication of long-term hydrophilic surfaces of poly(dimethyl siloxane) using 2-hydroxy ethyl methacrylate. Sens Actuators B Chem. 2007;120:719–723. [Google Scholar]
- Boulmedais F, Frisch B, Etienne O, et al. Polyelectrolyte multilayer films with pegylated polypeptides as a new type of anti-microbial protection for biomaterials. Biomaterials. 2004;25:2003–2011. doi: 10.1016/j.biomaterials.2003.08.039. [DOI] [PubMed] [Google Scholar]
- Boxshall K, Wu MH, Cui Z, et al. Simple surface treatments to modify protein adsorption and cell attachment properties within a poly(dimethylsiloxane) micro-bioreactor. Surf Interface Anal. 2006;38:198–201. [Google Scholar]
- Brink C, Osterberg E, Holmberg K, et al. Using poly(ethylene imine) to graft poly(ethylene glycol) or polysaccharide to polystyrene. Colloids Surf. 1992;66:149–156. [Google Scholar]
- Brown AA, Khan NS, Steinbock L, et al. Synthesis of oligo(ethylene glycol) methacrylate polymer brushes. Eur Polym J. 2005;41:1757–1765. [Google Scholar]
- Cecchet F, De Meersman B, Demoustier-Champagne S, et al. One step growth of protein antifouling surfaces: monolayers of poly(ethylene oxide) (PEO) derivatives on oxidized and hydrogen-passivated silicon surfaces. Langmuir. 2006;22:1173–1181. doi: 10.1021/la052507z. [DOI] [PubMed] [Google Scholar]
- Chen IJ, Lindner E. The stability of radio-frequency plasma-treated polydimethylsiloxane surfaces. Langmuir. 2007;23:3118–3122. doi: 10.1021/la0627720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb KA, Dolnik V, Novotny M. Electrophoretic separations of proteins in capillaries with hydrolytically stable surface-structures. Anal Chem. 1990;62:2478–2483. doi: 10.1021/ac00221a013. [DOI] [PubMed] [Google Scholar]
- Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- Deval J, Umali TA, Lan EH, et al. Reconfigurable hydrophobic/hydrophilic surfaces in microelectromechanical systems (MEMS) J Micromech Microeng. 2004;14:91–95. [Google Scholar]
- Dittrich PS, Tachikawa K, Manz A. Micro total analysis systems. Latest advancements and trends. Anal Chem. 2006;78:3887–3907. doi: 10.1021/ac0605602. [DOI] [PubMed] [Google Scholar]
- Doherty EAS, Meagher RJ, Albarghouthi MN, et al. Microchannel wall coatings for protein separations by capillary and chip electrophoresis. Electrophoresis. 2003;24:34–54. doi: 10.1002/elps.200390029. [DOI] [PubMed] [Google Scholar]
- Dolnik V. Wall coating for capillary electrophoresis on microchips. Electrophoresis. 2004;25:3589–3601. doi: 10.1002/elps.200406113. [DOI] [PubMed] [Google Scholar]
- Dolnik V. Capillary electrophoresis of proteins 2003–2005. Electrophoresis. 2006;27:126–141. doi: 10.1002/elps.200500567. [DOI] [PubMed] [Google Scholar]
- Dou YH, Bao N, Xu JJ, et al. Separation of proteins on surface-modified poly(dimethylsiloxane) microfluidic devices. Electrophoresis. 2004;25:3024–3031. doi: 10.1002/elps.200405986. [DOI] [PubMed] [Google Scholar]
- Dubas ST, Schlenoff JB. Factors controlling the growth of polyelectrolyte multilayers. Macromolecules. 1999;32:8153–8160. [Google Scholar]
- Duffy DC, McDonald JC, Schueller OJA, et al. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane) Anal Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- Ebara M, Hoffman JM, Hoffman AS, et al. Switchable surface traps for injectable bead-based chromatography in PDMS microfluidic channels. Lab Chip. 2006;6:843–848. doi: 10.1039/b515128g. [DOI] [PubMed] [Google Scholar]
