Summary
Multiple Sclerosis (MS) is an inflammatory demyelinating disorder of the central nervous system (CNS). Since current treatments are aimed at nonspecifically down-regulating inflammation and natural mechanisms of repair and remyelination within the CNS are inadequate for recovery of function, MS patients presently only have available treatments that delay symptom progression. The complex nature of this disease means that only multifaceted treatments hold the promise of a cure. Recent studies indicate that the ER stress response, a cellular pathway that allows a cell to survive and recover from a stressful event, could be elicited to help the myelin-generating and myelin-support cells of the CNS survive inflammatory insult.
Keywords: Cell survival and apoptosis, Demyelination and remyelination, Experimental Autoimmune Encephalomyelitis, ER stress response, Multiple Sclerosis, Myelin Repair, Oligodendrocytes and OPCs, Unfolded protein response
Multiple Sclerosis and its therapies
The etiology and mechanisms of action of MS are incompletely understood. Disease strikes during early adulthood and results in some combination of symptoms that can include vision problems, fatigue, vertigo, tingling and/or numbness of limbs, motor loss, bladder/bowel dysfunction, motor dysfunction, and cognitive dysfunction. Disease is manifest in either a relapsing-remitting or a chronic-progressive manner, or a combination of the two. The varied and complicated disease pattern of MS has made it difficult to understand its pathogenesis, and it is likely that what is defined as MS may actually include several disparate diseases [1,2]. Though the underlying cause of MS is unknown, it is believed that that the disease is autoimmune in nature, and that via mechanism(s) triggered by a combination of genetic and environmental (microbial, longitudinal/latitudinal, nutritional, etc.) factors, T cells that are self-reactive to myelin epitopes become activated and destroy myelin within the CNS [3]. Within the inflamed CNS, these myelin-reactive T cells themselves, along with other inflammatory cells and mediators, are believed to damage axons that have been stripped of their myelin. The loss of axon conductivity via myelin loss and direct damage to unprotected axons result in the clinical symptoms of MS [4–6]. Currently, MS therapies are nonspecifically immunosuppressive. Therapies that more specifically target the cells and molecules mediating damage within the diseased CNS are preferable, and various potential therapies of this kind are always under investigation [7]. Since the body’s natural mechanisms of CNS repair are inefficient, therapies aimed exclusively at halting the inflammatory process would, at best, only stop disease progression. The future of MS treatments is likely to involve integrated therapies that not only control inflammation within the CNS, but also promote the body’s natural mechanisms of protection, regulation and repair. Such treatments, which would stop the inflammatory response as well as encourage remyelination of demyelinated axons would hopefully lead to the restoration of normal axon activity and clinical recovery.
One novel possible therapeutic target, only very recently connected with MS, is the endoplasmic reticulum (ER) stress response (ERSR, also known as the unfolded protein response, or UPR). This response is a mechanism of cellular preservation and recovery from protein buildup and mis-folding within the ER which can be induced under chronic inflammatory conditions. This cellular pathway, which effectively buys the cell time and induces synthesis of helpful proteins required to recover from stress, eventually leads to apoptosis if cellular homeostasis cannot be achieved. Thus, any therapy that induces this pathway must be designed with great care.
The ER stress response
Sources of cellular stress are various and include viral infection; UV irradiation; oxidative stress; nutrient, heme, or amino acid deprivation; and starvation of other factors important for cellular function. Any one of these stressors, or even normal activity in some cell types, can cause a buildup of proteins within the ER of a cell. The ER carries out the task of folding, modifying, and exporting proteins. When excessive cellular activity results in buildup of proteins within the ER so that proteins are being folded in a rushed and imperfect manner, the ERSR is triggered. Generally, the ERSR elicits inhibition of the translation of a majority of cellular proteins, while up-regulating the translation of a few key proteins, including chaperones, ER-associated degradation (ERAD) factors, those important for amino acid biosynthesis, and those that otherwise aid in dealing with un- or mis-folded protein buildup.
Current knowledge of the precise mechanisms through which the ERSR functions has been discussed in detail elsewhere [8–10]. Here, we will summarize the ERSR with particular attention paid to key molecules and those proteins that we or others have found to have possible links with MS pathogenesis or potential treatments.