- Eddington DT, Puccinelli JP, Beebe DJ. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens Actuators B Chem. 2006;114:170–172. [Google Scholar]
- Edmondson S, Osborne VL, Huck WTS. Polymer brushes via surface-initiated polymerizations. Chem Soc Rev. 2004;33:14–22. doi: 10.1039/b210143m. [DOI] [PubMed] [Google Scholar]
- Effenhauser CS, Bruin GJM, Paulus A, et al. Integrated capillary electrophoresis on flexible silicone microdevices: analysis of DNA restriction fragments and detection of single DNA molecules on microchips. Anal Chem. 1997;69:3451–3457. doi: 10.1021/ac9703919. [DOI] [PubMed] [Google Scholar]
- Efimenko K, Wallace WE, Genzer J. Surface modification of Sylgard-184 poly(dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J Colloid Interface Sci. 2002;254:306–315. doi: 10.1006/jcis.2002.8594. [DOI] [PubMed] [Google Scholar]
- Ejaz M, Yamamoto S, Ohno K, et al. Controlled graft polymerization of methyl methacrylate on silicon substrate by the combined use of the Langmuir–Blodgett and atom transfer radical polymerization techniques. Macromolecules. 1998;31:5934–5936. [Google Scholar]
- Emoto K, Van Alstine JM, Harris JM. Stability of poly(ethylene glycol) graft coatings. Langmuir. 1998;14:2722–2729. [Google Scholar]
- Fendler JH. Chemical self-assembly for electronic applications. Chem Mater. 2001;13:3196–3210. [Google Scholar]
- Feng W, Chen RX, Brash JL, et al. Surface-initiated atom transfer radical polymerization of oligo(ethylene glycol) methacrylate: effect of solvent on graft density. Macromol Rapid Commun. 2005;26:1383–1388. [Google Scholar]
- Feng W, Zhu SP, Ishihara K, et al. Adsorption of fibrinogen and lysozyme on silicon grafted with poly(2-methacryloyloxyethyl phosphorylcholine) via surface-initiated atom transfer radical polymerization. Langmuir. 2005;21:5980–5987. doi: 10.1021/la050277i. [DOI] [PubMed] [Google Scholar]
- Feng W, Brash JL, Zhu SP. Non-biofouling materials prepared by atom transfer radical polymerization grafting of 2-methacryloloxyethyl phosphorylcholine: separate effects of graft density and chain length on protein repulsion. Biomaterials. 2006;27:847–855. doi: 10.1016/j.biomaterials.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gates BD, Xu QB, Stewart M, et al. New approaches to nanofabrication: molding, printing, and other techniques. Chem Rev. 2005;105:1171–1196. doi: 10.1021/cr030076o. [DOI] [PubMed] [Google Scholar]
- Genzer J, Efimenko K. Recent developments in superhydrophobic surfaces and their relevance to marine fouling: a review. Biofouling. 2006;22:339–360. doi: 10.1080/08927010600980223. [DOI] [PubMed] [Google Scholar]
- Gilges M, Kleemiss MH, Schomburg G. Capillary zone electrophoresis separations of basic and acidic proteins using poly(vinyl alcohol) coatings in fused-silica capillaries. Anal Chem. 1994;66:2038–2046. [Google Scholar]
- Goda T, Konno T, Takai M, et al. Biomimetic phosphorylcholine polymer grafting from polydimethylsiloxane surface using photo-induced polymerization. Biomaterials. 2006;27:5151–5160. doi: 10.1016/j.biomaterials.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Gooding JJ, Mearns F, Yang WR, et al. Self-assembled monolayers into the 21(st) century: recent advances and applications. Electroanalysis. 2003;15:81–96. [Google Scholar]
- Gorochovceva N, Naderi A, Dedinaite A, et al. Chitosan-N-poly(ethylene glycol) brush copolymers: Synthesis and adsorption on silica surface. Eur Polym J. 2005;41:2653–2662. [Google Scholar]