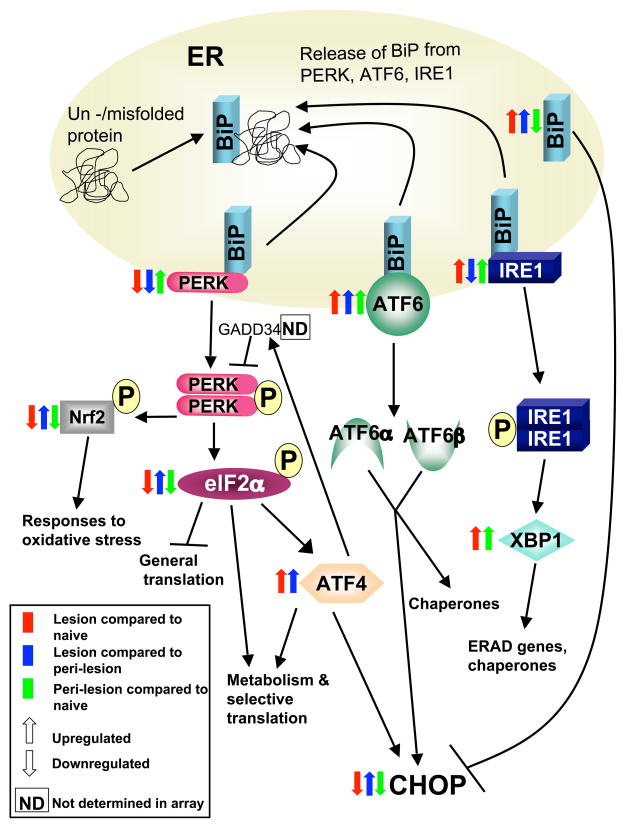
The ERSR master regulator, BiP (also known as GRP78), is normally bound to 3 signal transducers: inositol requiring kinase 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK) (Figure 1). When BiP becomes associated with unfolded protein, it releases IRE1, ATF6, and PERK, each of which initiate an arm of the ERSR [11,12]. IRE1 dimerizes upon release, auto-phosphorylates, and, via X box binding protein 1 (XBP1), initiates translation of protein degrading factors and chaperones [13]. ATF6 is cleaved into ATF6α and ATF6β within the golgi, and both subunits subsequently traffic to the nucleus and initiate expression of proteins that will aid in handling the overload within the ER, as well as expression of the pro-apoptotic factor C/EBP-homologous protein CHOP [14,15]. PERK also dimerizes and auto-phosphorylates after release from BiP. Activated PERK phosphorylates Nrf2, which is important in the antioxidant response, and eukaryotic translation initiation factor 2, alpha subunit (elf2α), which is a key substrate that simultaneously inhibits translation of most proteins, while initiating translation of proteins that will allow proper folding or degradation of excess and mis-folded proteins [16,17]. Elf2α also activates ATF4, which helps with metabolism so that the cell can attempt to recover from stress, but which also activates CHOP. While there are other pro-apoptotic molecules that are induced during the ER stress response, CHOP is required for ER stress-induced apoptosis [18]. The ERSR initiates a positive feedback loop to further release BiP from its bound proteins, and, once released, BiP inhibits the action of CHOP [19]. The purpose of the ER stress response is to protect the cell and give it a chance to recover from over-extension. However, in the interest of the organism as a whole, a cell that cannot recover from ER stress efficiently must die, thus the highly regulated expression of CHOP. Therefore, it is the balance between pro- and anti-apoptotic factors, the levels and timing of expression of which are determined by cell type, how long a cell has been undergoing ER stress, and how quickly and efficiently it can recover, that dictate whether a cell survives an ER stress event. The complex nature of the ERSR thus poses a challenge to any attempt to manipulate it during disease.
Figure 1. Expression levels of genes encoding endoplasmic reticulum stress response (ERSR) proteins are altered in experimental autoimmune encephalomyelitis (EAE) lesions.
This schematic diagram of the ERSR pathway includes how expression levels of several of the genes encoding ERSR proteins are altered during EAE. EAE was induced by priming SJL mice with proteolipid protein (PLP) 139–151 peptide emulsified in Freund’s complete adjuvant. At the peak of disease, lesion and peri-lesion regions of spinal cords from EAE mice, as well as comparable regions from the spinal cords of naïve SJL mice, were collected by laser dissection, and a gene array was performed. The color and direction of the arrows indicate which genes were up- or down-regulated for each region in comparison to the others. Lesions consist of multiple cell types and therefore these data may not give an accurate picture at a cellular level, and it is quite clear that the changes in gene expression within the pathway are heterogeneous. However, a trend for upregulation of genes that are important in activating pro-apoptotic signals such as ATF4 and ATF6 was evident within EAE lesion compared to peri-lesion and naive controls. Many of the genes in the PERK part of the ERSR pathway were downregulated in lesions. Further work at a protein level is required to confirm these data.
The ERSR has been linked to several diseases other than MS, including type 1 and 2 diabetes, Alzheimer’s disease, Parkinson’s disease, and others [20–25]. PERK−/− mice, as well as humans possessing a particular mutation in the gene encoding PERK (a condition known as Wolfram Syndrome) develop diabetes following β cell apoptosis that is due to an inability to cope with ER stress in pancreatic islets [20,26]. Type 2 diabetes could also occur during ER stress, because peripheral resistance to insulin results in a higher level of insulin production, followed by an overwhelmed β cell ER [20,27]. In neurological diseases such as Parkinson’s and Alzheimer’s, cell death within the CNS can be associated with a failure of the ERSR to deal with mis-folded proteins or, in some cases, genetic mutations that affect the ERSR [24,25]. In MS, disease pathogenesis is not believed to be associated with such mutations. There are aspects and outcomes of the ERSR that could contribute to the pathological mechanisms of MS, and there are those that could counteract them. The induction of apoptosis, for example, would be a desirable outcome with respect to inflammatory cells infiltrating the CNS during disease, but not with respect to CNS resident cells such as oligodendrocyte progenitor cells (OPC), oligodendrocytes, and neurons. Therefore, it is important to determine whether, and to what extent and within which cell types, the ERSR is induced during MS.
ERSR in MS lesions
Potential triggers of the ERSR in MS
There are various potential triggers of the ERSR prior to the onset of or during MS. These include viral infections, stimulation from all of the inflammatory mediators present, many of which have been documented to be ERSR-inducers [28–30], or high ER protein load, which could occur in a cell attempting to remyelinate axons. Recent studies of RNA and protein levels have shown that indeed, ERSR components can be found in an elevated state in and around MS lesions, compared to normal tissues [31,32].
ERSR proteins can be detected in and around MS lesions
BiP, XBP1, and CHOP have been studied histologically and found to be variously expressed within MS lesions, compared to non-affected white matter from MS patients as well as normal controls. A perfunctory morphological analysis of cell type-specific expression of these markers indicated that these proteins were expressed in macrophages, astrocytes, and oligodendrocytes [32]. More specifically, the pro-apoptotic molecule CHOP, which was primarily present only in areas of ongoing demyelination, was expressed in most CD3+ T cells and macrophages, and in some astrocytes and oligodendrocytes [32]. There were CHOP+ cells that did not fall into any of these categories, as well as cells in each category that were not CHOP+, suggesting that the microenvironment must play some role in determining whether and to what extent a cell will undergo the ERSR. The non-homogenous expression of ERSR proteins in specific cellular subsets may need to be addressed before clinical applications involving the ERSR are developed.
In human MS tissue, the edges of lesions tended to show expression of BiP as well as CHOP, while the centers of many lesions were CHOP+ but BiP-, perhaps suggesting that the drop in BiP at the center of a lesion signifies the dominant expression of CHOP and resultant apoptosis, and implying that a steady expression of BiP to counteract CHOP may allow survival of cells within the center of a lesion [32]. Effective control of these molecules by manipulating the ERSR, may prevent the growth or expansion of lesions.
Harnessing the ERSR as a potential therapy in MS
A clear application for ERSR as a therapy for MS is promotion of the survival of oligodendrocytes and OPCs from inflammatory insult so that they can maintain their protective myelin-mediated contact to axons, or attempt to repair demyelinated axons, respectively. In MS, evidence of remyelinating activity is variably present, with some patients exhibiting little remyelination and others exhibiting a high level [33]. However, even in patients with the highest levels, remyelinating capacity appears to be insufficient to abrogate persistent clinical symptoms. It is generally accepted that remyelination is carried out by oligodendrocytes recently differentiated from OPCs. For remyelination to occur, OPCs must migrate to the demyelinated area, then proliferate and mature into oligodendrocytes, which will contact demyelinated axons and wrap them with myelin sheaths [34]. Mature oligodendrocytes appear to lose most if not all of their remyelinating abilities, but remain to maintain previously deposited myelin [35]. It is critical to determine what effect the ERSR has on OPCs as well as on mature myelinating oligodendrocytes if it is hoped that triggering the ERSR may encourage remyelination itself, or may be used in conjunction with other therapies that induce remyelination.