- Haeberle S, Zengerle R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip. 2007;7:1094–1110. doi: 10.1039/b706364b. [DOI] [PubMed] [Google Scholar]
- Harris JM. Poly(ethylene glycol) chemistry: biotechnical and biomedical applications. New York: Plenum Press; 1992. [Google Scholar]
- Harris JM, Zalipsky S. Poly(ethylene glycol): chemistry and biological application. Washington, DC: American Chemical Society; 1997. [Google Scholar]
- Harrison DJ, Fluri K, Seiler K, et al. Micromachining a miniaturized capillary electrophoresis-based chemical-analysis system on a chip. Science. 1993;261:895–897. doi: 10.1126/science.261.5123.895. [DOI] [PubMed] [Google Scholar]
- He QG, Liu ZC, Xiao PF, et al. Preparation of hydrophilic poly(dimethylsiloxane) stamps by plasma-induced grafting. Langmuir. 2003;19:6982–6986. [Google Scholar]
- Hellmich W, Regtmeier J, Duong TT, et al. Poly(oxyethylene) based surface coatings for poly(dimethylsiloxane) microchannels. Langmuir. 2005;21:7551–7557. doi: 10.1021/la0510432. [DOI] [PubMed] [Google Scholar]
- Heuberger R, Sukhorukov G, Voros J, et al. Biofunctional polyelectrolyte multilayers and microcapsules: control of non-specific and bio-specific protein adsorption. Adv Funct Mater. 2005;15:357–366. [Google Scholar]
- Hillborg H, Gedde UW. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer. 1998;39:1991–1998. [Google Scholar]
- Hillborg H, Tomczak N, Olah A, et al. Nanoscale hydrophobic recovery: a chemical force microscopy study of UV/ozone-treated cross-linked poly(dimethylsiloxane) Langmuir. 2004;20:785–794. doi: 10.1021/la035552k. [DOI] [PubMed] [Google Scholar]
- Hjerten S. High-performance electrophoresis—elimination of electroendosmosis and solute adsorption. J Chromatogr. 1985;347:191–198. [Google Scholar]
- Ho CM, Tai YC. Micro-electro-mechanical-systems (MEMS) and fluid flows. Annu Rev Fluid Mech. 1998;30:579–612. [Google Scholar]
- Hu SW, Ren XQ, Bachman M, et al. Surface modification of poly(dimethylsiloxane) microfluidic devices by ultraviolet polymer grafting. Anal Chem. 2002;74:4117–4123. doi: 10.1021/ac025700w. [DOI] [PubMed] [Google Scholar]
- Hu SW, Ren XQ, Bachman M, et al. Surface-directed, graft polymerization within microfluidic channels. Anal Chem. 2004;76:1865–1870. doi: 10.1021/ac049937z. [DOI] [PubMed] [Google Scholar]
- Huang NP, Michel R, Voros J, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir. 2001;17:489–498. [Google Scholar]
- Huang WX, Kim JB, Bruening ML, et al. Functionalization of surfaces by water-accelerated atom-transfer radical polymerization of hydroxyethyl methacrylate and subsequent derivatization. Macromolecules. 2002;35:1175–1179. [Google Scholar]
- Huang B, Wu HK, Kim S, et al. Coating of poly(dimethylsiloxane) with n-dodecyl-beta-D-maltoside to minimize nonspecific protein adsorption. Lab Chip. 2005;5:1005–1007. doi: 10.1039/b509251e. [DOI] [PubMed] [Google Scholar]
- Huang TT, Mosier NS, Ladisch MR. Surface engineering of microchannel walls for protein separation and directed microfluidic flow. J Sep Sci. 2006;29:1733–1742. doi: 10.1002/jssc.200600150. [DOI] [PubMed] [Google Scholar]
- Huber DL, Manginell RP, Samara MA, et al. Programmed adsorption and release of proteins in a microfluidic device. Science. 2003;301:352–354. doi: 10.1126/science.1080759. [DOI] [PubMed] [Google Scholar]
- Hunt HC, Wilkinson JS. Optofluidic integration for microanalysis. Microfluid Nanofluid. 2008;4:53–79. doi: 10.1007/s10404-007-0223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husseman M, Malmstrom EE, McNamara M, et al. Controlled synthesis of polymer brushes by “Living” free radical polymerization techniques. Macromolecules. 1999;32:1424–1431. [Google Scholar]
- Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym J. 1990;22:355–360. [Google Scholar]
- Ishihara K, Nomura H, Mihara T, et al. Why do phospholipid polymers reduce protein adsorption? J Biomed Mater Res. 1998;39:323–330. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Janssen D, De Palma R, Verlaak S, et al. Static solvent contact angle measurements, surface free energy and wettability determination of various self-assembled monolayers on silicon dioxide. Thin Solid Films. 2006;515:1433–1438. [Google Scholar]
- Jones DM, Brown AA, Huck WTS. Surface-initiated polymerizations in aqueous media: effect of initiator density. Langmuir. 2002;18:1265–1269. [Google Scholar]
- Karkhaneh A, Mirzadeh H, Ghaffariyeh AR. Simultaneous graft copolymerization of 2-hydroxyethyl methacrylate and acrylic acid onto polydimethylsiloxane surfaces using a two-step plasma treatment. J Appl Polym Sci. 2007;105:2208–2217. [Google Scholar]
- Katayama H, Ishihama Y, Asakawa N. Stable cationic capillary coating with successive multiple ionic polymer layers for capillary electrophoresis. Anal Chem. 1998;70:5272–5277. doi: 10.1021/ac980522l. [DOI] [PubMed] [Google Scholar]
- Kim J, Chaudhury MK, Owen MJ. Hydrophobic recovery of polydimethylsiloxane elastomer exposed to partial electrical discharge. J Colloid Interface Sci. 2000;226:231–236. doi: 10.1016/j.jcis.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Koc Y, de Mello AJ, McHale G, et al. Nano-scale superhydrophobicity: suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip. 2008;8:582–586. doi: 10.1039/b716509a. [DOI] [PubMed] [Google Scholar]
- Lee SW, Laibinis PE. Protein-resistant coatings for glass and metal oxide surfaces derived from oligo(ethylene glycol)-terminated alkyltrichlorosilanes. Biomaterials. 1998;19:1669–1675. doi: 10.1016/s0142-9612(98)00044-1. [DOI] [PubMed] [Google Scholar]
- Lee S, Voros J. An aqueous-based surface modification of poly(dimethylsiloxane) with poly(ethylene glycol) to prevent biofouling. Langmuir. 2005;21:11957–11962. doi: 10.1021/la051932p. [DOI] [PubMed] [Google Scholar]
- Lee J, Moon H, Fowler J, et al. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens Actuators A Phys. 2002;95:259–268. [Google Scholar]
- Lee JN, Park C, Whitesides GM. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal Chem. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- Li D. Electrokinetics in microfluidics. Burlington: Elsevier; 2004. [Google Scholar]
- Liu JK, Lee ML. Permanent surface modification of polymeric capillary electrophoresis microchips for protein and peptide analysis. Electrophoresis. 2006;27:3533–3546. doi: 10.1002/elps.200600082. [DOI] [PubMed] [Google Scholar]
- Liu VA, Jastromb WE, Bhatia SN. Engineering protein and cell adhesivity using PEO-terminated triblock polymers. J Biomed Mater Res. 2002;60:126–134. doi: 10.1002/jbm.10005. [DOI] [PubMed] [Google Scholar]
- Lou XH, He P, Okelo GO, et al. Radical polymerization in biosensing. Anal Bioanal Chem. 2006;386:525–531. doi: 10.1007/s00216-006-0576-1. [DOI] [PubMed] [Google Scholar]