With regard to the presence of oligodendrocytes, there are basically two types of MS lesions: those in which few oligodendrocytes remain, and some OPCs are present but appear to be inefficient at remyelination; and those with surviving oligodendrocytes, to which OPCs are recruited and significant remyelination occurs [36]. For the former lesion, treatment would be aimed at preventing total oligodendrocyte loss, while the latter lesions are presumably the ones that can potentially be more completely repaired.
The ERSR is protective in a murine model of MS
In contrast to viewpoint that ERSR is a deleterious response that is likely to induce CHOP-mediated apoptosis in myelinating cells and neurons, or at the very least is merely indicative of damage [32], a more recent publication has viewed the ERSR in MS in a more positive light. Using the murine experimental autoimmune encephalomyelitis (EAE) model of MS, our group in collaboration with the Popko lab found that triggering the ERSR via CNS-specific expression of IFN-γ was protective, and that protection was mediated by enhanced survival of oligodendrocytes via the activation of PERK [37]. In this case, IFN-γ expression was induced before the onset of EAE. It was concluded that the ERSR should be explored from a therapeutic perspective, and these findings were the first to point to a potential benefit of inducing the ERSR during MS.
IFN-γ expression in the CNS has also been associated with increased levels of death in OPCs in mice with an ongoing demyelinating insult (EAE or cuprizone-induced demyelination) [38]. The discrepancy between these two findings, both of which are from the same group utilizing the same transgenic mouse strain, appears to be due to the particular timing of the presence of IFN-γ, as well as the metabolic state of the cells, i.e., how much of a protein load needs to be handled. In the study in which CNS IFN-γ was protective, IFN-γ expression was induced prior to disease onset, while in the other study, IFN-γ expression was induced after extensive demyelination had occurred, and remyelination was presumably ensuing. If an OPC is already attempting to myelinate axons, its activity and the levels of protein present may already have triggered the ERSR to some extent, and further induction by IFN-γ may exceed the threshold above which apoptosis is induced. This would be particularly applicable if the mechanism through which IFN-γ induces ER stress involves protein overproduction, though it is uncertain whether this is the case. On the other hand, an oligodendrocyte that is maintaining myelin, which does not require a high protein expression level, would not experience the adverse ERSR [37,39]. Regardless, in both cases, a deficiency in PERK worsened disease outcome, indicating that this ERSR component is induced and protective to some extent in inflammatory demyelinating disorders [37,38].
From another perspective, the timing of stress could be critically important, and indeed there is evidence that a small amount of ER stress will protect from subsequent stress that is more intense [40], so triggering the ERSR before the onset of EAE may have been protective in part for this reason. All of these factors add another level of complexity to the induction and effects of the ERSR as a possible therapy for demyelinating disease.
In order to determine the potential of ERSR-based therapy, several things must be determined: a) the effects of ERSR-triggering therapy on cells other than oligodendrocytes and OPCs, including neurons, other CNS-resident cells, as well as peripheral inflammatory cells present in MS/EAE lesions; b) the possibly additive or synergistic effects on the ERSR of other complicating factors, such as the presence of a microbial infection during or around the time of treatment (particularly as such infections have been associated with relapses and/or exacerbation of symptoms in MS); and c) the best way in which to trigger the ERSR, as administration of IFN-γ could have varied effects.
Cell type- and region-specific differences in response to ER stress
The ERSR has different effects in different cell types. For example, virus-induced ER stress in a neuron leads to apoptosis much more readily than the same viral trigger in an astrocyte, a difference that can be attributed to a higher level of BiP and other proteins that counteract CHOP within astrocytes [41]. In fact, astrocytes have a protein, old astrocyte specifically induced substance (OASIS), which aids astrocytes resist ER stress-induced apoptosis [42]. Although damaged neurons upregulate the protein BBF2H7 for the same purpose, they appear not to do so in response to every ER stress trigger [43]. More study of the induction of and resistance to ER stress-related apoptosis in neurons is required. As mentioned above, it appears that OPCs and oligodendrocytes have differential responses to ER stress as well, with the former exhibiting more susceptibility to the induction of apoptosis [37,38]. Whether cellular differences in response to stress are due to deficiencies in certain cells, distinct regulatory mechanisms, or differences in cellular activity/maturation is not clear. Additionally, regional (microenvironmental) differences in the extent to which a cell can resist ERSR-induced apoptosis have been reported [41]. Thus, it is critical to determine how the various cell types present in and around an MS lesion, at their various stages of activation and maturity, will react to stress.