- Love JC, Estroff LA, Kriebel JK, et al. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem Rev. 2005;105:1103–1169. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- Lucke A, Tessmar J, Schnell E, et al. Biodegradable poly(D, L-lactic acid)-poly(ethylene glycol)-monomethyl ether diblock copolymers: structures and surface properties relevant to their use as biomaterials. Biomaterials. 2000;21:2361–2370. doi: 10.1016/s0142-9612(00)00103-4. [DOI] [PubMed] [Google Scholar]
- Madou M, Zoval J, Jia GY, et al. Lab on a CD. Annu Rev Biomed Eng. 2006;8:601–628. doi: 10.1146/annurev.bioeng.8.061505.095758. [DOI] [PubMed] [Google Scholar]
- Makamba H, Kim JH, Lim K, et al. Surface modification of poly(dimethylsiloxane) microchannels. Electrophoresis. 2003;24:3607–3619. doi: 10.1002/elps.200305627. [DOI] [PubMed] [Google Scholar]
- Makamba H, Hsieh YY, Sung WC, et al. Stable permanently hydrophilic protein-resistant thin-film coatings on poly(dimethylsiloxane) substrates by electrostatic self-assembly and chemical cross-linking. Anal Chem. 2005;77:3971–3978. doi: 10.1021/ac0502706. [DOI] [PubMed] [Google Scholar]
- Mansky P, Liu Y, Huang E, et al. Controlling polymer-surface interactions with random copolymer brushes. Science. 1997;275:1458–1460. [Google Scholar]
- Manz A, Harrison DJ, Verpoorte EMJ, et al. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems—capillary electrophoresis on a chip. J Chromatogr. 1992;593:253–258. [Google Scholar]
- Martwiset S, Koh AE, Chen W. Nonfouling characteristics of dextran-containing surfaces. Langmuir. 2006;22:8192–8196. doi: 10.1021/la061064b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martynova L, Locascio LE, Gaitan M, et al. Fabrication of plastic microfluid channels by imprinting methods. Anal Chem. 1997;69:4783–4789. doi: 10.1021/ac970558y. [DOI] [PubMed] [Google Scholar]
- Matyjaszewski K, Miller PJ, Shukla N, et al. Polymers at interfaces: using atom transfer radical polymerization in the controlled growth of homopolymers and block copolymers from silicon surfaces in the absence of untethered sacrificial initiator. Macromolecules. 1999;32:8716–8724. [Google Scholar]
- McCormick RM, Nelson RJ, AlonsoAmigo MG, et al. Microchannel electrophoretic separations of DNA in injection-molded plastic substrates. Anal Chem. 1997;69:2626–2630. doi: 10.1021/ac9701997. [DOI] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, et al. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Michel R, Lussi JW, Csucs G, et al. Selective molecular assembly patterning: a new approach to micro- and nanochemical patterning of surfaces for biological applications. Langmuir. 2002;18:3281–3287. [Google Scholar]
- Moritani T, Yoon K, Rafailovich M, et al. DNA capillary electrophoresis using poly(vinyl alcohol). I. Inner capillary coating. Electrophoresis. 2003;24:2764–2771. doi: 10.1002/elps.200305546. [DOI] [PubMed] [Google Scholar]
- Muck A, Svatos A. Chemical modification of polymeric microchip devices. Talanta. 2007;74:333–341. doi: 10.1016/j.talanta.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Raghavan S, Tan JL, et al. Degradation of micropatterned surfaces by cell-dependent and -independent processes. Langmuir. 2003;19:1493–1499. [Google Scholar]
- Ng JMK, Gitlin I, Stroock AD, et al. Components for integrated poly(dimethylsiloxane) microfluidic systems. Electrophoresis. 2002;23:3461–3473. doi: 10.1002/1522-2683(200210)23:20<3461::AID-ELPS3461>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nnebe IM, Tilton RD, Schneider JW. Direct force measurement of the stability of poly(ethylene glycol)-polyethylenimine graft films. J Colloid Interface Sci. 2004;276:306–316. doi: 10.1016/j.jcis.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Orhan JB, Parashar VK, Flueckiger J, et al. Internal modification of poly(dimethylsiloxane) microchannels with a borosilicate glass coating. Langmuir. 2008;24:9154–9161. doi: 10.1021/la801317x. [DOI] [PubMed] [Google Scholar]
- Osterberg E, Bergstrom K, Holmberg K, et al. Protein-rejecting ability of surface-bound dextran in end-on and side-on configurations—comparison to peg. J Biomed Mater Res. 1995;29:741–747. doi: 10.1002/jbm.820290610. [DOI] [PubMed] [Google Scholar]
- Pallandre A, de Lambert B, Attia R, et al. Surface treatment and characterization: perspectives to electrophoresis and lab-on-chips. Electrophoresis. 2006;27:584–610. doi: 10.1002/elps.200500761. [DOI] [PubMed] [Google Scholar]
- Papra A, Bernard A, Juncker D, et al. Microfluidic networks made of poly(dimethylsiloxane), Si, and Au coated with polyethylene glycol for patterning proteins onto surfaces. Langmuir. 2001;17:4090–4095. [Google Scholar]
- Papra A, Gadegaard N, Larsen NB. Characterization of ultrathin poly(ethylene glycol) monolayers on silicon substrates. Langmuir. 2001;17:1457–1460. [Google Scholar]
- Prakash S, Long TM, Selby JC, et al. “Click” modification of silica surfaces and glass microfluidic channels. Anal Chem. 2007;79:1661–1667. doi: 10.1021/ac061824n. [DOI] [PubMed] [Google Scholar]
- Prucker O, Ruhe J. Mechanism of radical chain polymerizations initiated by azo compounds covalently bound to the surface of spherical particles. Macromolecules. 1998;31:602–613. [Google Scholar]
- Prucker O, Ruhe J. Synthesis of poly(styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules. 1998;31:592–601. [Google Scholar]
- Pruden KG, Beaudoin SP. Downstream microwave ammonia plasma treatment of polydimethylsiloxane. J Vac Sci Technol A. 2005;23:208–214. [Google Scholar]
- Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- Reyes DR, Iossifidis D, Auroux PA, et al. Micro total analysis systems. 1. Introduction, theory, and technology. Anal Chem. 2002;74:2623–2636. doi: 10.1021/ac0202435. [DOI] [PubMed] [Google Scholar]
- Riess G. Micellization of block copolymers. Prog Polym Sci. 2003;28:1107–1170. [Google Scholar]
- Roberts MA, Rossier JS, Bercier P, et al. UV laser machined polymer substrates for the development of microdiagnostic systems. Anal Chem. 1997;69:2035–2042. doi: 10.1021/ac961038q. [DOI] [PubMed] [Google Scholar]
- Roman GT, Culbertson CT. Surface engineering of poly(dimethylsiloxane) microfluidic devices using transition metal sol–gel chemistry. Langmuir. 2006;22:4445–4451. doi: 10.1021/la053085w. [DOI] [PubMed] [Google Scholar]
- Roman GT, Hlaus T, Bass KJ, et al. Sol–gel modified poly(dimethylsiloxane) microfluidic devices with high electroosmotic mobilities and hydrophilic channel wall characteristics. Anal Chem. 2005;77:1414–1422. doi: 10.1021/ac048811z. [DOI] [PubMed] [Google Scholar]
- Ruiz-Taylor LA, Martin TL, Zaugg FG, et al. Monolayers of derivatized poly(L-lysine)-grafted poly(ethylene glycol) on metal oxides as a class of biomolecular interfaces. Proc Natl Acad Sci USA. 2001;98:852–857. doi: 10.1073/pnas.98.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem AK, Cannizzaro SM, Davies MC, et al. Synthesis and characterisation of a degradable poly(lactic acid)–poly(ethylene glycol) copolymer with biotinylated end groups. Biomacromolecules. 2001;2:575–580. doi: 10.1021/bm010030+. [DOI] [PubMed] [Google Scholar]