Unless ER stress can be induced only in specific cell types during human disease, the extent of its effects on other relevant cells must be determined. It is critically important to remember that in the MS lesion are present inflammatory, disease-driving cells whose survival could also be aided by the ERSR, to the patient’s detriment. Therefore, it would be ideal to differentially target cellular subsets in such away that pro-apoptotic ERSR signals are induced in inflammatory cells, while a pro-survival balance is kept in cells such as oligodendrocytes and OPCs.
ERSR and viral infections
It is well established that the ERSR is triggered in various viral infections. This response is largely detrimental to a virus due to the general shut-down of host protein synthesis that occurs, though at least one virus, human cytomegalovirus, has adapted to the fact that it triggers the ERSR and turned it to its advantage [44].
There is a strong possibility that MS itself is triggered by a viral infection, perhaps by virus-specific immune cells long after viral clearance, or perhaps during acute or latent infection [3]. Relapses in symptoms are strongly correlative with viral infections as well, so it is possible that in humans, other triggers of ER stress are occurring in MS patients during relapse [45]. As discussed above, if a cell is already undergoing some level of the ERSR, a further trigger could tip the balance to apoptosis. Conversely, a low level of virus-induced stress present before a more intense stress trigger could actually be protective against cell death [40]. Also, if a virus is already manipulating the ERSR in some way, its activities could have implications for the effects of an ERSR-inducing treatment.
Strategies for eliciting the ERSR during disease
IFN-γ is a pleiotropic cytokine produced by both T cells and natural killer (NK) cells during inflammatory events. It is generally regarded as an inflammatory cytokine that is deleterious during autoimmune diseases such as MS, though there is also considerable evidence that it is required for regulation and dampening of the immune response [46–48]. IFN-γ has also been shown to induce a protective anti-apoptotic effect in oligodendrocytes [49]. Lin et al. showed that this cytokine elicited PERK activation, resulting in oligodendrocyte survival in the EAE model [37]. However, administration of IFN-γ to MS patients results in exacerbation of disease, and it is therefore an impractical treatment [50]. Lin et al. did not observe any effects on the immune system of IFN-γ expression in the CNS prior to EAE in their model, and were able to attribute the protective effects of CNS-specific expression of IFN-γ to the activation of PERK [37]. Therefore, other means of activating the ERSR as a whole, or PERK specifically, should be investigated as potential therapeutic agents. There are multiple points along the ERSR pathway that can be targeted, and any potential agents must first be tested in animal models. As a first step in this direction, it should be determined how various ERSR genes and proteins are altered in EAE.
Identifying future ERSR therapeutic targets
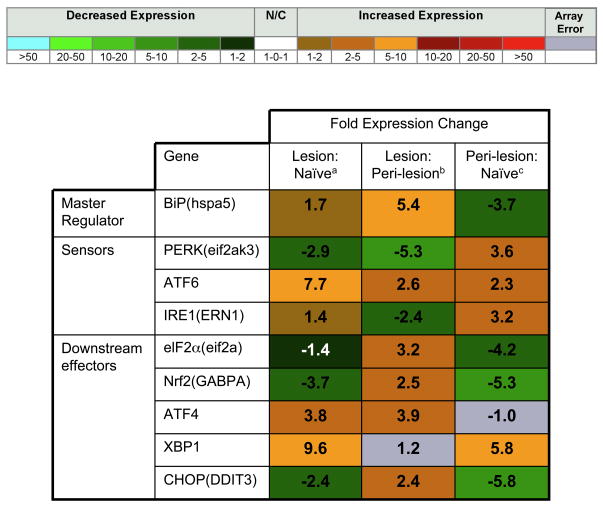
To better understand the role of ERSR during EAE, and in attempt to uncover novel targets, we have recently carried out a gene array analysis of laser dissected regions of the naïve spinal cord, lesions from the spinal cord of mice at the peak of EAE, and peri-lesion regions (adjacent to inflammatory demyelinating lesions) from the same EAE mice. Our results have identified several ERSR-related genes that could be of interest from a therapeutic perspective. Figure 2 shows the fold changes in ERSR genes that were analyzed in the array, while Figure 1 indicates where the protein associated with each gene falls in the ERSR pathway, and whether these genes are up- or down-regulated. We found that ATF6 was highly upregulated in EAE lesions, both in comparison to naïve tissue and peri-lesion tissue, and that XBP1 was highly upregulated in both peri-lesions and lesions to a similar degree, compared to naïve tissue. The PERK arm of the ERSR, however, was generally down-regulated, with the exception of ATF4, which was upregulated in lesions. Since ATF4, ATF6, and XBP1 all induce CHOP, we propose that cells within lesions may be undergoing CHOP-induced apoptosis. Without the important translational, metabolic, and anti-oxidative effects mediated by molecules downstream of PERK and elf2α, many of the beneficial aspects of the ERSR may not be active, and the balance may be tipped drastically toward apoptosis in cells within lesions (Figure 1). However, the actual gene expression of CHOP in lesions, though decreased compared to peri-lesions, was below that in naïve tissue, so further exploration of the presence of CHOP, particularly over time, will be important. If PERK is indeed too low in cells within EAE lesions, then, as found by Lin et al. [37], exogenous sources of PERK activation would be protective by allowing PERK’s downstream targets to counteract ER stress more efficiently within the CNS.
Figure 2. Expression of ERSR Proteins in EAE and Normal Tissue.
Fold expression changes in mRNA of the indicated genes (name in parentheses indicates name of gene as it appeared on the array, if it differs from the commonly used name) in spinal cord lesions from EAE mice compared to naïve spinal cord regionsa, lesions from EAE mice compared to peri-lesion regions from EAE miceb, and peri-lesion regions from EAE mice compared to naïve regionsc. Gray boxes indicate that p<0.01 among triplicate data values for a single sample, and fold change values therefore could not be reliably determined.
The reasons for an increase in some proteins of the ERSR while other are decreased are not clear, but further exploration of this disparity may give us important insights into EAE pathogenesis. In addition, we have recently taken lesions, as well as whole spinal cords from EAE mice, to perform a protein array. The relative amounts of the various proteins within the ERSR pathway will also provide insight into possible therapeutic targets, particularly with regard to the PERK arm of this cellular response. We view these results as a starting point for future experimental work that will be aimed at investigating the effects on disease, as well as different strategies for the induction, of PERK or molecules downstream of PERK. Other proteins in this pathway or others that could be induced or inhibited for therapeutic benefit could also be identified in this manner.
Conclusions & Future Perspective
Manipulation of the ERSR has potential as a therapeutic tool for MS. However, a more complete molecular understanding of the potential effects of using this varied and sensitive response as a therapeutic target is required prior to initiating clinical studies. Will this therapy be reasonably effective in protecting from demyelination/enhancing remyelination – are the appropriate CNS populations affected in the appropriate manner, how will inflammatory cells within the CNS of an MS patient respond to an ERSR trigger, how will a potential virus infection affect the ERSR?
It would be beneficial to be able to induce only certain components of the ERSR, or to induce only a partial ERSR. Treatments that would inhibit CHOP while inducing the protective cellular mechanisms of the ERSR would be ideal. Alternatively, delivery of ERSR-inducing agents to certain cell types would also be beneficial. This may however not be necessary depending on the trigger used and how different cell types respond to a particular trigger.
It is apparent that curative therapies for MS have to both inhibit inflammation, preferably in an antigen-specific manner, as well as stimulate myelin repair [51]. It is important to consider that evidence exists suggesting a certain amount of inflammation, probably in the form of certain inflammatory cytokines such as TNF and IL-1β, may be required for initiation remyelination [34,52,53]. Whatever triggers elicit the ERSR may or may not be able to contribute to the proliferation and maturation of OPCs required for remyelinating activity. Therefore, multifaceted therapies that include specific inhibition of disease-inducing inflammatory cells, along with stimulation of the ERSR for survival of oligodendrocytes and perhaps some endogenous or exogenous inflammatory signal to stimulate remyelination by OPCs, could be required for effective MS therapy.
Executive Summary.