- Schlapak R, Pammer P, Armitage D, et al. Glass surfaces grafted with high-density poly(ethylene glycol) as substrates for DNA oligonucleotide microarrays. Langmuir. 2006;22:277–285. doi: 10.1021/la0521793. [DOI] [PubMed] [Google Scholar]
- Schreiber F. Structure and growth of self-assembling monolayers. Prog Surf Sci. 2000;65:151–256. [Google Scholar]
- Senaratne W, Andruzzi L, Ober CK. Self-assembled monolayers and polymer brushes in biotechnology: current applications and future perspectives. Biomacromolecules. 2005;6:2427–2448. doi: 10.1021/bm050180a. [DOI] [PubMed] [Google Scholar]
- Shadpour H, Musyimi H, Chen JF, et al. Physiochemical properties of various polymer substrates and their effects on microchip electrophoresis performance. J Chromatogr A. 2006;1111:238–251. doi: 10.1016/j.chroma.2005.08.083. [DOI] [PubMed] [Google Scholar]
- Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- Sinclair J, Salem AK. Rapid localized cell trapping on biodegradable polymers using cell surface derivatization and microfluidic networking. Biomaterials. 2006;27:2090–2094. doi: 10.1016/j.biomaterials.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Slentz BE, Penner NA and Regnier FE (2002) Capillary electrochromatography of peptides on microfabricated poly(dimethylsiloxane) chips modified by cerium(IV)-catalyzed polymerization. J Chromatogr A 948:PII S0021-9673(01)01319-X [DOI] [PubMed]
- Song H, Chen DL, Ismagilov RF. Reactions in droplets in microflulidic channels. Angew Chem Int Edn. 2006;45:7336–7356. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone HA, Stroock AD, Ajdari A. Engineering flows in small devices: microfluidics toward a lab-on-a-chip. Annu Rev Fluid Mech. 2004;36:381–411. [Google Scholar]
- Sui GD, Wang JY, Lee CC, et al. Solution-phase surface modification in intact poly(dimethylsiloxane) microfluidic channels. Anal Chem. 2006;78:5543–5551. doi: 10.1021/ac060605z. [DOI] [PubMed] [Google Scholar]
- Sun TL, Tan H, Han D, et al. No platelet can adhere—largely improved blood compatibility on nanostructured superhydrophobic surfaces. Small. 2005;1:959–963. doi: 10.1002/smll.200500095. [DOI] [PubMed] [Google Scholar]
- Tan JL, Liu W, Nelson CM, et al. Simple approach to micropattern cells on common culture substrates by tuning substrate wettability. Tissue Eng. 2004;10:865–872. doi: 10.1089/1076327041348365. [DOI] [PubMed] [Google Scholar]
- Tan QG, Ji J, Zhao F, et al. Fabrication of thrombo resistant multilayer thin film on plasma treated poly (vinyl chloride) surface. J Mater Sci Mater Med. 2005;16:687–692. doi: 10.1007/s10856-005-2541-5. [DOI] [PubMed] [Google Scholar]
- Teh SY, Lin R, Hung LH, et al. Droplet microfluidics. Lab Chip. 2008;8:198–220. doi: 10.1039/b715524g. [DOI] [PubMed] [Google Scholar]
- Tessmar JK, Gopferich AM. Customized PEG-derived copolymers for tissue-engineering applications. Macromol Biosci. 2007;7:23–39. doi: 10.1002/mabi.200600096. [DOI] [PubMed] [Google Scholar]
- Thorsen T, Maerkl SJ, Quake SR. Microfluidic large-scale integration. Science. 2002;298:580–584. doi: 10.1126/science.1076996. [DOI] [PubMed] [Google Scholar]
- Towns JK, Regnier FE. Capillary electrophoretic separations of proteins using nonionic surfactant coatings. Anal Chem. 1991;63:1126–1132. doi: 10.1021/ac00011a013. [DOI] [PubMed] [Google Scholar]
- Tsujii Y, Ohno K, Yamamoto S, et al. Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization. Adv Polym Sci. 2006;197:1–45. [Google Scholar]