MS and its therapies
Current therapies for MS only downregulate the inflammatory responses acting primarily as immunosuppressants operating via unknown mechanisms
Therapies that are also aimed at helping the CNS resist demyelination and/or encourage remyelination need to be investigated
The ER stress response
This cellular pathway is triggered when proteins build up and/or undergo improper folding and modification within the ER
During this response, translation of most proteins is inhibited, while translation of protein chaperones and degraders, amino acid synthesis, and pro-survival molecules are upregulated in an attempt to recover from stress and properly fold or discard excess proteins
Eventually, if necessary, apoptosis is induced as a result of the ERSR
ERSR in MS lesions
There are many potential triggers of the ERSR during (or prior to) MS
ERSR proteins can be detected in MS lesions, with variations in the levels and types of proteins depending on the type and region of the lesion
Harnessing the ERSR as a potential therapy in MS
The ERSR is protective in a murine model of MS
There are cell type- and region-specific differences in response to ER stress
The ERSR is triggered by microbial infections, which may complicate matters within the context of MS treatment, as symptomatic relapses are often associated with infection
Strategies for eliciting the ERSR during disease must be approached with care, as they could have opposing effects on various cells and/or cellular processes
Identifying future ERSR-related therapeutic targets
We have identified several ERSR genes that are differentially expressed in EAE tissue using a microarray approach
PERK and several of its downstream effectors are particularly downregulated in EAE lesions compared to normal tissue, while molecules that activate CHOP are upregulated
Conclusions
All of the potential effects of using the ERSR in a therapeutic mode must be considered, as each of the cells present within the diseased CNS (including those cells contributing to inflammation and damage as well as those attempting to repair damage) will be affected in some way by the induction of this cellular response
The utilization of responses such as the ERSR, which is a natural mechanism of regulation and repair, would be preferable to more synthetic therapies, but it is important to have realistic and safe strategies for their administration
The ERSR may play multiple roles in diseases such as MS. Understanding these potential roles may be key to better understanding of this complicated disease
Though myelin-specific inflammatory cells clearly need to be suppressed or eliminated to slow or halt the progression of MS, some level of inflammation is likely to be necessary if oligodendrocytes and their progenitors are expected to actively remyelinate demyelinated axons. Therapeutic strategies that take all of these parameters into account could lead to more effective therapies for demyelinating disorders.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Lucchinetti CF, Parisi J, Bruck W. The pathology of multiple sclerosis. Neurol Clin. 2005;23:77–105. vi. doi: 10.1016/j.ncl.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Kantarci OH. Genetics and natural history of multiple sclerosis. Semin Neurol. 2008;28:7–16. doi: 10.1055/s-2007-1019125. [DOI] [PubMed] [Google Scholar]
- 3.Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008;7:268–277. doi: 10.1016/S1474-4422(08)70042-5. [DOI] [PubMed] [Google Scholar]
- 4.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120 (Pt 3):393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 6.Trapp BD, Ransohoff R, Rudick R. Axonal pathology in multiple sclerosis: relationship to neurologic disability. Curr Opin Neurol. 1999;12:295–302. doi: 10.1097/00019052-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Kohm AP, Turley DM, Miller SD. Targeting the TCR: T-cell receptor and peptide-specific tolerance-based strategies for restoring self-tolerance in CNS autoimmune disease. Int Rev Immunol. 2005;24:361–392. doi: 10.1080/08830180500371207. [DOI] [PubMed] [Google Scholar]
- 8.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- 10.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Developmental cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee KP, Dey M, Neculai D, Cao C, Dever TE, Sicheri F. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132:89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J, Rawson RB, Komuro R, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Shen J, Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- 16.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 17.de Haro C, Mendez R, Santoyo J. The eIF-2alpha kinases and the control of protein synthesis. FASEB J. 1996;10:1378–1387. doi: 10.1096/fasebj.10.12.8903508. [DOI] [PubMed] [Google Scholar]