- Ulman A. Formation and structure of self-assembled monolayers. Chem Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- Vickers JA, Caulum MM, Henry CS. Generation of hydrophilic poly(dimethylsiloxane) for high-performance microchip electrophoresis. Anal Chem. 2006;78:7446–7452. doi: 10.1021/ac0609632. [DOI] [PubMed] [Google Scholar]
- Vilkner T, Janasek D, Manz A. Micro total analysis systems. Recent developments. Anal Chem. 2004;76:3373–3385. doi: 10.1021/ac040063q. [DOI] [PubMed] [Google Scholar]
- Wang AJ, Xu JJ, Chen HY. Proteins modification of poly(dimethylsiloxane) microfluidic channels for the enhanced microchip electrophoresis. J Chromatogr A. 2006;1107:257–264. doi: 10.1016/j.chroma.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Wang YL, Bachman M, Sims CE, et al. Simple photografting method to chemically modify and micropattern the surface of SU-8 photoresist. Langmuir. 2006;22:2719–2725. doi: 10.1021/la053188e. [DOI] [PubMed] [Google Scholar]
- West J, Becker M, Tombrink S, et al. Micro total analysis systems: latest achievements. Anal Chem. 2008;80:4403–4419. doi: 10.1021/ac800680j. [DOI] [PubMed] [Google Scholar]
- Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- Whitesides GM, Ostuni E, Takayama S, et al. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- Wink T, vanZuilen SJ, Bult A, et al. Self-assembled monolayers for biosensors. Analyst. 1997;122:R43–R50. doi: 10.1039/a606964i. [DOI] [PubMed] [Google Scholar]
- Wu DP, Luo Y, Zhou XM, et al. Multilayer poly(vinyl alcohol)-adsorbed coating on poly(dimethylsiloxane) microfluidic chips for biopolymer separation. Electrophoresis. 2005;26:211–218. doi: 10.1002/elps.200406157. [DOI] [PubMed] [Google Scholar]
- Wu DP, Zhao BX, Dai ZP, et al. Grafting epoxy-modified hydrophilic polymers onto poly(dimethylsiloxane) microfluidic chip to resist nonspecific protein adsorption. Lab Chip. 2006;6:942–947. doi: 10.1039/b600765a. [DOI] [PubMed] [Google Scholar]
- Wu MH, Urban JPG, Cui Z, et al. Development of PDMS microbioreactor with well-defined and homogenous culture environment for chondrocyte 3-D culture. Biomed Microdevices. 2006;8:331–340. doi: 10.1007/s10544-006-9597-y. [DOI] [PubMed] [Google Scholar]
- Wu DP, Qin JH, Lin BC. Self-assembled epoxy-modified polymer coating on a poly(dimethylsiloxane) microchip for EOF inhibition and biopolymers separation. Lab Chip. 2007;7:1490–1496. doi: 10.1039/b708877a. [DOI] [PubMed] [Google Scholar]
- Xiao DQ, Zhang H, Wirth M. Chemical modification of the surface of poly(dimethylsiloxane) by atom-transfer radical polymerization of acrylamide. Langmuir. 2002;18:9971–9976. [Google Scholar]
- Xiao DQ, Van Le T, Wirth MJ. Surface modification of the channels of poly(dimethylsiloxane) microfluidic chips with polyacrylamide for fast electrophoretic separations of proteins. Anal Chem. 2004;76:2055–2061. doi: 10.1021/ac035254s. [DOI] [PubMed] [Google Scholar]
- Youngblood JP, Andruzzi L, Ober CK, et al. Coatings based on side-chain ether-linked poly(ethylene glycol) and fluorocarbon polymers for the control of marine biofouling. Biofouling. 2003;19:91–98. doi: 10.1080/0892701021000053381. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chao T, Chen SF, et al. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir. 2006;22:10072–10077. doi: 10.1021/la062175d. [DOI] [PubMed] [Google Scholar]
- Zhao B, Brittain WJ. Polymer brushes: surface-immobilized macromolecules. Prog Polym Sci. 2000;25:677–710. [Google Scholar]