- 18.Zinszner H, Kuroda M, Wang X, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XZ, Lawson B, Brewer JW, et al. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lipson KL, Fonseca SG, Urano F. Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr Mol Med. 2006;6:71–77. doi: 10.2174/156652406775574613. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell. 2001;105:891–902. doi: 10.1016/s0092-8674(01)00407-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxidants & redox signaling. 2007;9:553–561. doi: 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- 23.Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Katayama T, Imaizumi K, Sato N, et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 25.Katayama T, Imaizumi K, Honda A, et al. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer’s disease-linked presenilin-1 mutations. J Biol Chem. 2001;276:43446–43454. doi: 10.1074/jbc.M104096200. [DOI] [PubMed] [Google Scholar]
- 26.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 27.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara K, Oyadomari S, Gotoh T, Kohsaka S, Nakayama H, Mori M. Induction of CHOP and apoptosis by nitric oxide in p53-deficient microglial cells. FEBS Lett. 2001;506:135–139. doi: 10.1016/s0014-5793(01)02898-8. [DOI] [PubMed] [Google Scholar]
- 30.Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mycko MP, Papoian R, Boschert U, Raine CS, Selmaj KW. Microarray gene expression profiling of chronic active and inactive lesions in multiple sclerosis. Clin Neurol Neurosurg. 2004;106:223–229. doi: 10.1016/j.clineuro.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 32*.Mhaille AN, McQuaid S, Windebank A, et al. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2008;67:200–211. doi: 10.1097/NEN.0b013e318165b239. This is the first study investigating the presence of ERSR proteins in tissue from MS patients. [DOI] [PubMed] [Google Scholar]
- 33.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 34*.Chari DM. Remyelination in multiple sclerosis. Int Rev Neurobiol. 2007;79:589–620. doi: 10.1016/S0074-7742(07)79026-8. This comprehensive review gives much insight into the mechanisms of and requirements for remyelination in MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keirstead HS, Blakemore WF. Identification of post-mitotic oligodendrocytes incapable of remyelination within the demyelinated adult spinal cord. J Neuropathol Exp Neurol. 1997;56:1191–1201. doi: 10.1097/00005072-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122 (Pt 12):2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 37**.Lin W, Bailey SL, Ho H, et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. Induced expression of IFN-γ in the CNS inhibited EAE through oligodendrocyte survival. The effect that was mediated via PERK activation. These results were the first to indicate that the induction of the ERSR may be a therapeutic target for MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. Induced expression of IFN-γ in the CNS activated the ERSR, which in turn exacerbated ongoing demyelinating disorders via decreased OPC survival, highlighting the different effects the ERSR can have in different situations and the care that must be taken with potential ERSR-associated therapies. [DOI] [PubMed] [Google Scholar]
- 39.Lees JR, Cross AH. A little stress is good: IFN-gamma, demyelination, and multiple sclerosis. J Clin Invest. 2007;117:297–299. doi: 10.1172/JCI31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278:29317–29326. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- 41*.Williams BL, Lipkin WI. Endoplasmic reticulum stress and neurodegeneration in rats neonatally infected with borna disease virus. J Virol. 2006;80:8613–8626. doi: 10.1128/JVI.00836-06. ER stress has vastly different effects on different CNS cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito A, Hino S, Murakami T, Kondo S, Imaizumi K. A novel ER stress transducer, OASIS, expressed in astrocytes. Antioxidants & redox signaling. 2007;9:563–571. doi: 10.1089/ars.2006.1520. [DOI] [PubMed] [Google Scholar]
- 43.Kondo S, Saito A, Hino S, et al. BBF2H7, a novel transmembrane bZIP transcription factor, is a new type of endoplasmic reticulum stress transducer. Mol Cell Biol. 2007;27:1716–1729. doi: 10.1128/MCB.01552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Isler JA, Skalet AH, Alwine JC. Human cytomegalovirus infection activates and regulates the unfolded protein response. J Virol. 2005;79:6890–6899. doi: 10.1128/JVI.79.11.6890-6899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67:652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- 46.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 47.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 48.Gao X, Gillig TA, Ye P, D’Ercole AJ, Matsushima GK, Popko B. Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci. 2000;16:338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- 49.Balabanov R, Strand K, Goswami R, et al. Interferon-gamma-oligodendrocyte interactions in the regulation of experimental autoimmune encephalomyelitis. J Neurosci. 2007;27:2013–2024. doi: 10.1523/JNEUROSCI.4689-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 51**.Frederick TL, Miller SD. The future of MS therapy - combining antigen-specific immunotherapy with myelin repair strategies. Future Neurology. 2006;1:489–503. This review highlights the recent literature with regard to the need for integrative therapies for MS that involve both down-regulation of inflammation and repair of the damaged CNS. [Google Scholar]
- 52.Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 53.Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]